Dental Prostheses Materials: Corrosion Behavior of Co-Cr-W Alloys Processed by SLM Technique
Abstract
1. Introduction
2. Materials and Methods
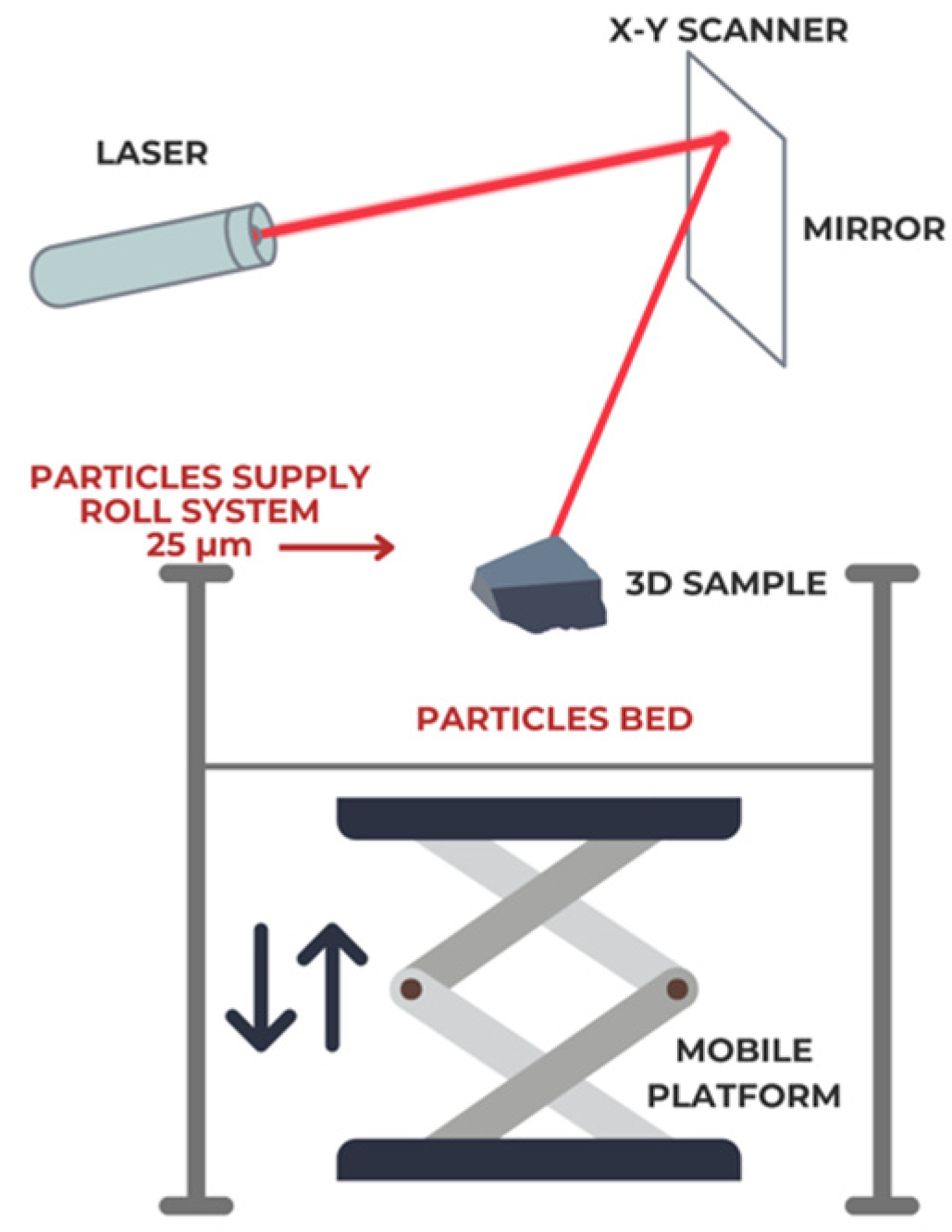
2.1. Specimens’ Fabrication
