Enhancing Tensile Bond Strength of Glass Fiber Posts Using Chitosan as a Coupling Agent: A Novel Approach for Improved Dental Restorations
Abstract
1. Introduction
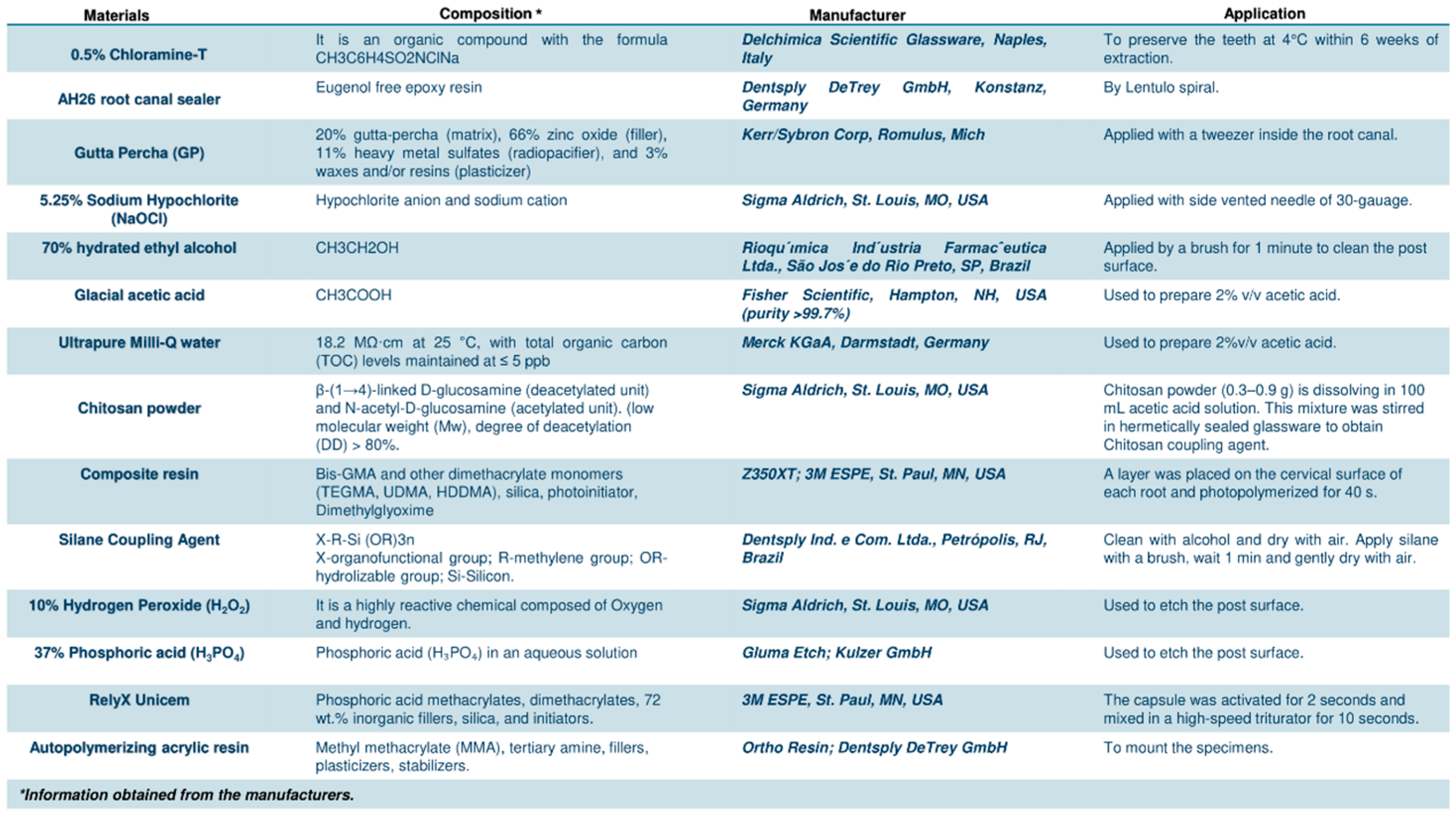
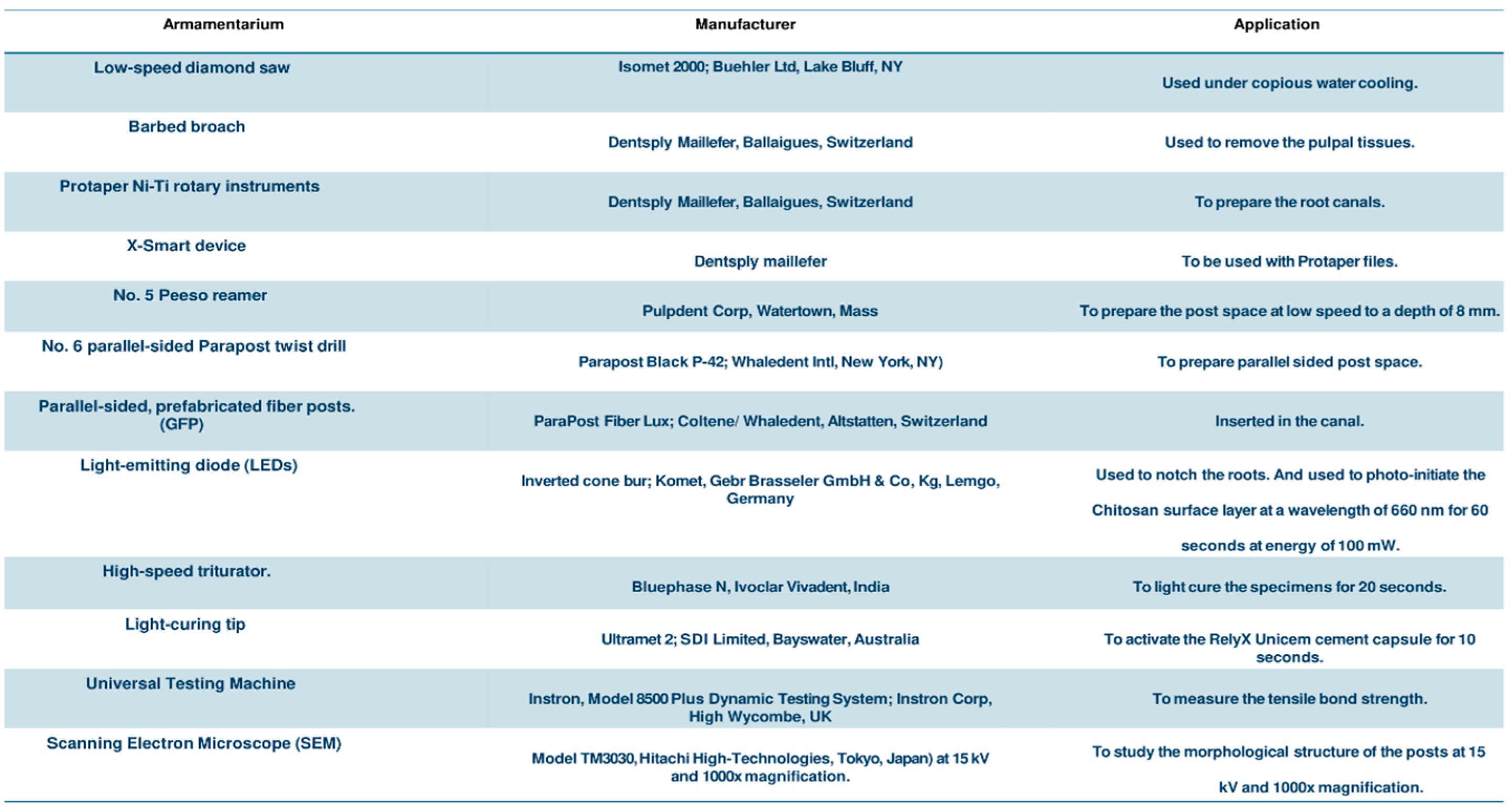
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Size Estimation
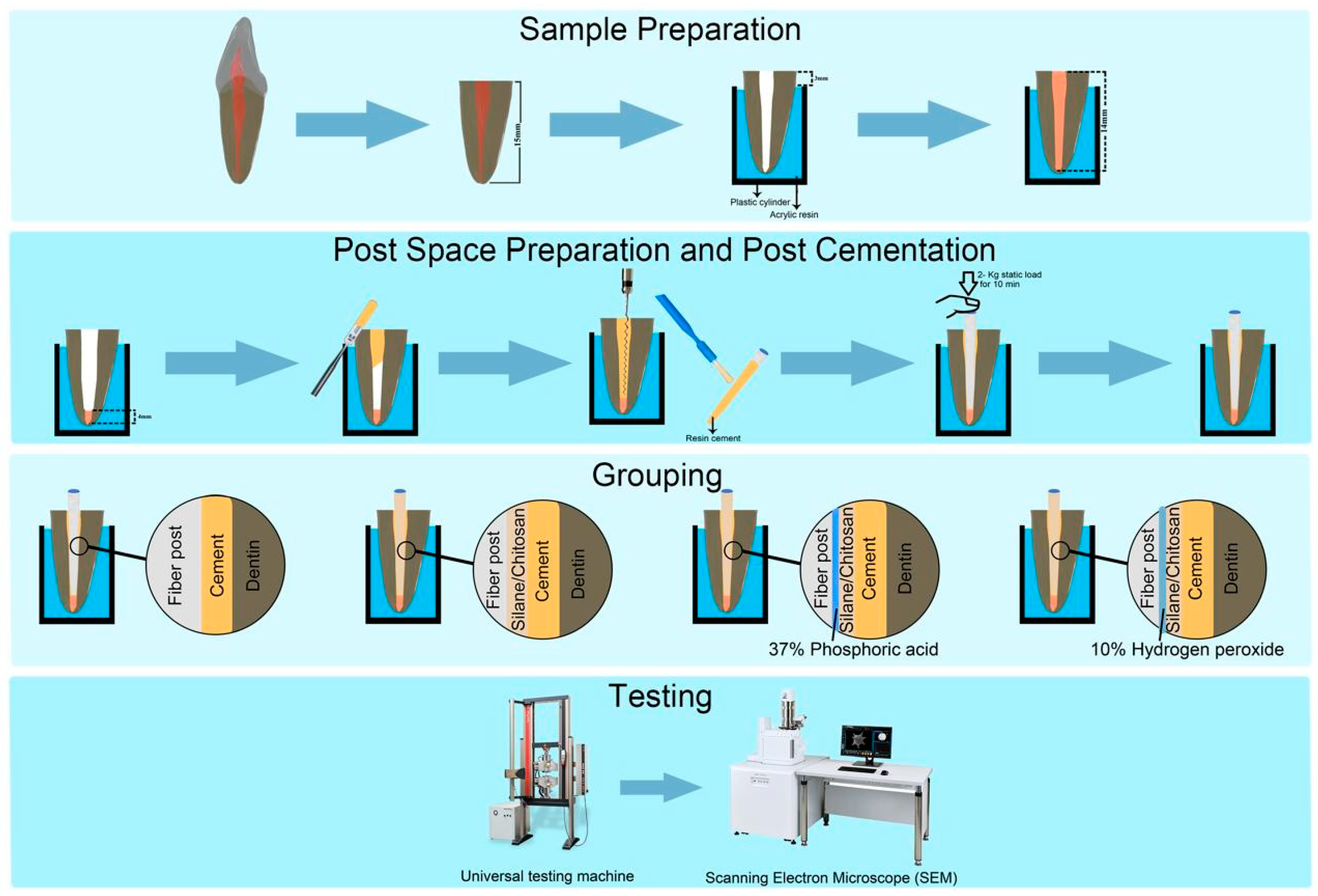
