The Antimicrobial Effect of the Incorporation of Inorganic Substances into Heat-Cured Denture Base Resins—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
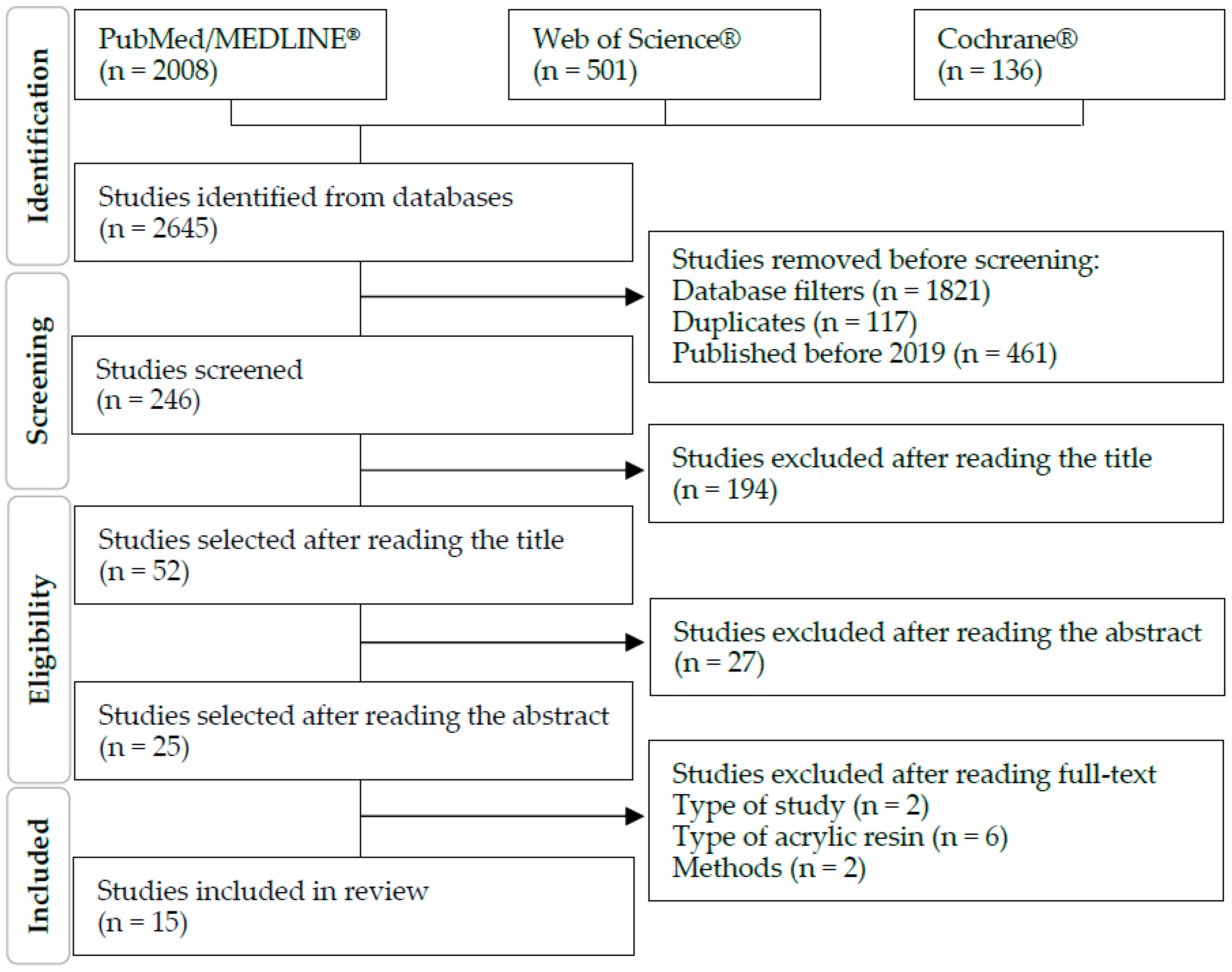
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
- Is it clear in the study what is the “cause” and what is the “effect” (i.e., there is no confusion about which variable comes first)?
- Were the participants included in any comparisons similar?
- Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest?
- Was there a control group?
- Were there multiple measurements of the outcome both pre- and post-intervention/exposure?
