From Detection to Treatment: Nanomaterial-Based Biosensors Transforming Prosthetic Dentistry and Oral Health Care: A Scoping Review
Abstract
1. Introduction
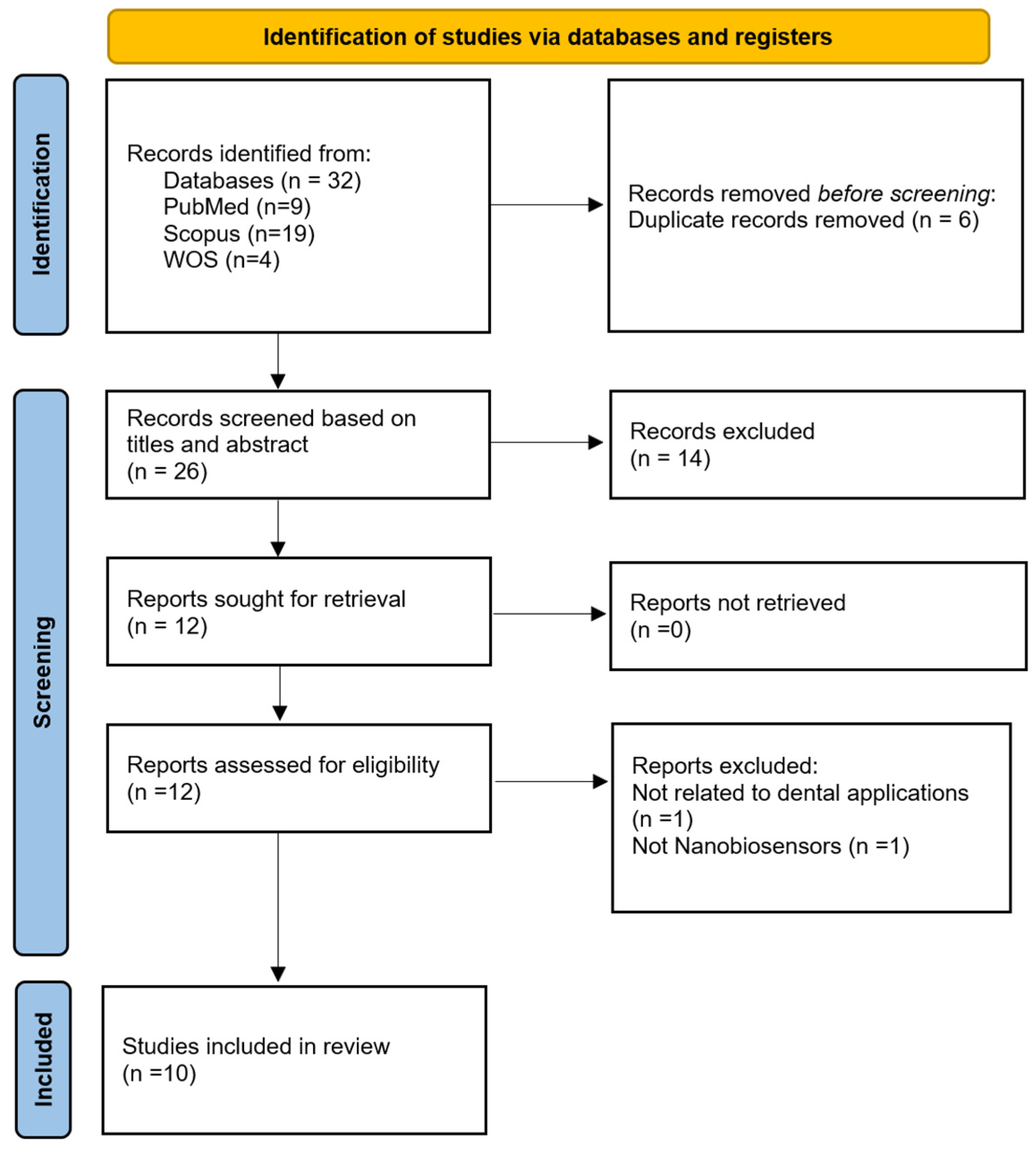
2. Materials and Methods
2.1. Search Strategy
2.2. Screening Process
2.3. Data Extraction
3. Nanomaterials: The Building Blocks of Advanced Dental Biosensors
3.1. Nanobiosensors for Periodontal Health
3.1.1. Metal Nanoparticles
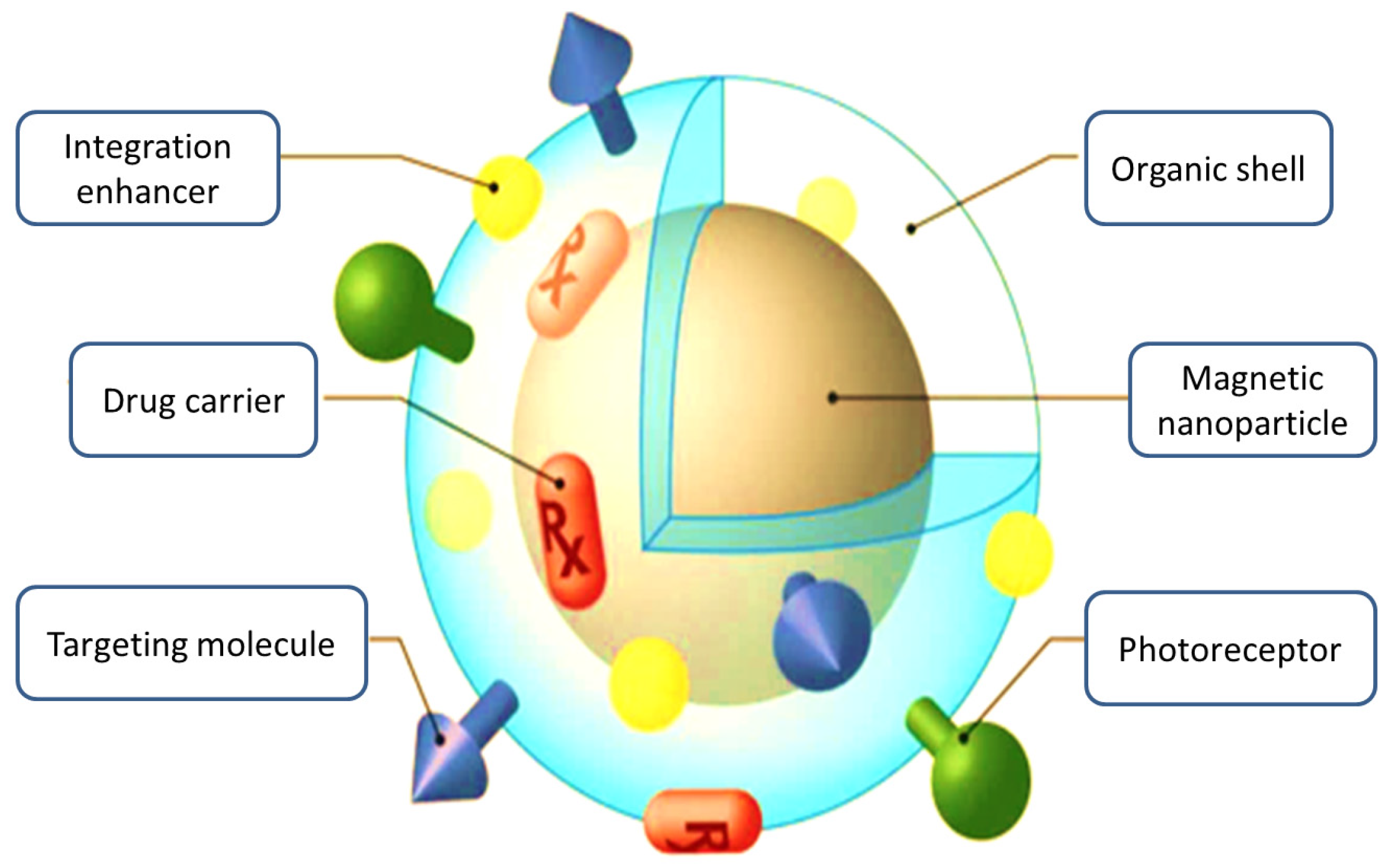
3.1.2. Magnetic Nanoparticles
3.1.3. Carbon-Based Nanomaterials
3.1.4. Quantum Dots
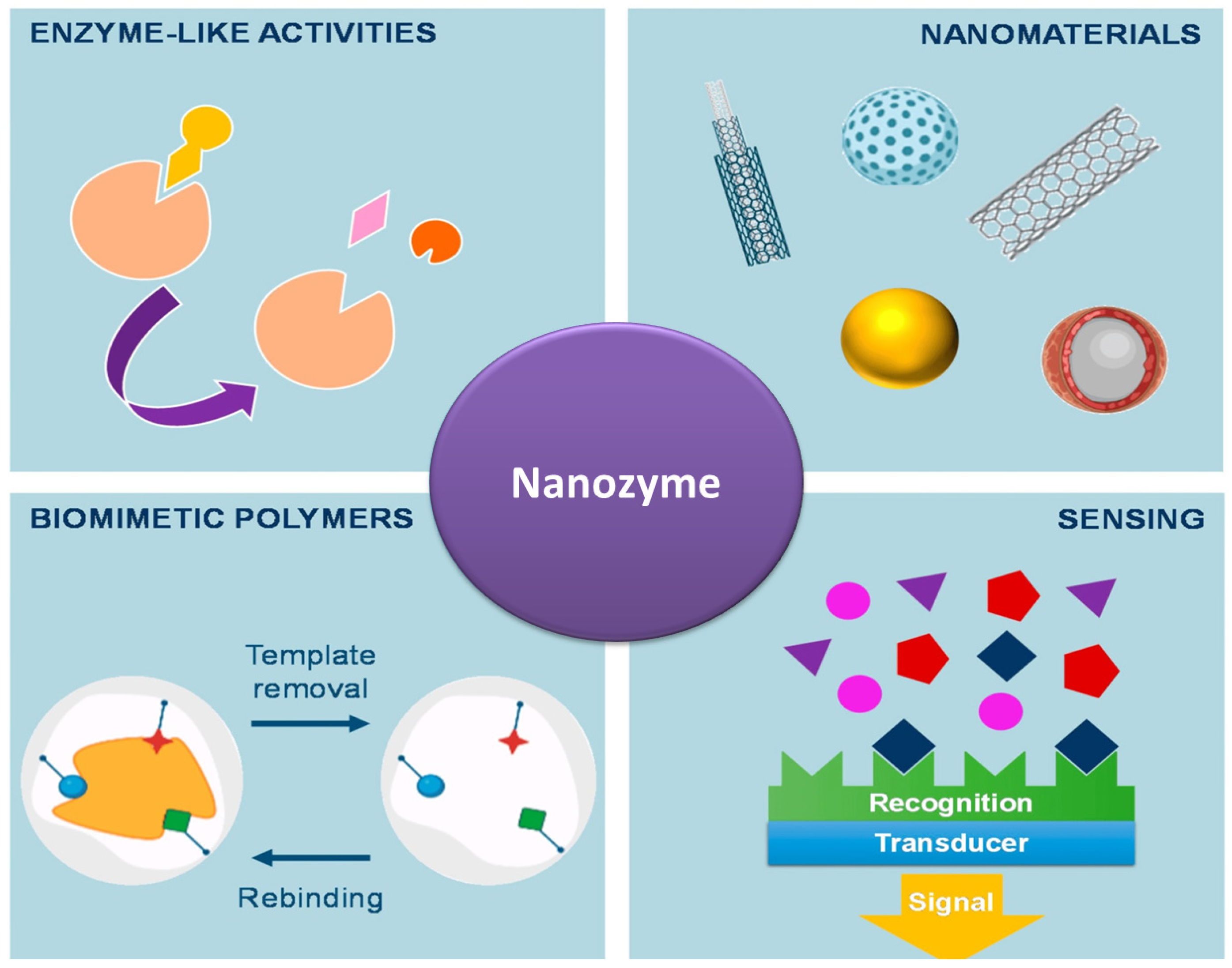
3.1.5. Nanozymes
