Abstract
Objectives: Traditional 2D cell cultures on flat surfaces fail to replicate 3D environments, affecting cellular morphology and function. Various 3D techniques (e.g., spheroids, organoids, organs-on-chips, 3D bioprinting) have been used for disease modelling and drug testing, but their application in hard tissues remains challenging. This study aimed to develop a biocompatible 3D culture method for bone tissue organoids using human bone marrow-derived stem cells (hBMSCs) and hydrogels. Methods: hBMSCs were isolated from human jawbone marrow. The control group was cultured under 2D conditions, whereas the experimental group was cultured in a 3D hydrogel environment. In vitro analyses, including flow cytometry and RNA sequencing, were performed. Quantitative data were statistically analysed at a 0.05 level of significance. Results: hBMSCs cultured in 3D hydrogel conditions indicated enhanced reproducibility, increased cell viability, and significant osteogenic differentiation. Genes such as MMP-13, LPL, and SP7 showed substantially higher expression in 3D cultures, with protein-level confirmation by Western blot. These findings suggest that 3D culture more effectively supports the natural growth and differentiation of hBMSCs. Conclusions: Culturing hBMSCs in a 3D environment more closely mimics in vivo conditions, thus promoting the expression and activity of critical proteins involved in hBMSC differentiation.
1. Introduction
In traditional two-dimensional (2D) cultures, cells grow as a monolayer on a flat surface coated with cell adhesion materials [1,2]. This method is straightforward and cost-effective, making it widely used in biotechnology and medicine for toxicity analysis, drug development, and biological activity assessment. However, this approach differs from the natural environment of the body, where cells proliferate in layers and actively interact with each other and their surroundings. These differences can affect cellular gene expression, often resulting in low similarity to in vivo conditions, and cause significant variations in cell differentiation, growth rates, mechanical stimuli responses, and drug responsiveness [3,4,5,6,7,8]. In contrast, 3D cell culture replicates the body’s environment in a laboratory. Therefore, it is a highly biocompatible experimental method capable of producing results that are unattainable with 2D cell cultures or direct in vivo methods. In 3D cultures, cells are cultured within a gel-like matrix or a hard scaffold, such as a bone. These structures can be stimulated by the local environment, as observed in vivo, through active cell–cell and cell–environment interactions [9,10]. Additionally, 3D cultures can be used to study cellular topology, gene expression, signalling, and metabolism [11,12,13,14,15,16,17,18].
Bone marrow mesenchymal stem cells (BMSCs) are multipotent adult stem cells that differentiate into various lineages, including chondrocytes, osteoblasts, and adipocytes [19,20,21,22,23,24]. BMSCs can directly differentiate into osteoblasts that can further differentiate into mature osteocytes or transform into lipid-storing cells [25,26,27]. BMSCs secrete various growth factors, such as bone morphometric proteins (BMPs), vascular endothelial growth factor (VEGF), and transforming growth factor-beta that support tissue repair and regeneration therapy [19,28,29]. BMSCs are relatively easy to obtain and possess self-renewal capabilities [30]. Additionally, the autologous transplantation of BMSCs causes minimal trauma, no rejection, and almost no complications after transplantation [31]. Owing to these advantages, BMSCs are used to treat large bone defects in humans [32]. In a rat model, BMSCs can accelerate ossification and restore bone mechanical properties in osteoporotic fractures [33,34]. Additionally, bone morphogenetic protein 2/VEGF co-expressing BMSC sheets facilitate bone regeneration in critical-sized calvarial defects in mice [35]. These findings indicate that BMSCs have significant potential to enhance bone healing.
Hydrogels—3D cross-linked polymer networks—serve as excellent scaffolds for cell culture by mimicking the extracellular matrix (ECM) [36]. These hydrogels facilitate 3D cell culture using the surrounding cells in a natural ECM environment [37]. Using these hydrogels, we aimed to cultivate BMSCs in a 3D set-up to assess the differences compared to 2D cultures and confirm that 3D methods replicate the in vivo environment.
Recently, 3D culture techniques using hydrogels or Matrigel have successfully developed soft tissue models, such as those for the brain, lungs, liver, prostate, fallopian tubes, and oesophagus, for disease modelling and drug testing [38]. Despite significant efforts in 3D bone tissue culture, replicating the complex bone structure remains challenging because of its ongoing remodelling and interaction with collagen and minerals [39,40]. Despite these challenges, a definitive 3D method for culturing bone replacements is yet to be developed. The purpose of this study was to establish a biocompatible 3D culture method for the development of bone tissue organoids using human bone marrow-derived stem cells (hBMSCs) and hydrogels. This study hypothesised that culturing human BMSCs (hBMSCs) from the jawbone marrow in a 3D hydrogel environment may exhibit gene and protein expression distinct from those observed in 2D cultures. This study assessed the technical reproducibility, cell viability, and differentiation potential of hBMSCs in the 3D hydrogel culture and compared it to the 2D method to assess whether 3D culture methods can support natural cellular growth and differentiation.
2. Materials and Methods
2.1. Isolation and Culture of hBMSCs
Primary hBMSCs were isolated from the jawbone tissue harvested from patients who visited the Department of Oral and Maxillofacial Surgery, Seoul National University Dental Hospital, and underwent wisdom tooth extraction surgery. The procedures adhered to the Declaration of Helsinki and were approved by the Institutional Review Board of Seoul National University Dental Hospital (IRB number CRI24007). After obtaining written informed consent, jawbone specimens were collected during surgery, kept on ice, transported to the laboratory, and washed 2–3 times with 1× phosphate-buffered saline (PBS) in a sterile environment. The bone marrow was flushed out using alpha Minimum Essential Medium (12571-063; Gibco, Waltham, MA, USA) supplemented with 10% foetal bovine serum (#SFBU30-2597; Equitec Bio Inc., Kerrville, TX, USA) and 1% penicillin/streptomycin (15140-122; Gibco). The cells were collected into a 1 mL tube and centrifuged (500 rcf, 4 °C, 5 min, HANIL SCIENCE INDUSTRIAL, combi-5I4R). The supernatant was discarded and erythrocytes were lysed using 1× RBC lysis buffer (#420301; BioLegend, San Diego, CA, USA). After 3 min of lysis, the cells were washed with 1× PBS and collected by centrifugation (500 rcf, 5 min, 4 °C). The supernatant was discarded, and the cell pellet was suspended in a culture medium, filtered through a 100 μm nylon filter (Millipore, Burlington, MA, USA), and cultured in dishes at 37 °C with 5% CO2. Primary hBMSCs were subcultured and used for 2D vs. 3D comparison experiments at passage 3.