- Was follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analysed?
- Were the outcomes of participants included in any comparisons measured in the same way?
- Were outcomes measured in a reliable way?
- Was appropriate statistical analysis used?
3. Results
3.1. Study Characteristics
3.1.1. Fungi
3.1.2. Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S.; Vinesh, E.; Selvi, D.T.; Kannan, R.K.; Jayakumar, A.; Dinakaran, J. Prevalence of Candida among Denture Wearers and Nondenture Wearers. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. S1), S702–S705. [Google Scholar] [CrossRef] [PubMed]
- Farid, D.A.M.; Zahari, N.A.H.; Said, Z.; Ghazali, M.I.M.; Hao-Ern, L.; Zol, S.M.; Aldhuwayhi, S.; Alauddin, M.S. Modification of Polymer Based Dentures on Biological Properties: Current Update, Status, and Findings. Int. J. Mol. Sci. 2022, 23, 10426. [Google Scholar] [CrossRef]
- Walczak, K.; Schierz, G.; Basche, S.; Petto, C.; Boening, K.; Wieckiewicz, M. Antifungal and Surface Properties of Chitosan-Salts Modified PMMA Denture Base Material. Molecules 2020, 25, 5899. [Google Scholar] [CrossRef]
- Mathur, V.P.; Dhillon, J.K. Dental Caries: A Disease Which Needs Attention. Indian J. Pediatr. 2018, 85, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Taebunpakul, P.; Jirawechwongsakul, P. Palatal Inflammation and the Presence of Candida in Denture-Wearing Patients. J. Int. Soc. Prev. Community Dent. 2021, 11, 272–280. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Saleh Al-Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111382. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Paes, S.M.; Pupo, Y.M.; Cavenago, B.C.; Fonseca-Silva, T.; Santos, C.C.O. Cryopreservation of mesenchymal stem cells derived from dental pulp: A systematic review. Restor. Dent. Endod. 2021, 46, e26. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Albanese, G.; De Giorgi, M.L.; Corsalini, M.; Rinaldi, R. Silver Nanoparticles Addition in Poly(Methyl Methacrylate) Dental Matrix: Topographic and Antimycotic Studies. Int. J. Mol. Sci. 2019, 20, 4691. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.N.d.S.; Sala, R.L.; Fernandes, R.A.; Xavier, T.P.O.; Cruz, S.A.; Paranhos, C.M.; Monteiro, D.R.; Barbosa, D.B.; Delbem, A.C.B.; de Camargo, E.R. Effect of synthetic colloidal nanoparticles in acrylic resin of dental use. Eur. Polym. J. 2019, 112, 531–538. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Mathew, T.A.; Mozetič, M.; Jose, J.; Thomas, S.; Kalarikkal, N. Development of biocompatible and biofilm resistant silver-poly(methylmethacrylate) nanocomposites for stomatognathic rehabilitation. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 186–199. [Google Scholar] [CrossRef]
