Citric Acid-Based Solutions as Decontaminant Mouthwash in Titanium and Dental Prostheses Materials in Implantoplasty Processes
Abstract
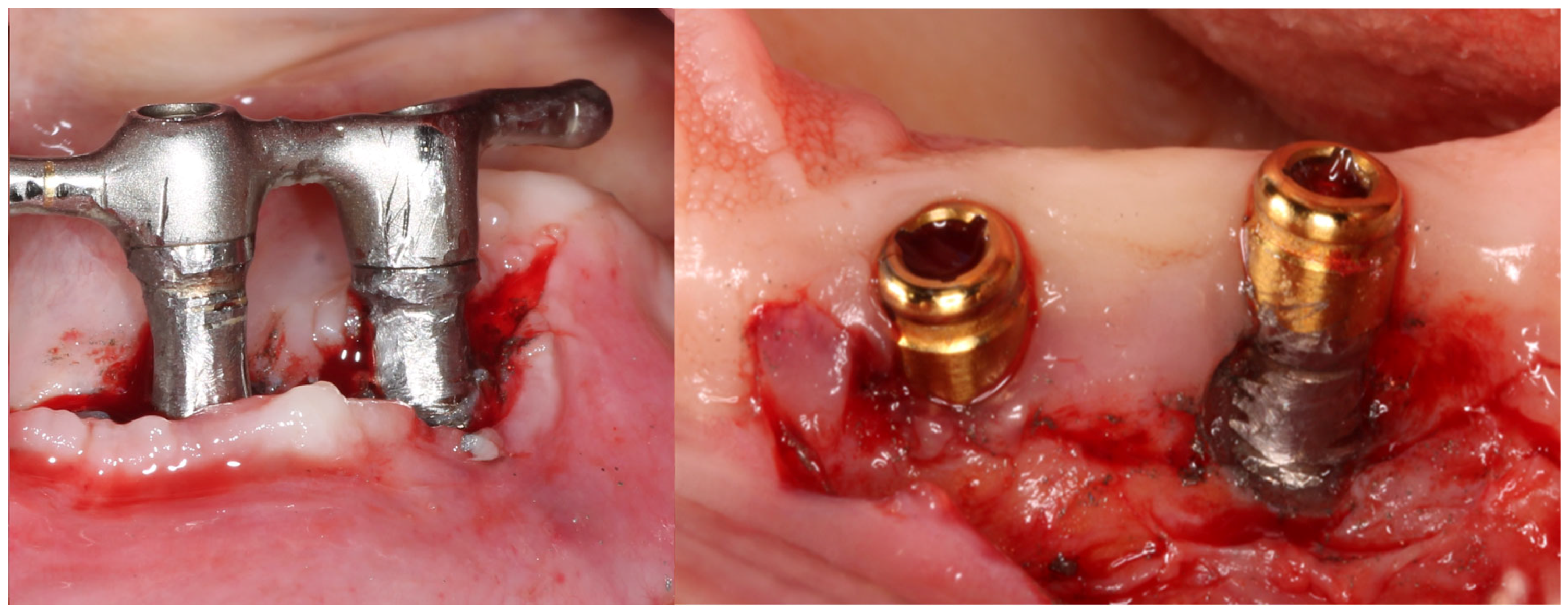
1. Introduction
2. Materials and Methods
2.1. Materials
- Ti: commercially pure Titanium (Ti), grade 3.
- Zr: zirconia (ZrO2 with 2.5% in weight of yttria) (Y2O3).
- Composite formed by polymethyl methacrylate (PMMA) with feldspar CaAl2Si2O8 with 38% in volume.
- CrCo: the chromium content was 30 wt%, the Mo content was 7 wt% and the W content was 0.1 wt%; cobalt was the balance.
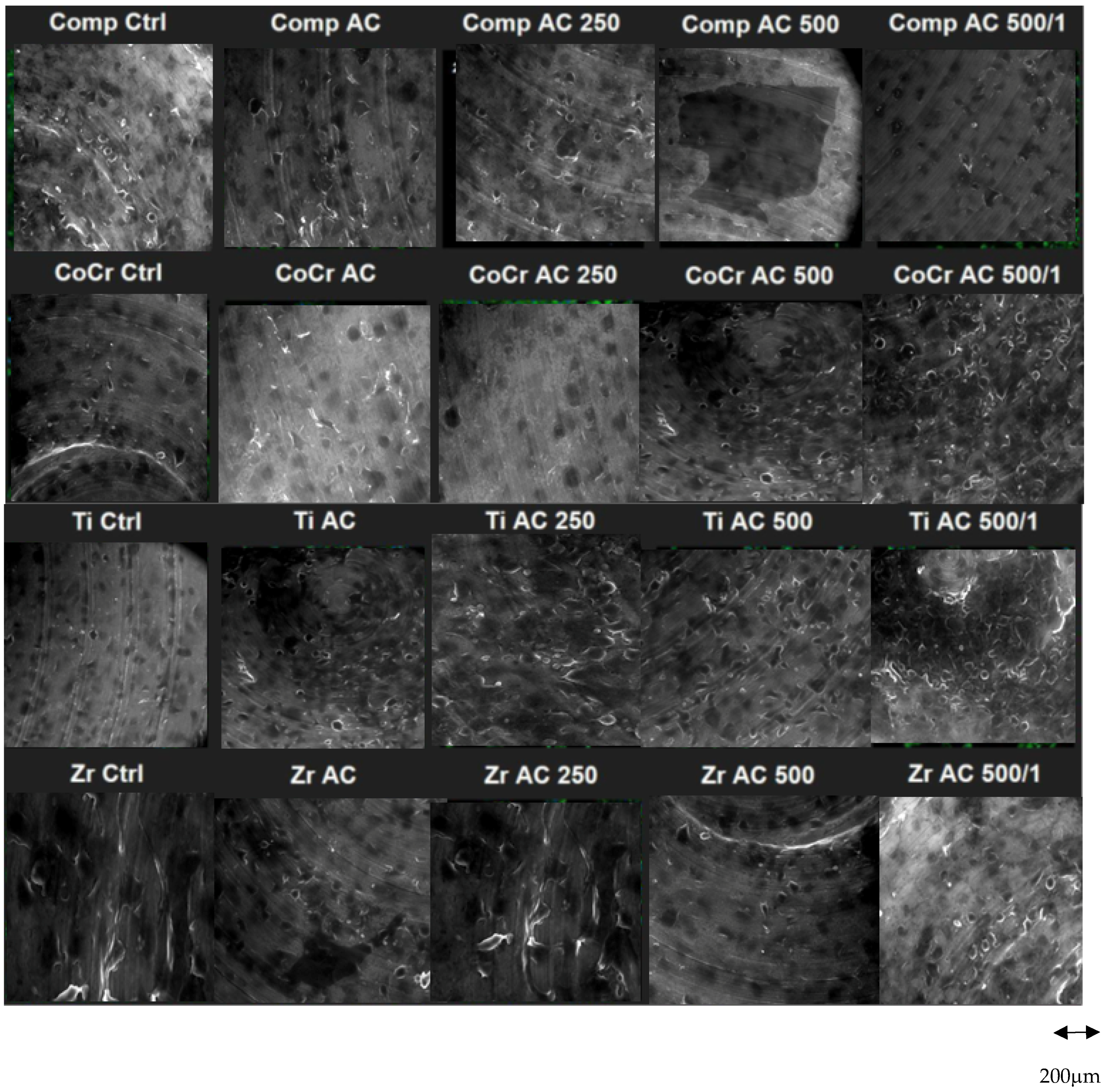
2.2. Roughness Analysis
2.3. Wettability
2.4. Fibroblast Culture
2.5. Osteoblasts Culture
2.6. Immunofluorescence
2.7. Bacterial Culture
2.8. Statistical Analysis
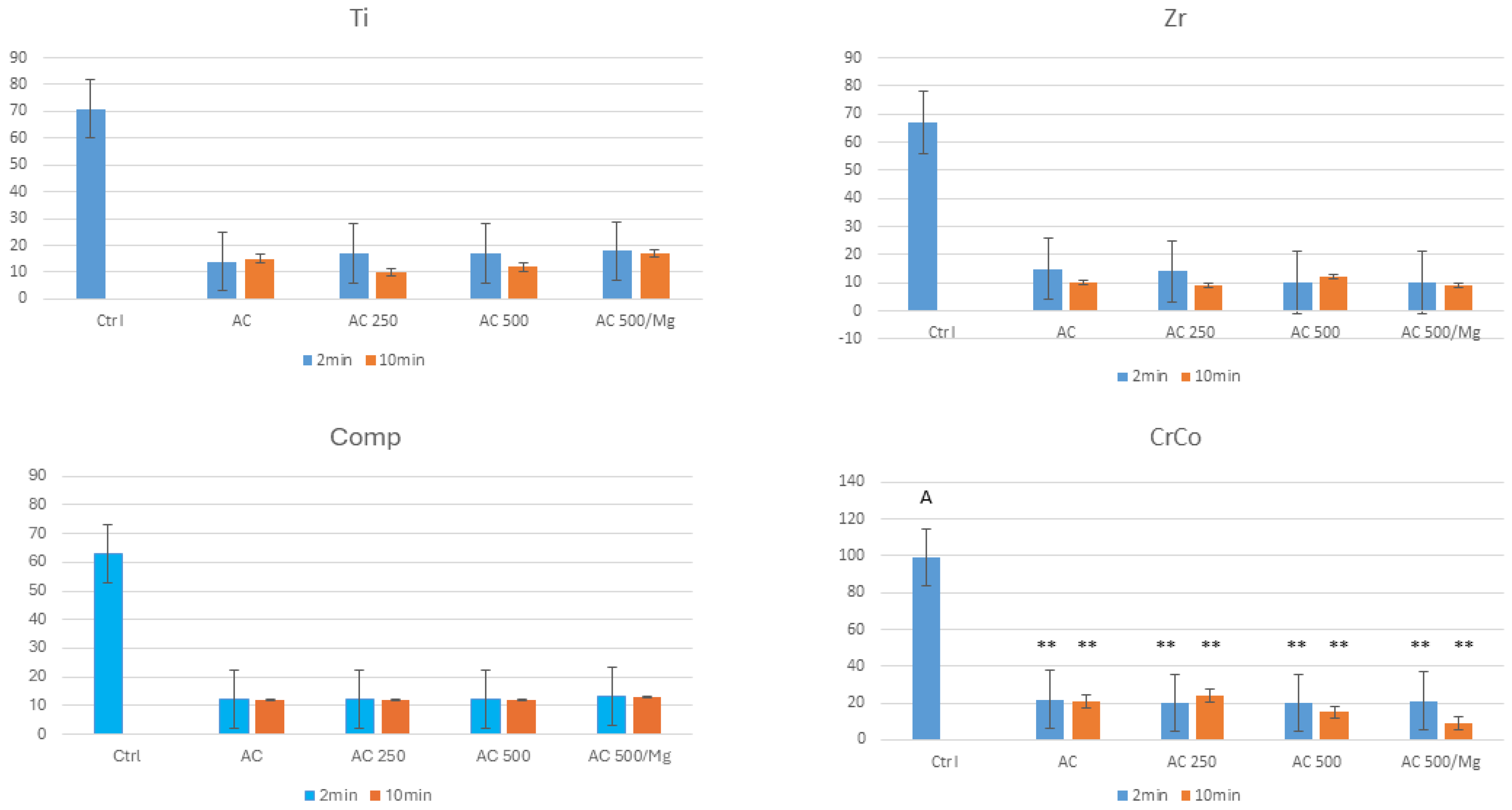
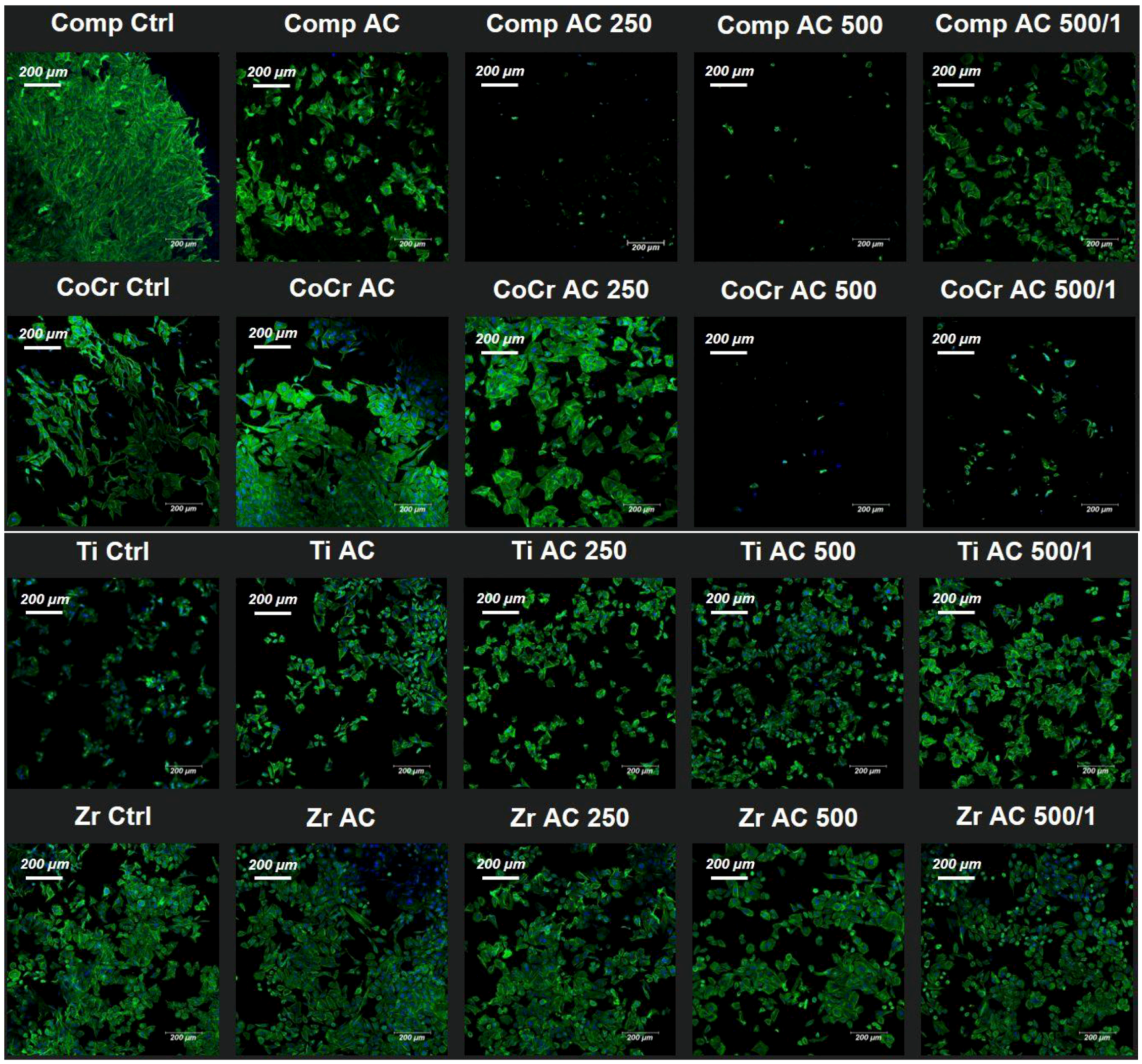
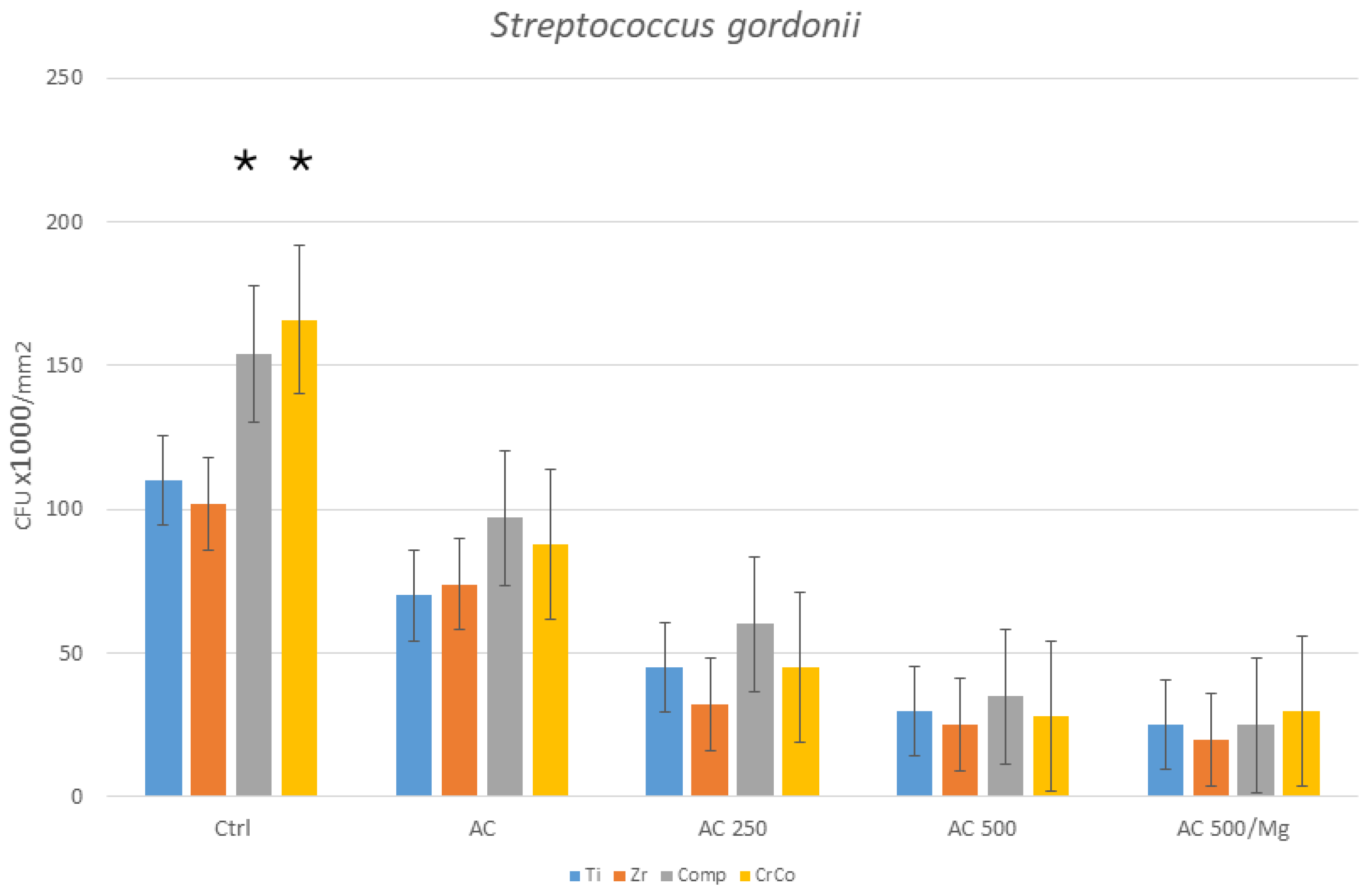
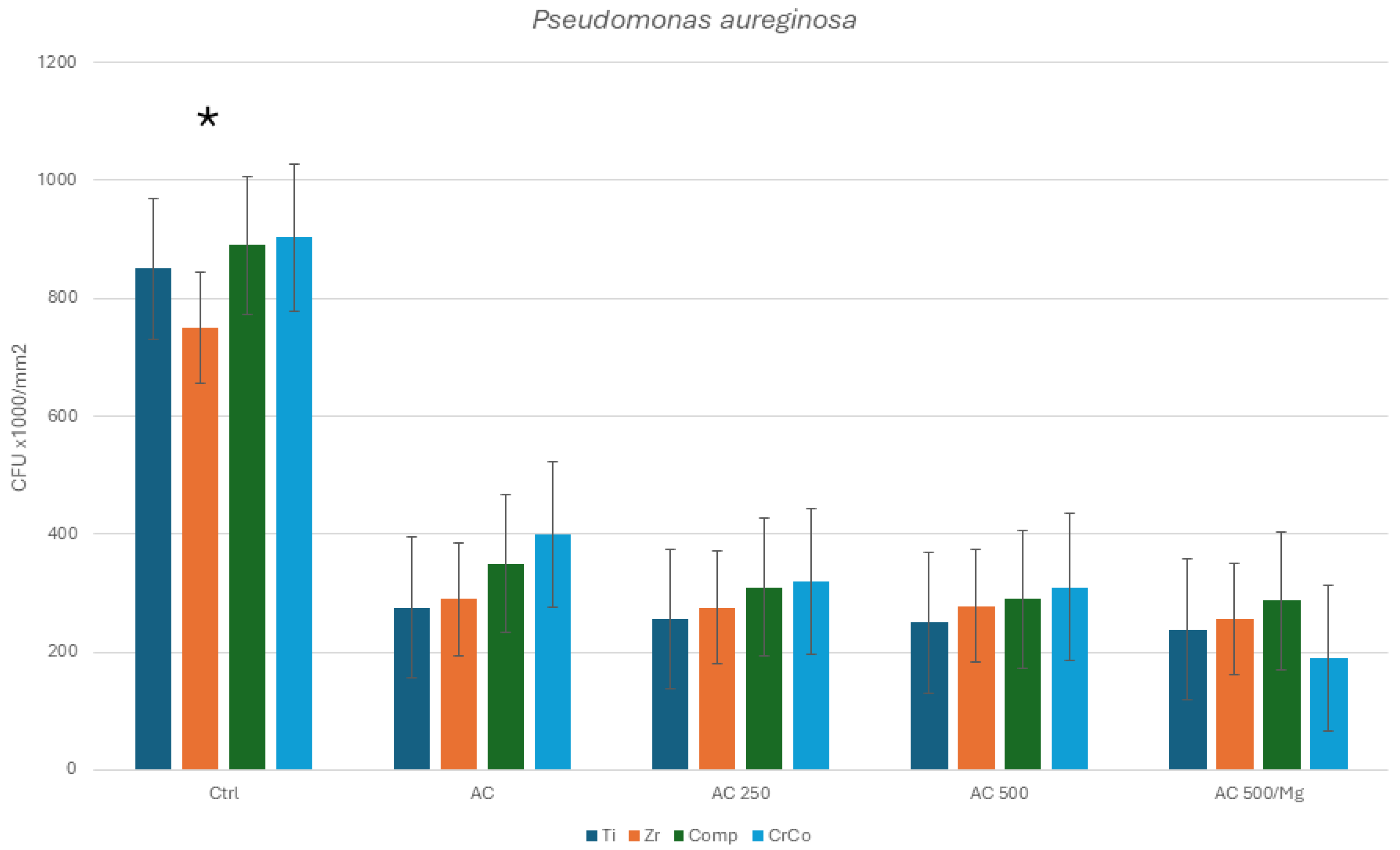
3. Results
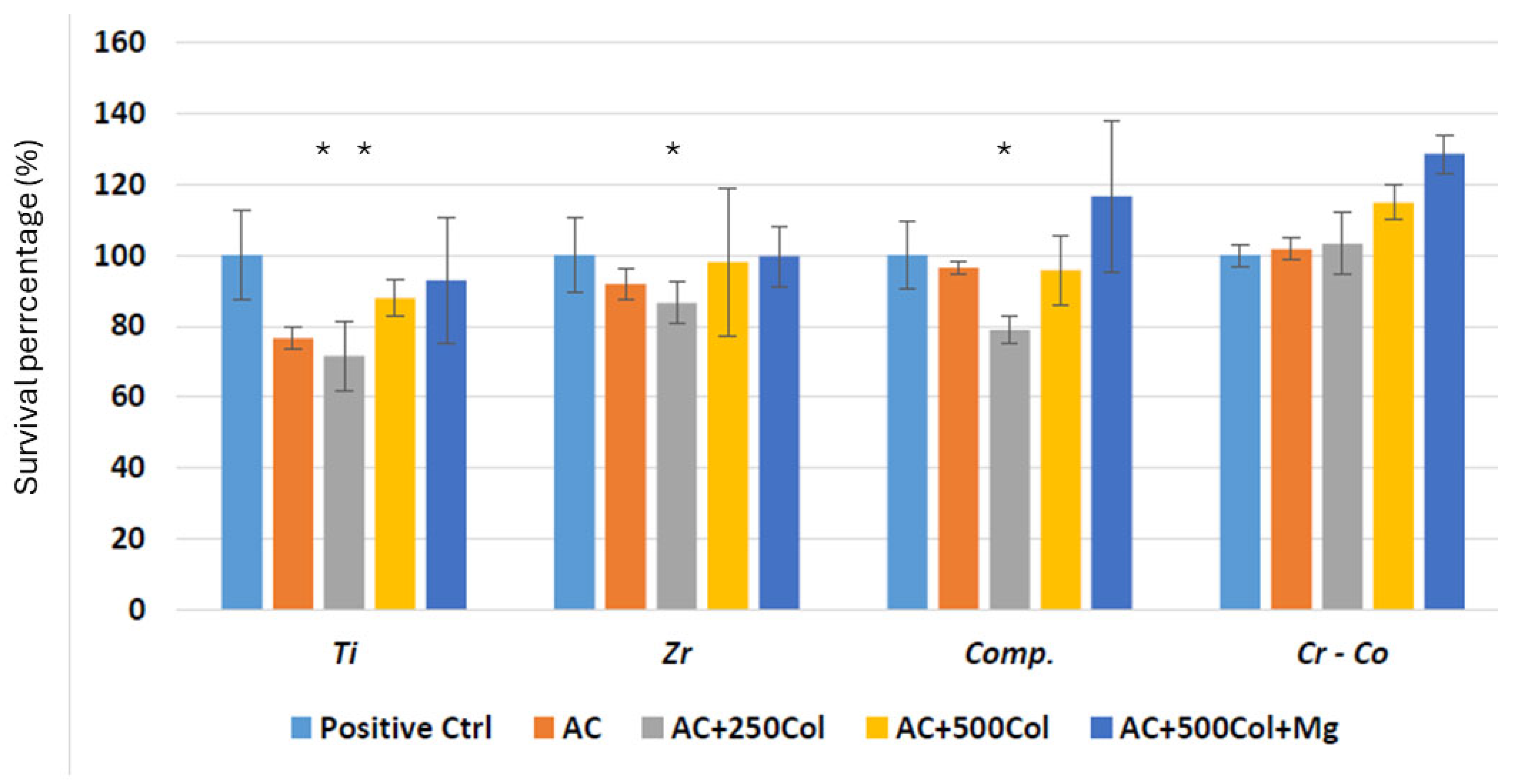
Bacterial Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates, Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment, Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006, 33, 283–289. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 199–214. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodont. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontology 2000 2019, 81, 29–40. [Google Scholar] [CrossRef]
- Englezos, E.; Cosyn, J.; Koole, S.; Jacquet, W.; De Bruyn, H. Resective Treatment of Peri-implantitis: Clinical and Radiographic Outcomes After 2 Years. Int. J. Periodontics Restor. Dent. 2018, 38, 729–735. [Google Scholar] [CrossRef]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implant. Res. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Monje, A.; Schwarz, F. Principles of Combined Surgical Therapy for the Management of Peri-Implantitis. Clin. Adv. Periodontics 2022, 12, 57–63. [Google Scholar] [CrossRef]