3.1.6. Integrated Nanobiosensor Platforms
Upconversion Nanoparticle-Based Lateral Flow Immunoassay (LFIS)
SERS-Based Magnetic Microfluidic Sensor
Advanced Nano–Bio Interfaces
3.2. Nanobiosensors for Management of Oral Cancer
3.2.1. MoS2-ZnO Nanocomposite Immunosensor
3.2.2. Biowaste-Derived Triboelectric Nanogenerator
3.2.3. Nanocomposite-Based Biosensors
3.2.4. Gold Nanorod Multiplex Bioanalytical Assay
3.2.5. Magnetite Nanoparticles
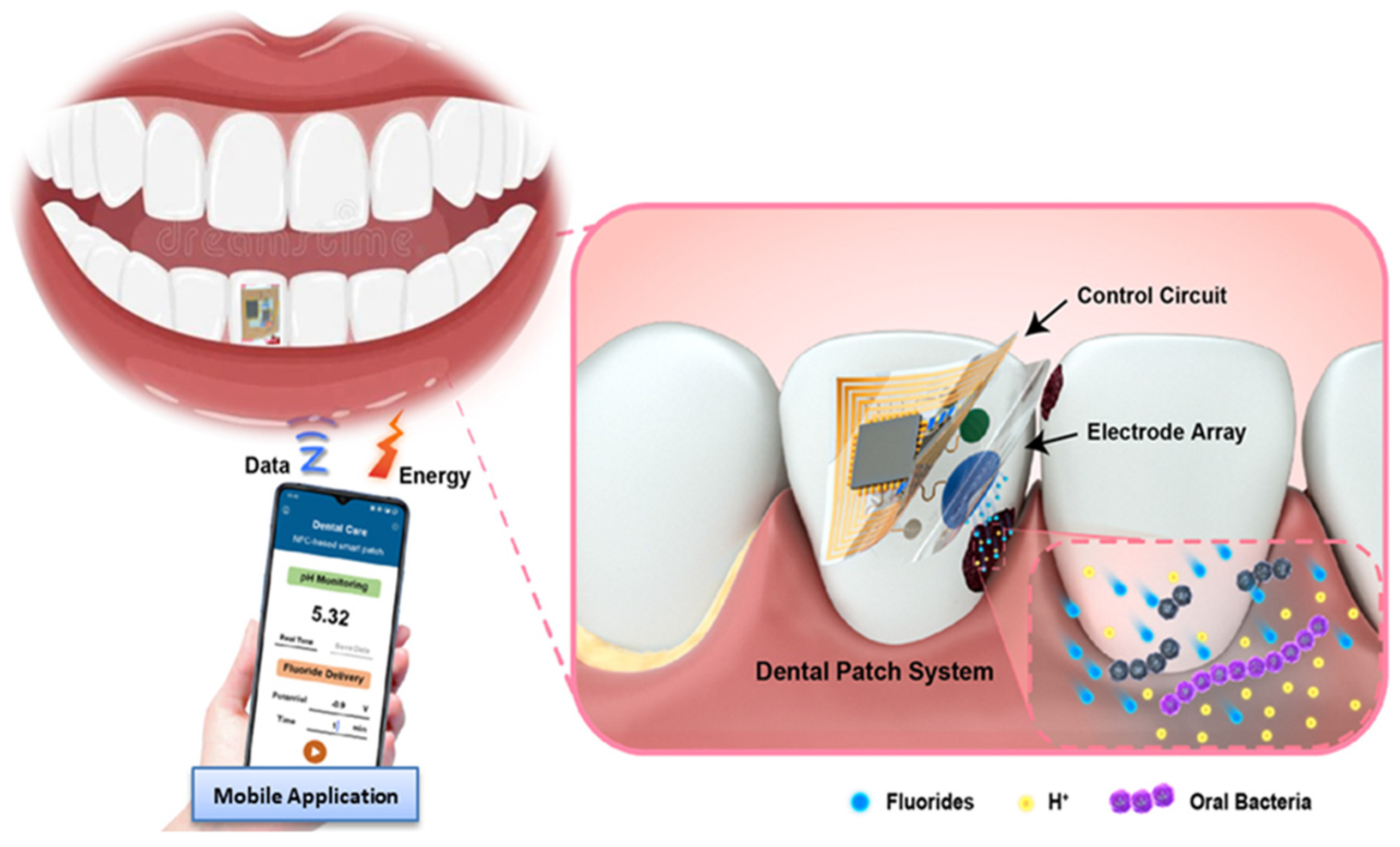
3.3. Integrated Nanobiosensors for Detection of Dental Caries
3.3.1. Metal Nanoparticles
3.3.2. Carbon-Based Nanomaterials
3.3.3. Quantum Dots
3.3.4. Integrated Nanobiosensor Platforms
Nanoparticle-Based Targeting and Detection of Microcavities
Nanomaterial-Based Electrochemical Biosensors
3.4. Nanosensors for Dental Implant Monitoring and Maintenance
3.4.1. Metal Nanoparticles
3.4.2. Magnetic Nanoparticles (MNPs)
3.4.3. Carbon-Based Nanobiosensors
3.4.4. Quantum Dot-Based Nanosensors
3.4.5. Integrated Nanobiosensor Platforms
4. Limitations
5. Challenges and Future Perspectives
6. Conclusions
- Nanomaterial-based biosensors are poised to revolutionize dental theranostics, offering unprecedented opportunities for early disease detection and personalized treatment.