- Giti, R.; Zomorodian, K.; Firouzmandi, M.; Zareshahrabadi, Z.; Rahmannasab, S. Antimicrobial Activity of Thermocycled Polymethyl Methacrylate Resin Reinforced with Titanium Dioxide and Copper Oxide Nanoparticles. Int. J. Dent. 2021, 2021, 6690806. [Google Scholar] [CrossRef]
- Pinheiro, M.C.R.; Carneiro, J.A.O.; Pithon, M.M.; Martinez, E.F. Thermopolymerized Acrylic Resin Immersed or Incorporated with Silver Nanoparticle: Microbiological, Cytotoxic and Mechanical Effect. Mat. Res. 2021, 24, e20200115. [Google Scholar] [CrossRef]
- Alzayyat, S.T.; Almutiri, G.A.; Aljandan, J.K.; Algarzai, R.M.; Khan, S.Q.; Akhtar, S.; Matin, A.; Gad, M.M. Antifungal Efficacy and Physical Properties of Poly (methylmethacrylate) Denture Base Material Reinforced with SiO2 Nanoparticles. J. Prosthodont. 2021, 30, 500–508. [Google Scholar] [CrossRef]
- Takamiya, A.S.; Monteiro, D.R.; Gorup, L.F.; Silva, E.A.; Camargo, E.R.; Gomes-Filho, J.E.; De Oliveira, S.H.P.; Barbosa, D.B. Biocompatible silver nanoparticles incorporated in acrylic resin for dental application inhibit Candida albicans biofilm. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111341. [Google Scholar] [CrossRef]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; Al Ghamdi, M.A.; Khan, S.Q.; Akhtar, S.; Al Eraky, D.M.; Al-Harbi, F.A. Effect of Low Nanodiamond Concentrations and Polymerization Techniques on Physical Properties and Antifungal Activities of Denture Base Resin. Polymers 2021, 13, 4331. [Google Scholar] [CrossRef] [PubMed]
- Hazim, R.H.; Fatalla, A.A. The Effect of Tellurium Oxide Micro Particles Incorporation into PMMA on Candida albicans Adherence. J. Res. Med. Dent. Sci. 2021, 9, 129–135. [Google Scholar]
- Ivanovic, V.; Popovic, D.; Petrovic, S.; Rudolf, R.; Majerič, P.; Lazarevic, M.; Djordjevic, I.; Lazic, V.; Radunovic, M. Unraveling the Antibiofilm Activity of a New Nanogold Resin for Dentures and Epithesis. Pharmaceutics 2022, 14, 1513. [Google Scholar] [CrossRef] [PubMed]
- Gligorijević, N.; Mihajlov-Krstev, T.; Kostić, M.; Nikolić, L.; Stanković, N.; Nikolić, V.; Dinić, A.; Igić, M.; Bernstein, N. Antimicrobial Properties of Silver-Modified Denture Base Resins. Nanomaterials 2022, 12, 2453. [Google Scholar] [CrossRef]
- Ismaeil, M.A.; Ebrahim, M. Antifungal effect of acrylic resin denture base containing different types of nanomaterials: A comparative study. J. Int. Oral. Health 2023, 15, 78–83. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; Valente, M.L.D.C.; Sessa, J.P.N.; Gubitoso, B.; Schiavon, M.A.; Dos Reis, A.C. Adhesion of biofilm, surface characteristics, and mechanical properties of antimicrobial denture base resin. J. Adv. Prosthodont. 2023, 15, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Marić, I.; Zore, A.; Rojko, F.; Škapin, A.S.; Štukelj, R.; Učakar, A.; Vidrih, R.; Veselinović, V.; Gotić, M.; Bohinc, K. Antifungal Effect of Polymethyl Methacrylate Resin Base with Embedded Au Nanoparticles. Nanomaterials 2023, 13, 2128. [Google Scholar] [CrossRef]