- Bertl, K.; Isidor, F.; von Steyern, P.V.; Stavropoulos, A. Does implantoplasty affect the failure strength of narrow and regular diameter implants? A laboratory study. Clin. Oral Investig. 2021, 25, 2203–2211. [Google Scholar] [CrossRef]
- Leitão-Almeida, B.; Camps-Font, O.; Correia, A.; Mir-Mari, J.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of bone loss on the fracture resistance of narrow dental implants after implantoplasty. An in vitro study. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e611–e618. [Google Scholar] [CrossRef] [PubMed]
- Hentenaar DF, M.; De Waal YC, M.; Stewart, R.E.; Van Winkelhoff, A.J.; Meijer HJ, A.; Raghoebar, G.M. Erythritol airpolishing in the non-surgical treatment of peri-implantitis: A randomized controlled trial. Clin. Oral Implant. Res. 2021, 32, 840–852. [Google Scholar] [CrossRef]
- Tomasi, C.; Regidor, E.; Ortiz-Vigón, A.; Derks, J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 340–356. [Google Scholar] [CrossRef]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant. Dent. 2015, 1, 22. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansåker, A.M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2022, 35 (Suppl. S8), 305–315. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol. 2022, 93, 110–122. [Google Scholar] [CrossRef]
- Barrak, F.N.; Li, S.; Muntane, A.M.; Jones, J.R. Particle release from implantoplasty of dental implants and impact on cells. Int. J. Implant. Dent. 2020, 6, 50. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.A.; Gay-Escoda, C.; Valmaseda-Castellon, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosión behavior of Ti6Al4V particles obtained by Implatoplasty. An in vivo study. Part. II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials 2021, 14, 6507. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.; Proubasta, I.; Vazquez, J. Fundamentos de biomecánica y biomateriales. In Fundamentos de Biomecánica y Biomateriales; Ergon: Barcelona, Spain, 1997; pp. 125–132. [Google Scholar]
