Load-Bearing Capacity of Lithium Silicate Derivates Applied as Ultra-Thin Occlusal Veneers on Molars
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation and Group Allocation
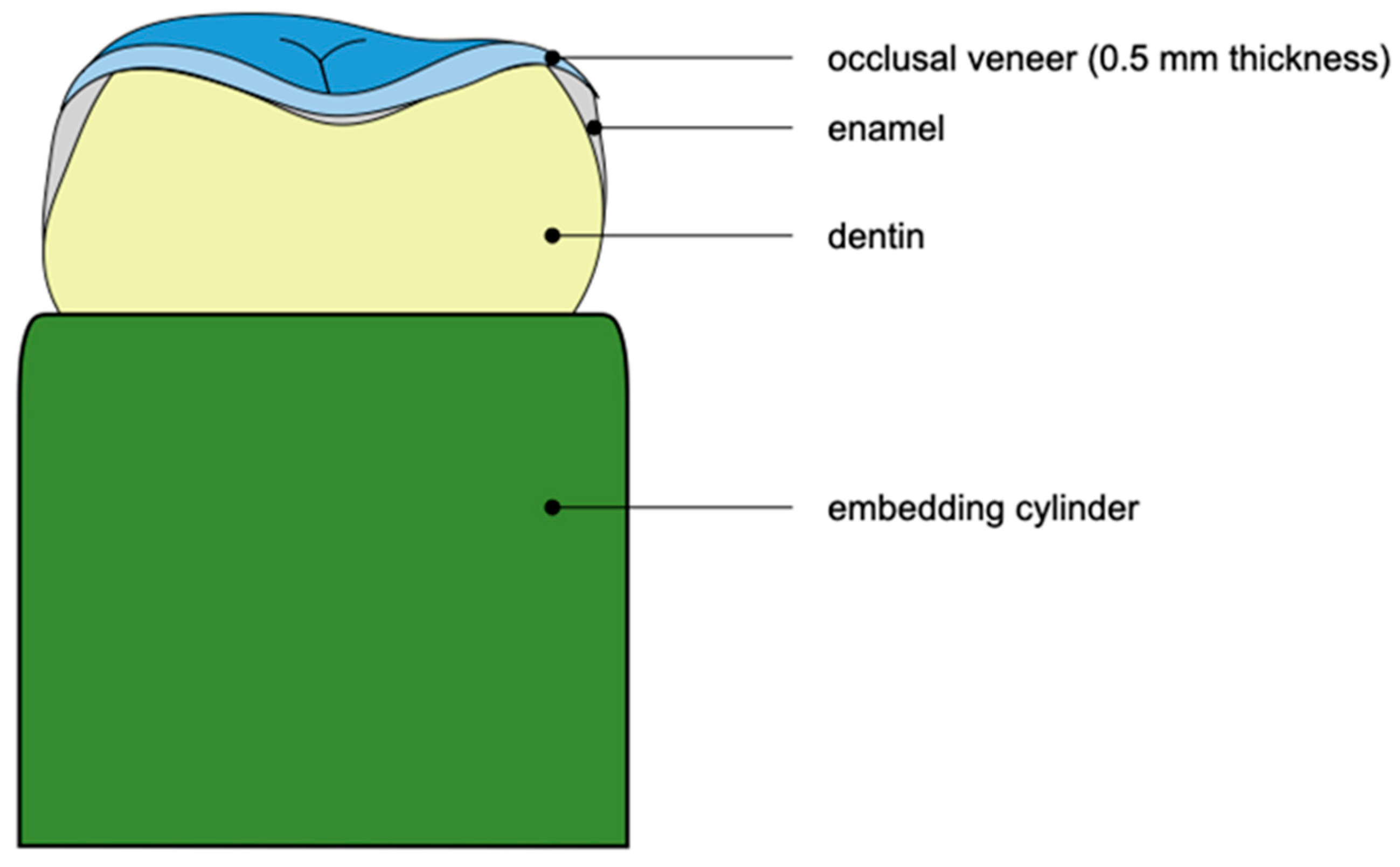
2.2. Specimen Preparation
2.3. Scanning Procedure and Digital Restoration Design
2.4. Fabrication of Restorations
2.5. Cementation of Restorations
2.6. Aging of Specimens
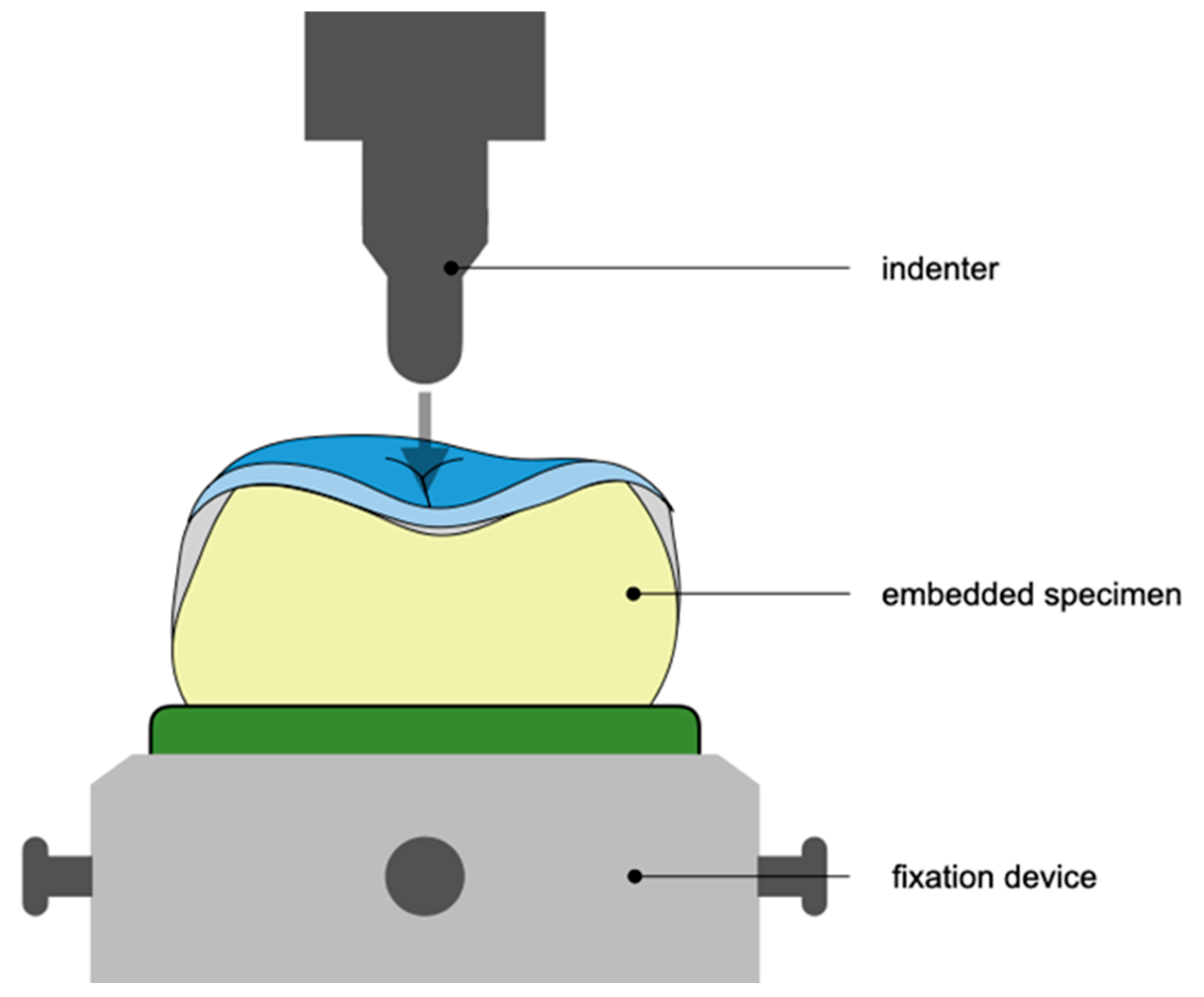
2.7. Static Loading of Specimens
2.8. Statistical Analysis
3. Results
3.1. Fatigue Resistance
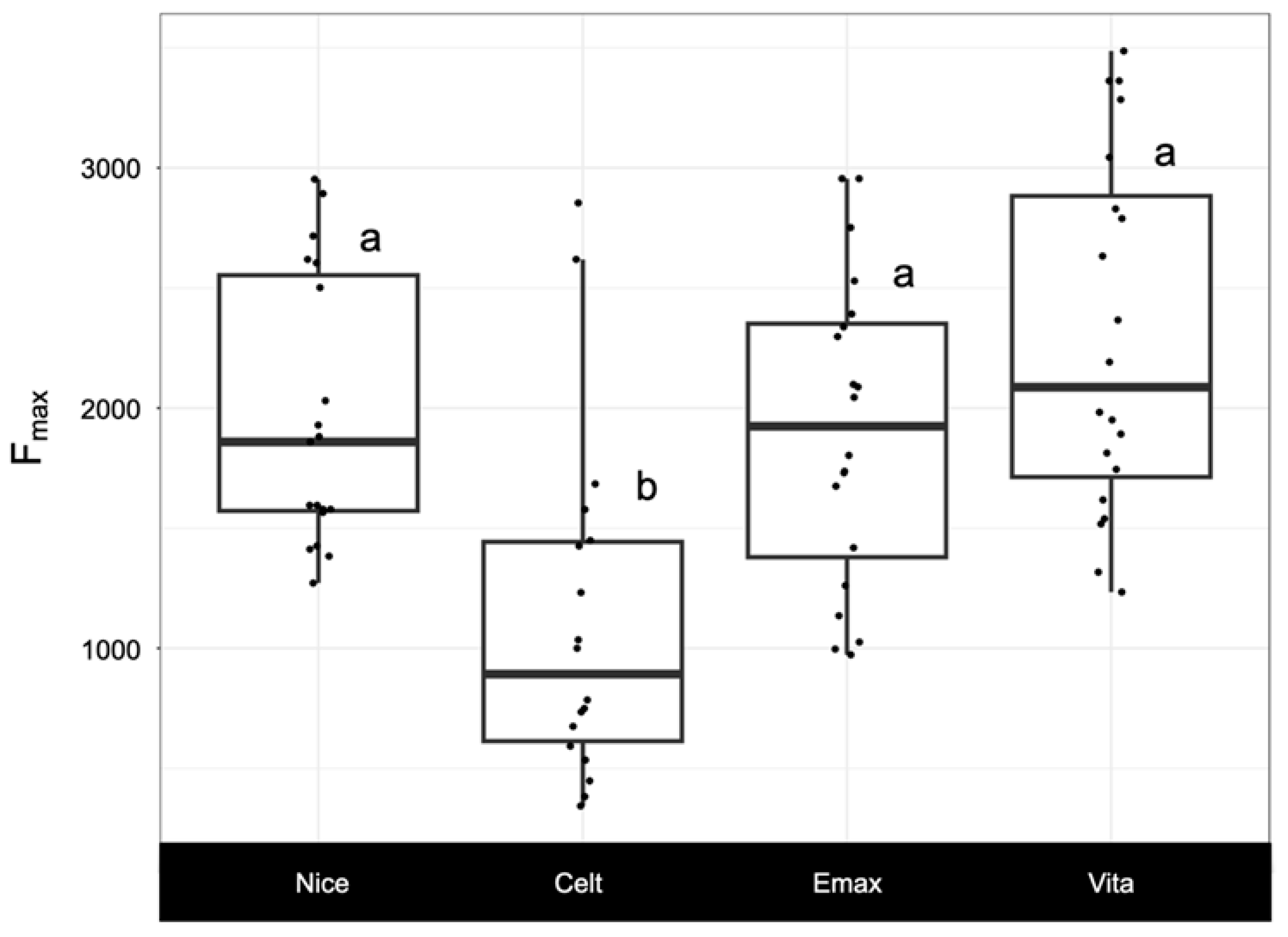
3.2. Load-Bearing Capacity Fmax
3.3. Failure Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lussi, A.; Carvalho, T.S. Erosive tooth wear: A multifactorial condition of growing concern and increasing knowledge. Monogr. Oral Sci. 2014, 25, 1–15. [Google Scholar] [PubMed]
- Jaeggi, T.; Lussi, A. Prevalence, incidence and distribution of erosion. Monogr. Oral Sci. 2006, 20, 44–65. [Google Scholar] [PubMed]
- Lee, A.; He, L.H.; Lyons, K.; Swain, M.V. Tooth wear and wear investigations in dentistry. J. Oral Rehabil. 2012, 39, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Bazos, P.; Magne, P. Bio-emulation: Biomimetically emulating nature utilizing a histo-anatomic approach; structural analysis. Eur. J. Esthet. Dent. 2011, 6, 8–19. [Google Scholar] [PubMed]
