Prevalence of Osteoporosis and Vitamin D Levels in Patients Undergoing Total Hip Arthroplasty: Insights from a Single-Center Experience in Italy
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, P.L.; Hsu, C.J.; Ma, Y.G.; Liu, D.; Peng, R.; Xu, X.H.; Lu, H.D. Prevalence and treatment rate of osteoporosis in patients undergoing total knee and hip arthroplasty: A systematic review and meta-analysis. Arch. Osteoporos. 2022, 17, 16. [Google Scholar] [CrossRef]
- Krenzlin, H.; Schmidt, L.; Jankovic, D.; Schulze, C.; Brockmann, M.A.; Ringel, F.; Keric, N. Impact of Sarcopenia and Bone Mineral Density on Implant Failure after Dorsal Instrumentation in Patients with Osteoporotic Vertebral Fractures. Medicina 2022, 58, 748. [Google Scholar] [CrossRef] [PubMed]
- Aro, H.T.; Alm, J.J.; Moritz, N.; Mäkinen, T.J.; Lankinen, P. Low BMD affects initial stability and delays stem osseointegration in cementless total hip arthroplasty in women: A 2-year RSA study of 39 patients. Acta Orthop. 2012, 83, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.G.; Weber, P.; Steinbrück, A.; Hua, X.; Jansson, V.; Schmidutz, F. Periprosthetic bone remodelling of short-stem total hip arthroplasty: A systematic review. Int. Orthop. 2018, 42, 2077–2086. [Google Scholar] [CrossRef]
- Bernatz, J.T.; Brooks, A.E.; Squire, M.W.; Illgen, R.I., 2nd; Binkley, N.C.; Anderson, P.A. Osteoporosis Is Common and Undertreated Prior to Total Joint Arthroplasty. J. Arthroplast. 2019, 34, 1347–1353. [Google Scholar] [CrossRef]
- Delsmann, M.M.; Strahl, A.; Mühlenfeld, M.; Jandl, N.M.; Beil, F.T.; Ries, C.; Rolvien, T. High prevalence and undertreatment of osteoporosis in elderly patients undergoing total hip arthroplasty. Osteoporos. Int. 2021, 32, 1661–1668. [Google Scholar] [CrossRef]
- Yu, J.S.; Krishna, N.G.; Fox, M.G.; Blankenbaker, D.G.; Frick, M.A.; Jawetz, S.T.; Li, G.; Reitman, C.; Said, N.; Stensby, J.D.; et al. ACR Appropriateness Criteria® Osteoporosis and Bone Mineral Density: 2022 Update. J. Am. Coll. Radiol. 2022, 19, S417–S432. [Google Scholar] [CrossRef]
- Glowacki, J.; Hurwitz, S.; Thornhill, T.S.; Kelly, M.; LeBoff, M.S. Osteoporosis and vitamin-D deficiency among postmenopausal women with osteoarthritis undergoing total hip arthroplasty. J. Bone Jt. Surg. 2003, 85, 2371–2377. [Google Scholar] [CrossRef]
- Mäkinen, T.J.; Alm, J.J.; Laine, H.; Svedström, E.; Aro, H.T. The incidence of osteopenia and osteoporosis in women with hip osteoarthritis scheduled for cementless total joint replacement. Bone 2007, 40, 1041–1047. [Google Scholar] [CrossRef]