- Gil, F.J.; Planell, J.A. Aplicaciones biomédicas del titanio v sus aleaciones. Biomecánica 1993, 1, 34–43. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Cordeiro, J.M.; Lima, C.V.; Barão, V.A.R. Citric acid reduces oral biofilm and influences the electrochemical behavior of titanium: An in situ and in vitro study. J. Periodontol. 2019, 90, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Książek, E. Citric Acid: Properties, Microbial Production, and Applications in Industries. Molecules 2024, 29, 22. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Pires, J.M.; Souza, J.G.S.; Lima, C.V.; Bertolini, M.M.; Rangel, E.C.; Barão, V.A.R. Optimizing citric acid protocol to control implant-related infections: An in vitro and in situ study. J. Periodontal Res. 2021, 56, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.C.P.D.; Abreu, L.G.; Andrade, E.J.; Asquino, N.; Esteves Lima, R.P. Effectiveness of antimicrobial photodynamic therapy in the treatment of peri-implantitis: Systematic review and meta-analysis. Lasers Med. Sci. 2024, 39, 186. [Google Scholar] [CrossRef]
- Wang, S.; Xu, C.; Yu, S.; Wu, X.; Jie, Z.; Dai, H. Citric acid enhances the physical properties, cytocompatibility and osteogenesis of magnesium calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2019, 94, 42–50. [Google Scholar] [CrossRef]
- Brynhildsen, L.; Rosswall, T. Effects of cadmium, copper, magnesium, and zinc on the decomposition of citrate by a Klebsiella sp. Appl. Environ. Microbiol. 1989, 55, 1375–1379. [Google Scholar] [CrossRef]
- Gil, F.J.; Solano, E.; Pena, J.; Engel, E.; Mendoza, A.; Planell, J.A. Microstructural, mechanical and citotoxicity evaluation of different NiTi and NiTiCu shape memory alloys. J. Mater. Sci. Mater. Med. 2004, 15, 1181–1185. [Google Scholar] [CrossRef]