- Loomans, B.; Opdam, N.; Attin, T.; Bartlett, D.; Edelhoff, D.; Frankenberger, R.; Benic, G.; Ramseyer, S.; Wetselaar, P.; Sterenborg, B.; et al. Severe tooth wear: European consensus statement on management guidelines. J. Adhes. Dent. 2017, 19, 111–119. [Google Scholar]
- Ioannidis, A.; Pitta, J.; Panadero, R.A.; Chantler, J.G.M.; Villar, S.F.; Fonseca, M.; Lozano, J.G.; Hämmerle, C.H.F.; Hey, J.; Karasan, D.; et al. Rehabilitation Strategies and Occlusal Vertical Dimension Considerations in the Management of Worn Dentitions: Consensus Statement From SSRD, SEPES, and PROSEC Conference on Minimally Invasive Restorations. J. Esthet. Restor. Dent. 2025, 1–5. [Google Scholar] [CrossRef]
- Edelhoff, D.; Sorensen, J.A. Tooth structure removal associated with various preparation designs for anterior teeth. J. Prosthet. Dent. 2002, 87, 503–509. [Google Scholar] [CrossRef]
- Kontakiotis, E.G.; Filippatos, C.G.; Stefopoulos, S.; Tzanetakis, G.N. A prospective study of the incidence of asymptomatic pulp necrosis following crown preparation. Int. Endod. J. 2015, 48, 512–517. [Google Scholar] [CrossRef]
