Abstract
Background/Objectives: Bovine pericardial valve conduits (PVCs) are commonly used for right ventricular outflow tract reconstruction in both pediatric and adult patients. Calcification, particularly prevalent in children and young adults, is a leading cause of conduit failure and is affected by the chemical composition of the treated biomaterials. In this study, we aimed to compare the structural changes in diepoxy-treated (DE-PVCs) and glutaraldehyde-treated PVCs (GA-PVCs) and to identify factors contributing to tissue mineralization in a large animal model. Methods: Pulmonary artery replacement was performed in minipigs (33–88 kg) using twelve DE-PVCs and four GA-PVCs. After six months, the animals were euthanized, and the explanted PVCs underwent macroscopic and microscopic examination. Results: Large calcium deposits formed along conduit joining suture (CJS) lines in all PVCs, regardless of the cross-linking agent. Mineral clusters surrounded the multifilament braided thread, and its fibers were encrusted with hydroxyapatite crystals. In DE-PVCs, no mineralization occurred outside the suture lines, and they showed successful integration and graft vitalization with a uniform neointima and well-developed endothelial monolayer. GA-PVCs developed a rigid external capsule, foci of collagen fiber calcification within the walls, and neointimal hyperplasia with limited endothelial coverage. Conclusions: In PVCs, calcification predominantly occurs along the CJS lines, where the multifilament suture acts as a nucleation site for hydroxyapatite crystals. DE treatment prevents collagen mineralization, unlike GA, and offers better integration, reduced neointimal hyperplasia, and a well-developed endothelial layer. These findings suggest that DE-PVCs may be a superior option for pediatric cardiac surgery by reducing calcification and improving conduit durability. Overall, the results will help optimize PVC manufacturing strategies to lower the risk of conduit failure.
1. Introduction
In pediatric cardiac surgery, right ventricular outflow tract (RVOT) reconstruction represents the most frequently performed valve replacement procedure [1]. Various types of valve conduits are used for RVOT reconstruction and pulmonary artery (PA) replacement. Pulmonary allografts remain the preferred conduits for RVOT reconstruction and are recommended for use in both children and adults (for example, in the Ross procedure or for pulmonary artery replacement in grown-up patients with congenital heart disease) [2,3,4]. Nevertheless, the use of pulmonary artery allografts—especially those of small diameter—is limited due to their restricted availability, which results from various economic and legal factors. In particular, until recently, allograft banks were not available in Russia [5]. As a result, the acute shortage of small-sized pulmonary allografts has led to the widespread use of conduits made from xenogenic biomaterials in children [6]. Bovine pericardium has been used in heart valve prostheses since the 1970s [7]. Bovine pericardial valve conduits (PVC) and prostheses have become widely used in cardiac surgery [8]. Chemically treated bovine pericardium has several benefits, such as availability, low cost, the ability to manufacture a prosthesis of different sizes, and acceptable physical characteristics.
Although children eventually outgrow their bioprosthetic heart transplants due to the grafts’ inability to grow, structural valve deterioration often leads to conduit dysfunction much earlier [3,9,10]. Calcification remains a primary failure mechanism in bovine pericardial valve conduits. Alongside mechanical factors, immune responses, and patient metabolic characteristics, the chemical composition of treated biomaterials is a key determinant influencing calcification [9,11,12,13,14]. Glutaraldehyde (GA) is the most commonly used preservation agent for xenogeneic tissues [8,15]. It efficiently cross-links collagen and conceals xenoantigens, improving tissue stability and reducing immune responses [6,14]. During tissue processing, GA reacts with primary amine groups in lysine, hydroxylysine, and N-terminal amino acid residues of collagen, forming pyridinium cross-links via Schiff base formation [8]. However, glutaraldehyde cross-linking of tissue proteins disrupts the charge balance and promotes the binding of inorganic phosphate from the surrounding environment. This leads to the formation of negatively charged complexes that attract calcium ions and facilitate crystal nucleation [16,17]. Another potential issue with GA-processing is the presence of free aldehyde residues in treated tissues or their release from polymeric cross-links [15,16,18]. Both GA released from prosthetic tissues post-implantation and aldehydes covalently bound to proteins can exert cytotoxic effects on host cells and provoke an immune response to the prosthetic tissue [19,20,21]. Ongoing concerns regarding the negative effects of glutaraldehyde are driving efforts to develop improved strategies for treating xenogeneic tissue.
Next-generation protocols for chemical treatment incorporate ethylene glycol diglycidyl ether (diepoxide, DE) [22]. We have previously demonstrated that substituting the preservative agent with DE significantly reduces the calcification rate in various biomaterials [23,24]. The most pronounced anti-calcification effect was observed in bovine pericardium treated with diepoxide. In a subcutaneous rat implantation study, DE-treated bovine pericardium exhibited no calcium accumulation even 90 days post-implantation [17]. However, in clinical settings, we observe dysfunction of DE-treated bioprosthetic heart valves due to calcification [9,22]. In both children and adults, mineralization of the walls and leaflets of the DE-treated PVC leads to stenosis at the graft valve level and ultimately to its failure [25,26]. Despite encouraging experimental results, DE-treated PVC is not fully protected against tissue mineralization, and the underlying mechanisms of this process remain unclear. This is a significant concern in pediatric patients, as children have a greater predisposition to mineralization due to a more reactive immune system and higher calcium metabolism compared to adults [27,28].
This work focused on determining the structural changes in DE-treated PVC compared to GA-treated PVC and to identify the factors that contribute to tissue mineralization in a porcine model over a six-month period.
2. Materials and Methods
2.1. Study Design
All experimental procedures were conducted in compliance with EU Directive 2010/63/EU for animal experiments and received approval from the Ethics Committee of the E. Meshalkin National Medical Research Center.
The study included 16 healthy minipigs (Institute of Cytology and Genetics, Novosibirsk, Russia), with body weights ranging from 33 to 88 kg (Table 1).

Table 1.
Pre- and intraoperative characteristics across groups.
The minipigs were randomly assigned to two groups in sequential order using a random number generator (Figure 1). In all animals, the main PA was grafted with PVC under cardiopulmonary bypass (CPB). The study group (n = 12) underwent implantation of diepoxy-treated bovine pericardial valved conduits (DE-PVC), while the control group (n = 4) received glutaraldehyde-treated bovine pericardial valved conduits (GA-PVC). The size of the control group was limited in accordance with ethical guidelines for laboratory animal care and 3R principles of experimental biomedical research [29]. Bioprosthetic heart valves made from GA-treated pericardium have a long history of use in cardiac surgery and are generally well-studied. The follow-up period was 6 months. All animals underwent transesophageal echocardiography (TEE) three times—intraoperatively and at 3 and 6 months after implantation. Upon completion of the six-month follow-up, the animals were euthanized, and all implanted conduits were explanted for comprehensive macroscopic and microscopic examination. The primary outcome was the localization and extent of calcification. Secondary outcomes included graft integration, neointimal thickness and area of coverage, and detection of an endothelial layer.
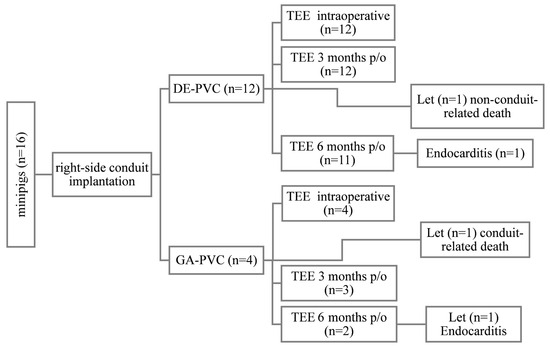
Figure 1.
Study design. DE-PVC, diepoxy-treated bovine pericardial valved conduit; GA-PVC, glutaraldehyde-treated bovine pericardial valved conduit; let, lethal outcome; p/o, postoperative; TEE, transesophageal echocardiography.
2.2. Bovine Pericardial Valved Conduits
Commercial reactive solutions were utilized as cross-linking agents following the manufacturer’s guidelines: 97% DE (N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, SB RAS, Novosibirsk, Russian Federation) and 25% GA (catalog No. 253857, Panreac Quimica SLU, Barcelona, Spain).
Fresh bovine pericardium was collected from healthy animals immediately after slaughter, cleaned of surrounding tissues, and rinsed multiple times with 0.9% NaCl (Solopharm, St. Petersburg, Russia). Preservation was performed using two methods:
- (1)
- For 14 days at room temperature in a 5% buffered DE solution (0.05 M phosphate buffer, pH 7.4), with the solution changed once on day 2;
- (2)
- For 21 days at room temperature in a 0.625% buffered GA solution (0.05 M phosphate buffer, pH 7.4), with the solution replaced on days 2 and 7.