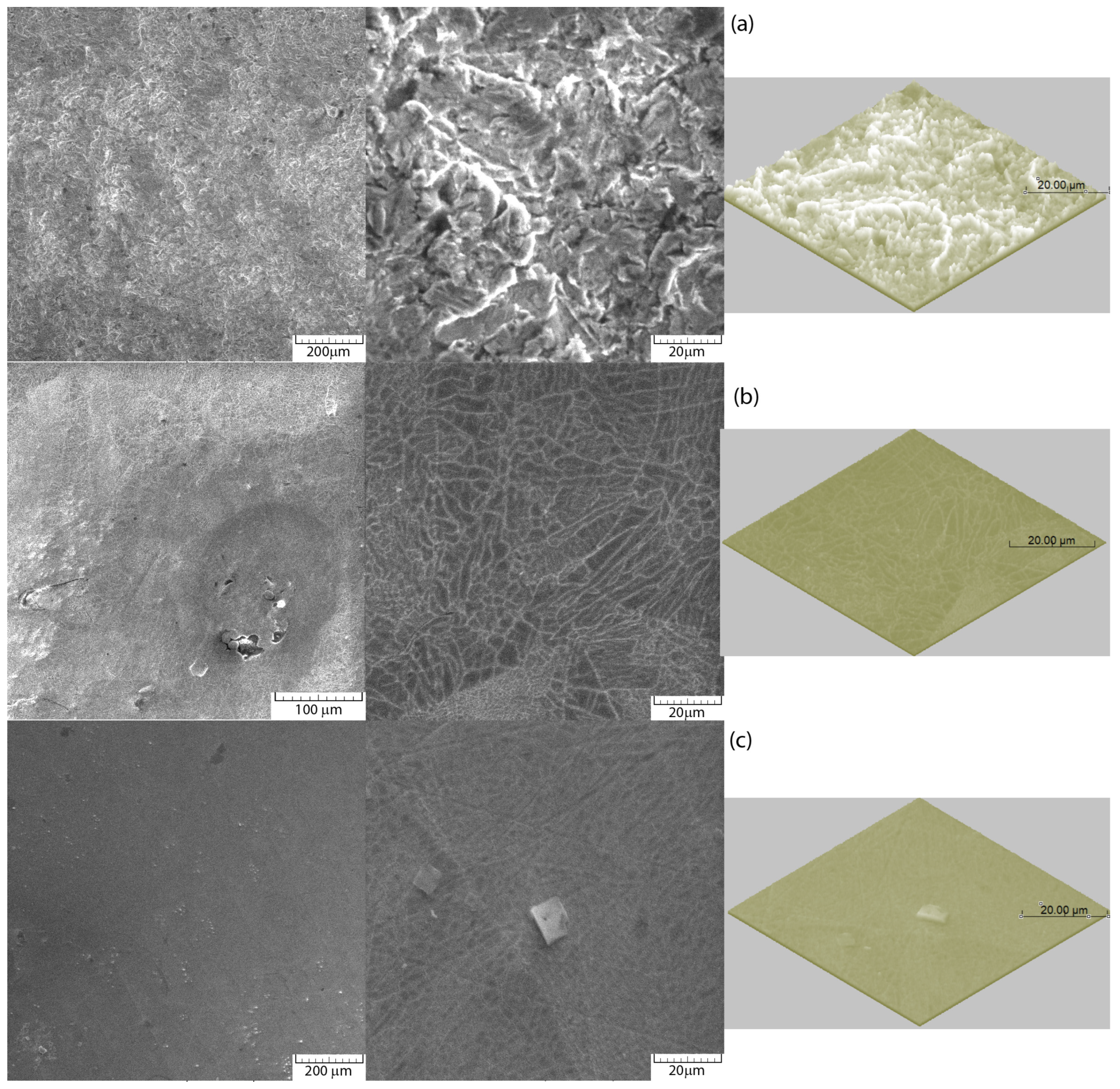
- Group A: Non-sandblasted samples.
- Group B: Samples sandblasted with alumina (Al2O3) particles.
- Group C: Samples successively sandblasted with alumina (Al2O3) particles followed by glass particles.
