Influence of Simulated Skin Color on the Accuracy of Face Scans
Abstract
1. Introduction
2. Materials and Methods
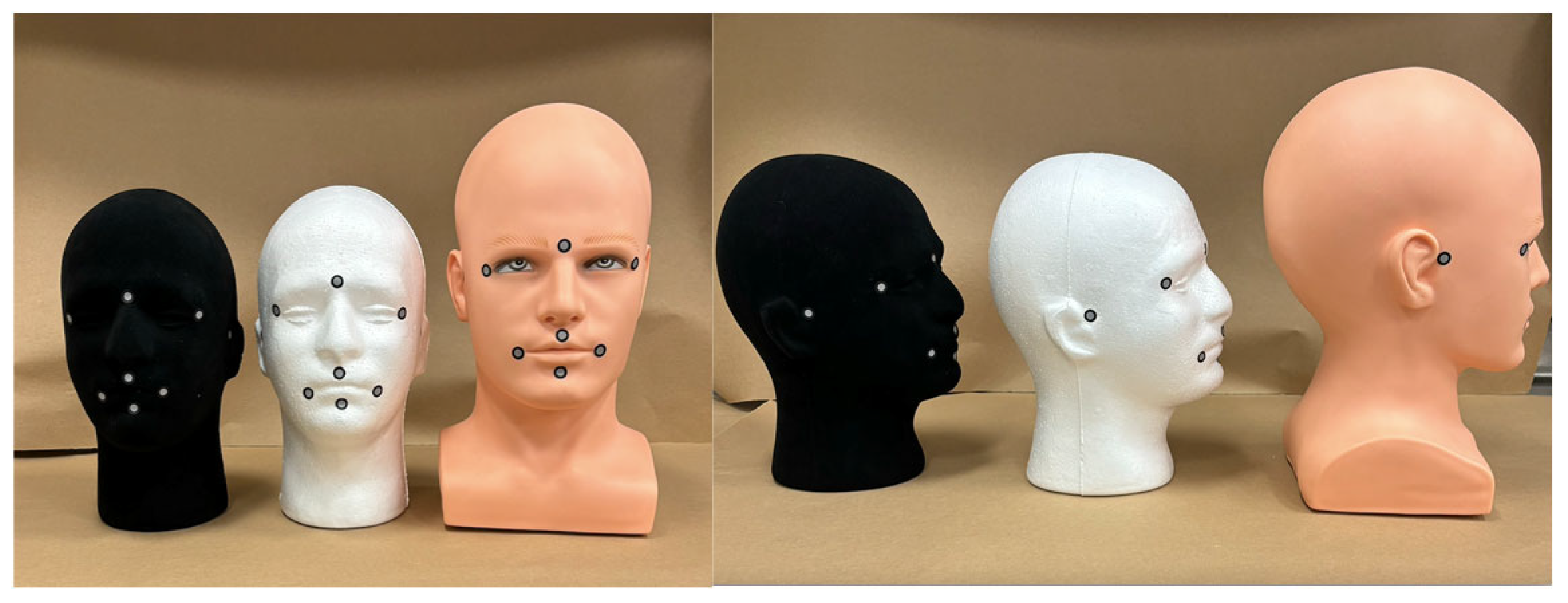
2.1. Groups and Sample Size
2.2. Scanning Procedure and Post-Processing
- Digital trimming: Reduced noise and redundant information.
- Fusion: Merged all captured data into a single unified model.
- Isolation: Removed unrelated/background data not connected to the main model.
- Overlap: Detected and eliminated overlapping data points.
- Smooth: Removed noise and duplicated data to create a cleaner model.
- Simplify: Compressed the data to reduce the overall file size.
- Mesh: Enhanced the model’s quality by controlling point density and detail.
- Fill Holes: Repaired areas with missing data to ensure uniformity.
- Texture Mapping: Helped replicate surface textures.
- File export: Files were exported as standard tessellation language (STL) for comparative evaluation of point clouds.