Following conservation, the material was carefully selected for conduit fabrication. A thickness “topographic map” (Figure 2A,B) was created for each bovine pericardium flap using a MELAZ-Cardio laser device (Lazerus LLC, Novosibirsk, Russia). Conduit components were precisely cut from areas with specific thicknesses: 0.3–0.35 mm for leaflets and 0.7–1.0 mm for the graft wall (Figure 2C). These parts were joined with continuous wrap sutures using polyester braided Ethibond 5/0 thread (Ethicon Inc., Somerville, NJ, USA)—referred to as conduit joining sutures (CJS) (Figure 2D–F). Completed conduits were sealed in individual hermetic packages and stored in a proprietary biocidal solution [30].
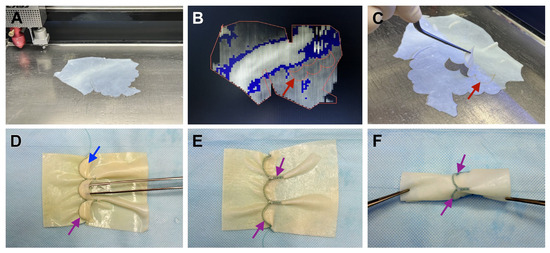
Figure 2.
PVC fabrication process: (A)—preparation of treated bovine pericardium for cutting; (B)—“topographic map” of the flap thicknesses, laser marking of graft components at the required thickness; (C)—cut conduit components; (D)—inner side of the conduit with leaflets; (E)—outer side of the conduit, with sutures brought out; (F)—final view of the completed conduit. The red arrow indicates to the graft component, the purple arrow—to formed conduit joining sutures, the blue arrow—to the leaflet.
2.3. Conduit Implantation
Preoperative preparation of the animal, anesthetic management, CPB, and the surgical technique for conduit implantation were conducted according to the previously described method [23].
The size of the PVC was chosen based on the animal’s weight and the diameter of its native PA trunk. Prior to implantation, the conduit was thoroughly rinsed with sterile 0.9% NaCl, with the solution being changed three times at 20 min intervals. The conduits were implanted on a beating heart with the support of normothermic, full-flow CPB. Both the proximal and distal anastomoses were performed using continuous sutures with monofilament thread 6/0 (PremiCron, B. Braun, Melsungen, Germany) (Figure 3). In all cases, the conduit underwent double sterility checks to ensure its cleanliness and safety for use.
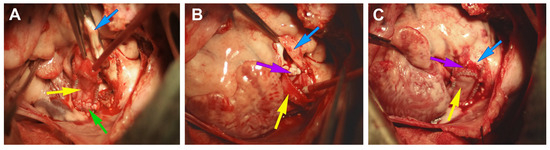
Figure 3.
Implantation of PVC in the pulmonary position in the experiment. (A)—creation of the distal anastomosis between the PA and PVC; (B)—formation of the proximal anastomosis; (C)—general view of the implanted conduit. The yellow arrow refers to the PVC, the blue arrow to the PA, the purple arrow to the formed proximal anastomosis, and the green arrow to the distal anastomosis.
2.4. Postoperative Treatment
For the first seven postoperative days, the minipigs received nadroparin calcium at a dose of 0.3 mL twice daily. Antiplatelet therapy with clopidogrel (75 mg/day) and acetylsalicylic acid (75 mg/day) was then initiated and continued throughout the subsequent follow-up. For antibiotic therapy, amoxicillin clavulanate (8.75 mg/kg; Synulox, Haupt Pharma Latina S.r.L., Borgo San Michele, Italy) was administered for seven days. After six months of follow-up, the animals were first sedated with Zoletil-100 (5–7 mg/kg; Virbac Sante Animale, Carros, France), then euthanized with a super-therapeutic dose of sodium thiopental.
2.5. TEE
To evaluate hemodynamic parameters, TEE (Phillips, Bothell, DC, USA) was performed intraoperatively (at 0 months) and at 3 and 6 months following conduit implantation. Prior to each assessment, the animals were weighed and sedated using Zoletil-100 (5–7 mg/kg). Proximal and distal pressure gradients were derived from flow velocity measurements. Valve insufficiency was graded on a scale from 0 to 3: 0 indicating none or trivial regurgitation, 1 for minor regurgitation, 2 for moderate regurgitation, and 3 for severe regurgitation.
2.6. Macro- and Microscopic Evaluation of Explanted Conduits
Following euthanasia, PVCs were surgically excised along with adjacent native PA tissues. Macroscopic examination of conduit walls, leaflets, and surrounding tissues was performed to evaluate structural abnormalities, including thrombosis, calcification, deformation, stenosis, and aneurysms. All explanted conduits were preserved in 10% neutral buffered formalin. For histological analysis, longitudinal tissue samples of the conduits were cut along their entire length, including the leaflet and both anastomotic sites. The tissues were then dehydrated and embedded in paraffin using an automated laboratory tissue processor (MT Point, St. Petersburg, Russia). For histological analysis, 6 µm tissue sections were stained using hematoxylin and eosin (H&E; Biovitrum, St. Petersburg, Russia), Von Kossa (Biovitrum, St. Petersburg, Russia), and Russell–Movat pentachrome (Diapath, Martinengo, Italy) staining kits, following the manufacturers’ protocols.
2.7. Statistical Analysis
Data analysis was conducted using the STATA software package (version 13.0; StataCorp LP, College Station, TX, USA). Continuous variables are presented as median (Me) and interquartile range (IQR), while categorical variables are expressed as counts and percentages. Due to the small sample size, we used non-parametric statistical methods. Comparative analyses between groups were performed using the Mann–Whitney U test and Fisher’s exact test. The changes in the indicator over the observation period were evaluated using Friedman’s ANOVA. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Postoperative Period
The implantation of all PVCs was completed successfully, with no intraoperative complications observed. All minipigs were extubated within 1.5 to 3 h after surgery. Following weaning from CPB, only three animals required inotropic support (Table 1).
During the follow-up period, three animals died: one in the DE-PVC group and two in the GA-PVC group. In one case from the DE-PVC group, a non-conduit-related death occurred 2.5 months after implantation. One animal in the GA-PVC group was withdrawn from the experiment after 4.8 months due to progressive heart failure associated with prosthesis dysfunction. Another animal in the GA-PVC group was diagnosed with prosthetic infective endocarditis and was withdrawn from the experiment 5.6 months post-implantation (Figure 1). All remaining minipigs survived to the end of the 6-month follow-up period without developing any prosthesis-related complications. During this time, the animals showed significant weight gain, with a median increase of 16.5 kg (IQR 7.5–22 kg), p < 0.001.
3.2. TEE Findings
During the follow-up, as the animals grew, there was an observed increase in the pressure gradient in both the proximal and distal regions of the conduit (Table 2).

Table 2.
Echocardiographic findings at follow-up for the entire cohort.
Among the 13 minipigs that completed the follow-up period, 6 (46.2%) exhibited an increase in the peak pressure gradient across the PVC to more than 50 mmHg, with a corresponding mean gradient exceeding 30 mmHg (Figure 4); in the DE-PVC group, four animals (36.4%); in the GA-PVC group, two animals (100%). Among the seven remaining animals in the DE-PVC group that completed the experiment, the peak/mean pressure gradients in the proximal region were 12 mmHg (IQR 10–28 mmHg)/7 mmHg (IQR 6.5–17 mmHg). In the distal region, these values were 32 mmHg (IQR 25–37 mmHg) and 21 mmHg (IQR 14–23 mmHg), respectively. No thrombosis or significant valve insufficiency was detected (Figure 5).
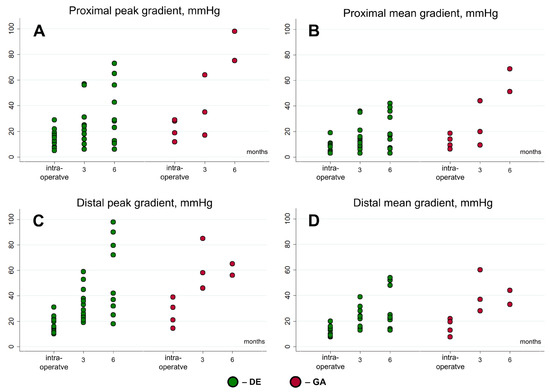
Figure 4.