2.2. Hydrogel 3D Culture
The insert was placed in a 12-well culture plate (#665613; Greiner Bio-One, Kremsmünster, Austria) and pre-wetted with 1x PBS. Approximately 0.8 × 106 cells were suspended in the culture medium, and hydrogel (VHM03; TheWell Bioscience, North Brunswick, NJ, USA) was added after aspirating the PBS. Following a 10–15 min incubation at 20–25 °C, the outer and inner media were added, and the cells were incubated at 37 °C with 5% CO2. The cells were cultured in a 3D hydrogel environment for 21 days, and the medium was changed every 2–3 days.
2.3. Recovery of the 3D-Cultured Cells
On day 21 of incubation, the hydrogels were washed twice with Dulbecco’s PBS. Subsequently, 1 mL of cell recovery solution (MS03-100; TheWell Bioscience) was added to the wells and the hydrogel was broken into small pieces by gentle pipetting. The hydrogel fragments were transferred to a 15 mL conical tube containing 5 mL of the cell recovery solution, mixed by pipetting 3–5 times, and incubated in a 37 °C water bath for 2–3 min. The cells were centrifuged (100× g, 5 min, room temperature), and the cell pellet was collected.
2.4. Two-Dimensional Culture
The cells for the 3D culture were seeded in a 12-well culture plate and cultured for 21 days, with 4–6 subcultures. Upon harvest, the cells had a passage number of 7–9.
2.5. Flow Cytometry
The cultured cells were harvested, placed in a 3 mL flow cytometry tube, and washed once with 1x PBS. After discarding the supernatant, Ghost Dye (#13-0870-T100, violet 510; TONBO Biosciences, San Diego, CA, USA) was added to remove dead cells and incubated for 30 min at 4 °C. Following washing with fluorescence-activated cell sorting buffer, the cells were stained with fluorochrome-labelled monoclonal antibodies against CD90 (#328107; BioLegend), CD73 (#12-0739-42; Invitrogen, Waltham, MA, USA), CD105 (#323217; BioLegend), CD45 (#563716; BD Biosciences, Franklin Lakes, NJ, USA), CD34 (#378603; BioLegend), and human leukocyte antigen-DR (HLA-DR) (#307609; BioLegend), and incubated for 30 min at 4 °C. Data were acquired using an LSR Fortessa X-20 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analysed using FlowJo v10.7.2_CL. Live cells were gated by excluding Ghost Dye-stained dead cells.
2.6. Live/Dead Assay for 3D-Cultured Cells
The LIVE/DEAD assay kit (Cat# BM01; TheWell Bioscience) was used at 20–25 °C. A reagent (2 µL) was added per 100 μL of the total volume in each well. The cells were incubated at 37 °C for 5–10 min. The images were captured using a confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany).
2.7. RNA Sequencing (RNA-seq) Analysis
RNA-seq was performed using the Novoseq6000 platform. RNA samples with RNA integrity number values > 4.0 were used for downstream library construction. Messenger RNA was isolated from total RNA using poly T oligo-attached magnetic beads. After fragmentation, first-strand cDNA was synthesised using random hexamer primers, followed by second-strand cDNA synthesis using either dUTP for the directional library or dTTP for the nondirectional library. A nondirectional library was prepared through end repair, A-tailing, adapter ligation, size selection, amplification, and purification. The library was quantified using Qubit and real-time polymerase chain reaction, and a bioanalyzer to detect the size distribution. The quantified libraries were pooled and sequenced on Illumina platforms based on effective library concentration and data quantity. Each sequencing library produced at least 20 million unique mapped reads. This analysis was repeated three times.
2.8. Immunoblot Analysis
Cells harvested from the 2D and 3D cultures were washed twice with ice-cold PBS and dissolved in 60 mL of cell lysis buffer. Subsequently, the samples were sonicated and centrifuged for 25 min. The supernatants were boiled with 5× sodium dodecyl sulphate (SDS) sample buffer for 5 min, resolved on SDS-polyacrylamide gel electrophoresis (10%) gels, and transferred to polyvinylidene fluoride membranes that were incubated overnight at 4 °C with the following primary antibodies—matrix metalloproteinase-13 (MMP-13) (#69926; Cell Signaling Technology, Danvers, MA, USA), lipoprotein lipase (LPL) (AF7197; R&D Systems, Minneapolis, MN, USA), specificity protein-7 (SP7) (ab209484; Abcam, Cambridge, UK), and glyceraldehyde 3-phosphate dehydrogenase (K200057M; Solarbio, Beijing, China). After six washes in Tris-buffered saline containing 0.1% Tween 20, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at 20–25 °C. The blots were washed and visualised by chemiluminescence using an ECL Western blot detection system (PRN3243; Amersham, Buckinghamshire, UK). Quantitation was performed using densitometry with ImageJ software (version 1.54, National Institutes of Health). This analysis was repeated three times.
2.9. Phalloidin Staining
For phalloidin staining, hBMSCs were cultured in a 2D or 3D environment and washed with cold PBS after removing the culture medium. The cells were fixed with 4% paraformaldehyde (HPFA0410; BYLABS, Hanam, Korea) for 20 min and washed with cold PBS. Subsequently, the cells were treated with 3% bovine serum albumin (A3294; Sigma, St. Louis, MO, USA) and 0.1% Triton X-100 in PBS for 20 min. The cells were stained with a phalloidin mixture (A12379, 1:1000; Invitrogen) in the dark for 1 h at 20–25 °C with gentle agitation. Subsequently, the cells were washed twice with cold PBS and stained with 4′,6-diamidino-2-phenylindole (D1306; Invitrogen) for 15 min. Finally, the cells were mounted on a Fluorescence Mounting Medium (AB104135; Abcam) and imaged using a confocal microscope (Zeiss).