- Valderhaug, J.; Jokstad, A.; Ambjørnsen, E.; Norheim, P.W. Assessment of the periapical and clinical status of crowned teeth over 25 years. J. Dent. 1997, 25, 97–105. [Google Scholar] [CrossRef]
- Albelasy, E.H.; Hamama, H.H.; Tsoi, J.K.H.; Mahmoud, S.H. Fracture resistance of cad/cam occlusal veneers: A systematic review of laboratory studies. J. Mech. Behav. Biomed. Mater. 2020, 110, 103948. [Google Scholar] [CrossRef]
- Zumstein, K.; Fiscalini, L.; Al-Haj Husain, N.; Evci, E.; Özcan, M.; Ioannidis, A. Load-bearing capacity of pressable lithium disilicates applied as ultra-thin occlusal veneers on molars. J. Mech. Behav. Biomed. Mater. 2022, 136, 105520. [Google Scholar] [CrossRef] [PubMed]
- Paqué, P.N.; Gantner, C.; Mätzener, K.J.; Özcan, M.; Ioannidis, A. Load-bearing capacity, internal accuracy and time-efficiency of heat-pressed, milled and 3D-printed lithium disilicate ultra-thin occlusal veneers. Dent. Mater. 2024, 40, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Jurado, C.A.; Lee, D.; Ramirez, P.; Cortes-Treviño, D.A.; Tsujimoto, A. Fracture Resistance of Chairside CAD/CAM Lithium Disilicate-reinforced Ceramic Occlusal Veneers with and Without Margin and Full Coverage Crowns. Oper. Dent. 2024, 49, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Mörikofer, N.; Benic, G.I.; Park, J.M.; Özcan, M.; Hüsler, J.; Ioannidis, A. Relationship