- Watanabe, N.; Miyatake, K.; Takada, R.; Ogawa, T.; Amano, Y.; Jinno, T.; Koga, H.; Yoshii, T.; Okawa, A. The prevalence and treatment of osteoporosis in patients undergoing total hip arthroplasty and the levels of biochemical markers of bone turnover. Bone Jt. Res. 2022, 11, 873–880. [Google Scholar] [CrossRef]
- Garnero, P. Biomarkers for osteoporosis management: Utility in diagnosis, fracture risk prediction and therapy monitoring. Mol. Diagn. Ther. 2008, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Ma, J.; Feng, K.; Liu, Z.; Chen, L.; Jia, H.; Ma, X. Reference markers of bone turnover for prediction of fracture: A meta-analysis. J. Orthop. Surg. Res. 2019, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ma, Y.; Chen, D.; Li, M.; Li, Z.; Deng, Z.; Zheng, Q.; Fu, G. Bone turnover biomarkers predict one-year all-cause mortality and walking ability in geriatric hip fracture patients. Bone 2023, 177, 116922. [Google Scholar] [CrossRef]
- Okpara, C.; Negm, A.; Adachi, J.D.; Armstrong, D.; Atkinson, S.; Avram, V.; de Beer, J.; Hladysh, G.; Ioannidis, G.; Kennedy, C.; et al. Getting fit for hip and knee replacement: The Fit-Joints multimodal intervention for frail patients with osteoarthritis—A pilot randomized controlled trial. J. Frailty Aging 2025, 14, 100028. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1994; Volume 843, pp. 1–129. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Lingard, E.A.; Mitchell, S.Y.; Francis, R.M.; Rawlings, D.; Peaston, R.; Birrell, F.N.; McCaskie, A.W. The prevalence of osteoporosis in patients with severe hip and knee osteoarthritis awaiting joint arthroplasty. Age Ageing 2010, 39, 234–239. [Google Scholar] [CrossRef]
- Dequeker, J.; Aerssens, J.; Luyten, F.P. Osteoarthritis and osteoporosis: Clinical and research evidence of inverse relationship. Aging Clin. Exp. Res. 2003, 15, 426–439. [Google Scholar] [CrossRef]
- Paranhos-Neto, F.P.; Vieira Neto, L.; Madeira, M.; Moraes, A.B.; Mendonça, L.M.C.; Lima, I.C.B.; Chagas, C.L.R.; Lira, D.A.; Spitz, J.F.; Guimarães, J.A.M.; et al. Vitamin D deficiency is associated with cortical bone loss and fractures in the elderly. Eur. J. Endocrinol. 2019, 181, 509–517. [Google Scholar] [CrossRef]
- Segheto, K.J.; Pereira, M.; Silva, D.C.G.D.; Carvalho, C.J.D.; Massardi, F.R.; Kakehasi, A.M.; Juvanhol, L.L.; Longo, G.Z. Vitamin D and bone health in adults: A systematic review and meta-analysis. Ciência Saúde Coletiva 2021, 26, 3221–3244. [Google Scholar] [CrossRef]