2.10. Statistical Analysis
Statistical analyses were conducted using Prism 8 (GraphPad, Boston, MA, USA), and the data are presented as the mean ± standard deviation. The statistical significance between groups was calculated using Student’s t-test. Statistical significance was set at p < 0.05 (* p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001).
3. Results
3.1. Experimental Process and Characterisation of hBMSCs
We isolated BMSCs from human jawbone fragments and cultured them up to passage 3 to generate sufficient quantities for experimentation. For the 2D culture, 2.2 × 106 cells were seeded in a 100 mm dish, whereas 0.8 × 106 cells were mixed with the hydrogel for the 3D culture and placed into an insert (Figure 1A). The cells were cultured for 21 days, during which the 2D-cultured cells were subcultured three–four times, reaching passages 6–7 by day 21. To confirm the identity of cells as mesenchymal stem cells (MSCs), we performed flow cytometry analysis. CD34, CD45, and HLA-DR were used as haematopoietic markers, whereas CD73, CD90, and CD105 served as stem cell markers. The cells isolated from the human jawbone exhibited minimal expression of haematopoietic markers and were positive for stem cell markers. Notably, CD105 expression was lower than that of the other two stem cell markers, but higher than that of the haematopoietic markers (Figure 1B). The expression of CD105 can be affected by various biological and environmental factors. CD105 is highly expressed in the undifferentiated state and tends to decrease as differentiation progresses. When MSCs are cultured in a spherical three-dimensional manner in a U-shaped bottom or 96-inch ultra-low attachment well plate, CD105 expression decreases [41]. One study also finds that CD105 expression decreases when cultured for a long period in a medium containing serum. We believe that the reason for the decrease in CD105 in our study is due to long-term culturing [42]. These results indicated that the cells used in the experiment were stem cells.
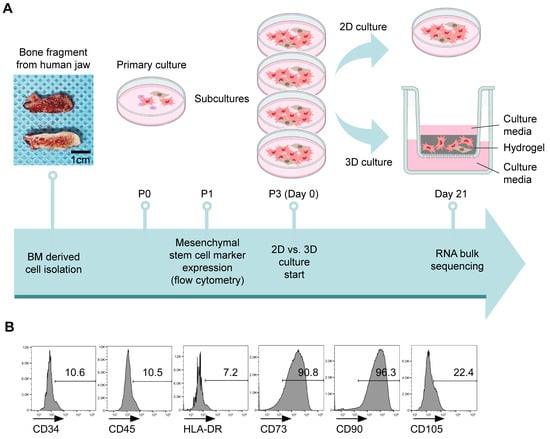
Figure 1.
Experimental procedure and characterisation of human bone marrow-derived stem cells (hBMSCs). (A) Overview of the 3D and 2D culture processes following hBMSC isolation. (B) Flow cytometry analysis confirmed that human jawbone marrow-isolated cells are mesenchymal stem cells (MSCs) and exhibited positive expression of MSC markers (CD73, CD90, and CD105) and negative expression of the endothelial marker (CD34) and immune cell markers (CD45 and HLA-DR). The histograms represent three independent experiments. Numbers indicate the frequency of positive staining for each surface marker. The data represent the mean ± standard deviation of three independent experiments.
3.2. Differential Gene Expression Between 2D- and 3D-Cultured hBMSCs
On day 21 of culture, 2D- and 3D-cultured cells were observed under a microscope to assess their morphology in various environments. The 2D-cultured cells were elongated, whereas the 3D-cultured cells were round, with numerous branches connecting them to the neighbouring cells (Figure 2A (2D, 3D)). Because 3D cultures do not support monolayer growth, a live/dead assay was conducted to assess cell viability within the 3D matrix. At day 21, although a few dead cells were observed, the majority remained alive (Figure 2A (3D (live/dead)).
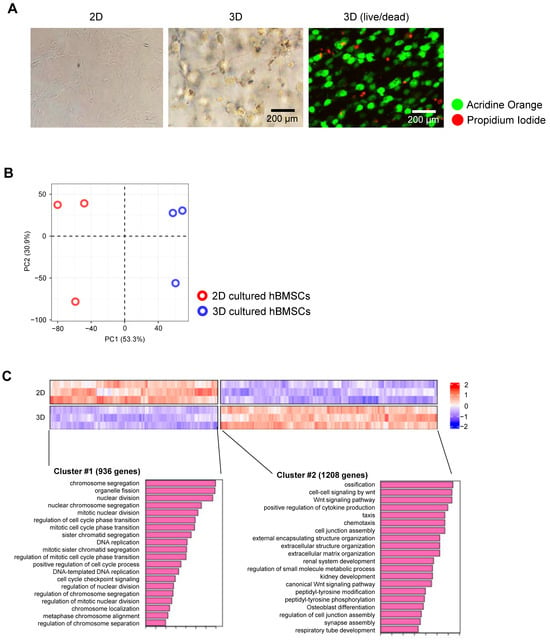
Figure 2.
Characterisation of 3D-cultured human bone marrow-derived stem cells (hBMSCs). (A) Images of hBMSCs cultured for 21 days: 2D culture, 3D culture (Digital Inverted Fluorescence Microscope; Nikon), and 3D live/dead imaging (LSM 700; ZEISS). (B) Principal component analysis plots of RNA sequencing (RNA-seq) data demonstrating different characteristics of 2D- and 3D-cultured hBMSCs based on gene expression levels. (n = 3). (C) Heatmap of gene expression using RNA-seq for differentially expressed genes (DEGs) between 2D- and 3D-cultured hBMSCs, with red and blue indicating higher and lower expression levels, respectively. DEGs are defined as genes with a log2 fold-change value of <−1 or >1 and false discovery rate-adjusted (adjusted) p < 0.05. Clusters #1 and #2 represent genes that are highly expressed in 2D and 3D cultures, respectively.