2.3. Teeth Selection and Post Space Preparation
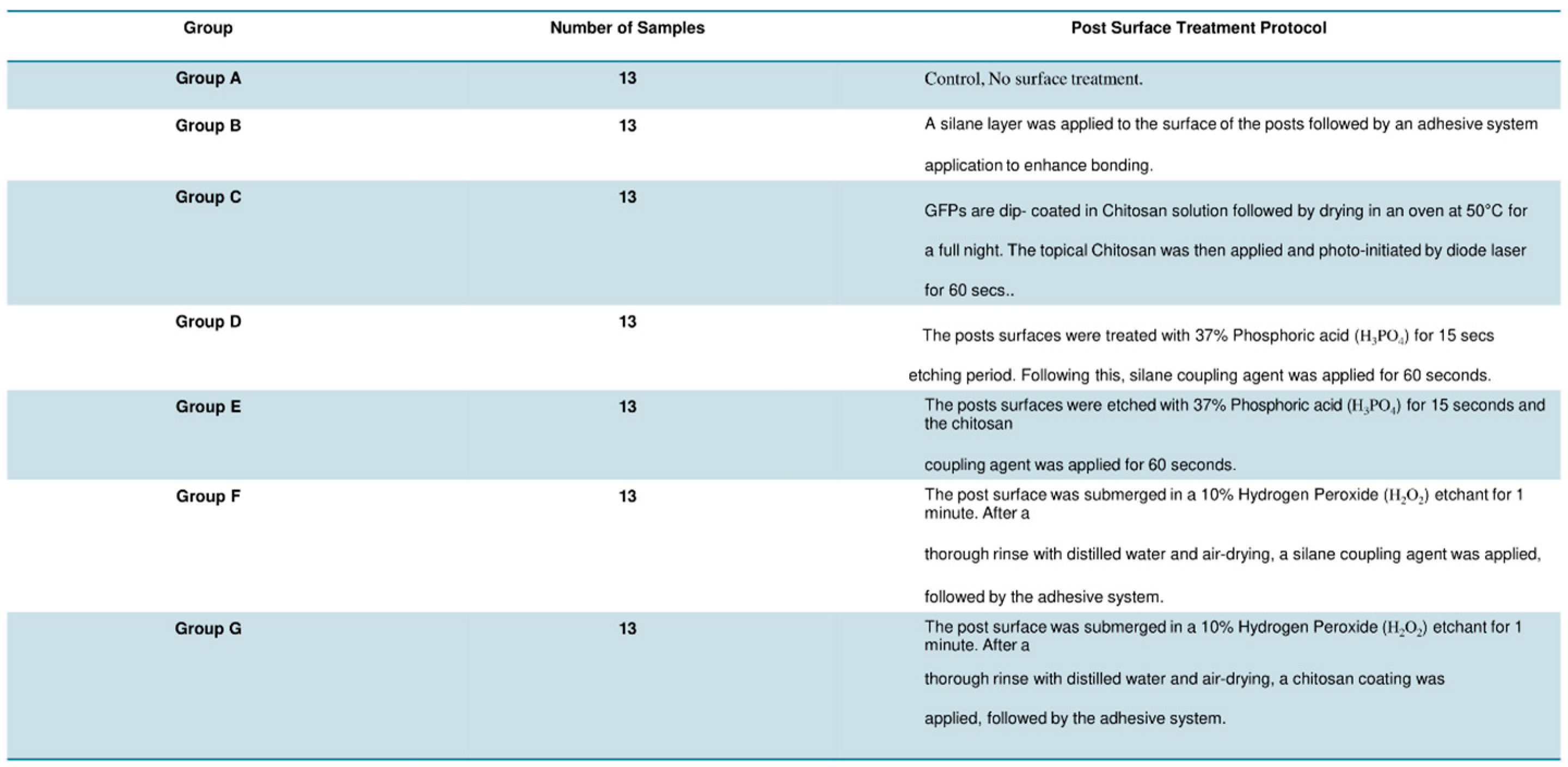
2.4. Sample Grouping and Post Surface Treatment
2.5. Post Cementation
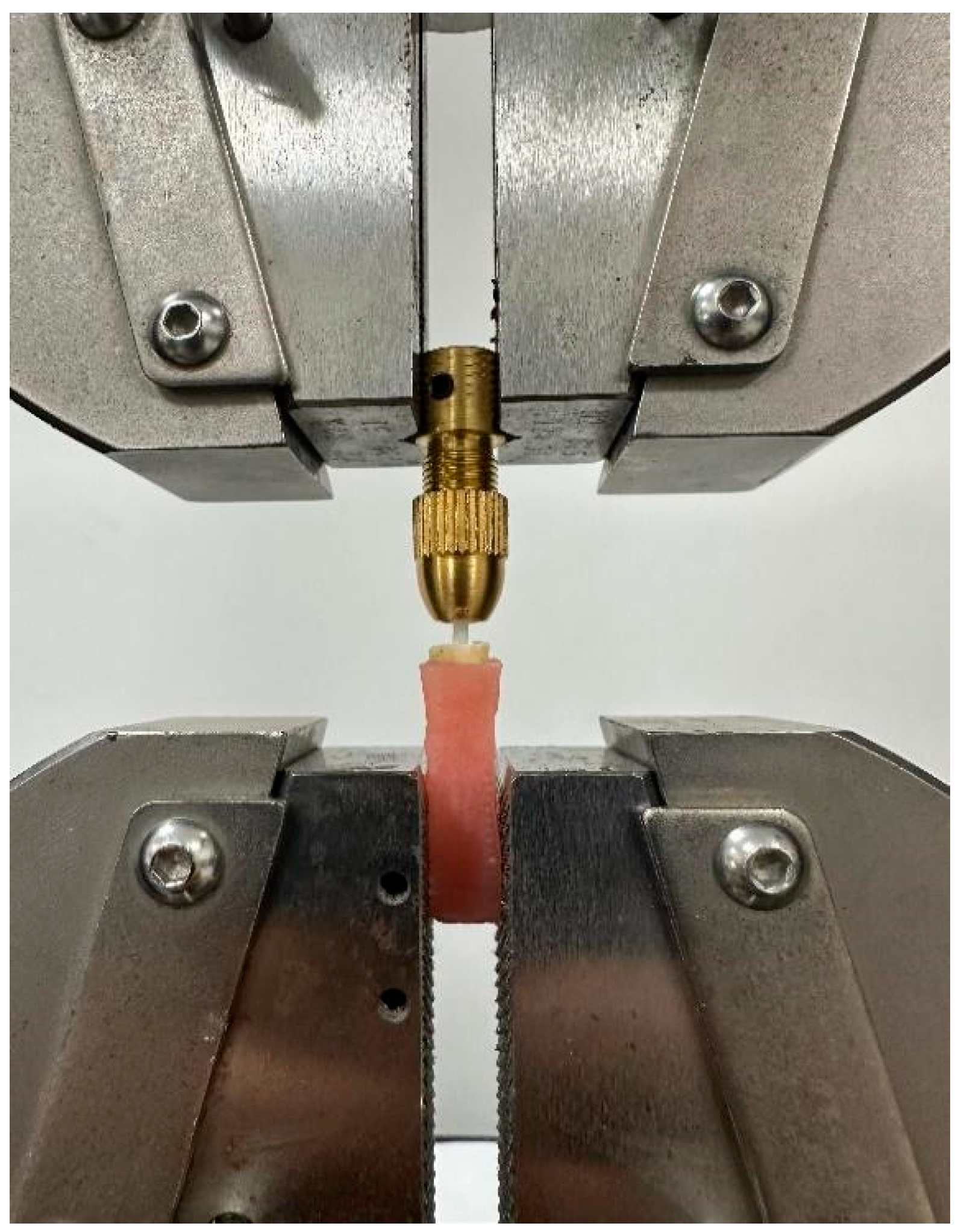
2.6. Tensile Bond Strength Testing
2.7. Scanning Electron Microscopy and Failure Mode Analysis
2.8. Statistical Analysis
3. Results
SEM Analysis
4. Discussion
5. Conclusions
- The incorporation of chitosan as a coupling agent for the surface treatment of GFP demonstrates a significant enhancement in tensile bond strength when compared to traditional silane coupling agents.
- The highest tensile bond strength was achieved when the posts underwent prior surface conditioning with a hydrogen peroxide etchant.
- These findings suggest that utilizing chitosan in conjunction with appropriate surface conditioning can positively influence the longevity of glass fiber posts, reducing the likelihood of post failure.
- This study highlights the potential of using chitosan as a natural biopolymer alternative to synthetic coupling agents to develop more effective bonding strategies for dental restorations.