- Bruyère, O.; Cavalier, E.; Reginster, J.Y. Vitamin D and osteosarcopenia: An update from epidemiological studies. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Minisola, S.; Cianferotti, L.; Biondi, P.; Cipriani, C.; Fossi, C.; Franceschelli, F.; Giusti, F.; Leoncini, G.; Pepe, J.; Bischoff-Ferrari, H.A.; et al. Correction of vitamin D status by calcidiol: Pharmacokinetic profile, safety, and biochemical effects on bone and mineral metabolism of daily and weekly dosage regimens. Osteoporos. Int. 2017, 28, 3239–3249. [Google Scholar] [CrossRef]
- Sitta, M.C.; Cassis, S.V.; Horie, N.C.; Moyses, R.M.; Jorgetti, V.; Garcez-Leme, L.E. Osteomalacia and vitamin D deficiency in the elderly. Clinics 2009, 64, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Birinci, M.; Hakyemez, Ö.S.; Geçkalan, M.A.; Mutlu, M.; Yildiz, F.; Bilgen, Ö.F.; Azboy, İ. Effect of Vitamin D Deficiency on Periprosthetic Joint Infection and Complications After Primary Total Joint Arthroplasty. J. Arthroplast. 2024, 39, S151–S157. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, G.; Lepetsos, P.; Perrea, D.N.; Iliopoulos, D.C.; Nikolaou, V.S. Possible Roles of Vitamin D in Bone Grafting. Cureus 2021, 13, e14688. [Google Scholar] [CrossRef]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Kelly, J.; Lin, A.; Wang, C.J.; Park, S.; Nishimura, I. Vitamin D and bone physiology: Demonstration of vitamin D deficiency in an implant osseointegration rat model. J. Prosthodont. 2009, 18, 473–478. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef]
- Mei, Z.; Hu, H.; Zou, Y.; Li, D. The role of vitamin D in menopausal women’s health. Front. Physiol. 2023, 14, 1211896. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Chen, C.; Wang, Q.; Yang, C.; Qiu, J.; Li, J.; Liu, X.; Zhang, Y.; Liu, L.; et al. Interaction of estradiol and vitamin D with low skeletal muscle mass among middle-aged and elderly women. BMC Women’s Health 2023, 23, 491. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yuan, H.; Ma, G.; Cao, H. Bone-muscle crosstalk under physiological and pathological conditions. Cell. Mol. Life Sci. 2024, 81, 310. [Google Scholar] [CrossRef]
- Karsenty, G.; Mera, P. Molecular bases of the crosstalk between bone and muscle. Bone 2018, 115, 43–49. [Google Scholar] [CrossRef]
- Lim, J.; Onozawa, M.; Saad, M.; Ong, T.A.; Malek, R.; Akaza, H.; A-CaP (Asian Prostate Cancer) Study; J-CaP (Japan Prostate Cancer Study Group); M-CaP (Malaysia Prostate Cancer Study Group). Recent trend of androgen deprivation therapy in newly diagnosed prostate cancer patients: Comparing between high- and middle-income Asian countries. Cancer Sci. 2021, 112, 2071–2080. [Google Scholar] [CrossRef]