Because of the variations in cell shape between 2D and 3D cultures, we hypothesised that gene expression levels may vary based on the culture conditions. To explore this, we performed bulk RNA-seq to identify differences in gene expression under each culture method. The principal component analysis (PCA) plot results indicated distinct genetic differences between the 2D and 3D cultures (Figure 2B). Global heatmap analysis revealed that genes expressed under the two culture conditions were clustered into two distinct groups. Cluster #1 contained genes largely expressed in 2D cultures and were primarily associated with fundamental processes of cell division, such as organelle fission, nuclear division, ribosome biogenesis, and DNA replication. In contrast, cluster #2 comprised genes primarily expressed in 3D cultures, including various genes associated with ossification, the Wnt cell–cell signalling pathway, bone development, and bone and skeletal morphogenesis (Figure 2C). These findings confirmed that 2D and 3D culture environments are fundamentally distinct, causing variations in both cell shape and gene expression.
3.3. Adipogenic and Osteogenic Differentiation-Associated Proteins Highly Expressed in 3D Hydrogel Culture
The global heatmap results confirmed that the 3D culture method enhanced cell differentiation towards bone formation through a bone-forming signalling pathway. Subsequently, we determined whether the protein expression levels of specific genes differed between 2D and 3D cultures. The bar plot results indicated that MMP-13, SP7, and LPL were expressed at significantly higher levels in the 3D cultures than in the 2D cultures (Figure 3A). Western blot analysis confirmed these results at the protein level, revealing a similar trend. In 3D cultures, MMP-13 expression was 47 times higher, LPL was 3.4 times higher, and SP7 was 19 times higher than that in 2D cultures (Figure 3B).
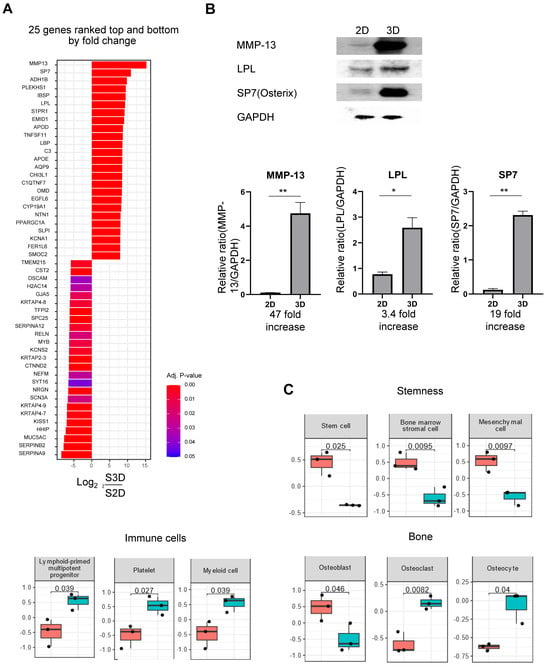
Figure 3.
Comparison of protein expression levels in 3D and 2D cultures. (A) Bar plot of gene expression levels obtained from RNA sequencing (RNA-seq) analysis, illustrating 25 upregulated and 25 downregulated genes in 3D samples compared to that in 2D samples; right and left bars represent upregulated and downregulated genes, respectively. (B) Western blot analysis of protein expression levels of matrix metalloproteinase 13 (MMP-13), lipoprotein lipase (LPL), Sp7 transcription factor (SP7), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in 2D- and 3D-cultured human bone marrow-derived stem cells (hBMSCs). The relative protein expression levels of MMP-13, LPL, and SP7 normalised to GAPDH levels are presented as bar graphs. Quantitative data are presented as the mean ± standard deviation (n = 3). * p < 0.05 and ** p < 0.01. (C) Box plots from RNA-seq analysis indicating alterations in the expression levels of stemness, immune cells, and bone-related genes between 2D- and 3D-cultured hBMSCs, as determined using cell marker enrichment analysis. Statistically significant differences are observed. The p-value is indicated on each plot for comparison between 2D and 3D cultures.
Subsequently, we confirmed alterations in cellular types through cell marker enrichment. Cells cultured for 21 days using the 2D method maintained their stem cell properties, whereas 3D-cultured cells exhibited a reduction in these properties. Further, 2D-cultured cells primarily exhibited osteoblast characteristics, whereas 3D-cultured cells exhibited both osteoclast and osteocyte characteristics. Additionally, in the 3D culture, immune-related cells, such as lymphoid-primed multipotent progenitor cells, myeloid cells, and platelets, were higher than those in the 2D culture (Figure 3C). In summary, these results indicated that in 2D culture, the majority of cells do not actively differentiate and remain in the stem cell state, whereas in 3D culture, stem cells differentiate into bone- and immune-related cells.
3.4. Effects of Three-Dimensional Culture Environment on Cell Morphology
Because the 3D culture system facilitates 360º cell-to-cell interaction with hBMSCs, we hypothesised that the cellular morphology of the cultured cells may differ from that of the conventional 2D culture environment. Additionally, among the proteins highly expressed in 3D cultures, SP7 is a primary transcription factor that induces the differentiation of osteoblasts into osteocytes [43]. Osteocytes—known for their unique dendrite-like structures—were induced by SP7 [44]. We used phalloidin staining to confirm dendrite formation. As illustrated in Figure 4, the cells cultured in a 2D environment did not form branches, whereas those cultured in a 3D environment formed branches and established numerous connections with neighbouring cells (Figure 4A–C). A closer examination of the 3D-cultured cells revealed the formation of numerous dendrite-like branches around them (Figure 4B). The length of the dendrites varied from 30 to 150 μM depending on the intercellular distance, and the number of dendrites per cell was approximately 7 to 10. These results indicated that the 3D culture environment induces morphological alterations in cells by secreting various factors, thereby enhancing cell-to-cell interactions.