2.2. Microstructural Characterization and Chemical Composition Analysis
2.3. Electrochemical Corrosion Testing
2.4. Statistical Analysis
3. Results
3.1. Microstructure and Chemical Composition
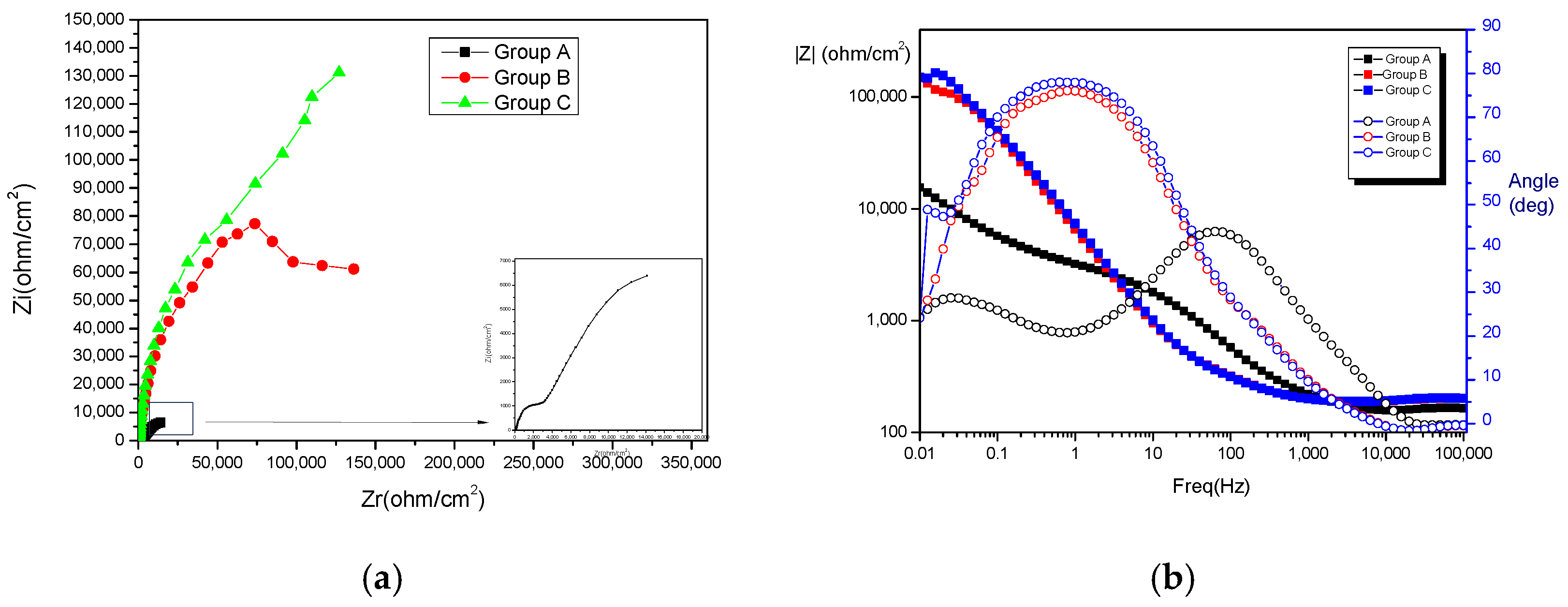
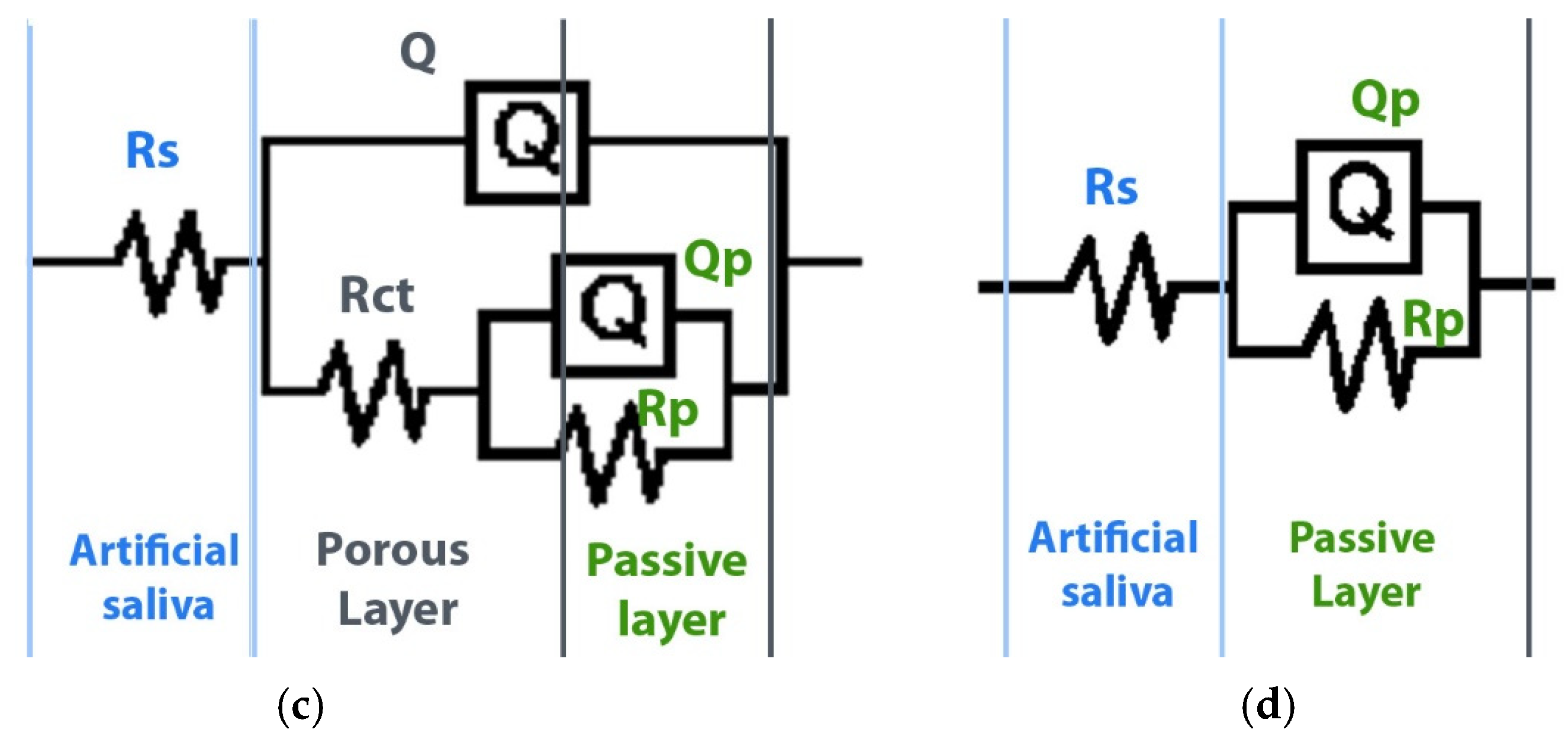
3.2. Electrochemical Corrosion Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xin, X.Z.; Chen, J.; Xiang, N.; Wei, B. Surface properties and corrosion behavior of Co-Cr alloy fabricated with selective laser melting technique. Cell Biochem. Biophys. 2013, 67, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Gómez-Polo, M.; Park, S.H.; Barmak, A.B.; Özcan, M. Adhesion of veneering porcelain to cobalt-chromium dental alloys processed with casting, milling, and additive manufacturing methods: A systematic review and meta-analysis. J. Prosthet. Dent. 2022, 128, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Antanasova, M.; Kocjan, A.; Kovac, J.; Zuzek, B.; Jevnikar, P. Influence of thermo-mechanical cycling on porcelain bonding to cobalt-chromium and titanium dental alloys fabricated by casting, milling, and selective laser melting. J. Prosthodont. Res. 2018, 62, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Cheng, Y. Stiffness and strength tailoring of cobalt chromium graded cellular structures for stress-shielding reduction. Mater. Des. 2017, 114, 633–641. [Google Scholar] [CrossRef]
- Kassapidou, M.; Stenport, V.F.; Hjalmarsson, L.; Johansson, C.B. Cobalt-chromium alloys in fixed prosthodontics in Sweden. Acta Biomater. Odontol. Scand. 2017, 3, 53–62. [Google Scholar] [CrossRef]
