Abstract
The aim of this systematic scoping review was to provide scientific evidence on the efficacy and methods of application of hyaluronic acid (HA) in the coverage of gingival recessions in terms of recession depth (RD) reduction, clinical attachment level (CAL) gain and probing depth (PD). An electronic search of the literature on the main databases was conducted. Initially, 405 articles were identified. Finally, four studies were included after the review process. It was not possible to perform a meta-analysis of the articles selected because of the differences among the surgical treatments and commercial formulations and compositions of HA. Both randomized controlled trials in this research examined type 1 gingival recessions treated with a coronally advanced flap. In the selected case series, recessions were treated with either a modified coronally advanced tunnel or laterally closed tunnel combined with a subepithelial connective tissue graft and HA. No significant variation was found in terms of PD. Modifications of CAL are connected to variations of RD; however, RD reduction is similar to the control group. HA seems to improve the clinical outcomes of gingival recession coverage in the short term, but the magnitude is limited. Formulations, surgical techniques and application methods are heterogeneous.
1. Introduction
The gingival recession defect is defined as the apical shift of the gingival margin with respect to the cemento-enamel junction [1]. This condition is associated with dentinal hypersensitivity, higher occurrence of carious or non-carious cervical lesions and moderate difficulties in performing proper oral hygiene measures [2]. Patients may also have a relevant perception of a visible gingival recession, especially in maxillary esthetic areas. As periodontal health was defined as the main variable able to influence smile patterns, this condition can consequently affect the perceived oral health-related quality of life. Patients can become reluctant to express their emotions and smile, and can appear more insecure, introverted and unsatisfied [3].
Plenty of mucogingival surgical procedures and strategies have been suggested to conjugate the increasing stringent esthetic demand of the restoration with a healthy and functional periodontal anatomy. In the literature, several root covering procedures (RCPs) are described alone or in combination with subepithelial connective tissue grafts (sCTGs) or soft tissue substitutes. Surgical techniques used in the treatment of gingival recessions can be classified as pedicle soft-tissue graft procedures and free soft-tissue graft procedures [4]. Currently, the most frequently used approaches for both single and multiple gingival recessions are coronally advanced flaps (CAF) and tunnel techniques. In addition, the use of an enamel matrix derivative (EMD) has been proposed to improve the effectiveness of soft tissue coverage of exposed root surfaces [5]. Studies showed that CAF combined with sCTG or EMD increases the probability of achieving complete root coverage (CRC) in Miller class I and II [6] and Cairo type I [7] recession defects compared to CAF alone [8,9,10]. The satisfying results achieved with the use of EMD have encouraged clinicians to introduce another organic molecule in periodontal therapy, namely hyaluronic acid (HA). This molecule seems to enhance the clinical outcomes in terms of clinical attachment level (CAL) gain and a reduction in bleeding on probing (BoP) following surgical and non-surgical periodontal procedures [11].
HA is a linear glycosaminoglycan (GAG) consisting of repeated units of D-glucoronic acid (1-B-3) N-Acetyl –D-glucosamine (1-B4) [12]. First isolated in the early twentieth century [13], this molecule is ubiquitously distributed in vertebrated tissues in different concentrations and molecular weights [14]. HA is able to bind to a large amount of water, forming a highly viscous gel. Biochemically, HA inhibits tissue breakdown by activating metalloproteinase inhibitors [15]. Furthermore, HA stimulates cell migration and differentiation during the development and repair of hard and soft tissues [16]. Due to their unique biological and physico-chemical properties and their safety profile, native HA and many of its derivatives represent interesting biomaterials for a variety of medical and cosmetic applications [17] including periodontal therapy [18]. Such enthusiasm concerning the beneficial properties of this molecule led Pini Prato et al. to publish a case series reporting the use of HA in mucogingival surgery [19]. Nowadays, although the potential and role of this molecule is not entirely defined, hyaluronan has been applied in different ways and in many oral surgery procedures demonstrating gratifying results [20]. The topical application of HA may lead to additional clinical benefits when used as an adjunctive to non-surgical and surgical periodontal therapy. To date, only a few clinical studies have investigated the effectiveness of HA applications in the surgical treatment of gingival recession coverage. In general, better results of the chosen surgical technique were observed when HA was applied additionally during the surgical procedure [21,22]. However, to the best of our knowledge, the evidence related to the effectiveness of such molecules in gingival recession treatment is still lacking. Therefore, the aim of this systematic scoping review was to provide further scientific evidence on the efficacy and methods of application of HA in soft tissue coverage of single and multiple gingival recessions in terms of recession depth (RD) and probing depth (PD) reduction, and clinical attachment level (CAL) gain.