- Correa, S.; Matamala, L.; González, J.P.; de la Fuente, M.; Miranda, H.; Olivares, B.; Maureira, M.; Agüero, A.; Gómez, L.; Lee, X.; et al. Development of novel antimicrobial acrylic denture modified with copper nanoparticles. J. Prosthodont. Res. 2024, 68, 156–165. [Google Scholar] [CrossRef]
- Garcia, A.A.M.N.; Sugio, C.Y.C.; de Azevedo-Silva, L.J.; Gomes, A.C.G.; Batista, A.U.D.; Porto, V.C.; Soares, S.; Neppelenbroek, K.H. Nanoparticle-modified PMMA to prevent denture stomatitis: A systematic review. Arch. Microbiol. 2021, 204, 75. [Google Scholar] [CrossRef]
- An, S.; Evans, J.L.; Hamlet, S.; Love, R.M. Overview of incorporation of inorganic antimicrobial materials in denture base resin: A scoping review. J. Prosthet. Dent. 2023, 130, 202–211. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, Y.; He, G.X.; Katagori, N.; Chen, H. A comparison of conventional methods for the quantification of bacterial cells after exposure to metal oxide nanoparticles. BMC Microbiol. 2014, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Roza, M.P.; Colli, K.G.; Dalben, Y.R.; Maifrede, S.B.; Valiatti, T.B.; Novo, V.M.; Cayô, R.; Grão-Velloso, T.R.; Gonçalves, S.S. Candida-associated denture stomatitis: Clinical, epidemiological, and microbiological features. Braz. J. Microbiol. 2023, 54, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.Z.; Khan, S.B. Antimicrobial Efficacy of Silver Nanoparticles against Candida Albicans. Materials 2022, 15, 5666. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef]
- Bangera, M.K.; Kotian, R.; Madhyastha, P. Effects of silver nanoparticle-based antimicrobial formulations on the properties of denture polymer: A systematic review and meta-analysis of in vitro studies. J. Prosthet. Dent. 2023, 129, 310–321. [Google Scholar] [CrossRef]
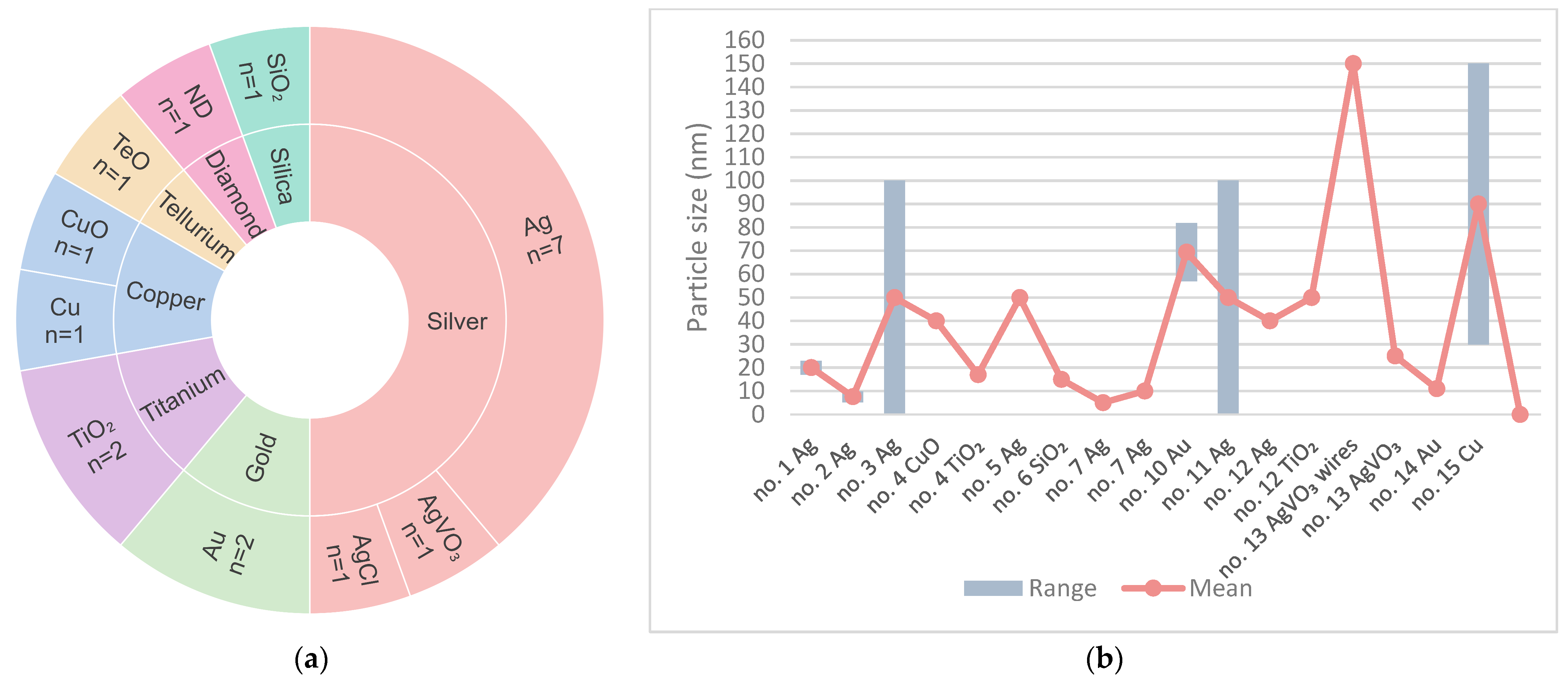
| No. | Country | 1st Author, Year | Title | Journal |
|---|---|---|---|---|
| 1 [15] | Italy | De Matteis V, 2019 | Silver Nanoparticles Addition in Poly(Methyl Methacrylate) Dental Matrix: Topographic and Antimycotic Studies | Int J Mol Sci |
| 2 [16] | Brazil | Souza Neto FN, 2019 | Effect of synthetic colloidal nanoparticles in acrylic resin of dental use | Eur Polym J |
| 3 [17] | India | Gopalakrishnan S, 2020 | Development of biocompatible and biofilm-resistant silver-poly(methylmethacrylate) nanocomposites for stomatognathic rehabilitation | Int J Polym Mater Polym Biomater |
| 4 [18] | Iran | Giti R, 2021 | Antimicrobial Activity of Thermocycled Polymethyl Methacrylate Resin Reinforced with Titanium Dioxide and Copper Oxide Nanoparticles | Int J Dent |
| 5 [19] | Brazil | Pinheiro MCR, 2021 | Thermopolymerized Acrylic Resin Immersed or Incorporated with Silver Nanoparticle: Microbiological, Cytotoxic and Mechanical Effect | Mat Res |