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Tsintsadze, N.; Margvelashvili-Malament, M.; Natto, Z.S.; Ferrari, M. Comparing survival rates of endodontically treated teeth restored either with glass-fiber-reinforced or metal posts: A systematic review and meta-analyses. J. Prosthet. Dent. 2022, 131, 567–578. [Google Scholar] [CrossRef]
- Ben Suleiman, A.; Desai, S.; Tepperman, A.; Chvartszaid, D.; Malkhassian, G.; Habsha, E.; Barzilay, I.; Azarpazhooh, A. The Outcomes of Endodontically Treated Teeth Restored with Custom-Made Cast Post-and-Core Restorations: A Retrospective Cohort Study. J. Endod. 2024, 50, 316–328. [Google Scholar] [CrossRef]
- Oulghazi, I.; El Yamani, A.; Morchad, B. Factors influencing vertical radicular fractures in teeth supported by metallic dental core: A scoping review. Clin. Cosmet. Investig. Dent. 2024, 16, 101–114. [Google Scholar] [CrossRef]
- Sachi, S.; Varada, S.; Jitha, J.; Nair, S.V. Reattachment of Fractured Maxillary Central Incisor with Fibre Post. Kerala Dent. J. 2024, 47, 32–36. [Google Scholar] [CrossRef]
- de Matos, L.M.R.; Silva, M.L.; Cordeiro, T.O.; Cardoso, S.d.A.M.; Campos, D.E.S.; de Muniz, I.A.F.; Barros, S.A.d.L.; Seraidarian, P.I. Clinical and laboratorial performance of rehabilitation of endodontically treated teeth: A systematic review. J. Esthet. Restor. Dent. 2024, 36, 1281–1300. [Google Scholar] [CrossRef]
- Hawthan, M.; Larsson, C.; Chrcanovic, B.R. Survival of fixed prosthetic restorations on vital and nonvital teeth: A systematic review. J. Prosthodont. 2024, 33, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Generali, L.; Veneri, F.; Forabosco, E.; Cavani, F.; Piergianni, V.; Sassatelli, P.; Checchi, V.; Pedullà, E. Push-out bond strength and SEM fractographical analysis of hollow fibre posts used with self-adhesive resin cement: A pilot study. Odontology 2024, 112, 158–168. [Google Scholar] [CrossRef]
- Cheniski, D.A.; Freire, A.; Camargo, E.S.; da Costa, R.G.; de Souza, E.M.; Rached, R.N. Bond strength of prefabricated and CAD-CAM milled glass fiber post-and-cores luted with conventional, universal, and self-adhesive composite resin cement. J. Prosthet. Dent. 2024, 131, 251.e1–251.e7. [Google Scholar] [CrossRef]
- Rajnics, Z.; Pammer, D.; Kőnig-Péter, A.; Turzó, K.; Marada, G.; Radnai, M. Push-Out Bond Strength of Glass Fiber Endodontic Posts with Different Diameters. Materials 2024, 17, 1492. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.; Albaqami, M.; Naji, Z.; Al-Khunein, Y.; Alsubaie, K.; Alqahtani, A.; Al-Thobity, A.M. Impact of different surface treatment methods on bond strength between fiber post and composite core material. Saudi Dent. J. 2021, 33, 334–341. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Fernandes, V.; Correia, A.; Miller, P.; Carvalho, O.; Silva, F.; Özcan, M.; Henriques, B. Surface modification of glass fiber-reinforced composite posts to enhance their bond strength to resin-matrix cements: An integrative review. Clin. Oral Investig. 2022, 26, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.; Khan, A.S.; Velo, M.M.d.A.C.; Panda, S.; Zavattini, A.; Rizzante, F.A.P.; Arbildo Vega, H.I.; Sauro, S.; Lukomska-Szymanska, M. Effects of Surface Treatments of Glass Fiber-Reinforced Post on Bond Strength to Root Dentine: A Systematic Review. Materials 2020, 13, 1967. [Google Scholar] [CrossRef] [PubMed]
- Simões, Í.I.N.; Moreira da Silva, E.; Penelas, A.G.; Poskus, L.T.; Botelho Dos Santos, G.; Guimarães, J.G.A. Evaluation of recementation protocols for dislodged glass fiber posts. Int. J. Prosthodont. 2023, 36, 650. [Google Scholar] [CrossRef] [PubMed]
- Machry, R.V.; Fontana, P.E.; Bohrer, T.C.; Valandro, L.F.; Kaizer, O.B. Effect of Different Surface Treatments of Resin Relined Fiber Posts Cemented With Self-adhesive Resin Cement on Push-out and Microtensile Bond Strength Tests. Oper. Dent. 2020, 45, E185–E195. [Google Scholar] [CrossRef]
- Santander-Rengifo, F.M.; Castillo-Andamayo, D.E.; Tay, L.Y.; López-Gurreonero, C.; Cornejo-Pinto, A.; Cervantes-Ganoza, L.A.; Cayo-Rojas, C.F. Bond Strength and Failure Mode of Glass Fiber Posts with Different Surface Treatments Prior to Silanization: An In Vitro Comparative Study. J. Int. Soc. Prev. Community Dent. 2023, 13, 42–53. [Google Scholar] [CrossRef]