Peak and mean gradients at proximal and distal areas of conduits at follow-up. GA, glutaraldehyde-treated bovine pericardial conduit; DE, diepoxy-treated bovine pericardial conduit.
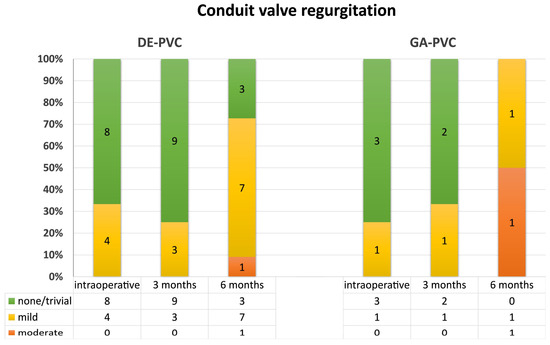
Figure 5.
Conduit valve regurgitation at follow-up. GA-PVC, glutaraldehyde-treated bovine pericardial conduit; DE-PVC, diepoxy-treated bovine pericardial conduit.
3.3. Macroscopic Evaluation of Explanted Conduits
All DE-PVCs remained free from deformation and kinking and were well integrated into the native pulmonary artery, despite exhibiting moderate wall stiffness. The inner surfaces of the grafts were clean and smooth, with no signs of calcification or thrombi (Figure 6A,B). In the majority of cases (n = 7; 60.0%), a relatively thin layer of neointima formed on the inner wall surface. We also observed neointimal overgrowth involving the leaflets on both the inflow and outflow surfaces (Figure 6A). The leaflets remained mobile and free from deformations, fenestrations, ruptures or large foci of calcification. In two DE-PVCs, intimal hyperplasia was observed along the distal anastomosis, particularly at the lesser curvature, resulting in an increased gradient within the conduits. In one case, a significant mismatch between the prosthesis and the animal was noted, due to the minipig’s active growth during the follow-up period. In another animal, prosthetic infective endocarditis was identified at autopsy.
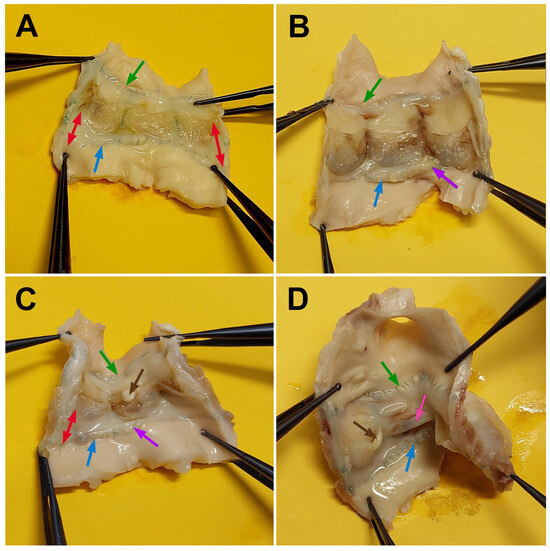
Figure 6.
Macrophotographs of explanted conduits after a 6-month follow-up period. (A,B)—DE-PVC; (C,D)—GA-PVC. The double red arrow indicates the extension of neointima from the conduit wall to the leaflets. The green arrow points to the distal anastomosis, the blue arrow to the proximal anastomosis, the purple arrow to hyperplastic neointima along the proximal anastomosis line, the brown arrow to calcium deposits, and the pink arrow to leaflet adhesion.
Compared to DE-PVC, GA-PVCs exhibited a stiffer wall and extensive calcium deposits within the wall and on some leaflets. The luminal surface of the conduits was covered by an irregular neointima involving the leaflets (Figure 6C). The greatest neointimal thickening was observed at both the proximal and distal anastomoses, causing an elevated gradient within the graft during the follow-up. The leaflets were thickened, and calcium deposits were present. Both of these factors contributed to an increased gradient in the proximal area. In one case, complete adhesion of a cusp to the conduit wall was observed (Figure 6D). One animal, which died during the follow-up period from progressive heart failure, had conduit leaflets covered with hyperplastic neointima. This resulted in limited mobility and fixation of the cusps in a semi-open position.
In the two cases of infective endocarditis, the infection was strictly localized to the conduit itself, remaining confined within the boundaries of the proximal and distal anastomoses.
3.4. Histological Analysis of Explanted Conduits
The DE-PVC wall and leaflets retained their correct fiber orientation (Figure 7C), despite some localized collagen fragmentation. In all cases of DE-PVC implantation, the biomaterial integrated successfully. The outer surface of the graft was enveloped by dense connective tissue, which formed fibrous strands that penetrated deeply into the conduit wall. Neither deforming fibrosis of the graft wall nor rigid external capsules were observed. In the majority of explanted DE-PVCs (66.7%), migration of myofibroblastic cells into the graft wall (Figure 7C) was noted, along with areas of neoangiogenesis originating from the developing neoadventitia. In the walls of some conduits, small isolated foci of inflammatory cell infiltration were observed, consisting of lymphocytes and neutrophils. However, no foreign body giant cells were detected in any of the animals. The inner surface of the DE-PVC was lined with a relatively uniform neointimal layer, spanning from the anastomoses to both surfaces of the conduit leaflets. Typically, the neointimal thickness on the DE-PVC wall ranged from ~220–410 µm, and on the leaflets from ~120–300 µm (Table 3). In all cases, fibroblasts were evenly distributed throughout the neointimal layer. A well-developed endothelial layer completely covered the conduit wall, including the cusps (Figure 7A,E). The graft sinuses were devoid of thrombi.
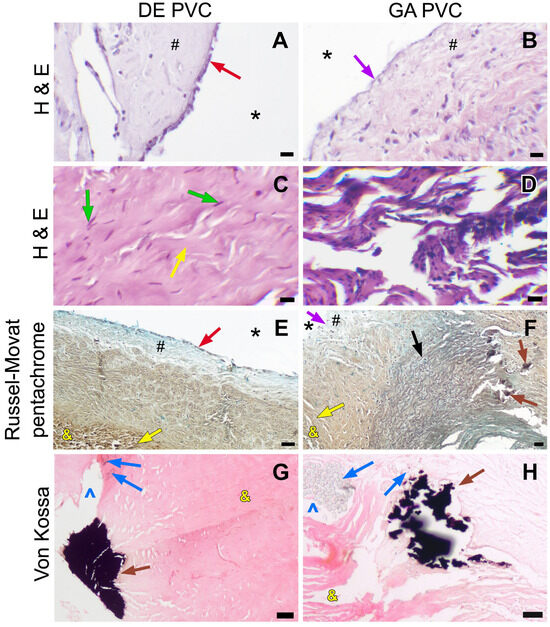
Figure 7.
Light microscopy images of explanted conduits after a 6-month follow-up period. (A,B)—H&E staining, the conduit luminal surface, ×400 magnification, 10 µm bar; (C,D)—H&E staining, the conduit wall structure, ×400 magnification, 10 µm bar; (E,F)—Russell–Movat pentachrome staining, overview images, ×40 magnification, 50 µm bar; (G,H)—Von Kossa staining, conduit calcification, ×100 magnification, 50 µm bar. Red arrow indicates the endothelial monolayer, purple arrow—absence of endothelium, green arrow—migrating fibroblasts, yellow arrow—collagen fibers, brown arrow—calcification focus, black arrow—native pulmonary artery, blue arrow—multifilament thread, #—neointima, *—conduit lumen, &—conduit tissue, ^—suture hole.

Table 3.
Description of the neointimal layer and endothelialization of explanted conduits (except for prosthetic infective endocarditis).
In three cases, neointimal hyperplasia was observed at both the proximal and distal anastomoses, with a thickness ranging from ~750–1010 µm (Table 3). Two of them had a hemodynamically significant gradient, while in one case, there was a graft–patient mismatch. In these DE-PVCs, fibrous bands extended from the neointima into the graft wall, and foci of deforming fibrosis were observed in the anastomotic regions. Furthermore, neoangiogenesis was present within the neointimal layers of these grafts.
No mineralization was observed in the DE-PVC wall and leaflets outside the suture line (Figure 7G). However, in the majority of cases (83.3%), large calcium clusters were found along the CJSs that secured the leaflets and formed the conduit body (Figure 8B,D).