- Eleven evaluations were completed without markers and eleven evaluations were completed with markers for each mannequin group for each scanner. For a total of one hundred and thirty-two evaluations.
2.3. Global Deviations
2.4. CRIS Guidelines
3. Results
3.1. Global Deviations for the Infrared Scanner in Mannequins with and Without Markers
3.2. Global Deviations for the Blue-Light Scanner in Mannequins with and Without Markers
3.3. Statistical Comparison of Infrared Scans with and Without Markers
3.4. Statistical Comparison of Blue-Light Scans with and Without Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Bornstein, M.M.; Hsung, R.T.-C.; Ajmera, D.H.; Leung, Y.Y.; Gu, M. Frontiers in Three-Dimensional Surface Imaging Systems for 3D Face Acquisition in Craniofacial Research and Practice: An Updated Literature Review. Diagnostics 2024, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Bootvong, K.; Liu, Z.; McGrath, C.; Hagg, U.; Wong, R.W.K.; Bendeus, M.; Yeung, S. Virtual Model Analysis as an Alternative Approach to Plaster Model Analysis: Reliability and Validity. Eur. J. Orthod. 2010, 32, 589–595. [Google Scholar] [CrossRef]
- Santoro, M.; Galkin, S.; Teredesai, M.; Nicolay, O.F.; Cangialosi, T.J. Comparison of Measurements Made on Digital and Plaster Models. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Salloum, R. Revolutionizing Dentistry: Exploring the Potential of Facial Scanners for Precise Treatment Planning and Enhanced Patient Outcomes. J. Prosthet. Dent. 2024, 132, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Berisha, F.; Bronzino, I.; Kamnoedboon, P.; Leles, C.R. Reliability of a Face Scanner in Measuring the Vertical Dimension of Occlusion. J. Dent. 2024, 146, 105016. [Google Scholar] [CrossRef]
- Quinzi, V.; Polizzi, A.; Ronsivalle, V.; Santonocito, S.; Conforte, C.; Manenti, R.J.; Isola, G.; Lo Giudice, A. Facial Scanning Accuracy with Stereophotogrammetry and Smartphone Technology in Children: A Systematic Review. Children 2022, 9, 1390. [Google Scholar] [CrossRef]
- Raffone, C.; Gianfreda, F.; Pompeo, M.G.; Antonacci, D.; Bollero, P.; Canullo, L. Chairside Virtual Patient Protocol. Part 2: Management of Multiple Face Scans and Alignment Predictability. J. Dent. 2022, 122, 104123. [Google Scholar] [CrossRef] [PubMed]
- Lippold, C.; Liu, X.; Wangdo, K.; Drerup, B.; Schreiber, K.; Kirschneck, C.; Moiseenko, T.; Danesh, G. Facial Landmark Localization by Curvature Maps and Profile Analysis. Head Face Med. 2014, 10, 54. [Google Scholar] [CrossRef]
- Leung, M.Y.; Lo, J.; Leung, Y.Y. Accuracy of Different Modalities to Record Natural Head Position in 3 Dimensions: A Systematic Review. J. Oral Maxillofac. Surg. 2016, 74, 2261–2284. [Google Scholar] [CrossRef]
- Probst, J.; Dritsas, K.; Halazonetis, D.; Ren, Y.; Katsaros, C.; Gkantidis, N. Precision of a Hand-Held 3D Surface Scanner in Dry and Wet Skeletal Surfaces: An Ex Vivo Study. Diagnostics 2022, 12, 2251. [Google Scholar] [CrossRef]
- Xia, S.; Guo, S.; Li, J.; Istook, C. Comparison of Different Body Measurement Techniques: 3D Stationary Scanner, 3D Handheld Scanner, and Tape Measurement. J. Text. Inst. 2019, 110, 1103–1113. [Google Scholar] [CrossRef]
- Renard, G.; Leid, J. Les dangers de la lumière bleue: La vérité! J. Fr. D’Ophtalmol. 2016, 39, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Schmit, J.; Olszak, A. High-Precision Shape Measurement by White-Light Interferometry with Real-Time Scanner Error Correction. Appl. Opt. 2002, 41, 5943. [Google Scholar] [CrossRef]