- Majeti, C.; Veeramachaneni, C.; Morisetty, P.K.; Rao, S.A.; Tummala, M. A simplified etching technique to improve the adhesion of fiber post. J. Adv. Prosthodont. 2014, 6, 295–301. [Google Scholar] [CrossRef]
- Mosharraf, R.; Ranjbarian, P. Effects of post surface conditioning before silanization on bond strength between fiber post and resin cement. J. Adv. Prosthodont. 2013, 5, 126–132. [Google Scholar] [CrossRef]
- Aldegheishem, A.; Barakat, R.; Alrefaei, N.A.L.H.; Alhussain, G.; Alkoblan, K.; Al-Madi, E.M. Fracture Resistance of Endodontically Treated Teeth Restored Using Multifiber Posts Compared with Single Fiber Posts. Int. J. Polym. Sci. 2024, 2024, 1–6. [Google Scholar] [CrossRef]
- Meimandinezhad, M.; Bidhendi, H.M.; Zadeh, E.T.; Anzabi, R.M.; Asadi, H.; Fathi, A.; Gholami, M. Comparison of different surface treatment methods on flexural strength and elasticity modules of quartz and glass fiber-based posts: A narrative review. J. Fam. Med. Prim. Care 2022, 11, 7616–7620. [Google Scholar] [CrossRef]
- Fernandes, V.; Silva, F.; Henriques, B.; Özcan, M.; Carvalho, O.; Souza, J.C.M. Laser-texturing and traditional surface modification to improve the adhesion of glass fiber-reinforced composite posts to resin cements. Int. J. Adhes. Adhes. 2024, 131, 103645. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent Progress in Silane Coupling Agent with Its Emerging Applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Leme, A.A.; Pinho, A.L.; de Gonçalves, L.; Correr-Sobrinho, L.; Sinhoreti, M.A. Effects of silane application on luting fiber posts using self-adhesive resin cement. J. Adhes. Dent. 2013, 15, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Adwani, S.; Elsubeihi, E.; Zebari, A.; Aljanahi, M.; Moharamzadeh, K.; Elbishari, H. Effect of Different Silane Coupling Agents on the Bond Strength between Hydrogen Peroxide-Etched Epoxy-Based- Fiber-Reinforced Post and Composite Resin Core. Dent. J. 2023, 11, 142. [Google Scholar] [CrossRef]
- Saini, S.; Yadav, R.; Sonwal, S.; Meena, A.; Huh, Y.S.; Brambilla, E.; Ionescu, A.C. Tribological, mechanical, and thermal properties of nano tricalcium phosphate and silver particulates reinforced Bis-GMA/TEGDMA dental resin composites. Tribol. Int. 2024, 199, 110010. [Google Scholar] [CrossRef]
- Yadav, R.; Saini, S.; Sonwal, S.; Meena, A.; Huh, Y.S.; Brambilla, E.; Ionescu, A.C. Optimization and ranking of dental restorative composites byENTROPY-VIKOR and VIKOR-MATLAB. Polym. Adv. Technol. 2024, 35, e6526. [Google Scholar] [CrossRef]
- Hameed, H.; Khan, M.A.; Paiva-Santos, A.C.; Ereej, N.; Faheem, S. Chitin: A versatile biopolymer-based functional therapy for cartilage regeneration. Int. J. Biol. Macromol. 2024, 265, 131120. [Google Scholar] [CrossRef]
- Malhotra, H.; Gautam, R.K. Biomedical applications of chitosan and its derivatives. In Biopolymers for Biomedical Applications; Annu, Ed.; Wiley: Hoboken, NJ, USA, 2024; pp. 25–51. ISBN 9781119865025. [Google Scholar]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Seidi, F.; Youssefi Azarfam, M.; Khodadadi Yazdi, M.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J.; et al. Biopolymer-based composites for tissue engineering applications: A basis for future opportunities. Compos. Part B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, J.; Huang, Z.; Mai, S. Promotion effect of carboxymethyl chitosan on dental caries via intrafibrillar mineralization of collagen and dentin remineralization. Materials 2022, 15, 4835. [Google Scholar] [CrossRef]
- Nishanthine, C.; Miglani, R.; R, I.; Poorni, S.; Srinivasan, M.R.; Robaian, A.; Albar, N.H.M.; Alhaidary, S.F.R.; Binalrimal, S.; Almalki, A.; et al. Evaluation of Fluoride Release in Chitosan-Modified Glass Ionomer Cements. Int. Dent. J. 2022, 72, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Abedian, Z.; Jenabian, N.; Moghadamnia, A.A.; Zabihi, E.; Tashakorian, H.; Rajabnia, M.; Sadighian, F.; Bijani, A. Antibacterial activity of high-molecular-weight and low-molecular-weight chitosan upon oral pathogens. J. Conserv. Dent. 2019, 22, 169–174. [Google Scholar] [CrossRef]