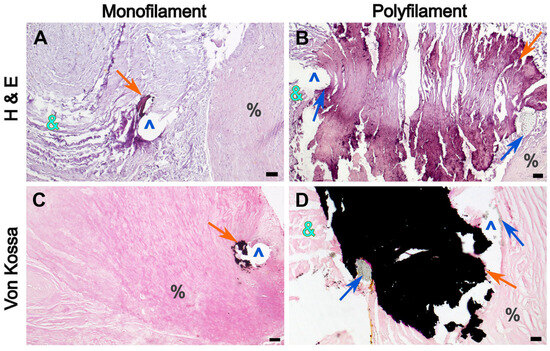
Figure 8.
Microphotographs of DE-PVC showing calcification foci at the suture lines. (A,C)—the distal anastomosis area secured with a 6/0 monofilament thread; (B,D)—the conduit joining suture area secured with a 5/0 multifilament thread. (A,B)—H&E staining, magnification ×40, bar 50 µm; (C,D)—Von Kossa staining, magnification ×40, bar 50 µm. Orange arrow indicates mineralization areas, blue arrow—multifilament thread fibers, &—conduit tissue, ^—suture hole, %—pulmonary artery tissue.
The braided threads were incorporated into calcium clusters, and furthermore, the multifilament fibers were encrusted with hydroxyapatite crystals (Figure 9A). In contrast, small calcium deposits were found along monofilament sutures in both the DE-PVC and native PA tissues (Figure 8A,C).
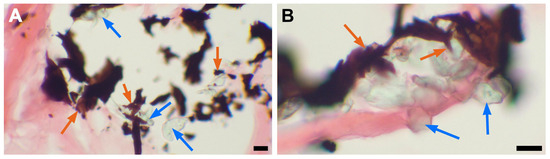
Figure 9.
Incrustation of multifilament suture fibers with hydroxyapatite crystals. (A)—DE-PVC stained with von Kossa staining, ×400 magnification; bar 10 µm; (B)—GA-PVC stained with von Kossa staining, ×400 magnification; bar 10 µm. Blue arrows point to multifilament suture fibers, orange arrows point to mineralization sites.
The explanted GA-PVCs showed several notable structural differences from the DE-PVCs. Firstly, all GA-PVCs were characterized by a thick, dense, and rigid fibrous external capsule that adhered tightly to the conduit. Fibrous deformation was observed in the graft tissue, with fibrous tissue strands extending into the wall from both the external capsule and the deep layers of the neointima. Notably, these samples typically showed collagen fiber fragmentation and fraying (Figure 7D). The migration of fibroblast cells was not pronounced, with only isolated myofibroblastic cells detected on the adventitial side of the graft wall. Inflammatory cells, including lymphocytes and macrophages, were present, but there was no evidence of foreign body giant cells. Secondly, compared to the DE-PVC group, the neointimal layer in GA-PVCs was thicker and covered the inner surface unevenly. Its thickness ranged from ~300 µm to ~850 µm on the conduit wall and from ~220 µm to ~500 µm on the leaflets (Table 3). The greatest thickness of the neointima was observed in the area of the distal anastomosis, exceeding 1850 µm. The hyperplastic neointima overlapped the native pulmonary artery tissue (~150 µm). Additionally, in two explanted GA-PVCs, areas of chondroid metaplasia with foci of calcification were identified. The chondroid tissue was localized subneointimally, both in the graft wall and in the native PA near the anastomotic region (Figure 10). Furthermore, GA-PVCs demonstrated poorer endothelial coverage. Endothelial cells partially covered the neointima at both the distal and proximal anastomotic sites. However, the endothelial layer was absent on most of the conduit body wall and on the cusps (Figure 7B). Mural thrombi were detected in the sinuses of one GA-PVC. Finally, calcification in GA-PVCs was more extensive. In all cases, large calcium deposits were consistently found along the CJSs of the graft (Figure 7H). Calcium clusters were directly linked to the multifilament suture fibers (Figure 9B). Foci of mineralized collagen fibers were identified within the thickness of the GA-PVC wall (Figure 7D,H). Calcium deposits were also identified in both the graft wall and the PA tissue, specifically along the proximal and distal anastomotic lines (Figure 7F).
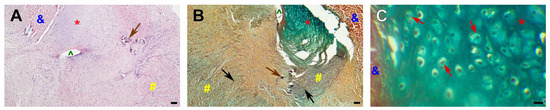
Figure 10.
Chondroid metaplasia in the GA-PVC wall. (A)—H&E-stained GA-PVC section, overview images, ×40 magnification; bar 50 µm; (B)—Russell–Movat pentachrome-stained GA-PVC section, overview images, ×40 magnification; bar 50 µm; (C)—Russell–Movat pentachrome-stained GA-PVC section, chondroid metaplasia, ×400 magnification; bar 10 µm. Black arrows point to elastin fibers, brown arrows—calcium deposits, red arrows—cartilage-like cells, #—native pulmonary artery, *—chondroid metaplasia, &—conduit tissue; ^—suture hole.
In two animals with prosthetic infective endocarditis, thrombotic masses accompanied by extensive inflammatory infiltration and bacterial colonies were identified on both the inflow and outflow sides of the conduit walls and leaflets. The conduit walls exhibited degenerative changes, including a decrease in collagen fibers, focal necrosis, and productive inflammation characterized by a significant presence of neutrophils and macrophages. In the anastomotic zones, granulation tissue with neovascularization, sclerosis, and infiltration of lymphocytes and histiocytes were observed. The inflammatory response led to the development of foci of deforming sclerosis and the formation of an isolating fibrous capsule from adjacent tissues.
4. Discussion
This study aimed to analyze structural changes in PVC treated with DE and GA, as well as identify factors contributing to bovine pericardium mineralization in a porcine model over a 6-month period.
Previous research has shown that the type of cross-linking agent significantly affects the degree of calcification in valved bioprostheses. Glutaraldehyde is widely used for biomaterial preservation, but GA-treated tissues are susceptible to early calcification [24,31,32]. At the same time, a significant advantage of epoxy compound treatment is that it results in a lower propensity for tissue mineralization [23,24,33,34,35]. This is attributed to the fact that diepoxides form specific bonds by interacting with free residues of primary and secondary amino and hydroxyl groups in collagen [36,37]. These bonds exhibit high chemical mobility and, owing to their two oxygen atoms, confer hydrophilicity to the diepoxide bond, thereby inhibiting calcium and phosphorus ion interactions in the parafibrillar space [37]. In a rodent model, DE-treated bovine pericardium showed no calcium deposition [17,24]. Although the experimental results are promising, clinical data reveal a contrasting outcome. Analysis of pulmonary graft performance identified calcification of DE-PVC (Figure 11) as a primary cause of prosthesis dysfunction [25,26]. This discrepancy between experimental findings and clinical observations suggests that DE treatment does not completely prevent PVC calcification, and that additional factors contribute to the nucleation of hydroxyapatite crystals.
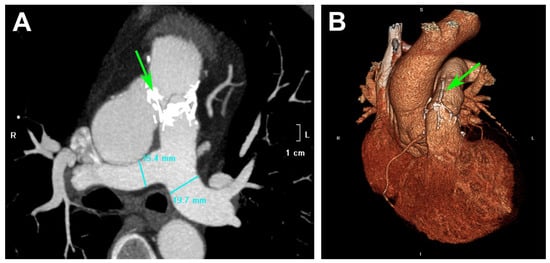
Figure 11.
CT images of a patient after RVOT reconstruction with DE-PVC: (A)—multiplanar reconstruction; (B)—3D volume rendering technique. Green arrows indicate calcium deposits located at the conduit joining sutures of the DE-PVC. (Data sourced from the archive of the E. Meshalkin National Medical Research Center.).
In this experimental study, we simulated conditions closely mimicking the actual application of the conduit, specifically its implantation in the PA position. The results confirmed mineralization of PVC, but the extent and distribution of calcium accumulation varied depending on the treatment of the bovine pericardium. Most DE-PVCs exhibited large, mature calcium deposits along the CJSs, where multifilament fibers were encrusted with hydroxyapatite crystals. Similar microscopic findings were observed in all explanted GA-PVCs. The data suggest that the multifilament braided suture initiates calcium accumulation, regardless of the treatment applied to the bovine pericardium. The void spaces within the braid of the multifilament braided suture create capillary effects and potential sites for cell accumulation [38]. The impregnation of threads with blood plasma proteins and the sedimentation of formed elements in cavities appear to initiate the nucleation process of hydroxyapatite crystals [39,40,41]. At the same time, tissue mineralization around the monofilament suture was less pronounced. In the DE-PVC group, both the proximal and distal anastomosis lines were almost completely free of calcium inclusions. Small mineral deposits were observed around a few stitches in the graft and pulmonary artery wall. This advantage of monofilament threads over multifilament braided sutures is due to their fewer potential nucleation zones [42].