2. Materials and Methods
The present systematic scoping review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines with the purpose of mapping the evidence on a topic and identifying major concepts and knowledge gaps [23].
2.1. Study Registration
The protocol of the present scoping review was registered at the National Institute for Health Research PROSPERO, International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/PROSPERO (accessed on 25 July 2022)) with the registration ID: CRD42022346135.
2.2. Focused Question
The main question was “In the surgical treatment of gingival recessions, did the use of hyaluronic acid improve healing?”. The primary outcome was RD reduction, while the secondary outcomes were PD assessment and CAL gain. An adaptation of the PICO (Population, Intervention, Comparison and Outcome) model was used to construct a targeted question consisting of a PEO (Population, Exposure and Outcome) framework to determine the association between a particular exposure and the outcomes [24] (Table 1). This strategy was developed to perform qualitative systematic reviews of health care interventions [25], including oral surgery procedures [26].

Table 1.
PEO framework.
2.3. Sources of Evidence
An electronic search of the literature on PubMed through the MEDLINE, Scopus, EMBASE and Web of Science databases was conducted. The search aimed to find relevant information on the use of hyaluronic acid in the surgical treatment of gingival recession defects. The years from 1968 to May 2022 were considered in all databases.
2.4. Search Strategies
For all libraries, a combination of specific keywords, medical topic titles [MeSH] and other non-indexed terms, such as MeSH, was used to identify all relevant studies according to the precise directions of the PEO query. Articles were selected on electronic databases using the following terms: (Hyaluron* acid) AND ((mucogingival surgery) OR (periodontal surgery) OR (recession coverage) OR (gingival recession*) OR (root coverage)). Additional screening of the reference lists of all pertinent articles was performed but no additional relevant studies were identified. No filters were applied to any search string during the electronic research.
2.5. Eligibility Criteria
2.5.1. Inclusion Criteria
All sources of evidence had to meet specific inclusion criteria to be included. Only articles written in English, on humans and without time exclusion criteria until May 2022 were screened. Studies included in the screening procedure were randomized controlled trials (RCTs), controlled clinical trials (CCTs), retrospective and prospective case–control studies. No limitations were imposed on population characteristics, number of patients, age or systemic conditions. Studies reporting on the healing of the surgical treatment of gingival recession defects with the adjunctive application of HA were included.
2.5.2. Exclusion Criteria
All studies that did not meet the inclusion criteria were excluded, such as
- in vitro and in vivo animal studies;
- articles written in a language other than English;
- case series, case reports and literature reviews;
- studies that did not report the surgical treatment of gingival recessions in combination with HA;
- studies that performed periodontal regeneration in conjunction with HA.
2.5.3. Selection of Sources of Evidence
Two reviewers (F.E.S and M.T.), working independently, completed the preliminary screening of titles and abstracts of all included articles with a Cohen’s K of 0.7 (substantial agreement). Full-text articles were independently evaluated, and selections were compared between the two researchers. The final list and any disagreement between the two researchers were taken to the attention of a third and fourth researcher (M.M. and P.P.P.). Duplicate articles in the databases were identified and removed using EndNote Web reference manager software (Clarivate Analytics, Philadelphia, PA, USA).
3. Results
Search and Selection Results
Initially, 154 articles were identified in EMBASE, 104 in PubMed, 69 in SCOPUS and 78 in Web of Science. After duplicate removal, 214 articles remained for the screening phase. Following the evaluation of titles and abstracts, 196 publications were excluded. The full texts of the remaining 19 articles were read thoroughly. Overall, 14 studies had to be excluded after careful examination of the full text as they did not meet the above-mentioned inclusion criteria. Nandanwar’s article [27], although reporting the use of HA in RCP, was excluded because of clinical parameters that could not be compared with other studies (relative attachment level and relative gingival margin level). Finally, four studies [21,22,28,29] were included after the review process. The selection strategy was conducted as shown in the flowchart in Figure 1.
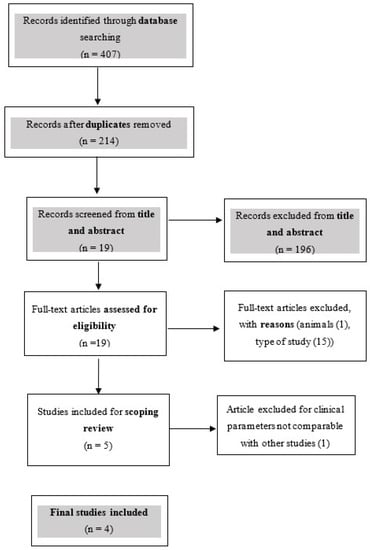
Figure 1.