- Soares, F.M.D.S.; Santana, A.I.D.C.; dos Santos, L.B.F.; Gomes, P.A.M.C.; Monteiro, E.D.S.; Coimbra, M.E.R.; Elias, C.N. Influence of oral pH Environment in the Corrosion Resistance of Cr-Co-Mo alloy Used for Dentistry Prosthetic Components. Mater. Res. 2019, 22, e20190330. [Google Scholar] [CrossRef]
- Sarantopoulos, D.M.; Beck, K.A.; Holsen, R.; Berzins, D.W. Corrosion of CoCr and NiCr dental alloys alloyed with palladium. J. Prosthet. Dent. 2011, 105, 35–43. [Google Scholar] [CrossRef]
- Akbar, M.; Brewer, J.M.; Grant, M.H. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J. Immunotoxicol. 2011, 8, 140–149. [Google Scholar] [CrossRef]
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 2020, 10, 1151. [Google Scholar] [CrossRef]
- Savencu, C.E.; Costea, L.V.; Dan, M.L.; Porojan, L. Corrosion Behaviour of Co-Cr Dental Alloys Processed by Alternative CAD/CAM Technologies in Artificial Saliva Solutions. Int. J. Electrochem. Sci. 2018, 13, 3588–3600. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef] [PubMed]
- Strub, J.R.; Rekow, E.D.; Witkowski, S. Computer-aided design and fabrication of dental restorations: Current systems and future possibilities. J. Am. Dent. Assoc. 2006, 137, 1289–1296. [Google Scholar] [CrossRef]
- Lavanya, M. A Brief Insight into Microbial Corrosion and its Mitigation with Eco-friendly Inhibitors. J. Bio Tribo Corros. 2021, 7, 125. [Google Scholar] [CrossRef]
- Musa, A.Y.; Behazin, M.; Wren, J.C. Potentiostatic Oxide Growth Kinetics on Ni-Cr and Co-Cr Alloys: Potential and pH De-pendences. Electrochim. Acta 2015, 162, 185–197. [Google Scholar] [CrossRef]
- Nierlich, J.; Papageorgiou, S.N.; Bourauel, C.; Hültenschmidt, R.; Bayer, S.; Stark, H.; Keilig, L. Corrosion behavior of dental alloys used for retention elements in prosthodontics. Eur. J. Oral Sci. 2016, 124, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef] [PubMed]