between internal accuracy and load-bearing capacity of minimally invasive lithium disilicate occlusal veneers. Int. J. Prosthodont. 2021, 34, 365–372. [Google Scholar] [CrossRef]
- Alkadi, L.; Ruse, N.D. Fracture toughness of two lithium disilicate dental glass ceramics. J. Prosthet. Dent. 2016, 116, 591–596. [Google Scholar] [CrossRef]
- Traini, T.; Sinjari, B.; Pascetta, R.; Serafini, N.; Perfetti, G.; Trisi, P.; Caputi, S. The zirconia-reinforced lithium silicate ceramic: Lights and shadows of a new material. Dent. Mater. J. 2016, 35, 748–755. [Google Scholar] [CrossRef]
- Krithikadatta, J.; Gopikrishna, V.; Datta, M. CRIS Guidelines (Checklist for Reporting In-vitro Studies): A concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. J. Conserv. Dent. 2014, 17, 301–304. [Google Scholar] [CrossRef]
- Maeder, M.; Pasic, P.; Ender, A.; Özcan, M.; Benic, G.I.; Ioannidis, A. Load-bearing capacities of ultra-thin occlusal veneers bonded to dentin. J. Mech. Behav. Biomed. Mater. 2019, 95, 165–171. [Google Scholar] [CrossRef]
- Human Research Ordinance (810.301), Art. 30. Available online: https://www.fedlex.admin.ch/eli/cc/2013/642/en (accessed on 3 March 2025).