Cyclic mechanical stress is another probable cause of the pronounced calcification in the suture that secures the leaflets in PVC. The commissure zones and leaflet bases experience increased mechanical stress [43,44], leading to damage of the extracellular matrix [45,46]. Microdamage to collagen fibers exposes chemically active sites, which become nucleation zones for calcium and phosphate ions diffusing into the tissue [18,47,48,49]. Zareian et al. [46] observed calcium deposits along the suture lines of all valves tested under mechanical stress in a calcifying solution. They found that mechanical damage to the extracellular matrix caused by external forces increased calcium deposition and accelerated mineralization deeper within the tissue. Additionally, in PVCs, the leaflet attachment area creates a fold in the bovine pericardium (Figure 2), which may promote tissue mineralization [50].
Based on these findings, it can be inferred that using biomaterials that do not require suture fixation of the leaflets and have a native valve may reduce mineralization and improve the durability of prostheses. Examples of such xenogenic materials include the bovine jugular vein and porcine aortic root. Although commercial products made from these materials are used for RVOT reconstruction, they have specific causes of dysfunction and their durability remains suboptimal [9,51,52].
We did not observe any mineralization foci in the DE-PVC wall and leaflets outside the suture line. In contrast, the GA-PVC wall showed areas of collagen fiber calcification within its thickness. This suggests that in GA-PVC, calcium accumulation begins not only at the suture line but also within the tissue due to collagen calcification. Analysis of explanted failed heart valve prostheses revealed similar findings, showing that mineralization in bioprosthetic tissue begins within the chemically fixed bovine pericardium [44]. The results from the rat model also showed that calcification of GA-treated bovine pericardium spreads “from the inside out” [17]. Calcium phosphate deposits initially formed in the collagen fibers of the deep tissue layer and then gradually extended to the outer layers. This process was accompanied by the accumulation of hydroxyapatite crystals and subsequent tissue damage. Given the irreversible growth of calcium clusters [53], large mineral deposits are expected to form in GA-PVC tissue outside the CJS area in the short term, leading to early graft dysfunction.
In our experimental work, we observed not only reduced mineralization but also structural advantages in DE-preserved valves. Most DE-PVCs exhibited signs of successful graft integration and tissue revitalization. This included non-deforming connective tissue infiltration, myofibroblast migration into the graft wall, and peripheral neoangiogenesis. These findings are significant because bovine pericardium typically has low inherent cellular content, which makes the observed cellular and vascular integration particularly advantageous [54,55]. We conclude that the identified cell population originates from the migration of cellular components from the recipient tissues. At the same time, in the GA-PVC wall, we found isolated myofibroblast-like cells, predominantly localized on the external side of the graft. The low level of cell migration is caused by the cytotoxic properties of GA [21,56,57]. Additionally, a dense, rigid fibrous capsule was detected around the GA-PVC in all cases, indicating a host response aimed at encapsulating and isolating the conduit from surrounding tissues. Strands of coarse fibrous tissue from the outer capsule and neointimal layer deeply penetrated the GA-PVC wall. This process, combined with internal mineralization, resulted in a loss of elasticity and the formation of a rigid structure. Fibrous deformation is a common feature of GA-treated xenomaterials and is observed when they are used in both high- and low-pressure areas [23,58,59].
Neointimal hyperplasia, characteristic of the GA-PVCs, is considered by us as another mechanism for leveling the toxic effects of GA [60]. All GA-PVCs were characterized by an irregular neointimal layer with a greatest thickness at the anastomosis area. It was also extending to the wall of the native PA. Apparently, a sufficiently thick neointimal layer is required to isolate the GA-treated tissues and facilitate endothelial cell migration to this area [23,60]. Previous studies have shown that GA released from prosthetic tissues suppresses endothelial cell growth and metabolism [20,61,62,63]. In the GA-PVC group, endothelial cells were found exclusively in the anastomotic zones, where the neointimal layer was significantly thicker. In contrast, DE-PVCs exhibited a thinner and more uniform neointimal layer. A key advantage of DE-PVCs over the GA-PVC group was the presence of a well-developed endothelial layer that fully covered the neointima. Our results are consistent with data indicating lower cytotoxicity of epoxy compounds compared to GA [23,56,64]. In the DE-PVC group, neointimal hyperplasia was observed in three cases and linked to elevated blood flow turbulence caused by sizing mismatch or fibrotic deformation of the anastomotic regions. Geometric alterations in the suture line and increased conduit wall rigidity resulted in accelerated blood flow, which subsequently stimulated neointimal proliferation [65,66].
In two explanted GA-PVCs, foci of chondroid metaplasia were identified in areas of greatest neointimal proliferation. The precise mechanisms underlying this phenomenon remain unclear. Myofibroblasts are thought to play a central role, as they can differentiate into chondrocytes under the influence of the tissue-specific microenvironment [60,67,68]. Local hypoxia, tissue injury accompanied by inflammation, and mechanical stimuli caused by structural mismatches between the graft and native tissue initiate a signaling cascade that drives cartilage differentiation [60,69,70,71,72]. Subsequently, cartilage tissue can become a site of pathological calcification and eventually be replaced by heterotopic bone [67,69,71]. Our findings align with the concept described. Chondroid metaplasia in GA-PVCs was observed in the anastomotic regions, where marked neointimal hyperplasia extended into the graft tissue and the native PA. These areas were devoid of angiogenesis. Similar localization and microstructural changes have been reported in previous studies [58,60,72,73]. In the DE-PVC group, neither chondroid metaplasia nor foci of heterotopic ossification were observed, suggesting an indirect role of GA in this pathological process [23].
The data suggest that DE-PVCs may outperform GA-PVCs, as they demonstrate superior integration into the recipient’s pulmonary arterial bed and greater graft vitalization. Furthermore, their reduced propensity for neointimal hyperplasia and better endothelialization of the luminal surface are likely to improve the long-term durability of DE-PVCs.
Our study identified distinct features common to all PVCs, regardless of the type of cross-linking agent used. As previously noted, large calcium deposits were observed along the line of the CJS in all explanted PVCs. Another notable characteristic of PVC was the involvement of the graft leaflets into the neointimal overgrowth. In all cases, the neointima covered both the inflow and outflow surfaces of the leaflets, resulting in their thickening and, consequently, an increase in the conduit valve gradient. Conversely, bovine jugular vein grafts under similar experimental conditions did not exhibit neointimal overgrowth involving the leaflets [23]. One more finding of our study was the absence of a foreign body reaction in both the PVC wall and adjacent tissues. Regardless of the cross-linking agent used, bovine pericardium behaved as an inert biomaterial that did not provoke a foreign body response. This observation highlights the low immunogenicity of bovine pericardial tissue versus other biomaterials commonly used for RVOT reconstruction [23,74,75,76].
This animal study has several limitations that restrict direct extrapolation of its results to humans. The unique characteristics of the experimental model must be taken into account when translating the findings to clinical applications. Notably, our findings cannot be fully applied to patient groups with high pulmonary hypertension or anatomical abnormalities of the PA, as conduits were implanted in healthy animals with well-developed pulmonary arterial beds. However, similarities in cardiac anatomy and hemodynamics offer valuable insights into the conduit’s potential function in humans. An additional limitation arises from challenges in postoperative animal care, which may affect the incidence of infectious complications. In our study, two animals developed prosthetic endocarditis, causing conduit dysfunction. This complication is common in pulmonary conduit implantation studies with large animals and was mainly due to model-specific factors, challenges in maintaining hygiene and wound care, and the necessity for repeated injections during the follow-up [23,32,77,78]. Therefore, the observed endocarditis rate cannot be directly applied to the human population.
5. Conclusions
Our experimental study is the first to provide evidence that the primary sites of PVC calcification are the CJS lines, regardless of the type of bovine pericardial treatment. It appears that the multifilament braided thread serves as a nucleation site for hydroxyapatite crystal formation. This finding underscores the role of suture type as an additional initiator of mineralization, suggesting that excluding multifilament braided sutures from PVC manufacturing may help reduce the risk of early structural valve degeneration.