- Hancu, V.; Comaneanu, R.M.; Coman, C.; Filipescu, A.G.; Ghergic, D.L.; Cotrut, M.C. In Vitro Studies Regarding the Corrosion Resistance of NiCr and CoCr Types Dental Alloys. Rev. Chim. 2014, 65, 706–709. [Google Scholar]
- Wen, C. Structural Biomaterials: Properties, Characteristics, and Selection; Woodhead Publishing: Sawston, UK, 2021; pp. 33–59, 103–122. [Google Scholar]
- Mercieca, S.; Conti, M.C.; Buhagiar, J.; Camilleri, J. Assessment of corrosion resistance of cast cobalt- and nickel-chromium dental alloys in acidic environments. J. Appl. Biomater. Funct. Mater. 2018, 16, 47–54. [Google Scholar] [CrossRef]
- Ren, X.W.; Zeng, L.; Wei, Z.M.; Xin, X.Z.; Wei, B. Effects of multiple firings on metal-ceramic bond strength of Co-Cr alloy fabricated by selective laser melting. J. Prosthet. Dent. 2016, 115, 109–114. [Google Scholar] [CrossRef]
- Baciu, E.-R.; Cimpoeșu, R.; Vițalariu, A.; Baciu, C.; Cimpoeșu, N.; Sodor, A.; Zegan, G.; Murariu, A. Surface Analysis of 3D (SLM) Co–Cr–W Dental Metallic Materials. Appl. Sci. 2021, 11, 255. [Google Scholar] [CrossRef]
- Xing, X.; Hu, Q.; Liu, Y.; Wang, Y.; Cheng, H. Comparative analysis of the surface properties and corrosion resistance of Co-Cr dental alloys fabricated by different methods. J. Prosthet. Dent. 2022, 127, 497.e1–497.e11. [Google Scholar] [CrossRef] [PubMed]
- Bechir, F.; Bataga, S.M.; Ungureanu, E.; Vranceanu, D.M.; Pacurar, M.; Bechir, E.S.; Cotrut, C.M. Experimental Study Regarding the Behavior at Different pH of Two Types of Co-Cr Alloys Used for Prosthetic Restorations. Materials 2021, 14, 4635. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Jang, S.H.; Kim, Y.K.; Son, J.S.; Min, B.K.; Kim, K.H.; Kwon, T.Y. Microstructures and mechanical properties of Co-Cr dental alloys fabricated by three cad/cam-based processing techniques. Materials 2016, 9, 596. [Google Scholar] [CrossRef]