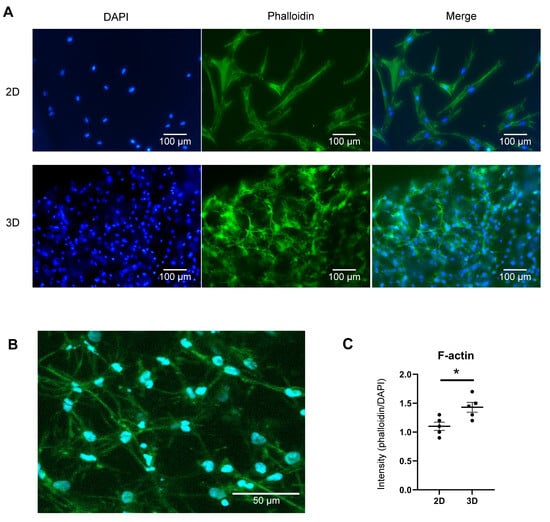
Figure 4.
Morphological differences between human bone marrow-derived stem cells (hBMSCs) in 2D and 3D cultures. (A) Immunofluorescence assay for phalloidin (green) and 4′,6-diamidino-2-phenylindole (DAPI) (blue). Compared to 2D-cultured cells, 3D-cultured hBMSCs are smaller and form multi-directional actin branches. Scale bars: 100 μm (Digital Inverted Fluorescence Microscope; Nikon). (B) Magnified image of F-actin formation in 3D-cultured cells (LSM 700; ZEISS). Numerous actin branches in 3D-cultured cells connect cells to one another and are anticipated to play a major role in cell-to-cell interactions. Scale bar: 50 μm (C) Quantification of F-actin, represented by the relative ratio of phalloidin to DAPI fluorescence levels. The graph demonstrates dot plots of five independent experiments (each dot represents the fluorescence intensity obtained from an individual image). Quantitative data are presented as the mean ± standard deviation (n = 5). * p < 0.05.
3.5. 3D-Cultured Cells Share Certain Characteristics with 2D-Cultured and Primary Ex Vivo Cells
Subsequently, we assessed which environment (3D or 2D) best mimicked the in vivo environment. To confirm this, we compared primary culture ex vivo cells with 2D- and 3D-cultured cells using bulk RNA-seq. Surprisingly, the PCA results revealed that primary culture ex vivo cells exhibited intermediate characteristics between 2D- and 3D-cultured cells (Figure 5A). When cell characteristics from the three different environments were compared, 2D-cultured cells expressed numerous genes associated with cytoskeleton formation, such as actin filament organisation, actin cytoskeleton organisation, and actomyosin structural organisation (Figure 5B (cluster #1)). Genes associated with axonogenesis, ECM organisation, bone development, cartilage development, and collagen fibril organisation were expressed in both 2D and 3D culture environments, with a higher abundance in 3D-cultured cells. Genes regulating cytokine production and cell chemotaxis were highly expressed in 3D-cultured cells and primary-cultured ex vivo cells (Figure 5B (cluster #5)). These results indicated that 3D-cultured cells exhibit the characteristics of intermediate cells, possessing the characteristics of both 2D-cultured and primary culture ex vivo cells.
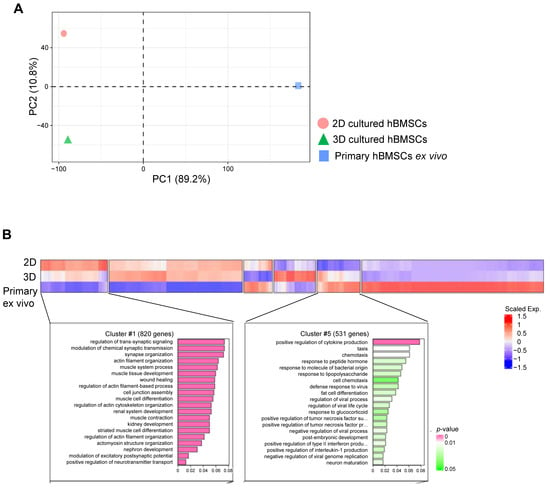
Figure 5.
RNA sequencing (RNA-seq) results comparing 2D and 3D cultures and primary ex vivo human bone marrow-derived stem cells (hBMSCs). (A) Principal component analysis plots of RNA-seq data demonstrating the characteristics of 2D- and 3D-cultured cells and hBMSCs based on their gene expression levels. Samples: 2D (n = 3), 3D (n = 3), and primary ex vivo hBMSCs (n = 2). (B) Heatmap of gene expression from RNA-seq data for differentially expressed genes (DEGs) among 2D- and 3D-cultured cells and primary ex vivo cells (hBMSCs), where red and blue indicate higher and lower expression levels, respectively. Cluster #1 represents genes highly expressed in 2D-cultured cells compared to those in 3D-cultured and primary ex vivo cells. Cluster #5 represents genes highly expressed in 3D-cultured and primary ex vivo cells compared to those in 2D-cultured cells. Note the similarity in gene expression levels between 3D-cultured and primary ex vivo cells.
4. Discussion
Many studies have used various types of hydrogels for 3D culture. Hydrogels play an important role in simulating ECM, but each component has its own advantages and disadvantages. Alginate hydrogel has the advantages of excellent biocompatibility and easy gelation, but has the disadvantages of poor mechanical strength and less cell adhesion [45]. Hyaluronic acid gel is an ECM component and has the advantages of excellent viscoelasticity, but has weak mechanical strength and rapid decomposition, making it unfavourable for long-term culture [46]. Collagen gel is the main ECM component and has excellent cell adhesion, but has the disadvantages of being temperature-sensitive and contractile, so its structure easily collapses [47]. Matrigel is a mixture similar to biological ECM containing laminin, collagen, etc., but cannot be used in human clinical applications due to safety issues, because it originates from a mouse tumour [37].