- Janson, O.; Gururaj, S.; Pujari-Palmer, S.; Karlsson Ott, M.; Strømme, M.; Engqvist, H. Titanium surface modification to enhance antibacterial and bioactive properties while retaining biocompatibility. Mater. Sci. Eng. C 2019, 96, 272–279. [Google Scholar] [CrossRef]
- Costa-Berenguer, X.; Garcia-Garcia, M.; Sanchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellon, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implant. Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- Francis, S.; Iaculli, F.; Perrotti, V.; Piattelli, A.; Quaranta, A. Titanium Surface Decontamination: A Systematic Review of In Vitro Comparative Studies. Int. J. Oral Maxillofac. Implant. 2022, 37, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Costochondral Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Lincks, J.; Lohmann, C.H.; Sylvia, V.L.; Cochran, D.L.; Blanchard, C.R.; Dean, D.D.; Schwartz, Z. Effect of surface roughness and composition on chondrocytes is dependent on cell maturation state. J. Orthop. Res. 1999, 17, 446–457. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 524–532. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Rolauffs, B.; Adolfsson, E.; Palmero, P.; Fürderer, T.; Altmann, B. Controlling osteoblast morphology and proliferation via surface micro-topographies of implant biomaterials. Sci. Rep. 2020, 10, 12810. [Google Scholar] [CrossRef]
- Esposito, M. Titanium for Dental Implants (I). In Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Charest, J.L.; Eliason, M.T.; García, A.J.; King, W.P. Combined microscale mechanical topography and chemical patterns on polymer cell culture substrates. Biomaterials 2006, 27, 2487–2494. [Google Scholar] [CrossRef]