- As we continue to refine these technologies, we stand on the brink of a new era in oral healthcare—one where diagnosis is rapid, precise, and minimally invasive.
- The integration of nanotechnology into dentistry not only advances our diagnostic capabilities but also provides the way for more effective, targeted therapies.
- As research progresses, these innovative tools promise to transform the landscape of oral health, ultimately leading to improved patient outcomes and a new standard of dental care.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| Au | Gold |
| AuNps | Gold Nanoparticles |
| Ag | Silver |
| CDs | Carbon Dots |
| CH3SH | Methyl Mercaptan |
| CNTs | Carbon Nanotubes |
| Fe2O3 | Iron Oxide |
| GCF | Gingival Crevicular Fluid |
| GNS | Gold Nanostructures |
| H2S | Hydrogen Sulfide |
| IL-1β | Interleukin-1 Beta |
| LFIS | Lateral Flow Immunoassay |
| LOD | Limit of Detection |
| MMPs | Matrix Metalloproteinases |
| QDs | Quantum Dots |
| SERS | Surface-Enhanced Raman Scattering |
| TNF-α | Tumor Necrosis Factor-Alpha |
| UCNPs | Upconversion Nanoparticles |
| ZnO | Zinc Oxide |
| OSCC | Oral Squamous Cell carcinoma |
References
- Taymour, N.; Haque, M.A.; Atia, G.A.N.; Mohamed, S.Z.; Rokaya, D.; Bajunaid, S.M.; Soliman, M.M.; Shalaby, H.K.; Barai, P.; Roy, M.; et al. Nanodiamond: A Promising Carbon-Based Nanomaterial for Therapeutic and Regenerative Dental Applications. ChemistrySelect 2024, 9, e202401328. [Google Scholar] [CrossRef]
- Atia, G.A.N.; Mohamed, S.Z.; Taymour, N.; Soliman, M.M.; Halim, H.A.; Shalaby, H.K.; Ghobashy, M.M.; Barai, P.; Haque, M.A.; Barai, H.R. Carbon Dots as Promising Carbon Nanomaterials for Diagnostics, Therapeutics, and Regenerative Orofacial Applications. J. Drug Deliv. Sci. Technol. 2025, 12, 100359. [Google Scholar] [CrossRef]
- Yudaev, P.; Tupikov, A.; Chistyakov, E. Organocyclophosphazenes and Materials Based on Them for Pharmaceuticals and Biomedicine. Biomolecules 2025, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Chen, Y.; Wang, Z.; Zhang, X.; Gao, F.; Shao, Z.; Wang, H. Graphene derivative based hydrogels in biomedical applications. J. Tissue Eng. 2024, 15, 20417314241282131. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2023, 13, 40. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical biosensors: A decade in review. Alex. Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Kiilerich-Pedersen, K.; Poulsen, C.R.; Jain, T.; Rozlosnik, N. Polymer based biosensor for rapid electrochemical detection of virus infection of human cells. Biosens. Bioelectron. 2011, 28, 386–392. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abd-elaziem, W.; Elsheikh, A. Nanoscale Advances Advancements in nanomaterials for nanosensors. Nanoscale Adv. 2024, 6, 4015–4046. [Google Scholar] [CrossRef]
- Saylan, Y.; Kılıç, S.; Denizli, A. Biosensing Applications of Molecularly Imprinted-Polymer-Based Nanomaterials. Processes 2024, 12, 177. [Google Scholar] [CrossRef]
- Gadelhak, Y.; Hafez, S.H.M.; Mohamed, H.F.M.; Abdel-hady, E.E. Nanomaterials-modified disposable electrodes and portable electrochemical systems for heavy metals detection in wastewater streams: A review. Microchem. J. 2023, 193, 109043. [Google Scholar] [CrossRef]
- Ranveer, A.; Nasipude, S.; Bagwan, S. Biosensor for environmental monitoring. Int. J. Innov. Eng. Res. Technol. 2015, 2, 1–8. [Google Scholar]
- Khan, Z.H.; Kermany, A.R.; Öchsner, A.; Iacopi, F. Mechanical and electromechanical properties of graphene and their potential application in MEMS. J. Phys. D Appl. Phys. 2017, 50, 053003. [Google Scholar] [CrossRef]
- Che, N.; Chik, E.; Mahdi, M.A. Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Bio-Sens. Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Vonnie, J.M.; Rovina, K.; Mariah, A.M.A.; Erna, K.H.; Felicia, W.X.L.; ‘Aqilah, M.N. Trends in Nanotechnology Techniques for Detecting Heavy Metals in Food and Contaminated Water: A Review. Int. J. Environ. Sci. Technol. 2023, 20, 8041–8072. [Google Scholar] [CrossRef]
- He, W.; You, M.; Li, Z.; Cao, L.; Xu, F.; Li, F.; Li, A. Upconversion nanoparticles-based lateral flow immunoassay for point-of-care diagnosis of periodontitis. Sens. Actuators B Chem. 2021, 334, 129673. [Google Scholar] [CrossRef]
- Saliev, T. The Advances in Biomedical Applications of Carbon Nanotubes. C 2019, 5, 29. [Google Scholar] [CrossRef]
- Lan, H.; Jamil, M.; Ke, G.; Dong, N. The role of nanoparticles and nanomaterials in cancer diagnosis and treatment: A comprehensive review. Am. J. Cancer Res. 2023, 13, 5751–5784. [Google Scholar]