- Li, D.; Zhuang, B.; Wang, X.; Wu, Z.; Wei, W.; Aladejana, J.T.; Hou, X.; Yves, K.G.; Xie, Y.; Liu, J. Chitosan used as a specific coupling agent to modify starch in preparation of adhesive film. J. Clean. Prod. 2020, 277, 123210. [Google Scholar] [CrossRef]
- Alzahrani, A.H.; Ebrahim, M.I.; Felemban, M.F.; Alqarni, A.A.; Algahtani, F.S.; Shawli, H.T.; Al Humayyani, N.; Meshni, A.A.; Al Moaleem, M.M. Nanoparticle Augmentation of Adhesive Systems: Impact on Tensile Strength in Fiberglass Post Placement within Root Dentin. Med. Sci. Monit. 2024, 30, e943502. [Google Scholar] [CrossRef] [PubMed]
- Alrabeah, G.; Binhassan, F.; Al Khaldi, S.; Al Saleh, A.; Al Habeeb, K.; Anwar, S.; Habib, S.R. Effect of Self-Adhesive Resin Cement Film Thickness on the Shear Bond Strength of Lithium Disilicate Ceramic–Cement–Tooth Triplex. Inorganics 2023, 12, 14. [Google Scholar] [CrossRef]
- Rifaat, S.; Aljami, A.; Alshehri, T.; Alameer, S.T.; Aldossary, A.; Almutairi, W.; Almaliki, M.N.; A Farooqi, F.; Taymour, N. The Effect of Coronal Pre-flaring and Type of Root Canal Irrigation on Working Length Accuracy Using Electronic Apex Locators. F1000Research 2023, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Mu, Y.; Setzer, F.C.; Lu, H.; Qu, T.; Yu, Q. Failure of fiber posts after cementation with different adhesives with or without silanization investigated by pullout tests and scanning electron microscopy. J. Endod. 2012, 38, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Rifaat, S.; Rahoma, A.; Alkhalifa, F.; AlQuraini, G.; Alsalman, Z.; Alwesaibi, Z.; Taymour, N. Push-Out Bond Strength of EndoSeal Mineral Trioxide Aggregate and AH Plus Sealers after Using Three Different Irrigation Protocols. Eur. J. Dent. 2023, 17, 76–81. [Google Scholar] [CrossRef]
- Souza, N.d.O.; Sousa, R.S.; Isolan, C.P.; Moraes, R.R.d.; Lima, G.d.S.; Lomonaco, D.; de Paula, D.M.; Alves, A.H.; Sabóia, V.d.P.; Feitosa, V.P. Intraradicular Dentin Biomodification with Natural Agents for Bonding Glass-fiber Posts. J. Adhes. Dent. 2021, 23, 223–230. [Google Scholar] [CrossRef]
- Kosan, E.; Prates-Soares, A.; Blunck, U.; Neumann, K.; Bitter, K. Root canal pre-treatment and adhesive system affect bond strength durability of fiber posts ex vivo. Clin. Oral Investig. 2021, 25, 6419–6434. [Google Scholar] [CrossRef]
- Lo Giudice, G.; Ferrari Cagidiaco, E.; Lo Giudice, R.; Puleio, F.; Nicita, F.; Calapaj, M. Evaluation of mechanical properties of a hollow endodontic post by three point test and SEM analysis: A pilot study. Materials 2019, 12, 1983. [Google Scholar] [CrossRef] [PubMed]
- Skupien, J.A.; Sarkis-Onofre, R.; Cenci, M.S.; Moraes, R.R.d.; Pereira-Cenci, T. A systematic review of factors associated with the retention of glass fiber posts. Braz. Oral Res. 2015, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, F.; Osorio, R.; Sadek, F.T.; Radovic, I.; Toledano, M.; Ferrari, M. Surface treatments for improving bond strength to prefabricated fiber posts: A literature review. Oper. Dent. 2008, 33, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vásquez, M.J.; Valenzuela-Buitimea, E.L.; Plascencia-Jatomea, M.; Encinas-Encinas, J.C.; Rodríguez-Félix, F.; Sánchez-Valdes, S.; Rosas-Burgos, E.C.; Ocaño-Higuera, V.M.; Graciano-Verdugo, A.Z. Functionalization of chitosan by a free radical reaction: Characterization, antioxidant and antibacterial potential. Carbohydr. Polym. 2017, 155, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Žigrayová, D.; Mikušová, V.; Mikuš, P. Advances in chitosan derivatives: Preparation, properties and applications in pharmacy and medicine. Gels 2024, 10, 701. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Demirkesen, I.; Colussi, R.; Roy, S.; Tabassum, N.; de Oliveira Filho, J.G.; Bist, Y.; Kumar, Y.; Nowacka, M.; Galus, S.; et al. Recent trends in the application of films and coatings based on starch, cellulose, chitin, chitosan, xanthan, gellan, pullulan, Arabic gum, alginate, pectin, and carrageenan in food packaging. Food Front. 2024, 5, 350–391. [Google Scholar] [CrossRef]