The hydrogel used in this research is a xeno-free synthetic polysaccharide-based hydrogel containing RGD (Arginine-Glycine-Aspartic acid) peptides. RGD is a cell adhesion motif present in fibronectin and vitronectin, and binds to integrin receptors on the cell surface to participate in cell adhesion, migration, differentiation, etc. The hydrogel containing RGD is a product that overcomes the limitations of non-adhesive hydrogels such as alginate and PEG, and prevents cell death through adhesion, thereby maintaining a high cell survival rate even in 3D culture, and stably maintaining cell shape and function. It is suitable for the differentiation of stem cells into osteocytes and chondrocytes because it induces differentiation into specific lines through integrin signalling. In addition, it does not contain animal-derived components, so it has the advantage of minimising the immunological response of cells and being suitable for xenograft research. When recovering cells in the gel, recovery solution can be used to efficiently recover them within 5–15 min, minimising cell damage.
In 2D cultures, genes associated with cell signalling, such as those involved in organelle fission, nuclear division, ribosome biogenesis, and DNA replication, were highly expressed. This indicates that the cells repeated the simple division- and proliferation-prone environments of the 2D culture system. In contrast, while the expression of these genes was lower in 3D cultures, genes associated with the ossification pathway, the cell–cell signalling pathway (Wnt), and the development and morphogenesis of the skeletal system were expressed at significantly higher levels. These findings indicated that the 3D culture environment induces the differentiation of BMSCs into osteocyte-like cells.
Interestingly, MMP-13, SP7, and LPL were significantly more highly expressed in the 3D hydrogel culture systems than in the 2D cultures. MMP-13 is crucial for wound healing, tissue remodelling, cartilage degradation, bone development, and ossification. It aids in the resorption of hypertrophic cartilage and the remodelling of the novel trabecular bone during long bone development [48,49,50]. MMP-13 regulates osteoclast number and/or activity, bone resorption, and bone mass [51]. High expression of LPL, a primary enzyme in triglyceride metabolism responsible for lipid clearance, utilisation, and storage, indicates that hBMSCs can differentiate into adipocytes [52]. These findings highlight the potential of 3D culture to facilitate diverse cell differentiations. SP7, a primary transcription factor required for osteoblast differentiation and bone formation, affects osteocyte differentiation [53,54]. SP7 variants are associated with alterations in bone density and fracture risk, whereas mutations can cause osteogenesis imperfecta [55]. Additionally, it controls osteocyte dendrite formation, contributing to the unique shape of osteocytes [44,55,56,57]. Our study revealed significantly higher SP7 expression levels in 3D cultures, indicating the differentiation of hBMSCs into osteocytes.
Distinct cellular morphology was observed in the 3D culture system, which was the primary finding of this study. In 2D cultures, the cells exhibited a typical fibroblast-like hBMSC shape. However, in 3D cultures, cells extended numerous actin filament branches in all directions and connected to numerous neighbouring cells, as demonstrated by actin filament staining. Actin filaments provide strength, facilitate cell–cell and cell–ECM connections, and serve as pathways for intracellular transport and scaffolds for generating force [58]. This indicates that the 3D culture environment enhanced both the cell–cell and cell–matrix networks. Additionally, phalloidin staining confirmed enhanced dendrite formation in cells within the 3D culture environment, resulting in dendrite-like structures. Although the 3D culture environment induced alterations in cellular morphology, it remains unclear whether these alterations were driven by direct cell–cell interactions or indirect interactions involving cell–matrix communication and secreted molecules. Further studies are required to explore whether substrate communication differs between 2D and 3D culture systems.
Comparison of hBMSCs cultured in the 3D hydrogel with those cultured in the 2D system revealed that the 3D environment enhanced MSC differentiation, potentially resembling that of living organisms. Additionally, when hBMSCs cultured ex vivo were compared to those cultured in 2D and 3D systems, the 3D-cultured cells exhibited gene expression profiles similar to those in the ex vivo conditions. These findings highlight the potential for broader applications of the 3D hydrogel culture method, such as assessing foreign body reactions to implants, conducting drug tests, and facilitating research on bone tissues. Moreover, this culture method provides a framework for producing bone organoids, thereby expanding their potential applications in regenerative medicine and tissue engineering.
5. Conclusions
This study demonstrated distinct differences in cell responses between 3D and 2D culture systems, including higher expression of MMP-13, SP7, and LPL—key factors in hard tissue physiology—and enhanced the formation of cell–cell and cell–matrix networks in the 3D environment. Culturing hBMSCs within a 3D hydrogel can facilitate their differentiation into osteocyte-like cells, potentially emulating more physiologically relevant conditions. Consequently, 3D culture environments may provide findings that more closely represent in vivo conditions compared to traditional 2D systems.
Author Contributions
Conceptualization, J.-Y.P. and I.-S.L.Y.; data curation, J.-Y.P. and I.-S.L.Y.; formal analysis, H.J.L.; funding acquisition, J.-Y.P. and I.-S.L.Y.; investigation, H.J.L. and J.-Y.P.; methodology, H.J.L., J.-Y.P. and I.-S.L.Y.; project administration, I.-S.L.Y.; resources, J.-Y.P. and I.-S.L.Y.; supervision, J.-Y.P. and I.-S.L.Y.; validation, L.N.L., S.K.S. and I.-S.L.Y.; visualisation, L.N.L., J.-Y.P. and I.-S.L.Y.; writing—original draft, H.J.L. and J.-Y.P.; writing—review and editing, L.N.L., S.K.S. and I.-S.L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) through grants funded by the Ministry of Science and ICT of the Korean government [No. 2021R1A2C200465011] and the Creative-Pioneering Researchers Program through Seoul National University [No. RS-2023-00252981].