- Rey, M.; Volpe, G.; Volpe, G. Light, Matter, Action: Shining Light on Active Matter. ACS Photonics 2023, 10, 1188–1201. [Google Scholar] [CrossRef]
- Mizuno, K.; Ishii, J.; Kishida, H.; Hayamizu, Y.; Yasuda, S.; Futaba, D.N.; Yumura, M.; Hata, K. A Black Body Absorber from Vertically Aligned Single-Walled Carbon Nanotubes. Proc. Natl. Acad. Sci. USA 2009, 106, 6044–6047. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Pan, T.-S.; Hsiao, W.-C.; Lin, W.-H.; Liu, Y.-L.; Su, T.-J.; Wang, S.-M. Using Contactless Facial Image Recognition Technology to Detect Blood Oxygen Saturation. Bioengineering 2023, 10, 524. [Google Scholar] [CrossRef]
- Petrides, G.; Clark, J.R.; Low, H.; Lovell, N.; Eviston, T.J. Three-Dimensional Scanners for Soft-Tissue Facial Assessment in Clinical Practice. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 605–614. [Google Scholar] [CrossRef]
- Major, M.; Mészáros, B.; Würsching, T.; Polyák, M.; Kammerhofer, G.; Németh, Z.; Szabó, G.; Nagy, K. Evaluation of a Structured Light Scanner for 3D Facial Imaging: A Comparative Study with Direct Anthropometry. Sensors 2024, 24, 5286. [Google Scholar] [CrossRef]
- Akl, M.A.; Mansour, D.E.; Zheng, F. The Role of Intraoral Scanners in the Shade Matching Process: A Systematic Review. J. Prosthodont. 2023, 32, 196–203. [Google Scholar] [CrossRef]
- Allred, S.R.; Olkkonen, M. The Effect of Background and Illumination on Color Identification of Real, 3D Objects. Front. Psychol. 2013, 4, 821. [Google Scholar] [CrossRef]
- Varda, K.; Bešlagić, E.; Zaimović-Uzunović, N.; Lemeš, S. Ambient Light and Object Color Influence on the 3D Scanning Process. In New Technologies, Development and Application V; Karabegović, I., Kovačević, A., Mandžuka, S., Eds.; Lecture Notes in Networks and Systems; Springer International Publishing: Cham, Switzerland, 2022; Volume 472, pp. 37–45. ISBN 978-3-031-05229-3. [Google Scholar]
- Krithikadatta, J.; Gopikrishna, V.; Datta, M. CRIS Guidelines (Checklist for Reporting In-vitro Studies): A concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. J. Conserv. Dent. 2014, 17, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Cui, X.; Wang, X.; Xie, H.; Chen, B. Light Absorption of Black Carbon Is Doubled at Mt. Tai and Typical Urban Area in North China. Sci. Total Environ. 2018, 635, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, U.; Sekerci, R.; Hizay, A.; Yildirim, Y.; Uysal, H. Assessment of reproducibility and reliability of facial expressions using 3D handheld scanner. J. Craniomaxillofac Surg. 2019, 47, 895–901. [Google Scholar] [CrossRef]
- Knoops, P.G.M.; Beaumont, C.A.A.; Borghi, A.; Rodriguez-Florez, N.; Breakey, R.W.F.; Rodgers, W.; Angullia, F.; Jeelani, N.U.O.; Schievano, S.; Dunaway, D.J. Comparison of Three-Dimensional Scanner Systems for Craniomaxillofacial Imaging. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Egri, L.; Nabati, H.; Yu, J.Y. RainbowTag: A Fiducial Marker System with a New Color Segmentation Algorithm. In Proceedings of the 2022 IEEE International Conference on Connected Vehicle and Expo (ICCVE), Lakeland, FL, USA, 7–9 March 2022; pp. 1–6. [Google Scholar]
- Dindaroğlu, F.; Kutlu, P.; Duran, G.; Görgülü, S.; Aslan, E. Accuracy and reliability of 3D stereophotogrammetry: A comparison to direct anthropometry and 2D photogrammetry. Angle Orthod. 2016, 86, 487–494. [Google Scholar] [CrossRef]
- Paschotta, R. Infrared Light. RP Photonics Encyclopedia. Available online: https://www.rp-photonics.com/infrared_light.html (accessed on 30 September 2024).