- Fink, A.; Puchwein, P.; Fahrleitner-Pammer, A.; Eder-Halbedl, M.; Bernhardt, G.A. Increased Early Postoperative Complication Rate after Osteoporotic Hip Fracture in Patients with Low 25 (OH) Vitamin D Levels. Nutrients 2024, 16, 1917. [Google Scholar] [CrossRef]
| Variables | Mean | Standard Deviation |
|---|---|---|
| Age (years) | 67.55 | 9.07 |
| Gender (M/F) | 35 M, 31 F | - |
| Hip Femoral Neck BMD (T-score) | −0.69 | 1.23 |
| Total Femur BMD (T-score) | −0.32 | 1.34 |
| Lumbar Spine BMD (T-score) | 0.18 | 1.76 |
| Vitamin D (ng/mL) | 19.33 | 9.76 |
| Parathyroid Hormone (PTH, pg/mL) | 84.42 | 39.38 |
| Pre-op Harris Hip Score (HHS) | 43.15 | 17.49 |
| Handgrip Strength (kg) | 22.59 | 10.94 |
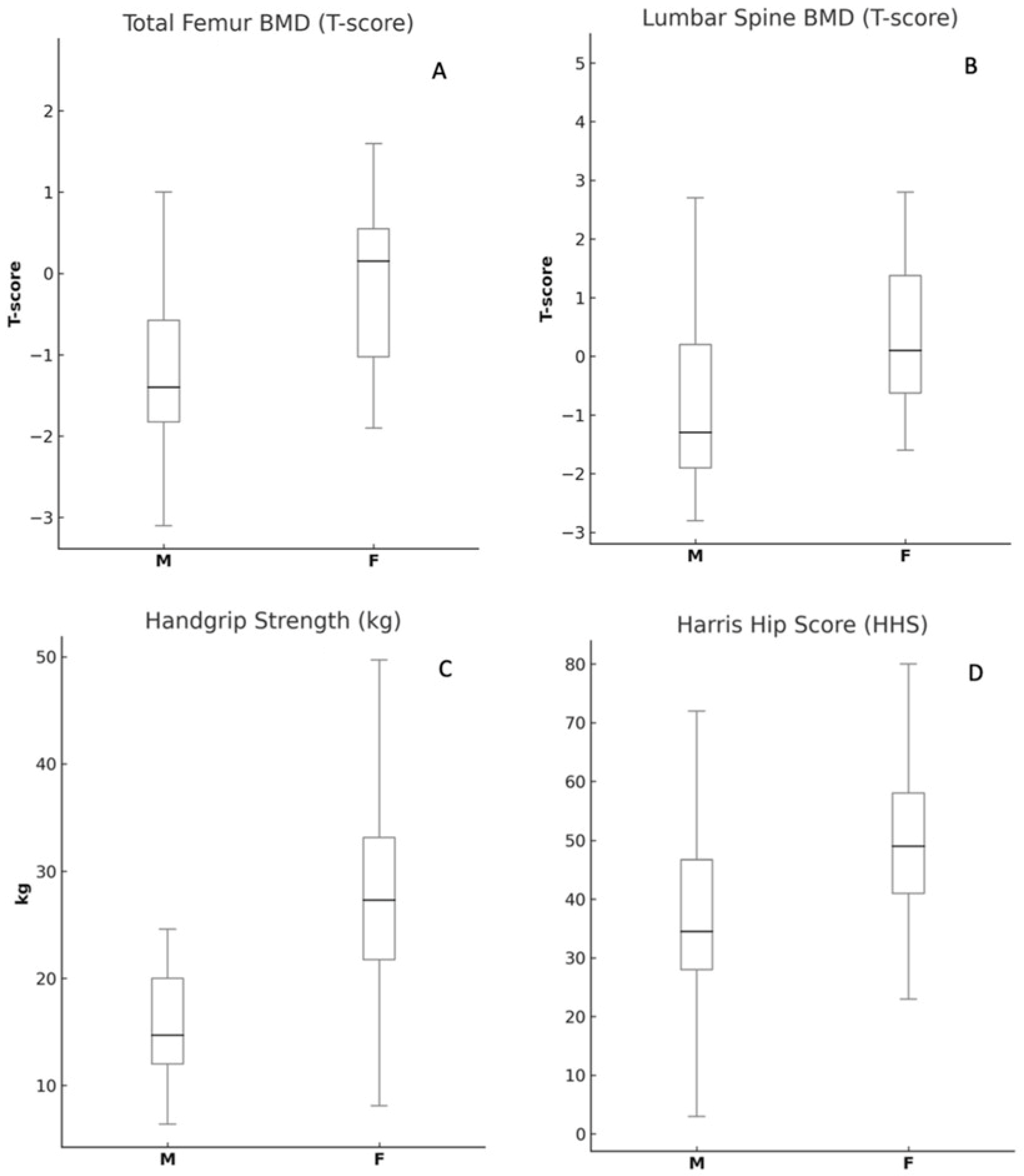
| Variables | Female (Mean ± SD) | Male (Mean ± SD) | p-Value |
|---|---|---|---|
| Age (years) | 69.0 ± 9.27 | 64.08 ± 9.15 | 0.07 |
| Femoral Neck BMD (T-score) | −0.99 ± 1.29 | −0.55 ± 1.28 | 0.26 |
| Total Femur BMD (T-score) | −0.98 ± 1.42 | −0.08 ± 1.04 | <0.05 * |
| Lumbar Spine BMD (T-score) | −0.66 ± 1.74 | 0.67 ± 1.59 | <0.05 * |
| Vitamin D (ng/mL) | 19.81 ± 9.03 | 18.91 ± 9.97 | 0.75 |
| Parathyroid Hormone (PTH, pg/mL) | 95.22 ± 47.03 | 78.26 ± 31.22 | 0.11 |
| Harris Hip Score (HHS) | 36.65 ± 19.41 | 46.16 ± 17.01 | 0.08 |
| Handgrip Strength (kg) | 17.05 ± 8.15 | 27.31 ± 10.34 | <0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smakaj, A.; Iundusi, R.; Chiavoghilefu, A.; Cardelli, T.; Rossi, D.; Raso, C.; Tarantino, U.; Gasbarra, E. Prevalence of Osteoporosis and Vitamin D Levels in Patients Undergoing Total Hip Arthroplasty: Insights from a Single-Center Experience in Italy. Prosthesis 2025, 7, 73. https://doi.org/10.3390/prosthesis7040073
Smakaj A, Iundusi R, Chiavoghilefu A, Cardelli T, Rossi D, Raso C, Tarantino U, Gasbarra E. Prevalence of Osteoporosis and Vitamin D Levels in Patients Undergoing Total Hip Arthroplasty: Insights from a Single-Center Experience in Italy. Prosthesis. 2025; 7(4):73. https://doi.org/10.3390/prosthesis7040073
Chicago/Turabian StyleSmakaj, Amarildo, Riccardo Iundusi, Angela Chiavoghilefu, Tommaso Cardelli, Danilo Rossi, Claudio Raso, Umberto Tarantino, and Elena Gasbarra. 2025. "Prevalence of Osteoporosis and Vitamin D Levels in Patients Undergoing Total Hip Arthroplasty: Insights from a Single-Center Experience in Italy" Prosthesis 7, no. 4: 73. https://doi.org/10.3390/prosthesis7040073
APA StyleSmakaj, A., Iundusi, R., Chiavoghilefu, A., Cardelli, T., Rossi, D., Raso, C., Tarantino, U., & Gasbarra, E. (2025). Prevalence of Osteoporosis and Vitamin D Levels in Patients Undergoing Total Hip Arthroplasty: Insights from a Single-Center Experience in Italy. Prosthesis, 7(4), 73. https://doi.org/10.3390/prosthesis7040073