| 6 [20] | Saudi Arabia | Alzayyat ST, 2021 | Antifungal Efficacy and Physical Properties of Poly(methylmethacrylate) Denture Base Material Reinforced with SiO(2)Nanoparticles | J Prosthodont |
| 7 [21] | Brazil | Takamiya AS, 2021 | Biocompatible silver nanoparticles incorporated in acrylic resin for dental application inhibit Candida albicans biofilm | Mater Sci Eng C Mater Biol Appl |
| 8 [22] | Saudi Arabia | Fouda SM, 2021 | Effect of Low Nanodiamond Concentrations and Polymerization Techniques on Physical Properties and Antifungal Activities of Denture Base Resin | Polymers (Basel) |
| 9 [23] | Iraq | Hazim RH, 2021 | The Effect of Tellurium Oxide Micro Particles Incorporation into PMMA on Candida albicans Adherence | J Res Med Dent Sci |
| 10 [24] | Serbia | Ivanovic V, 2022 | Unraveling the Antibiofilm Activity of a New Nanogold Resin for Dentures and Epithesis | Pharmaceutics |
| 11 [25] | Serbia | Gligorijevic N, 2022 | Antimicrobial Properties of Silver-Modified Denture Base Resins | Nanomaterials (Basel) |
| 12 [26] | Saudi Arabia | Ismaeil MA, 2023 | Antifungal Effect of Acrylic Resin Denture Base Containing Different Types of Nanomaterials: A Comparative Study | J Int Oral Health |
| 13 [27] | Brazil | Teixeira ABV, 2023 | Adhesion of biofilm, surface characteristics, and mechanical properties of antimicrobial denture base resin | J Adv Prosthodont |
| 14 [14] | Slovenia | Marić I, 2023 | Antifungal Effect of Polymethyl Methacrylate Resin Base with Embedded Au Nanoparticles | Nanomaterials (Basel) |
| 15 [28] | Chile | Correa S, 2024 | Development of novel antimicrobial acrylic denture modified with copper nanoparticles | J Prosthodont Res |
| No. | PMMA | Samples (mm) | Particle Size | Concentration (%) | Microorganism |
|---|---|---|---|---|---|
| 1 | Paladon® 65 (Kulzer) Gemany | Disk Ø ≈ 20 | Ag 20 nm ± 3 | 3; 3.5 | C. albicans |
| 2 | Lucitone® 550 (Dentsply® Ind. e Com. Ltd.a.) Brazil | Coupon 60 × 10 × 3 | Ag 7.6 nm ± 2.3 | 0.05; 0.5; 5 | C. glabrata |
| 3 | Alfa Aesar. USA | Not specified | Ag <100 nm | 1; 2; 5;10 | S. mutans; C. albicans |
| 4 | SR Triplex Hot (Ivoclar Vivadent®) Liechtenstein | 150 (30 per group) disk 10 × 2 | CuO 40 nm | 2.5; 7.5 | C. albicans; C. dubliniensis; S. mutans; S. sobrinus; S. salivarius; S. sanguis |
| TiO2 17 nm | |||||
| 5 | Vipicril (Vipi® Ind. e Com. Ltd.a.) Brazil | 108 (27 per group) disk 15 × 2 | Ag 50 nm | 1; 2.5; 5 | C. albicans |
| 6 | Major Base 20 (Major Prodotti Dentari SPA®) Italy | 50 (10 per group) disk 15 × 2 | SiO2 15 nm | 0.05; 0.25; 0.5; 1 | C. albicans |
| 7 | Lucitone® 550 (Dentsply® Ind. e Com. Ltd.a.) Brazil | 63 (9 per group) disk 10 × 3 | Ag 5/10 nm | 0.05; 0.5; 5 | C. albicans |
| 8 | Major base 20 (Major Prodotti Dentari SPA®) Italy | 80 (20 per group) disk 15 × 2 | ND | 0.1; 0.25; 0.5 | C. albicans |