- McDonough, M. Blue Light. Encyclopedia Britannica. 2024. Available online: https://www.britannica.com/science/blue-light (accessed on 30 September 2024).
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef]








| Group | N | Mean | StDev | 95% Cl |
|---|---|---|---|---|
| RedWT (Test) | 10 | 0.1977 | 0.0317 | (0.1779, 0.2175) |
| RedWC (Control) | 10 | 0.17753 | 0.02781 | (0.15771, 0.19734) |
| RedPT (Test) | 10 | 0.13542 | 0.01310 | (0.05602, 0.21482) |
| RedPC (Control) | 10 | 0.3612 | 0.1685 | (0.2818, 0.4406) |
| RedBT (Test) | 10 | 0.3417 | 0.0506 | (0.2864, 0.3970) |
| RedBC (Control) | 10 | 0.5902 | 0.1063 | (0.5349, 0.6455) |
| Group | N | Mean | StDev | 95% Cl |
|---|---|---|---|---|
| BlueWT (Test) | 10 | 0.8280 | 0.45934 | (0.1965, 1.9653) |
| BlueWC (Control) | 10 | 1.19940 | 0.330039 | (0.55231, 1.5223) |
| BluePT (Test) | 10 | 2.26478 | 1.0608 | (1.4749, 4.4803) |
| BluePC (Control) | 10 | 2.98472 | 0.381317 | (2.15074, 3.45292) |
| BlueBT (Test) | 10 | - | - | - |
| BlueBC (Control) | 10 | - | - | - |
| Samples | T-Value | Adjusted p-Value |
|---|---|---|
| InfraRedWC (Control) vs. RedWT (Test) | −1.36 | 0.207 |
| InfraRedPC (Control) vs. RedPT (Test) | 4.23 | 0.001 |
| InfraRedBC (Control) vs. RedBT (Test) | 6.67 | 0.001 |
| Samples | T-Value | Adjusted p-Value |
|---|---|---|
| BlueWC (Control) vs. BlueWT (Test) | −1.81 | 0.148 |
| BluePC (Control) vs. BluePT (Test) | −1.88 | 0.092 |
| BlueBC (Control) vs. BlueBT (Test) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brintouch, I.; Ali, A.; Romanos, G.E.; Delgado-Ruiz, R.A. Influence of Simulated Skin Color on the Accuracy of Face Scans. Prosthesis 2024, 6, 1372-1382. https://doi.org/10.3390/prosthesis6060099
Brintouch I, Ali A, Romanos GE, Delgado-Ruiz RA. Influence of Simulated Skin Color on the Accuracy of Face Scans. Prosthesis. 2024; 6(6):1372-1382. https://doi.org/10.3390/prosthesis6060099
Chicago/Turabian StyleBrintouch, Ido, Aisha Ali, Georgios E. Romanos, and Rafael A. Delgado-Ruiz. 2024. "Influence of Simulated Skin Color on the Accuracy of Face Scans" Prosthesis 6, no. 6: 1372-1382. https://doi.org/10.3390/prosthesis6060099
APA StyleBrintouch, I., Ali, A., Romanos, G. E., & Delgado-Ruiz, R. A. (2024). Influence of Simulated Skin Color on the Accuracy of Face Scans. Prosthesis, 6(6), 1372-1382. https://doi.org/10.3390/prosthesis6060099