- World Medical Association (WMA). Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. In Proceedings of the 64th WMA General Assembly, Fortaleza, Brazil, 16–19 October 2013. [Google Scholar]
- Human Research Act (810.30), Art. 2 and 32, Human Research Ordinance (810.301), Art. 25. Available online: https://www.fedlex.admin.ch/eli/cc/2013/642/en (accessed on 3 March 2025).
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). R Package. 2014. Available online: https://CRAN.R-project.org/package=PMCMR (accessed on 3 March 2025).
- Graves, S.; Piepho, H.; Selzer, L. multcompView: Visualizations of Paired Comparisons. R Package Version 0.1-8. 2019. Available online: https://CRAN.R-project.org/package=multcompView (accessed on 3 March 2025).
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R Package for Fitting Distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Rigby, R.A.; Stasinopoulos, D.M. Generalized additive models for location, scale and shape, (with discussion). Appl. Statist. 2005, 54 Pt 3, 507–554. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 3 March 2025).
- Gurel, G.; Sesma, N.; Calamita, M.A.; Coachman, C.; Morimoto, S. Influence of enamel preservation on failure rates of porcelain laminate veneers. Int. J. Periodont. Restor. Dent. 2013, 33, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.F.; Stafford, G.D.; Harrison, A. Masticatory function—A review of the literature. 1. The form of the masticatory cycle. J. Oral Rehabil. 1975, 2, 281–301. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.; Douglas, W.H. An artificial oral environment for testing dental materials. IEEE Trans. Biomed. Eng. 1991, 38, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Mitsias, M.E.; Ludwig, K.; Kern, M. In vitro evaluation of a mechanical testing chewing simulator. Dent. Mater. 2009, 25, 494–499. [Google Scholar] [CrossRef]
- Edmonds, H.M.; Glowacka, H. The ontogeny of maximum bite force in humans. J. Anat. 2020, 237, 529–542. [Google Scholar] [CrossRef]
- Mavriqi, L.; Valente, F.; Murmura, G.; Sinjari, B.; Macrì, M.; Trubiani, O.; Caputi, S.; Traini, T. Lithium disilicate and zirconia reinforced lithium silicate glass-ceramics for CAD/CAM dental restorations: Biocompatibility, mechanical and microstructural properties after crystallization. J. Dent. 2022, 119, 104054. [Google Scholar] [CrossRef]
- Kelly, J.R.; Benetti, P. Ceramic materials in dentistry: Historical evolution and current practice. Aust. Dent. J. 2011, 56 (Suppl. S1), 84–96. [Google Scholar] [CrossRef]
- Weyhrauch, M.; Igiel, C.; Scheller, H.; Weibrich, G.; Lehmann, K.M. Fracture Strength of Monolithic All-Ceramic Crowns on Titanium Implant Abutments. Int. J. Oral Maxillofac. Implants 2016, 31, 304–309. [Google Scholar] [CrossRef]
- Ioannidis, A.; Bomze, D.; Hämmerle, C.H.F.; Hüsler, J.; Birrer, O.; Mühlemann, S. Load-bearing capacity of CAD/CAM 3D-printed zirconia, CAD/CAM milled zirconia, and heat-pressed lithium disilicate ultra-thin occlusal veneers on molars. Dent. Mater. 2020, 36, e109–e116. [Google Scholar] [CrossRef]
- Gierthmuehlen, P.C.; Jerg, A.; Fischer, J.B.; Bonfante, E.A.; Spitznagel, F.A. Posterior minimally invasive full-veneers: Effect of ceramic thicknesses, bonding substrate, and preparation designs on failure-load and -mode after fatigue. J. Esthet. Restor. Dent. 2022, 34, 145–153. [Google Scholar] [CrossRef]