- Mustafa, K.; Wennerberg, A.; Wroblewski, J.; Hultenby, K.; Lopez, B.S.; Arvidson, K. Determining optimal surface roughness of TiO(2) blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin. Oral Implant. Res. 2001, 12, 515–525. [Google Scholar] [CrossRef]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing, and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Nath, S.; Sugawara, Y.; Divakarla, K.; Das, T.; Manos, J.; Chrzanowski, W.; Matsushita, T.; Kokubo, T. Two-in-one biointerfaces—Antimicrobial and bioactive nanoporous gallium titanate layers for titanium implants. Nanomaterials 2017, 7, 229. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 538–545. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Neumann, A.; Ng, H.P.; Lapovok, R.; Kasper, C.; Lowe, T.C. Combined effect of grain refinement and surface modification of pure titanium on the attachment of mesenchymal stem cells and osteoblast-like SaOS-2 cells. Mater. Sci. Eng. C 2017, 71, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.S.; Zhou, W.S.; He, X.W.; Liu, W.; Bai, B.L.; Zhou, Q. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater. Sci. Eng. C 2016, 62, 226–232. [Google Scholar] [CrossRef]
- Mitchel, J.A.; Hoffman-Kim, D. Cellular scale anisotropic topography guides Schwann cell motility. PLoS ONE 2011, 6, e24316. [Google Scholar] [CrossRef]
- Huang, Q.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Yang, X.; Shen, Z.; Feng, Q. Effects of hierarchical micro/nano-topographies on the morphology, proliferation and differentiation of osteoblast-like cells. Colloids Surf. B Biointerfaces 2016, 145, 37–45. [Google Scholar] [CrossRef]
- ISO 10993—5 (55); Biological Evaluation of Medical Devices—Part 5, Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Bax, D.V.; Smalley, H.E.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Cellular response to collagen-elastin composite materials. Acta Biomater. 2019, 86, 158–170. [Google Scholar] [CrossRef]
- Yoon, B.H.; Kim, H.W.; Lee, S.H.; Bae, C.J.; Koh, Y.H.; Kong, Y.M.; Kim, H.E. Stability and cellular responses to fluorapatite-collagen composites. Biomaterials 2005, 26, 2957–2963. [Google Scholar] [CrossRef]