- Assis, H.C.; Nascimento, G.C.d.; Roperto, R.; Sousa-Neto, M.D.; Lopes-Olhê, F.C. Can Carbodiimide (EDC) and Chitosan Cross-linking Agents Effect the Longevity of Fiberglass Posts Luted with Different Types of Composite Cements to Root Dentin? J. Adhes. Dent. 2023, 25, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Rudd, C.; Parsons, A.; Sharmin, N.; Zhang, J.; Chen, W.; Ahmed, I. Chitosan as a coupling agent for phosphate glass fibre/polycaprolactone composites. Fibers 2018, 6, 97. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Elnaghy, A.M.; Mandorah, A.; Hassan, A.H.; Elshazli, A.; Elsaka, S. Effect of surface treatments on push-out bond strength of calcium silicate-based cements to fiber posts. BMC Oral Health 2021, 21, 131. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; Lima, D.M.; Carvalho, E.M.; Rodrigues, V.P.; Alves, C.M.C. Effect of Glass Fiber Post Surface Treatment on Bond Strength of a Self-Adhesive Resin Cement: An “In Vitro” Study. Int. J. Dent. 2021, 2021, 8856657. [Google Scholar] [CrossRef] [PubMed]
- Rechia, B.C.d.N.; Bravo, R.P.; Oliveira, N.D.d.; Baratto Filho, F.; Gonzaga, C.C.; Storrer, C.L.M. Influence of different surface treatments of fiberglass posts on the bond strength to dentin. Braz. J. Oral Sci. 2017, 15, 158. [Google Scholar] [CrossRef]

| Means | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| (A) Control | 268.0892 a | 70.2765 | 19.491 | 225.62 | 310.556 | 185.98 | 371.71 | <0.001 |
| (B) Silane | 279.8954 a | 49.4014 | 13.701 | 250.04 | 309.748 | 204.99 | 351.34 | |
| (C) Chitosan | 300.3915 a,b | 51.2316 | 14.209 | 269.43 | 331.350 | 201.84 | 375.64 | |
| (D) H3PO4–Silane | 293.2931 b | 50.3383 | 13.961 | 262.87 | 323.712 | 203.21 | 369.28 | |
| (E) H3PO4–Chitosan | 330.6600 c | 46.5409 | 12.908 | 302.53 | 358.784 | 251.84 | 385.68 | |
| (F) H2O2–Silane | 326.3015 c | 36.0140 | 9.9885 | 304.53 | 348.064 | 280.28 | 396.02 | |
| (G) H2O2–Chitosan | 472.8469 | 34.9147 | 9.6836 | 451.74 | 493.945 | 414.11 | 508.22 | |
| Percentage of Failure Pattern Test Group | |||
|---|---|---|---|
| Adhesive% | Cohesive% | Mixed% | |
| Group (n = 13) | |||
| Group A (Control) | 10 (76.9%) | 3 (23.1%) | 0 (0.0%) |
| Group B (Silane) | 9 (69.2%) | 2 (15.4%) | 2 (15.4%) |
| Group C (Chitosan) | 3 (23.1%) | 5 (38.5%) | 5 (38.5%) |
| Group D (H3PO4–Silane) | 3 (23.1%) | 6 (46.2%) | 4 (30.8%) |
| Group E (H3PO4–Chitosan) | 4 (30.8%) | 9 (61.5%) | 0 (0%) |
| Group F (H2O2–Silane) | 3 (23.1%) | 7(53.8%) | 3 (23.1%) |
| Group G (H2O2–Chitosan) | 2 (15.4%) | 10 (76.9%) | 1 (7.7%) |
| n = 91 | 34 (37.4%) | 42 (46.2%) | 15 (16.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taymour, N.; Albin Hejji, M.H.; Alotaibi, M.F.; Alzahrani, R.A.; Almarzooq, A.M.; Shetty, A.C.; Rifaat, S. Enhancing Tensile Bond Strength of Glass Fiber Posts Using Chitosan as a Coupling Agent: A Novel Approach for Improved Dental Restorations. Prosthesis 2024, 6, 1561-1574. https://doi.org/10.3390/prosthesis6060112
Taymour N, Albin Hejji MH, Alotaibi MF, Alzahrani RA, Almarzooq AM, Shetty AC, Rifaat S. Enhancing Tensile Bond Strength of Glass Fiber Posts Using Chitosan as a Coupling Agent: A Novel Approach for Improved Dental Restorations. Prosthesis. 2024; 6(6):1561-1574. https://doi.org/10.3390/prosthesis6060112
Chicago/Turabian StyleTaymour, Noha, Mohammed Hashim Albin Hejji, Mohammed Faihan Alotaibi, Rakan Abdullah Alzahrani, Ali Mohammed Almarzooq, Ashwin C. Shetty, and Shimaa Rifaat. 2024. "Enhancing Tensile Bond Strength of Glass Fiber Posts Using Chitosan as a Coupling Agent: A Novel Approach for Improved Dental Restorations" Prosthesis 6, no. 6: 1561-1574. https://doi.org/10.3390/prosthesis6060112
APA StyleTaymour, N., Albin Hejji, M. H., Alotaibi, M. F., Alzahrani, R. A., Almarzooq, A. M., Shetty, A. C., & Rifaat, S. (2024). Enhancing Tensile Bond Strength of Glass Fiber Posts Using Chitosan as a Coupling Agent: A Novel Approach for Improved Dental Restorations. Prosthesis, 6(6), 1561-1574. https://doi.org/10.3390/prosthesis6060112