- Hooshiar, M.H.; Moghaddam, M.A.; Kiarashi, M.; Al-Hijazi, A.Y.; Hussein, A.F.; Alrikabi, H.A.; Salari, S.; Esmaelian, S.; Mesgari, H.; Yasamineh, S. Recent advances in nanomaterial-based biosensor for periodontitis detection. J. Biol. Eng. 2024, 18, 28. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Nayak, N.; Karmakar, S.; Chopra, A.; Arangaraju, R. Evolutionary History of Periodontitis and the Oral Microbiota—Lessons for the Future. Curr. Oral Health Rep. 2024, 11, 105–116. [Google Scholar] [CrossRef]
- D’Amico, E.; Aceto, G.M.; Petrini, M.; Cinquini, C.; D’Ercole, S.; Iezzi, G.; Pierfelice, T.V. How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy? Int. J. Mol. Sci. 2025, 26, 592. [Google Scholar] [CrossRef] [PubMed]
- Tokede, B.; Yansane, A.; Brandon, R.; Lin, G.H.; Lee, C.T.; White, J.; Jiang, X.; Lee, E.; Alsaffar, A.; Walji, M.; et al. The burden of diagnostic error in dentistry: A study on periodontal disease misclassification. J. Dent. 2024, 148, 105221. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Fontenele, R.C.; Lahoud, P.; Shujaat, S.; Bornstein, M.M. Radiographic diagnosis of periodontal diseases—Current evidence versus innovations. Periodontology 2000 2024, 95, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A. Diagnostic measures for monitoring and follow-up in periodontology and implant dentistry. Periodontology 2000 2024, 95, 129–155. [Google Scholar] [CrossRef]
- Kiarashi, M.; Mahamed, P.; Ghotbi, N.; Tadayonfard, A.; Nasiri, K.; Kazemi, P.; Badkoobeh, A.; Yasamineh, S.; Joudaki, A. Spotlight on therapeutic efficiency of green synthesis metals and their oxide nanoparticles in periodontitis. J. Nanobiotechnol. 2024, 22, 21. [Google Scholar] [CrossRef]
- Zhang, S.; Kong, N.; Wang, Z.; Zhang, Y.; Ni, C.; Li, L.; Wang, H.; Yang, M.; Yang, W.; Yan, F. Nanochemistry of gold: From surface engineering to dental healthcare applications. Chem. Soc. Rev. 2024, 53, 3656–3686. [Google Scholar] [CrossRef]
- Li, D.; Qiu, Y.; Zhang, S.; Zhang, M.; Chen, Z.; Chen, J. A Multifunctional Antibacterial and Osteogenic Nanomedicine: QAS-Modified Core-Shell Mesoporous Silica Containing Ag Nanoparticles. BioMed Res. Int. 2020, 2020, 4567049. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Gupta, G.; Kesharwani, P.; Shukla, R. Revolutionizing periodontic care: Nano Dentistry’s impact on inflammation management. J. Drug Deliv. Sci. Technol. 2024, 99, 105922. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Zhong, Y.; Li, R.; Deng, W.; Xiao, X.; Xu, Y.; Zhang, J.; Hu, X.; Wang, Y. A review of nanomaterials for biosensing applications. J. Mater. Chem. B 2023, 12, 1168–1193. [Google Scholar] [CrossRef]
- Yun, Y.H.; Eteshola, E.; Bhattacharya, A.; Dong, Z.; Shim, J.S.; Conforti, L.; Kim, D.; Schulz, M.J.; Ahn, C.H.; Watts, N. Tiny medicine: Nanomaterial-based biosensors. Sensors 2009, 9, 9275. [Google Scholar] [CrossRef]
- Sethuraman, S.; Ramalingam, K.; Ramani, P.; Kalaiyarasan, M. Nanomaterial Biosensors in Salivary Diagnosis of Oral Cancer: A Scoping Review. Cureus 2024, 16, e59779. [Google Scholar] [CrossRef]
- Faiz, N.; Sivaswamy, V.; Rohinikumar, S. Green Synthesis of Gold Nanoparticles Using Eucalyptus and Piper Longum and Antimicrobial Activity Evaluation. Indian J. Dent. Res. 2024, 35, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (Bio)sensors with biomedical applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Shaban, S.M.; Cho, S.Y.; Kim, D.H. Detection of Periodontal Disease Marker with Geometrical Transformation of Ag Nanoplates. Anal. Chem. 2023, 95, 2356–2365. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Kim, M.; Lee, A.; Ji, S.; Jang, M.; Yim, S.; Song, W.; Lee, S.S.; Yoon, D.H.; An, K.S. Nanometric lamination of zinc oxide nanofilms with gold nanoparticles for self-perceived periodontal disease sensors. Compos. Part B Eng. 2022, 230, 109490. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Z.; Liu, X.; Liu, L.; Jiang, S.; Zhang, Z.; Li, Y.; Pan, S. Activated silver nanoparticle-based platform for specific capture of Porphyromonas gingivalis in human saliva. Sens. Actuators B Chem. 2024, 403, 135171. [Google Scholar] [CrossRef]
- Tong, S.; Zhu, H.; Bao, G. Magnetic iron oxide nanoparticles for disease detection and therapy. Mater. Today 2019, 31, 86–99. [Google Scholar] [CrossRef]
- Gu, N.; Zhang, Z.; Li, Y. Adaptive iron-based magnetic nanomaterials of high performance for biomedical applications. Nano Res. 2022, 15, 1–17. [Google Scholar] [CrossRef]
- Palomar, Q.; Svärd, A.; Zeng, S.; Hu, Q.; Liu, F.; Aili, D.; Zhang, Z. Detection of gingipain activity using solid state nanopore sensors. Sens. Actuators B Chem. 2022, 368, 132209. [Google Scholar] [CrossRef]