- Sartori, M.; Giavaresi, G.; Parrilli, A.; Ferrari, A.; Aldini, N.N.; Morra, M.; Cassinelli, C.; Bollati, D.; Fini, M. Collagen type I coating stimulates bone regeneration and osteointegration of titanium implants in the osteopenic rat. Int. Orthop. 2015, 39, 2041–2052. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Scholz, M.; Baudisch, M.; Liese, J.; Wegner, K.; Frerich, B.; Lang, H. Guided Bone Regeneration Using Collagen Scaffolds, Growth Factors, and Periodontal Ligament Stem Cells for Treatment of Peri-Implant Bone Defects In Vivo. Stem Cells Int. 2017, 2017, 3548435. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A Histomorphometric Study Miniat. Pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin. Oral Implant. Res. 2014, 25, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef]
- Avila, G.; Misch, K.; Galindo-Moreno, P.; Wang, H.L. Implant surface treatment using biomimetic agents. Implant. Dent. 2009, 18, 17–26. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surf. B Biointerfaces 2017, 152, 367–375. [Google Scholar] [CrossRef]
- Gosau, M.; Hahnel, S.; Schwarz, F.; Gerlach, T.; Reichert, T.E.; Bürgers, R. Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Clin. Oral Implant. Res. 2010, 21, 866–872. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, R.; Sun, Q.; Zhuang, Z.; Zhang, Y.; Chen, S.; Han, B. Bio-inspired special wettability in oral antibacterial applications. Front. Bioeng. Biotechnol. 2022, 10, 1001616. [Google Scholar] [CrossRef]
- Hemmatian, T.; Lee, H.; Kim, J. Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates. Polymers 2021, 13, 223. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Román, J.; Padilla, S.; Doadrio, J.C.; Gil, F.J. Bioactivity and mechanical properties of SiO2–CaO–P2O5 glassceramics. J. Mater. Chem. 2005, 15, 1353–1359. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, X.; Wei, M. Biomimetic collagen/apatite coating formation on Ti6Al4V substrates. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2012, 100, 871–881. [Google Scholar] [CrossRef]