| 9 | Not identified | 25 (5 per group) disk 10 × 2 | TeO | 1; 3; 5; 7 | C. albicans |
| 10 | PMMA Biogal® (Galenika) Serbia | 48- 24 (6 per species) 24 (control) disk 5 × 2 | Au 69.4 nm ± 12.42 | 2 | S. aureus; E. coli; C. albicans; S. mitis |
| 11 | SR Triplex Hot (Ivoclar Vivadent®) Liechtenstein | 375 (75 per group) disk 10 × 2 | Ag <100 nm | 2; 5; 10 | S. aureus; C. albicans |
| AgCl 1 µm | 10 | ||||
| 12 | Major base, Trevalon/Universal Clear (Dentsply® Ind. e Com. Ltd.a.) Germany | 100 (20 per group) disk 10 × 2 | Ag 40 nm | 0.5; 1 | C. albicans |
| TiO2 50 nm | |||||
| 13 | Classic Dental Articles Ltd.a. Brazil | 9 Disk 9 × 1 | AgVO3 Wires: Ø = 150 nm Particles: 25 nm | 2.5; 5; 10 | C. albicans; C. glabrata; S. mutans |
| 14 | Ivoclar Vivadent® Liechtenstein | Coupon 10 × 10 × 3 | Au 11 | 20 | C. albicans |
| 15 | Acryl BH (GDF) Germany | Disk 10 × 4 | Cu 30 to 150 | 0.015; 0.045; 0.055; 0.06; 0.068 | C. albicans; S. mutans; A. actinomycetemcomitans; S. aureus |
| No. | Particle | Tests | Microorganism | Best Effect | Worst Effect |
|---|---|---|---|---|---|
| 1 | Silver (Ag) | Viability CFU assay | C. albicans | 3.5% Ag | 3% Ag |
| Circularity SEM assay | 3.5% Ag | - Control | |||
| Area covered Colonization assay | 3.5% Ag | - Control | |||
| 2 | Silver (Ag) | CFU assay | C. glabrata | No statistically relevant difference | |
| Biomass reduction CV assay | 0.05% Ag | 0.5% Ag | |||
| Metabolic activity reduction XTT assay | 0.05% Ag | 5% Ag | |||
| Micrographs of biofilms | 0.05% Ag 0.5% Ag | 5% Ag | |||
| 3 | Silver (Ag) | Cell count | S. mutans | 10% Ag | 1% Ag |
| CFU assay | C. albicans | 10% Ag | 1% Ag | ||
| Fluorescent microscopy | S. mutans | PMMA/Ag | - Control | ||
| 4 | Copper oxide (CuO) Titanium dioxide (TiO2) | Optical density | C. albicans | 7.5% TiO2 | 2.5% TiO2 |
| C. dubliniensis | 7.5% CuO | 2.5% TiO2 | |||
| S. mutans | |||||
| S. sobrinus | 7.5% CuO | 2.5% CuO | |||
| S. salivarius | 7.5% TiO2 | 2.5% TiO2 | |||
| S. sanguis | 7.5% CuO | 2.5% TiO2 2.5% CuO | |||
| Biofilm inhibition | C. albicans | 7.5% TiO2 | 2.5% TiO2 | ||
| C. dubliniensis | 7.5% CuO | 2.5% TiO2 | |||
| S. mutans | 7.5% CuO | 7.5% TiO2 | |||
| S. sobrinus | 7.5% CuO | 2.5% CuO | |||
| S. salivarius | 7.5% TiO2 | 2.5% TiO2 | |||
| S. sanguis | 7.5% CuO | 2.5% CuO | |||
| 5 | Silver (Ag) | Viability assay Absorbance | C. albicans | 1% Ag | 2.5% Ag 5% Ag |
| 6 | Silicon dioxide (SiO2) | Direct culture | C. albicans | 1% SiO2 | 0.05% SiO2 0.25% SiO2 |
| Slide count (CFU/mL) | 1% SiO2 | 0.05% SiO2 | |||
| 7 | Silver (Ag) | CFU assay | C. albicans | 0.5% Ag 0.05% Ag | 5% Ag |
| 8 | Diamond (ND) | CFU assay | C. albicans | 0.5% ND | 0.1% ND |
| 9 | Tellurium oxide (TeO) | Adherence test | C. albicans | 5% TeO 7% TeO | 1% TeO |
| 10 | Gold (Au) | CFU assay on discs | S. aureus | 2% Au | - Control |