- Ioannidis, A.; Muhlemann, S.; Ozcan, M.; Husler, J.; Hammerle, C.H.F.; Benic, G.I. Ultra-thin occlusal veneers bonded to enamel and made of ceramic or hybrid materials exhibit load-bearing capacities not different from conventional restorations. J. Mech. Behav. Biomed. Mater. 2019, 90, 433–440. [Google Scholar] [CrossRef]
- Schlichting, L.H.; Resende, T.H.; Reis, K.R.; Raybolt Dos Santos, A.; Correa, I.C.; Magne, P. Ultrathin CAD-CAM glass-ceramic and composite resin occlusal veneers for the treatment of severe dental erosion: An up to 3-year randomized clinical trial. J. Prosthet. Dent. 2022, 128, e1–e158. [Google Scholar] [CrossRef]
| Group | Restorative Material | Chemical Composition | Flexural Strength (MPa) |
|---|---|---|---|
| Nice | Lithium aluminosilicate ceramic reinforced with lithium disilicate (N!ce, Straumann AG, Basel, Switzerland) | SiO2(64–70); Li2O(10.5–12.15); Al2O3(10.5–11.5); Na2O(1–3); K2O(0–3); P2O5(3–8); ZrO2(0–0.5); CaO(1–2); Coloring oxides (0–9) | 350 MPa |
| Celt | Zirconia-reinforced lithium silicate (Celtra Duo, Dentsply Sirona, Hanau-Wolfgang, Germany) | SiO2(58); Li2O(18.5); ZrO2(10.1); P2O5(5); Ce2O3(2); Al2O3(1.9); TbO2(1) | 370 MPa |
| Emax | Lithium disilicate ceramic (IPS e.max CAD PrograMill; Ivoclar Vivadent, Schaan, Liechtenstein) | SiO2(57–80); Li2O(11–19); K2O(0–13); P2O5(0–11); ZrO2(0–8); ZnO(0–5); Al2O3(0–5); MgO (0–5); Coloring oxides (0–8) | 530 MPa |
| Vita | Zirconia-reinforced glass ceramic (VITA Suprinity PC, Vita Zahnfabrik, Bad Säckingen, Germany) | SiO2(56–64); Li2O(15–21); K2O(1–4); P2O5(3–8); Al2O3(1–4); ZrO2(8–12); Ce2O3(0–4); La2O3 (0.1); Pigments (0–6) | 420 MPa |
| Group | Mean ± SD | Median | Range (Min to Max) |
|---|---|---|---|
| Nice | 1968 ± 563 | 1859 | 1271 to 2953 |
| Celt | 1118 ± 721 | 892 | 343 to 2854 |
| Emax | 1910 ± 641 | 1924 | 973 to 2955 |
| Vita | 2298 ± 744 | 2087 | 1234 to 3486 |
| Group | n | Score 0 (%) | Score 1 (%) | Score 2 (%) | Score 3 (%) |
|---|---|---|---|---|---|
| Nice | 19 | 0 | 0 | 95 | 5 |
| Celt | 18 | 0 | 5 | 90 | 5 |
| Emax | 20 | 0 | 5 | 80 | 15 |
| Vita | 20 | 0 | 10 | 65 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiscalini, L.; Willi, L.; Wiedemeier, D.; Özcan, M.; Ioannidis, A. Load-Bearing Capacity of Lithium Silicate Derivates Applied as Ultra-Thin Occlusal Veneers on Molars. Prosthesis 2025, 7, 43. https://doi.org/10.3390/prosthesis7020043
Fiscalini L, Willi L, Wiedemeier D, Özcan M, Ioannidis A. Load-Bearing Capacity of Lithium Silicate Derivates Applied as Ultra-Thin Occlusal Veneers on Molars. Prosthesis. 2025; 7(2):43. https://doi.org/10.3390/prosthesis7020043
Chicago/Turabian StyleFiscalini, Lorenzo, Liana Willi, Daniel Wiedemeier, Mutlu Özcan, and Alexis Ioannidis. 2025. "Load-Bearing Capacity of Lithium Silicate Derivates Applied as Ultra-Thin Occlusal Veneers on Molars" Prosthesis 7, no. 2: 43. https://doi.org/10.3390/prosthesis7020043
APA StyleFiscalini, L., Willi, L., Wiedemeier, D., Özcan, M., & Ioannidis, A. (2025). Load-Bearing Capacity of Lithium Silicate Derivates Applied as Ultra-Thin Occlusal Veneers on Molars. Prosthesis, 7(2), 43. https://doi.org/10.3390/prosthesis7020043