- Belloni, A.; Argentieri, G.; Orilisi, G.; Notarstefano, V.; Giorgini, E.; D’Addazio, G.; Orsini, G.; Caputi, S.; Sinjari, B. New insights on collagen structural organization and spatial distribution around dental implants: A comparison between machined and laser-treated surfaces. J. Transl. Med. 2024, 22, 120. [Google Scholar] [CrossRef]
- Zhao, S.F.; Jiang, Q.H.; Peel, S.; Wang, X.X.; He, F.M. Effects of magnesium-substituted nanohydroxyapatite coating on implant osseointegration. Clin. Oral Implant. Res. 2013, 24 (Suppl. A100), 34–41. [Google Scholar] [CrossRef]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: A systematic review. Clin. Oral Implant. Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef]
- de Tapia, B.; Valles, C.; Ribeiro-Amaral, T.; Mor, C.; Herrera, D.; Sanz, M.; Nart, J. The adjunctive effect of a titanium brush in implant surface decontamination at peri-implantitis surgical regenerative interventions: A randomized controlled clinical trial. J. Clin. Periodontol. 2019, 46, 586–596. [Google Scholar] [CrossRef]
- Dostie, S.; Alkadi, L.T.; Owen, G.; Bi, J.; Shen, Y.; Haapasalo, M.; Larjava, H.S. Chemotherapeutic decontamination of dental implants colonized by mature multispecies oral biofilm. J. Clin. Periodontol. 2017, 44, 403–409. [Google Scholar] [CrossRef]
| Dissolution | Chemical Composition |
|---|---|
| 25% Citric acid (AC) | Citric acid 25% in volume (v) |
| 25% Citric acid + collagen 250 (AC 250) | Citric acid 25% (v) with 0.25 g collagen/L |
| 25% Citric acid + collagen 500 (AC 500) | Citric acid 25% (v) with 0.50 g collagen/L |
| 25% Citric acid + collagen 500 + 1% Mg (AC 500/Mg) | Citric acid 25% (v) with 0.50 g collagen/L and 10% Mg(NO3)2·6H2O |
| Treatment | Ti | Zr | Comp | CrCo |
|---|---|---|---|---|
| As-received | 0.15 ± 0.09 * | 0.10 ± 0.07 | 0.12 ± 0.09 * | 0.18 ± 0.09 * |