- Ali Alftaikhah, S.A.; Issrani, R.; Alnasser, M.; Almutairi, H.A.; Khattak, O.; Iqbal, A.; Prabhu, N. Salivary Biomarkers in Periodontitis: A Scoping Review. Cureus 2023, 15, e50207. [Google Scholar] [CrossRef] [PubMed]
- Nangare, S.; Patil, S.; Patil, S.; Khan, Z.; Patil, A.; Patil, P. Design of graphene quantum dots decorated MnO2 nanosheet based fluorescence turn “On-Off-On” nanoprobe for highly sensitive detection of lactoferrin. Inorg. Chem. Commun. 2022, 143, 109751. [Google Scholar] [CrossRef]
- Fu, C.; Brand, H.S.; Bikker, F.J. The applications of carbon dots in oral health: A scoping review. Oral Dis. 2024, 30, 1861–1872. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, C.; Mo, J.; Wang, X.; Wang, T.; Li, G.; Zhou, Q. Recent progress in carbon dots for anti-pathogen applications in oral cavity. Front. Cell. Infect. Microbiol. 2023, 13, 1251309. [Google Scholar] [CrossRef]
- Shamsabadi, A.; Haghighi, T.; Carvalho, S.; Frenette, L.C.; Stevens, M.M. The Nanozyme Revolution: Enhancing the Performance of Medical Biosensing Platforms. Adv. Mater. 2024, 36, 2300184. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Jiang, C.; Huang, Y. The recent development of nanozymes for targeting antibacterial, anticancer and antioxidant applications. RSC Adv. 2023, 13, 1539–1550. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Qian, K. Nanozymes: Applications in clinical biomarker detection. Interdiscip. Med. 2023, 1, e20230020. [Google Scholar] [CrossRef]
- Witkowska, E.; Łasica, A.M.; Niciński, K.; Potempa, J.; Kamińska, A. In Search of Spectroscopic Signatures of Periodontitis: A SERS-Based Magnetomicrofluidic Sensor for Detection of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. ACS Sens. 2021, 6, 1621–1635. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, M.; Cai, H. Editorial: Advanced nano-bio interfaces for biosensing and diagnostics. Front. Bioeng. Biotechnol. 2023, 11, 1296849. [Google Scholar] [CrossRef]
- Omara, W.S.; Alghamdi, M.A.; Taymour, N. Nanomaterials Based Sensors: From Preparation to Versatile Applications. In Recent Progress and Development on Nanostructures; Intech: London, UK, 2024; pp. 1–18. [Google Scholar] [CrossRef]
- Tranby, E.P.; Heaton, L.J.; Tomar, S.L.; Kelly, A.L.; Fager, G.L.; Backley, M.; Frantsve-Hawley, J. Oral Cancer Prevalence, Mortality, and Costs in Medicaid and Commercial Insurance Claims Data. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1849–1857. [Google Scholar] [CrossRef]
- Goldoni, R.; Scolaro, A.; Boccalari, E.; Dolci, C.; Scarano, A.; Inchingolo, F.; Ravazzani, P.; Muti, P.; Tartaglia, G. Malignancies and biosensors: A focus on oral cancer detection through salivary biomarkers. Biosensors 2021, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, B.; van de Ven, A.L.; Tam, J.O.; Gillenwater, A.M.; Sokolov, K.V.; Richards-Kortum, R.R.; Roy, K. Efficient mucosal delivery of optical contrast agents using imidazole-modified chitosan. J. Biomed. Opt. 2010, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Vetrivel, C.; Sivarasan, G.; Durairaj, K.; Ragavendran, C.; Kamaraj, C.; Karthika, S.; Lo, H.M. MoS2-ZnO Nanocomposite Mediated Immunosensor for Non-Invasive Electrochemical Detection of IL8 Oral Tumor Biomarker. Diagnostics 2023, 13, 1464. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Kim, H.G.; Achary, P.G.R.; Pakawanit, P.; Yang, Y.; Mishra, Y.K.; Kim, H.J. Sustainable Solutions for Oral Health Monitoring: Biowaste-Derived Triboelectric Nanogenerator. ACS Appl. Mater. Interfaces 2023, 15, 36096–36106. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.; Ganapathy, D.; Ramadoss, R.; Atchudan, R.; Arya, S.; Sundramoorthy, A.K. Biosensors for rapid and accurate determination of oral cancer. Oral Oncol. Rep. 2023, 5, 100021. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mukherjee, A.; Ethiraj, K.R. Gold nanorod-based multiplex bioanalytical assay for the detection of CYFRA 21-1 and CA-125: Towards oral cancer diagnostics. Anal. Methods 2022, 14, 3614–3622. [Google Scholar] [CrossRef]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Gorgoń, S.; Radoń, A.; Bajdak-Rusinek, K. Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef]
- Jungcharoen, P.; Thivakorakot, K.; Thientanukij, N.; Kosachunhanun, N.; Vichapattana, C.; Panaampon, J.; Saengboonmee, C. Magnetite nanoparticles: An emerging adjunctive tool for the improvement of cancer immunotherapy. Explor. Target. Anti-tumor Ther. 2024, 5, 316–331. [Google Scholar] [CrossRef]