We also observed that DE-treatment effectively prevents collagen calcification, unlike GA-treatment, suggesting a lower rate of mineralization in DE-PVC. Additional advantages of DE-PVC identified in this study include better integration into the pulmonary arterial bed, tissue vitalization, reduced neointimal hyperplasia, and a well-developed endothelial layer compared to GA-PVC. These findings indicate that DE-PVCs may offer improved performance and durability compared to GA-treated grafts. This is particularly important for children with congenital heart disease, as their ongoing growth requires multiple PA prosthesis replacements. Given the limitations of this experimental study, additional research is required to assess the clinical relevance of these findings.
Nonetheless, PVC calcification remains an unresolved challenge that requires further investigation and the development of innovative strategies for xenoconduit fabrication. The new insight into the role of suture material in calcium deposition enhances our understanding of tissue mineralization mechanisms and may help develop better solutions for reducing valve calcification.
Author Contributions
Conceptualization, N.R.N., A.A.D. and I.Y.Z.; methodology, N.R.N., Y.Y.K., E.V.K., E.V.B., T.P.T., O.Y.M. and A.V.B.-P.; formal analysis, N.R.N., A.A.D. and Y.Y.K.; investigation, N.R.N., A.A.D., E.V.K., Y.Y.K., E.V.B., Y.L.R., O.Y.M. and I.Y.Z.; writing—original draft preparation, N.R.N., A.A.D., E.V.K. and T.P.T.; writing—review and editing, I.Y.Z., Y.Y.K. and A.V.B.-P.; visualization, N.R.N. and A.A.D.; project administration, N.R.N., I.Y.Z. and A.V.B.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out within the state assignment of Ministry of Health of Russian Federation (theme No 124022500251-0). The funding source had no involvement in the study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of E. Meshalkin National Medical Research Center (protocol № 17 of ethical committee meeting, approved 14 June 2018).
Informed Consent Statement
Not applicable. Our study did not involve human participants, human tissues, or human data.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CJS | Conduit joining suture |
| CPB | Cardiopulmonary bypass |
| DE | Diepoxide, ethylene glycol diglycidyl ether |
| DE-PVC | Diepoxy-treated bovine pericardial valved conduit |
| GA | Glutaraldehyde |
| GA-PVC | Glutaraldehyde-treated bovine pericardial valved conduit |
| IQR | Interquartile range |
| Me | Median |
| PA | Pulmonary artery |
| PVC | Bovine pericardial valve conduit |
| RVOT | Right ventricular outflow tract |
| TEE | Transesophageal echocardiography |
References
- Herrmann, J.L.; Brown, J.W. Seven Decades of Valved Right Ventricular Outflow Tract Reconstruction: The Most Common Heart Procedure in Children. J. Thorac. Cardiovasc. Surg. 2020, 160, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Huyan, Y.; Chang, Y.; Song, J. Application of Homograft Valved Conduit in Cardiac Surgery. Front. Cardiovasc. Med. 2021, 8, 740871. [Google Scholar] [CrossRef]
- Hazekamp, M.G.; Barron, D.J.; Dangel, J.; Homfray, T.; Jongbloed, M.R.M.; Voges, I.; Anderson, R.H.; Belli, E.; Bellsham-Revell, H.R.; Herberg, U.; et al. Consensus Document on Optimal Management of Patients with Common Arterial Trunk. Eur. J. Cardio-Thorac. Surg. 2021, 60, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the Management of Adult Congenital Heart Disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Sharifulin, R.; Demin, I.; Karaskov, A.; Chernyavsky, A.; Bogachev-Prokophiev, A. What Are the Alternatives to Replace the Pulmonary Root in the Absence of Pulmonary Homografts? Ann Cardiothorac Surg 2021, 10, 524–526. [Google Scholar] [CrossRef]
- Harris, A.G.; Iacobazzi, D.; Caputo, M.; Bartoli-Leonard, F. Graft Rejection in Paediatric Congenital Heart Disease. Transl. Pediatr. 2023, 12, 1572–1591. [Google Scholar] [CrossRef]
- Koziarz, A.; Makhdoum, A.; Butany, J.; Ouzounian, M.; Chung, J. Modes of Bioprosthetic Valve Failure: A Narrative Review. Curr. Opin. Cardiol. 2020, 35, 123–132. [Google Scholar] [CrossRef]
- Attia, R.Q.; Raja, S.G. Surgical Pericardial Heart Valves: 50 Years of Evolution. Int. J. Surg. 2021, 94, 106121. [Google Scholar] [CrossRef]
- Nichay, N.R.; Zhuravleva, I.Y.; Kulyabin, Y.Y.; Timchenko, T.P.; Voitov, A.V.; Kuznetsova, E.V.; Soynov, I.A.; Zubritskiy, A.V.; Bogachev-Prokophiev, A.V.; Karaskov, A.M. In Search of the Best Xenogeneic Material for a Paediatric Conduit: An Analysis of Clinical Data. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 34–41. [Google Scholar] [CrossRef]
- Crago, M.; Winlaw, D.S.; Farajikhah, S.; Dehghani, F.; Naficy, S. Pediatric Pulmonary Valve Replacements: Clinical Challenges and Emerging Technologies. Bioeng. Transl. Med. 2023, 8, e10501. [Google Scholar] [CrossRef]
- Schoen, F.J.; Levy, R.J. Bioprosthetic Heart Valve Calcification: Clinicopathologic Correlations, Mechanisms, and Prevention. In Cardiovascular Calcification and Bone Mineralization; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 183–215. [Google Scholar] [CrossRef]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef]
- Manji, R.A.; Ekser, B.; Menkis, A.H.; Cooper, D.K.C. Bioprosthetic Heart Valves of the Future. Xenotransplantation 2014, 21, 1–10. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, Y.; Yim, W.Y.; Wang, S.; Xu, L.; Shi, J.; Qiao, W.; Dong, N. Mechanisms and Drug Therapies of Bioprosthetic Heart Valve Calcification. Front. Pharmacol. 2022, 13, 909801. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Herrero, E.; Paez, J.; Del Castillo-Olivares Ramos, J.L. Tissue Heart Valve Mineralization: Review of Calcification Mechanisms and Strategies for Prevention. J. Appl. Biomater. Biomech. 2005, 3, 67–82. [Google Scholar]
- Kim, K.M.; Herrera, G.A.; Battarbee, H.D. Role of Glutaraldehyde in Calcification of Porcine Aortic Valve Fibroblasts. Am. J. Pathol. 1999, 154, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, I.Y.; Karpova, E.V.; Dokuchaeva, A.A.; Titov, A.T.; Timchenko, T.P.; Vasilieva, M.B. Calcification of Various Bioprosthetic Materials in Rats: Is It Really Different? Int. J. Mol. Sci. 2023, 24, 7274. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.C.; Komninou, M.A.; Badria, A.F.; Korossis, S.; Koutsoukos, P.; Mavrilas, D. Calcification Assessment of Bioprosthetic Heart Valve Tissues Using an Improved In Vitro Model. IEEE Trans. Biomed. Eng. 2020, 67, 2453–2461. [Google Scholar] [CrossRef]
- Maizato, M.J.S.; Higa, O.Z.; Mathor, M.B.; Camillo, M.A.P.; Spencer, P.J.; Pitombo, R.N.; Zavaglia, C.A.C.; Leirner, A.A. Glutaraldehyde-treated Bovine Pericardium: Effects of Lyophilization on Cytotoxicity and Residual Aldehydes. Artif. Organs 2003, 27, 692–694. [Google Scholar] [CrossRef]
- Lopez-Moya, M.; Melgar-Lesmes, P.; Kolandaivelu, K.; de la Torre Hernández, J.M.; Edelman, E.R.; Balcells, M. Optimizing Glutaraldehyde-Fixed Tissue Heart Valves with Chondroitin Sulfate Hydrogel for Endothelialization and Shielding against Deterioration. Biomacromolecules 2018, 19, 1234–1244. [Google Scholar] [CrossRef]