| Implantoplasty (Ctrl) | 0.25 ± 0.15 * | 0.15 ± 0.05 | 0.28 ± 0.07 ** | 0.37 ± 0.10 ** |
| AC | 0.33 ± 0.13 ** | 0.17 ± 0.08 | 0.29 ± 0.09 ** | 0.40 ± 0.12 ** |
| AC 250 | 0.30 ± 0.10 ** | 0.14 ± 0.09 | 0.27 ± 0.07 ** | 0.43 ± 0.13 ** |
| AC 500 | 0.25 ± 0.11 ** | 0.17 ± 0.05 | 0.25 ± 0.08 ** | 0.44 ± 0.14 ** |
| AC 500/Mg | 0.27 ± 0.10 ** | 0.18 ± 0.04 | 0.26 ± 0.09 ** | 0.42 ± 0.15 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Garrido, P.; Fernández-Dominguez, P.; Fernández De La Fuente, L.; Manso De Gustin, B.; Varona, J.F.; Bosch, B.M.; Gil, J.; Fernández-Domínguez, M. Citric Acid-Based Solutions as Decontaminant Mouthwash in Titanium and Dental Prostheses Materials in Implantoplasty Processes. Prosthesis 2024, 6, 1211-1227. https://doi.org/10.3390/prosthesis6050087
Fernández-Garrido P, Fernández-Dominguez P, Fernández De La Fuente L, Manso De Gustin B, Varona JF, Bosch BM, Gil J, Fernández-Domínguez M. Citric Acid-Based Solutions as Decontaminant Mouthwash in Titanium and Dental Prostheses Materials in Implantoplasty Processes. Prosthesis. 2024; 6(5):1211-1227. https://doi.org/10.3390/prosthesis6050087
Chicago/Turabian StyleFernández-Garrido, Pilar, Pedro Fernández-Dominguez, Laura Fernández De La Fuente, Barbara Manso De Gustin, José Felipe Varona, Begoña M. Bosch, Javier Gil, and Manuel Fernández-Domínguez. 2024. "Citric Acid-Based Solutions as Decontaminant Mouthwash in Titanium and Dental Prostheses Materials in Implantoplasty Processes" Prosthesis 6, no. 5: 1211-1227. https://doi.org/10.3390/prosthesis6050087
APA StyleFernández-Garrido, P., Fernández-Dominguez, P., Fernández De La Fuente, L., Manso De Gustin, B., Varona, J. F., Bosch, B. M., Gil, J., & Fernández-Domínguez, M. (2024). Citric Acid-Based Solutions as Decontaminant Mouthwash in Titanium and Dental Prostheses Materials in Implantoplasty Processes. Prosthesis, 6(5), 1211-1227. https://doi.org/10.3390/prosthesis6050087