- Abdelaziz, M. Detection, Diagnosis, and Monitoring of Early Caries: The Future of Individualized Dental Care. Diagnostics 2023, 13, 3649. [Google Scholar] [CrossRef]
- Al Saffan, A.D. Current approaches to diagnosis of early proximal carious lesion: A literature review. Cureus 2023, 15, e43489. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in dentistry: A comprehensive review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Glowacka-Sobotta, A.; Ziental, D.; Czarczynska-Goslinska, B.; Michalak, M.; Wysocki, M.; Güzel, E.; Sobotta, L. Nanotechnology for Dentistry: Prospects and Applications. Nanomaterials 2023, 13, 2130. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Xu, V.W.; Yin, I.X.; Yu, O.Y.; Chu, C.-H. Metal and metal oxide nanoparticles in caries prevention: A review. Nanomaterials 2021, 11, 3446. [Google Scholar] [CrossRef]
- Patel, J.; Kumar, G.S.; Roy, H.; Maddiboyina, B.; Leporatti, S.; Bohara, R.A. From nature to nanomedicine: Bioengineered metallic nanoparticles bridge the gap for medical applications. Discov. Nano 2024, 19, 85. [Google Scholar] [CrossRef]
- Nafarrate-Valdez, R.A.; Martínez-Martínez, R.E.; Zaragoza-Contreras, E.A.; Áyala-Herrera, J.L.; Domínguez-Pérez, R.A.; Reyes-López, S.Y.; Donohue-Cornejo, A.; Cuevas-González, J.C.; Loyola-Rodríguez, J.P.; Espinosa-Cristóbal, L.F. Anti-Adherence and Antimicrobial Activities of Silver Nanoparticles against Serotypes C and K of Streptococcus mutans on Orthodontic Appliances. Medicina 2022, 58, 877. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Udduttulla, A.; Xu, V.W.; Chen, K.J.; Zhang, M.Y.; Chu, C.H. Use of Antimicrobial Nanoparticles for the Management of Dental Diseases. Nanomaterials 2025, 15, 209. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Mohammad Albar, N.H.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef]
- Ahalya, P.; Uloopi, K.S.; Vinay, C.; RojaRamya, K.S.; Alla, R.; RangaRaju, P. Evaluation of Dentin Remineralization with Zinc Oxide and Calcium Fluoride Nanoparticles—An In Vitro Study. Contemp. Clin. Dent. 2023, 14, 57–61. [Google Scholar] [CrossRef]
- Li, C.; Che, B.; Deng, L. Electrochemical biosensors based on carbon nanomaterials for diagnosis of human respiratory diseases. Biosensors 2022, 13, 12. [Google Scholar] [CrossRef]
- Xue, J.; Dong, H.; Ji, L.; Wang, Y.; Zhang, J. Peptide-Functionalized ZnSe:Mn Quantum Dots as Fluorescent Probes for Accurate Localization of Hidden Dental Lesion Sites. ACS Appl. Nano Mater. 2023, 6, 14431–14438. [Google Scholar] [CrossRef]
- Jones, N.A.; Chang, S.-R.; Troske, W.J.; Clarkson, B.H.; Lahann, J. Nanoparticle-Based Targeting and Detection of Microcavities. Adv. Healthc. Mater. 2017, 6, 1600883. [Google Scholar] [CrossRef] [PubMed]
- Mady, S.; Alzayyat, N. Nanotechnology in targeting and detection of microcavities. In Nanotechnology in Conservative Dentistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 29–47. ISBN 9780323902823. [Google Scholar]
- Eissa, S.; Tlili, C.; Mounir, B.A.; Kanoun, O. Editorial: Nanomaterials-based electrochemical biosensors. Front. Bioeng. Biotechnol. 2022, 10, 1592. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterial-Based Bacterial Sensors. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 329–359. [Google Scholar] [CrossRef]
- Taymour, N.; Fahmy, A.E.; Gepreel, M.A.H.; Kandil, S.; El-Fattah, A.A. Improved Mechanical Properties and Bioactivity of Silicate Based Bioceramics Reinforced Poly(ether-ether-ketone) Nanocomposites for Prosthetic Dental Implantology. Polymers 2022, 14, 1632. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.R.; Hong, M.H. Improved Biocompatibility and Osseointegration of Nanostructured Calcium-Incorporated Titanium Implant Surface Treatment (XPEED®). Materials 2024, 17, 2707. [Google Scholar] [CrossRef] [PubMed]
- Westas, E.; Svanborg, L.M.; Wallin, P.; Bauer, B.; Ericson, M.B.; Wennerberg, A.; Mustafa, K.; Andersson, M. Using QCM-D to study the adhesion of human gingival fibroblasts on implant surfaces. J. Biomed. Mater. Res.—Part A 2015, 103, 3139–3147. [Google Scholar] [CrossRef]
- Mujahid, M.H.; Upadhyay, T.K.; Khan, F.; Pandey, P.; Park, M.N.; Sharangi, A.B.; Saeed, M.; Upadhye, V.J.; Kim, B. Metallic and metal oxide-derived nanohybrid as a tool for biomedical applications. Biomed. Pharmacother. 2022, 155, 113791. [Google Scholar] [CrossRef]
- Alassy, H.; Parachuru, P.; Wolff, L. Peri-Implantitis Diagnosis and Prognosis Using Biomarkers in Peri-Implant Crevicular Fluid: A Narrative Review. Diagnostics 2019, 9, 214. [Google Scholar] [CrossRef]