- Umashankar, P.; Kumari, T.; Mohanan, P. Glutaraldehyde Treatment Elicits Toxic Response Compared to Decellularization in Bovine Pericardium. Toxicol. Int. 2012, 19, 51. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Glushkova, T.V.; Lobov, A.A.; Ovcharenko, E.A.; Zainullina, B.R.; Bogdanov, L.A.; Shishkova, D.K.; Markova, V.E.; Asanov, M.A.; Mukhamadiyarov, R.A.; et al. Proteolytic Degradation Is a Major Contributor to Bioprosthetic Heart Valve Failure. J. Am. Heart Assoc. 2023, 12, e028215. [Google Scholar] [CrossRef]
- Nichay, N.R.; Dokuchaeva, A.A.; Kulyabin, Y.Y.; Boyarkin, E.V.; Kuznetsova, E.V.; Rusakova, Y.L.; Murashov, I.S.; Vaver, A.A.; Bogachev-Prokophiev, A.V.; Zhuravleva, I.Y. Epoxy- versus Glutaraldehyde-Treated Bovine Jugular Vein Conduit for Pulmonary Valve Replacement: A Comparison of Morphological Changes in a Pig Model. Biomedicines 2023, 11, 3101. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, I.Y.; Nichay, N.R.; Kulyabin, Y.Y.; Timchenko, T.P.; Korobeinikov, A.A.; Polienko, Y.F.; Shatskaya, S.S.; Kuznetsova, E.V.; Voitov, A.V.; Bogachev-Prokophiev, A.V.; et al. In Search of the Best Xenogeneic Material for a Paediatric Conduit: An Experimental Study. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Nichay, N.R.; Zhuravleva, I.Y.; Kulyabin, Y.Y.; Zubritskiy, A.V.; Voitov, A.V.; Soynov, I.A.; Gorbatykh, A.V.; Bogachev-Prokophiev, A.V.; Karaskov, A.M. Diepoxy- Versus Glutaraldehyde-Treated Xenografts: Outcomes of Right Ventricular Outflow Tract Reconstruction in Children. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 56–64. [Google Scholar] [CrossRef]
- Sharifulin, R.; Bogachev-Prokophiev, A.; Demin, I.; Afanasyev, A.; Ovcharov, M.; Pivkin, A.; Sapegin, A.; Zhuravleva, I.; Karaskov, A. Allografts and Xenografts for Right Ventricular Outflow Tract Reconstruction in Ross Patients. Eur. J. Cardio-Thorac. Surg. 2021, 59, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Song, L. Calcium and Bone Metabolism Indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar] [CrossRef]
- Dowling, D.J.; Levy, O. Ontogeny of Early Life Immunity. Trends Immunol. 2014, 35, 299–310. [Google Scholar] [CrossRef]
- Verderio, P.; Lecchi, M.; Ciniselli, C.M.; Shishmani, B.; Apolone, G.; Manenti, G. 3Rs Principle and Legislative Decrees to Achieve High Standard of Animal Research. Animals 2023, 13, 277. [Google Scholar] [CrossRef]
- Zhuravleva, I.Y. Biocidal Composition for Aseptic Storage of Preserved Prosthetic Material from Tissues of Animal Origin. RU2580621C1, 10 April 2016. [Google Scholar]
- Grabenwöger, M.; Sider, J.; Fitzal, F.; Zelenka, C.; Windberger, U.; Grimm, M.; Moritz, A.; Böck, P.; Wolner, E. Impact of Glutaraldehyde on Calcification of Pericardial Bioprosthetic Heart Valve Material. Ann. Thorac. Surg. 1996, 62, 772–777. [Google Scholar] [CrossRef]
- Herijgers, P.; Ozaki, S.; Verbeken, E.; Van Lommel, A.; Meuris, B.; Lesaffre, E.; Daenen, W.; Flameng, W. Valved Jugular Vein Segments for Right Ventricular Outflow Tract Reconstruction in Young Sheep. J. Thorac. Cardiovasc. Surg. 2002, 124, 798–805. [Google Scholar] [CrossRef]
- Imamura, E.; Sawatani, O.; Koyanagi, H.; Noishiki, Y.; Miyata, T. Epoxy Compounds As a New Cross-Linking Agent for Porcine Aortic Leaflets: Subcutaneous Implant Studies in Rats. J. Card. Surg. 1989, 4, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Ma, J.; Tian, W.; Lei, X.; Long, S.; Xi, B. Prevention of Tissue Calcification on Bioprosthetic Heart Valve by Using Epoxy Compounds: A Study of Calcification Tests in Vitro and In Vivo. J. Biomed. Mater. Res. 1992, 26, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Noishiki, Y.; Kosuge, T.; Yamamoto, K.; Kondo, J.; Matsumoto, A. Use of a Bovine Jugular Vein Graft with Natural Valve for Right Ventricular Outflow Tract Reconstruction: A One-Year Animal Study. J. Thorac. Cardiovasc. Surg. 1997, 114, 224–233. [Google Scholar] [CrossRef]
- Zhuravleva, I.; Karpova, E.; Oparina, L.; Kabos, N.; Ksenofontov, A.; Zhuravleva, A.; Nichay, N.; Bogachev-Prokophiev, A.; Trofimov, B.; Karaskov, A. Bioprosthetic Xenopericardium Preserved with Di- and Penta-Epoxy Compounds: Molecular Cross-Linking Mechanisms, Surface Features and Mechanical Properties. Circ. Pathol. Card. Surg. 2018, 22, 56–68. [Google Scholar] [CrossRef]
- Sato, M.; Hiramatsu, Y.; Matsushita, S.; Sato, S.; Watanabe, Y.; Sakakibara, Y. Shrinkage Temperature and Anti-Calcification Property of Triglycidylamine-Crosslinked Autologous Tissue. J. Artif. Organs 2014, 17, 265–271. [Google Scholar] [CrossRef]
- Scott Taylor, M.; Shalaby, S.W. Sutures. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1010–1024. [Google Scholar] [CrossRef]
- Zhu, F.; Tong, Y.; Sheng, Z.; Yao, Y. Role of Dendritic Cells in the Host Response to Biomaterials and Their Signaling Pathways. Acta Biomater. 2019, 94, 132–144. [Google Scholar] [CrossRef]
- Ferrans, V.J.; Boyce, S.W.; Billingham, M.E.; Jones, M.; Ishihara, T.; Roberts, W.C. Calcific Deposits in Porcine Bioprostheses: Structure and Pathogenesis. Am. J. Cardiol. 1980, 46, 721–734. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, S.; Lan, H.; Meng, X.; Zhang, H.; Fan, Y.; Wang, Y.; Wang, C.; Wang, Z. Mapping the Calcification of Bovine Pericardium in Rat Model by Enhanced Micro-Computed Tomography. Biomaterials 2014, 35, 8305–8311. [Google Scholar] [CrossRef]
- Vaesken, A.; Pelle, A.; Pavon-Djavid, G.; Rancic, J.; Chakfe, N.; Heim, F. Heart Valves from Polyester Fibers: A Preliminary 6-Month in Vivo Study. Biomed. Eng./Biomed. Tech. 2018, 63, 271–278. [Google Scholar] [CrossRef]
- Liao, K.K.; Li, X.; John, R.; Amatya, D.M.; Joyce, L.D.; Park, S.J.; Bianco, R.; Bolman, R.M. Mechanical Stress: An Independent Determinant of Early Bioprosthetic Calcification in Humans. Ann. Thorac. Surg. 2008, 86, 491–495. [Google Scholar] [CrossRef]
- Tsolaki, E.; Corso, P.; Zboray, R.; Avaro, J.; Appel, C.; Liebi, M.; Bertazzo, S.; Heinisch, P.P.; Carrel, T.; Obrist, D.; et al. Multiscale Multimodal Characterization and Simulation of Structural Alterations in Failed Bioprosthetic Heart Valves. Acta Biomater. 2023, 169, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Motiwale, S.; Hsu, M.-C.; Sacks, M.S. Simulating the Time Evolving Geometry, Mechanical Properties, and Fibrous Structure of Bioprosthetic Heart Valve Leaflets under Cyclic Loading. J. Mech. Behav. Biomed. Mater. 2021, 123, 104745. [Google Scholar] [CrossRef] [PubMed]
- Zareian, R.; Tseng, J.-C.; Fraser, R.; Meganck, J.; Kilduff, M.; Sarraf, M.; Dvir, D.; Kheradvar, A. Effect of Stent Crimping on Calcification of Transcatheter Aortic Valves. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 64–73. [Google Scholar] [CrossRef]
- Schoen, F.J.; Levy, R.J. Calcification of Tissue Heart Valve Substitutes: Progress Toward Understanding and Prevention. Ann. Thorac. Surg. 2005, 79, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Badria, A.F.; Koutsoukos, P.G.; Mavrilas, D. Decellularized Tissue-Engineered Heart Valves Calcification: What Do Animal and Clinical Studies Tell Us? J. Mater. Sci. Mater. Med. 2020, 31, 132. [Google Scholar] [CrossRef]