- Lee, M.; Zakiyuddin, A.; Lee, K.; Park, C.J. Electrochemical characterization of passive film and corrosion in Co-rich high entropy alloys in Ringer’s solution. J. Mater. Res. Technol. 2024, 32, 649–660. [Google Scholar] [CrossRef]
- Konieczny, B.; Szczesio-Wlodarczyk, A.; Sokolowski, J.; Bociong, K. Challenges of Co–Cr Alloy Additive Manufacturing Methods in Dentistry—The Current State of Knowledge (Systematic Review). Materials 2020, 13, 3524. [Google Scholar] [CrossRef]
- Yung, K.C.; Wang, W.J.; Xiao, T.Y.; Choy, H.S. Laser polishing of additive manufactured Co-Cr components for controlling their wettability characteristics. Surf. Coat. Technol. 2018, 351, 89–98. [Google Scholar] [CrossRef]
- Qin, L.; Wu, H.; Guo, J.; Feng, X.; Dong, G.; Shao, J.; Zeng, Q.; Zhang, Y.; Qin, Y. Fabricating hierarchical micro and nano structures on implantable Co-Cr-Mo alloy for tissue engineering by one-step laser ablation. Colloid. Surf. B 2018, 161, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Yung, K.C.; Choy, H.S.; Xiao, T.Y.; Cai, Z.X. Effects of laser polishing on surface microstructure and corrosion resistance of additive manufactured Co-Cr alloys. Appl. Surf. Sci. 2018, 443, 167–175. [Google Scholar] [CrossRef]
- Pereira, A.L.C.; Mendonça, L.M.; Troconis, C.C.M.; Barão, V.A.R.; Porto Carreiro, A.D.F. Which metal surface treatment improves the bond strength between metal alloys and acrylic resin in removable partial dentures? A systematic review. J. Prosthet. Dent. 2023, 15, S0022-3913(23)00688-1. [Google Scholar] [CrossRef]