- Moaven, H.; Giacaman, A.; Beltrán, V.; Sam, Y.H.; Betancur, D.; Mainas, G.; Tarjomani, S.A.; Donos, N.; Sousa, V. Biomarker Expression of Peri-Implantitis Lesions before and after Treatment: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4085. [Google Scholar] [CrossRef]
- Kaefer, K.; Krüger, K.; Schlapp, F.; Uzun, H.; Celiksoy, S.; Flietel, B.; Heimann, A.; Schroeder, T.; Kempski, O.; Sönnichsen, C. Implantable Sensors Based on Gold Nanoparticles for Continuous Long-Term Concentration Monitoring in the Body. Nano Lett. 2021, 21, 3325–3330. [Google Scholar] [CrossRef]
- Navin, K.; Kurchania, R. Nanosensors based on magnetic materials. In Emerging Applications of Low Dimensional Magnets; CRC Press: Boca Raton, FL, USA, 2022; pp. 115–136. ISBN 9781003196952. [Google Scholar]
- Thomas, S. Nanomaterials in Dental Medicine; Materials Horizons: From Nature to Nanomaterials; Springer Nature: Singapore, 2023; ISBN 9789811987175. [Google Scholar]
- Behera, B.C.; Sarangi, S.N.; Sahoo, N.K.; Dash, S.P.; Tripathy, S.K. Magnetic Nanoparticles-Based Novel Sensors for Select Biomedical/Biological Science Applications. In Biomaterials-Based Sensors: Recent Advances and Applications; Kumar, P., Dash, S.K., Ray, S., Parween, S., Eds.; Springer Nature: Singapore, 2023; pp. 325–348. ISBN 978-981-19-8500-3. [Google Scholar]
- Buder, K.; Kaefer, K.; Flietel, B.; Uzun, H.; Schroeder, T.; Sönnichsen, C. Integrating Nanosensors into Macroporous Hydrogels for Implantation. ACS Appl. Bio Mater. 2022, 5, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, T.; Wang, H.; Wang, Z.; Hou, L.; Jiang, J.; Xu, T. Applications of nanomaterial technology in biosensing. J. Sci. Adv. Mater. Devices 2024, 9, 100694. [Google Scholar] [CrossRef]
- Das, P.; Deshmukh, R.S. Quantum dots: The trailblazers of early detection Pushpanjali. J. Oral Maxillofac. Pathol. 2024, 28, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Q.; Wang, Z.L.; Li, Z. Nanogenerator-Based Self-Powered Sensors for Wearable and Implantable Electronics. Research 2020, 2020, 8710686. [Google Scholar] [CrossRef]
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and their widespread impact on human health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]


| Parameter | Nanomaterial-Based Biosensors | Conventional Diagnostic Techniques |
|---|---|---|
| Specificity | High specificity for biomarkers (e.g., MMP-8, IL-1β) and pathogens (e.g., P. gingivalis) via functionalized nanomaterials [29]. | Moderate specificity: relies on clinical signs (e.g., probing depth, bleeding) or culture-based methods, which may miss early biomarkers [19]. |
| Turnaround Time | Rapid detection (<30 min) for real-time monitoring [30]. | Hours to days for laboratory results (e.g., ELISA, PCR, microbial cultures) [19]. |
| Integration into Workflow | Portable designs (e.g., microfluidic chips) enable chairside use but require training [31]. | Well-established in clinics (e.g., periodontal probes, radiographs) but limited to subjective assessments [19]. |
| Cost-Effectiveness | Higher initial development costs but lower per-test expenses. | Low initial costs but recurring expenses for reagents, lab processing, and specialist interpretation [29]. |
| Key Limitations | Biocompatibility concerns, scalability challenges, and need for standardized validation [19]. | Limited sensitivity for early diseases, invasive sampling (GCF), and inability to predict disease progression [19]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taymour, N.; Hassan, M.G.; AlGhamdi, M.A.; Omara, W.S. From Detection to Treatment: Nanomaterial-Based Biosensors Transforming Prosthetic Dentistry and Oral Health Care: A Scoping Review. Prosthesis 2025, 7, 51. https://doi.org/10.3390/prosthesis7030051
Taymour N, Hassan MG, AlGhamdi MA, Omara WS. From Detection to Treatment: Nanomaterial-Based Biosensors Transforming Prosthetic Dentistry and Oral Health Care: A Scoping Review. Prosthesis. 2025; 7(3):51. https://doi.org/10.3390/prosthesis7030051
Chicago/Turabian StyleTaymour, Noha, Mohamed G. Hassan, Maram A. AlGhamdi, and Wessam S. Omara. 2025. "From Detection to Treatment: Nanomaterial-Based Biosensors Transforming Prosthetic Dentistry and Oral Health Care: A Scoping Review" Prosthesis 7, no. 3: 51. https://doi.org/10.3390/prosthesis7030051
APA StyleTaymour, N., Hassan, M. G., AlGhamdi, M. A., & Omara, W. S. (2025). From Detection to Treatment: Nanomaterial-Based Biosensors Transforming Prosthetic Dentistry and Oral Health Care: A Scoping Review. Prosthesis, 7(3), 51. https://doi.org/10.3390/prosthesis7030051