Institutional Review Board Statement
Primary hBMSCs were isolated from the jawbone tissue harvested from patients who visited the Department of Oral and Maxillofacial Surgery, Seoul National University Dental Hospital, and underwent wisdom tooth extraction surgery. The procedures adhered to the Declaration of Helsinki and were approved by the Institutional Review Board of Seoul National University Dental Hospital (IRB number CRI24007).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
References
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Akther, F.; Little, P.J.; Li, Z.; Nguyen, N.; Ta, H.T. Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv. 2020, 10, 43682. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Özbolat, İ.T. 3D bioprinting for reconstituting the cancer microenvironment. NPJ Precis. Oncol. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Bissell, M.J.; Rizki, A.; Mian, I.S. Tissue architecture: The ultimate regulator of breast epithelial function. Curr. Opin. Cell Biol. 2003, 15, 753–762. [Google Scholar] [CrossRef]
- Hickman, J.A.; Graeser, R.; de Hoogt, R.; Vidic, S.; Brito, C.; Gutekunst, M.; van der Kuip, H. Three-dimensional models of cancer for pharmacology and cancer cell biology: Capturing tumor complexity in vitro/ex vivo. Biotechnol. J. 2014, 9, 1115–1128. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Moghimi, N.; Hosseini, S.A.; Dalan, A.B.; Mohammadrezaei, D.; Goldman, A.; Kohandel, M. Controlled tumor heterogeneity in a co-culture system by 3D bio-printed tumor-on-chip model. Sci. Rep. 2023, 13, 13648. [Google Scholar] [CrossRef]
- Cawkill, D.; Eaglestone, S.S. Evolution of cell-based reagent provision. Drug Discov. Today 2007, 12, 820–825. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Bouet, G.; Marchat, D.; Cruel, M.; Malaval, L.; Vico, L. In vitro three-dimensional bone tissue models: From cells to controlled and dynamic environment. Tissue Eng. Part B Rev. 2015, 21, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, F.; Moghe, P.V.; Toner, M.; Yarmush, M.L. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: Hepatocytes cultured in a sandwich configuration. FASEB J. 1996, 10, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- Frieboes, H.B.; Zheng, X.; Sun, C.H.; Tromberg, B.; Gatenby, R.; Cristini, V. An integrated computational/experimental model of tumor invasion. Cancer Res. 2006, 66, 1597–1604. [Google Scholar] [CrossRef]
- Ghosh, S.; Spagnoli, G.C.; Martin, I.; Ploegert, S.; Demougin, P.; Heberer, M.; Reschner, A. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: A high density oligonucleotide array study. J. Cell Physiol. 2005, 204, 522–531. [Google Scholar] [CrossRef]
- Marushima, H.; Shibata, S.I.; Asakura, T.; Matsuura, T.; Maehashi, H.; Ishii, Y.; Eda, H.; Aoki, K.; Lida, Y.; Morikawa, T.; et al. Three-dimensional culture promotes reconstitution of the tumor-specific hypoxic microenvironment under TGFβ stimulation. Int. J. Oncol. 2011, 39, 1327–1336. [Google Scholar] [CrossRef][Green Version]
- Powers, M.J.; Janigian, D.M.; Wack, K.E.; Baker, C.S.; Stolz, D.B.; Griffith, L.G. Functional behavior of primary rat liver cells in a three-dimensional perfused microarray bioreactor. Tissue Eng. 2002, 8, 499–513. [Google Scholar] [CrossRef]
- Semino, C.E.; Merok, J.R.; Crane, G.G.; Panagiotakos, G.; Zhang, S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation 2003, 71, 262–270. [Google Scholar] [CrossRef]
- Wolff, A.; Frank, M.; Staehlke, S.; Springer, A.; Hahn, O.; Meyer, J.; Peters, K. 3D spheroid cultivation alters the extent and progression of osteogenic differentiation of mesenchymal stem/stromal cells compared to 2D cultivation. Biomedicines 2023, 11, 1049. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Bicer, M.; Cottrell, G.S.; Widera, D. Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells. Stem Cell Res. Ther. 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Rasmussen, J.G.; Emmersen, J.; Pilgaard, L.; Fahlman, Å.; Brunberg, S.; Josefsson, J.; Arnemo, J.M.; Zarchar, V.; Swenson, J.E.; et al. Adipose-derived stem cells from the brown bear (Ursus arctos) spontaneously undergo chondrogenic and osteogenic differentiation in vitro. Stem Cell Res. 2011, 7, 89–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phinney, D.G.; Prockop, D.J. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair—Current views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Sunaga, J.; Nagata, S.; Nishio, M.; Fukuda, M.; Kamakura, T.; Sun, L.; Jin, Y.; Sakamoto, S.; Watanabe, A.; et al. 3D osteogenic differentiation of human iPSCs reveals the role of TGFβ signal in the transition from progenitors to osteoblasts and osteoblasts to osteocytes. Sci. Rep. 2023, 13, 1094. [Google Scholar] [CrossRef]
- Zheutlin, A.R.; Deshpande, S.S.; Nelson, N.S.; Kang, S.Y.; Gallagher, K.K.; Polyatskaya, Y.; Rodriguez, J.J.; Donneys, A.; Ranganathan, K.; Buchman, S.R. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: A histomorphometric evaluation. Cytotherapy 2016, 18, 664–672. [Google Scholar] [CrossRef]
- Pierce, J.L.; Begun, D.L.; Westendorf, J.J.; McGee-Lawrence, M.E. Defining osteoblast and adipocyte lineages in the bone marrow. Bone 2019, 118, 2–7. [Google Scholar] [CrossRef]
- Tang, X.T.; Wang, Z.; Wang, J.; Cui, S.; Xu, R.; Wang, Y. Functions and regulatory mechanisms of resting hematopoietic stem cells: A promising targeted therapeutic strategy. Stem Cell Res. Ther. 2023, 14, 73. [Google Scholar] [CrossRef]