| E. coli | |||||
| C. albicans | |||||
| S. mitis | |||||
| MTT assay | S. aureus | ||||
| E. coli | |||||
| C. albicans | |||||
| S. mitis | |||||
| SEM assay | S. aureus | ||||
| E. col | |||||
| C. albicans | |||||
| S. mitis | |||||
| CFU assay in the surrounding medium | S. aureus | No statistically relevant difference | |||
| E. coli | |||||
| C. albicans | |||||
| S. mitis | |||||
| 11 | Silver (Ag) | Inhibition zone | S. aureus | 10% Ag 10% AgCl | 2% Ag |
| C. albicans | 10% AgCl 10% Ag | 2% Ag 5% Ag | |||
| CFU assay | S. aureus | 10% Ag 10% AgCl 5% Ag | 2% Ag | ||
| C. albicans | |||||
| Microdilution method Minimum inhibitory concentrations | S. aureus | 10% AgCl | 10% Ag | ||
| C. albicans | |||||
| Microdilution method Minimum microbicidal concentrations | S. aureus | ||||
| C. albicans | |||||
| 12 | Silver (Ag) Titanium dioxide (TiO2) | Disc diffusion Antifungal activity | C. albicans | 1% Ag 1% TiO2 0.5% Ag | 0.5% TiO2 |
| Elution test Colony counts | 1% Ag 0.5% Ag 1% TiO2 | 0.5% TiO2 | |||
| 13 | Silver vanadate (AgVO3) | CFU assay | C. albicans In multispecies biofilm | - Control | 10% AgVO3 5% AgVO3 2.5% AgVO3 |
| C. glabrata In multispecies biofilm | 10% AgVO3 | 2.5% AgVO3 | |||
| S. mutans In multispecies biofilm | |||||
| Metabolic activity | Multispecies biofilm: C. albicans C. glabrata S. mutans | - Control | 10% AgVO3 | ||
| 14 | Gold (Au) | Yeast adhesion | C. albicans | 20% Au | - Control |
| 15 | Copper (Cu) | CFU assay | C. albicans | 0.045% Cu | 0.068% Cu |
| SEM assay | 0.045% Cu | - Control | |||
| Surface inhibitory capacity | A. actinomycetemcomitans | 0.045% Cu | - | ||
| S. aureus | |||||
| C. albicans | |||||
| S. mutans | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, M.; Salgado, H.; Correia, A.; Fonseca, P. The Antimicrobial Effect of the Incorporation of Inorganic Substances into Heat-Cured Denture Base Resins—A Systematic Review. Prosthesis 2024, 6, 1189-1201. https://doi.org/10.3390/prosthesis6050085
Lima M, Salgado H, Correia A, Fonseca P. The Antimicrobial Effect of the Incorporation of Inorganic Substances into Heat-Cured Denture Base Resins—A Systematic Review. Prosthesis. 2024; 6(5):1189-1201. https://doi.org/10.3390/prosthesis6050085
Chicago/Turabian StyleLima, Mariana, Helena Salgado, André Correia, and Patrícia Fonseca. 2024. "The Antimicrobial Effect of the Incorporation of Inorganic Substances into Heat-Cured Denture Base Resins—A Systematic Review" Prosthesis 6, no. 5: 1189-1201. https://doi.org/10.3390/prosthesis6050085
APA StyleLima, M., Salgado, H., Correia, A., & Fonseca, P. (2024). The Antimicrobial Effect of the Incorporation of Inorganic Substances into Heat-Cured Denture Base Resins—A Systematic Review. Prosthesis, 6(5), 1189-1201. https://doi.org/10.3390/prosthesis6050085