- Kaneko, T.; Hattori, M.; Hasegawa, K.; Yoshinari, M.; Kawada, E.; Oda, Y. Influence of finishing on the electrochemical properties of dental alloys. Bull. Tokyo Dent. Coll. 2000, 41, 49–57. [Google Scholar] [CrossRef][Green Version]
- Porojan, L.; Birdeanu, M.; Savencu, C.; Porojan, S. Surface characteristics and corrosion properties of Co-Cr dental alloys processed by Laser-based Methods. Rev. Chim. 2017, 28, 2538–2541. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S.; Ntasi, A.; Gaintatzopoulou, M.; Mueller, W.D.; Eliades, G.; Sherij, E.S.M.; Zinelis, S. Elemental, morphological, and corrosion characterization of different surface states of Co-Cr alloy for prosthodontic applications. Int. J. Electrochem. Sci. 2016, 11, 2982–2993. [Google Scholar] [CrossRef]

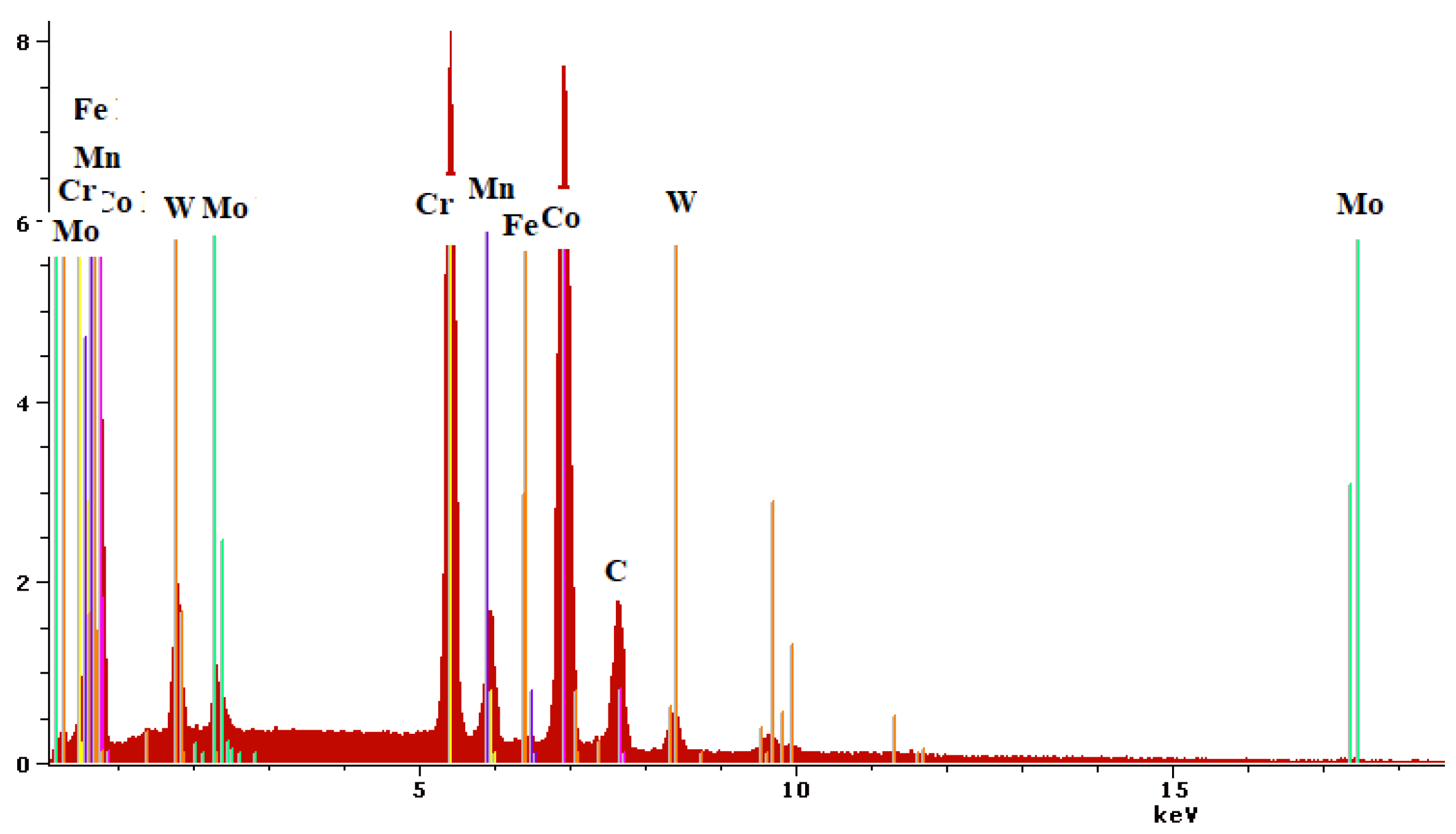



| Chemical Composition [Mass %] | |||
|---|---|---|---|
| Element | Powder | Sample | EDS Error |
| Co | 59.05 | 59.38 | 1.1 |
| Cr | 25.02 | 25.99 | 0.8 |
| W | 9.51 | 9.54 | 0.5 |
| Mo | 3.5 | 3.48 | 0.5 |
| Si | max. 1 | max. 1.16 | 0.1 |
| Other elements: C, Fe, Mn | max. 1.5 | 0.43 | 0.1 |
| Electrochemical Corrosion Parameters | Groups | |||
|---|---|---|---|---|
| A (n = 10) | B (n = 10) | C (n = 10) | p-Value | |
| Eoc (mV)/mean (SD) | −179.6 (17.5) | −146.9 (13.8) | −117.6 (10.9) | p < 0.001 * |
| Ecorr (mV)/mean (SD) | −331.6 (28.1) | −163.5 (19.6) | −63.6 (7.2) | p < 0.001 * |
| Jcorr (μA/cm2)/mean (SD) | 38.5 (5.1) | 13.5 (1.5) | 0.74 (0.03) | p < 0.001 * |
| βa (mV)/mean (SD) | 543.7 (42.2) | 424.7 (35.6) | 1021.03 (58.2) | p < 0.001 * |
| βc (mV)/mean (SD) | −440.7 (68.1) | −622.6 (73.5) | −40.1 (3.2) | p < 0.001 * |