- Whelan, A.; Williams, E.; Fitzpatrick, E.; Murphy, B.P.; Gunning, P.S.; O’Reilly, D.; Lally, C. Collagen Fibre-Mediated Mechanical Damage Increases Calcification of Bovine Pericardium for Use in Bioprosthetic Heart Valves. Acta Biomater. 2021, 128, 384–392. [Google Scholar] [CrossRef]
- Levy, R.J.; Schoen, F.J.; Levy, J.T.; Nelson, A.C.; Howard, S.L.; Oshry, L.J. Biologic Determinants of Dystrophic Calcification and Osteocalcin Deposition in Glutaraldehyde-Preserved Porcine Aortic Valve Leaflets Implanted Subcutaneously in Rats. Am. J. Pathol. 1983, 113, 143–155. [Google Scholar]
- Christ, T.; Paun, A.C.; Grubitzsch, H.; Holinski, S.; Falk, V.; Dushe, S. Long-Term Results after the Ross Procedure with the Decellularized AutoTissue Matrix P® Bioprosthesis Used for Pulmonary Valve Replacement. Eur. J. Cardio-Thorac. Surg. 2019, 55, 885–892. [Google Scholar] [CrossRef]
- Beckerman, Z.; De León, L.E.; Zea-Vera, R.; Mery, C.M.; Fraser, C.D. High Incidence of Late Infective Endocarditis in Bovine Jugular Vein Valved Conduits. J. Thorac. Cardiovasc. Surg. 2018, 156, 728–734. [Google Scholar] [CrossRef]
- Vidavsky, N.; Kunitake, J.A.M.R.; Estroff, L.A. Multiple Pathways for Pathological Calcification in the Human Body. Adv. Healthc. Mater. 2021, 10, 2001271. [Google Scholar] [CrossRef]
- Rodriguez, E.R.; Tan, C.D. Structure and Anatomy of the Human Pericardium. Prog. Cardiovasc. Dis. 2017, 59, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Anatomy and Physiology of the Pericardium. Cardiol. Clin. 2017, 35, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Nishi, C.; Nakajima, N.; Ikada, Y. In Vitro Evaluation of Cytotoxicity of Diepoxy Compounds Used for Biomaterial Modification. J. Biomed. Mater. Res. 1995, 29, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-W.; Huang, R.-N.; Huang, L.L.H.; Tsai, C.-C. In Vitro Evaluation of Cytotoxicity of a Naturally Occurring Cross-Linking Reagent for Biological Tissue Fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef]
- Gabbay, S.; Bortolotti, U.; Factor, S.; Shore, D.F.; Frater, R.W.M. Calcification of Implanted Xenograft Pericardium. J. Thorac. Cardiovasc. Surg. 1984, 87, 782–787. [Google Scholar] [CrossRef]
- Bell, D.; Prabhu, S.; Betts, K.; Justo, R.; Venugopal, P.; Karl, T.R.; Alphonso, N. Durability of Tissue-Engineered Bovine Pericardium (CardioCel®) for a Minimum of 24 Months When Used for the Repair of Congenital Heart Defects. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 284–290. [Google Scholar] [CrossRef]
- Chang, Y.; Liang, H.; Wei, H.; Chu, C.; Sung, H. Tissue Regeneration Patterns in Acellular Bovine Pericardia Implanted in a Canine Model as a Vascular Patch. J. Biomed. Mater. Res. A 2004, 69, 323–333. [Google Scholar] [CrossRef]
- Eybl, E.; Griesmacher, A.; Grimm, M.; Wolner, E. Toxic Effects of Aldehydes Released from Fixed Pericardium on Bovine Aortic Endothelial Cells. J. Biomed. Mater. Res. 1989, 23, 1355–1365. [Google Scholar] [CrossRef]
- Wiebe, D.; Megerman, J.; L’Italien, G.J.; Abbott, W.M. Glutaraldehyde Release from Vascular Prostheses of Biologic Origin. Surgery 1988, 104, 26–33. [Google Scholar]
- Grimm, M.; Eybl, E.; Grabenwöger, D.M.; Griesmacher, A.; Losert, U.; Böck, P.; Müller, M.M.; Wolner, E. Biocompatibility of Aldehyde-Fixed Bovine Pericardium. J. Thorac. Cardiovasc. Surg. 1991, 102, 195–201. [Google Scholar] [CrossRef]
- Bondarenko, N.A.; Surovtseva, M.A.; Lykov, A.P.; Kim, I.I.; Zhuravleva, I.Y.; Poveschenko, O.V. Cytotoxicity of Xenogeneic Pericardium Preserved by Epoxy Cross-Linking Agents. Sovrem. Tehnol. V Med. 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Lemson, M.S.; Tordoir, J.H.M.; Daemen, M.J.A.P.; Kitslaar, P.J.E.H.M. Intimal Hyperplasia in Vascular Grafts. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Berdajs, D.; Mosbahi, S.; Vos, J.; Charbonnier, D.; Hullin, R.; von Segesser, L.K. Fluid Dynamics Simulation of Right Ventricular Outflow Tract Oversizing. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 176–182. [Google Scholar] [CrossRef]
- Steiner, I.; Kašparová, P.; Kohout, A.; Dominik, J. Bone Formation in Cardiac Valves: A Histopathological Study of 128 Cases. Virchows. Archiv. 2007, 450, 653–657. [Google Scholar] [CrossRef]
- Mathieu, P.; Roussel, J.C.; Dagenais, F.; Anegon, I. Cartilaginous Metaplasia and Calcification in Aortic Allograft Is Associated with Transforming Growth Factor Β1 Expression. J. Thorac. Cardiovasc. Surg. 2003, 126, 1449–1454. [Google Scholar] [CrossRef]
- Mohler, E.R.; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone Formation and Inflammation in Cardiac Valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Fuery, M.A.; Liang, L.; Kaplan, F.S.; Mohler, E.R. Vascular Ossification: Pathology, Mechanisms, and Clinical Implications. Bone 2018, 109, 28–34. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Lin, H. The Hypoxic Microenvironment: A Driving Force for Heterotopic Ossification Progression. Cell Commun. Signal. 2020, 18, 20. [Google Scholar] [CrossRef]
- de Valence, S.; Tille, J.-C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long Term Performance of Polycaprolactone Vascular Grafts in a Rat Abdominal Aorta Replacement Model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef]
- Santibáñez-Salgado, J.; Olmos-Zúñiga, J.; Pérez-López, M.; Aboitiz-Rivera, C.; Gaxiola-Gaxiola, M.; Jasso-Victoria, R.; Sotres-Vega, A.; Baltazares-Lipp, M.; Pérez-Covarrubias, D.; Villalba-Caloca, J. Lyophilized Glutaraldehyde-Preserved Bovine Pericardium for Experimental Atrial Septal Defect Closure. Eur. Cell. Mater. 2010, 19, 158–165. [Google Scholar] [CrossRef]
- Findeisen, K.; Morticelli, L.; Goecke, T.; Kolbeck, L.; Ramm, R.; Höffler, H.; Brandes, G.; Korossis, S.; Haverich, A.; Hilfiker, A. Toward Acellular Xenogeneic Heart Valve Prostheses: Histological and Biomechanical Characterization of Decellularized and Enzymatically Deglycosylated Porcine Pulmonary Heart Valve Matrices. Xenotransplantation 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Bozso, S.J.; EL-Andari, R.; Al-Adra, D.; Moon, M.C.; Freed, D.H.; Nagendran, J.; Nagendran, J. A Review of the Immune Response Stimulated by Xenogenic Tissue Heart Valves. Scand. J. Immunol. 2021, 93, e13018. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, W.; Kim, K.; Lim, H.; Kim, Y.J. A Preclinical Trial of Perventricular Pulmonary Valve Implantation: Pericardial versus Aortic Porcine Valves Mounted on Self-expandable Stent. Artif. Organs 2021, 45, E89–E100. [Google Scholar] [CrossRef] [PubMed]
- Attmann, T.; Quaden, R.; Freistedt, A.; König, C.; Cremer, J.; Lutter, G. Percutaneous Heart Valve Replacement: Histology and Calcification Characteristics of Biological Valved Stents in Juvenile Sheep. Cardiovasc. Pathol. 2007, 16, 165–170. [Google Scholar] [CrossRef]
- Flameng, W.; Hermans, H.; Verbeken, E.; Meuris, B. A Randomized Assessment of an Advanced Tissue Preservation Technology in the Juvenile Sheep Model. J. Thorac. Cardiovasc. Surg. 2015, 149, 340–345. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).