- Undale, A.H.; Westendorf, J.J.; Yaszemski, M.J.; Khosla, S. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin. Proc. 2009, 84, 893–902. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, L.; Wang, S.; Huang, B.; Jing, Y.; Su, J. Bone marrow mesenchymal stromal cells: Identification, classification, and differentiation. Front. Cell Dev. Biol. 2022, 9, 787118. [Google Scholar] [CrossRef]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of human mesenchymal stem cells in regenerative therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef]
- Modugno, P.; Cilla, S.; Centritto, E.M.; Picone, V.; Maiorano, M.; Amatuzio, M.; Petrilli, M.P.; Fraticelli, V.; De Filippo, C.M.; Caradonna, E.; et al. Autologous bone marrow stem cells in patients with critical limb ischaemia not eligible for revascularization: A single centre experience. Angiology 2024, 75, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Jiang, X.; Si, Q.; Finne-Wistrand, A.; Liu, B.; Xue, Y.; Mustafa, K. Endochondral ossification induced by cell transplantation of endothelial cells and bone marrow stromal cells with copolymer scaffold using a rat calvarial defect model. Polymers 2021, 13, 1521. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, J.; Shu, B.; Xiao, Y.; Tang, D. Bone mesenchymal stem cell therapy for ovariectomized osteoporotic rats: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 556. [Google Scholar] [CrossRef]
- Guo, T.; Yuan, X.; Li, X.; Liu, Y.; Zhou, J. Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cell sheets co-expressing BMP2 and VEGF. J. Dent. Sci. 2023, 18, 135–144. [Google Scholar] [CrossRef]
- Pohlit, H.; Bohlin, J.; Katiyar, N.; Hilborn, J.; Tenje, M. Technology platform for facile handling of 3D hydrogel cell culture scaffolds. Sci. Rep. 2023, 13, 12829. [Google Scholar] [CrossRef]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Hatt, L.P.; Thompson, K.; Helms, J.A.; Stoddart, M.J.; Armiento, A.R. Clinically relevant preclinical animal models for testing novel cranio-maxillofacial bone 3D-printed biomaterials. Clin. Transl. Med. 2022, 12, e690. [Google Scholar] [CrossRef]
- Sharar, N.; Mahasneh, A.A.; Belharazem, D.; Ababneh, N.; Awidi, A. A descriptive study of the physical direct interaction between adipose tissue-mesenchymal stem cells and colo 205 cells: Impact on cancer cells stemness, and intracellular reactive oxygen species levels. Asian Pac. J. Cancer Prev. 2022, 23, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Krylova, T.A.; Musorina, A.S.; Zenin, V.V.; Poljanskaya, G.G. Cellular spheroids obtained from mesenchymal stem cells derived from bone marrow and limb muscle of early human embryo. Cell Tissue Biol. 2015, 9, 431–440. [Google Scholar] [CrossRef]
- Suske, G.; Bruford, E.; Philipsen, S. Mammalian SP/KLF transcription factors: Bring in the family. Genomics 2005, 85, 551–556. [Google Scholar] [CrossRef]
- Wang, J.S.; Kamath, T.; Mazur, C.M.; Mirzamohammadi, F.; Rotter, D.; Hojo, H.; Castro, C.D.; Tokavanich, N.; Patel, R.; Govea, N.; et al. Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin. Nat. Commun. 2021, 12, 6271. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef]
- Hinz, B.; McCulloch, C.A.; Coelho, N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp. Cell Res. 2019, 379, 119–128. [Google Scholar] [CrossRef]
- Khoswanto, C. Role of matrix metalloproteinases in bone regeneration: Narrative review. J. Oral Biol. Craniofac. Res. 2023, 13, 539–543. [Google Scholar] [CrossRef]
- Inada, M.; Wang, Y.; Byrne, M.H.; Rahman, M.U.; Miyaura, C.; López-Otín, C.; Krane, S.M. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 2004, 101, 17192–17197. [Google Scholar] [CrossRef]
- Stickens, D.; Behonick, D.J.; Ortega, N.; Heyer, B.; Hartenstein, B.; Yu, Y.; Fosang, A.J.; Schorpp-Kistner, M.; Angel, P.; Werb, Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004, 131, 5883–5895. [Google Scholar] [CrossRef]
- Ponte, F.; Kim, H.N.; Warren, A.; Iyer, S.; Han, L.; Mannen, E.; Gomez-Acevedo, H.; Nookaew, I.; Almeida, M.; Manolagas, S.C. Mmp13 deletion in mesenchymal cells increases bone mass and may attenuate the cortical bone loss caused by estrogen deficiency. Sci. Rep. 2022, 12, 10257. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, K.; Choi, S.H. Lipoprotein lipase: Is it a magic target for the treatment of hypertriglyceridemia. Endocrinol. Metab. 2022, 37, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; De Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Ma, Y.; Yang, J.; Tang, Y.; Jin, Y.; Li, L.; Ma, C. MiR-224-5p inhibits osteoblast differentiation and impairs bone formation by targeting Runx2 and Sp7. Cytotechnology 2023, 75, 505–516. [Google Scholar] [CrossRef]
- Lui, J.C.; Raimann, A.; Hojo, H.; Dong, L.; Roschger, P.; Kikani, B.; Wintergerst, U.; Fratzl-Zelman, N.; Jee, Y.H.; Haeusler, G.; et al. A neomorphic variant in SP7 alters sequence specificity and causes a high-turnover bone disorder. Nat. Commun. 2022, 13, 700. [Google Scholar] [CrossRef]
- Estrada, K.; Styrkarsdottir, U.; Evangelou, E.; Hsu, Y.H.; Duncan, E.L.; Ntzani, E.E.; Oei, L.; Albagha, O.M.; Amin, N.; Kemp, J.P.; et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012, 44, 491–501. [Google Scholar] [CrossRef]
- Rivadeneira, F.; Styrkársdottir, U.; Estrada, K.; Halldórsson, B.V.; Hsu, Y.H.; Richards, J.B.; Zillikens, M.C.; Kavvoura, F.K.; Amin, N.; Aulchenko, Y.S.; et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 2009, 41, 1199–1206. [Google Scholar] [CrossRef]
- Zonderland, J.; Moroni, L. Steering cell behavior through mechanobiology in 3D: A regenerative medicine perspective. Biomaterials 2021, 268, 120572. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).