| Rp (kΩ.cm2)/mean (SD) | 1.6 (0.1) | 5.5 (0.6) | 82.03 (6.7) | p < 0.001 * |
| CR (μm/year)/mean (SD) | 434.8 (41.6) | 156.1 (16.9) | 8.08 (0.8) | p < 0.001 * |
| Groups | Rs Ohm.cm2 | CPE | Rp Ohm.cm2 | CPE | Rct Ohm.cm2 | ||
|---|---|---|---|---|---|---|---|
| Qp × 10−5 Ssn/cm2 | n | Q1 × 10−5 Ssn/cm2 | n | ||||
| A | 155.9 | 1.933 | 0.71 | 310.9 | 33.53 | 0.66 | 20,420 |
| B | 204.3 | 3.189 | 0.83 | 19,920 | - | - | - |
| C | 203.6 | 2.898 | 0.84 | 30,970 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baciu, E.-R.; Bobu, L.; Cimpoeșu, R.; Budală, D.G.; Vasluianu, R.-I.; Gelețu, G.L.; Lupu, C.I.; Vițalariu, A.; Murariu, A. Dental Prostheses Materials: Corrosion Behavior of Co-Cr-W Alloys Processed by SLM Technique. Prosthesis 2025, 7, 27. https://doi.org/10.3390/prosthesis7020027
Baciu E-R, Bobu L, Cimpoeșu R, Budală DG, Vasluianu R-I, Gelețu GL, Lupu CI, Vițalariu A, Murariu A. Dental Prostheses Materials: Corrosion Behavior of Co-Cr-W Alloys Processed by SLM Technique. Prosthesis. 2025; 7(2):27. https://doi.org/10.3390/prosthesis7020027
Chicago/Turabian StyleBaciu, Elena-Raluca, Livia Bobu, Ramona Cimpoeșu, Dana Gabriela Budală, Roxana-Ionela Vasluianu, Gabriela Luminița Gelețu, Costin Iulian Lupu, Anca Vițalariu, and Alice Murariu. 2025. "Dental Prostheses Materials: Corrosion Behavior of Co-Cr-W Alloys Processed by SLM Technique" Prosthesis 7, no. 2: 27. https://doi.org/10.3390/prosthesis7020027
APA StyleBaciu, E.-R., Bobu, L., Cimpoeșu, R., Budală, D. G., Vasluianu, R.-I., Gelețu, G. L., Lupu, C. I., Vițalariu, A., & Murariu, A. (2025). Dental Prostheses Materials: Corrosion Behavior of Co-Cr-W Alloys Processed by SLM Technique. Prosthesis, 7(2), 27. https://doi.org/10.3390/prosthesis7020027