Flowchart summarizing the article selection process.
Data collection was performed using an electronic spreadsheet designed to express all relevant information on study characteristics and outcomes as tables in the results (Table 2 and Table 3). No meta-analysis of the articles included was performed because of the differences among surgical treatments, commercial formulations and compositions of HA employed in the studies. Hence, a qualitative descriptive statistical approach was used to present the data. The total number of included patients was 67. Overall, 15 patients presented multiple gingival recessions of RT1 and RT2 types, while 52 patients showed single gingival recessions of RT1 type. In total, 62 single gingival recessions were treated. In both included RCTs, the test group (25 total gingival recessions) was treated with CAF+HA, while the control group (25 total gingival recessions) received only CAF. Both RCTs assessed RD, PD and CAL, but with different follow-up periods [21,22]. Kumar et al. made clinical evaluations at 1, 3, 6, 12 and 24 weeks [22], while Pilloni et al. reported data at 18 months. Additionally, Pilloni also analyzed KT, CRC, MRC and VAS [21],. Another difference lies in the type of HA used, with Kumar et al. and Pilloni et al. employing Gengigel and Hyadent, respectively [21,22].

Table 2.
Summary of results.

Table 3.
Summary of results.
In the case series, gingival recession defects were treated with MCAT+sCTG+HA or LCT+sCTG+HA; however, the number of cases assigned to each surgical technique was not available [28,29]. In one case series, 12 single gingival recessions were treated [29], whereas the other reported data on 15 multiple gingival recessions [28]. Both case series used Hyadent HA and assessed RD, PD, CAL, KT, CRC and MRC; however, follow-up periods were different amongst them [28,29]. Guldener et al. scheduled follow-up recalls ranging from 6 to 30 months [29], while Lanzrain et al. reported follow-up periods ranging from 6 to 33 months. Furthermore, the latter study also evaluated RES [28].
In all studies included in this review, HA gel was applied on the root surface prior to suturing [21,22,28,29].
As previously mentioned, Pilloni also analyzed KT and VAS.
Seven days after surgical treatment, postoperative morbidity (pain intensity, discomfort and swelling) was evaluated using the visual analog scale (VAS), swelling and discomfort were statistically lower in the test group (p = 0.010 and p = 0.029, respectively), and no difference was found in pain intensity (p = 0.151) between the test and control groups.
At baseline in both the case and control groups, keratinized tissue (KT) was 2.0[1.0] mm; at 18 months in the test group, KT was 2.0[0.0] mm, while in the control group, KT was 2.0[1.0] mm; no differences were found between the test and control groups (p = 0.116).
Guldener and Lanzrein also evaluated KT. Guldener reported that at baseline KT was 1.6[0.9] mm, and at follow-up, it was 4.9[1.3], while Lanzrein observed that at baseline KT was 2.5[1.0] mm, and at follow-up, it was 3.7[0.8].
This article also evaluated the esthetic outcome of the surgical procedures using the root coverage esthetic score (RES). The mean RES was 7.9 ± 1.9.
4. Discussion
HA is a biodegradable, biocompatible and nontoxic linear polysaccharide found in extracellular matrices [12]. The major function of HA is to bind water and facilitate the transfer of essential metabolites, hence preserving the structural and homeostatic integrity of these tissues [18]. Recent in vitro and animal studies have shown that HA induces angiogenesis [34], stimulates clot formation [35], has bacteriostatic activity [36] significantly increases the tensile strength of granulation tissue [37] and stimulates osteogenesis [38] without interfering with the formation of new bone tissue [39]. These properties potentially decrease healing time and enhance wound stability [40]. Recently, the use of HA has been introduced in dentistry in cases of non-surgical treatment of periodontitis [41], as an adjunct to promote the healing of mouth ulcers and gingivitis [42] and in papilla regeneration [43]. On the surgical aspect, the application of HA has been associated with bone regeneration procedures including sinus lift and socket preservation, the surgical treatment of periodontal defects [44] and, recently, in post-extraction sockets [20,21,22,28,29,45,46].
Considering the surgical treatment of gingival recessions, both RCTs included in the present review treated RT1 defects with CAF. Conversely, either MCAT or LCT combined with sCTG and HA were applied in the case series. Lanzrein et al. [28] treated single RT1 [7] gingival recessions, while Guldener et al. [29] treated multiple adjacent RT1 and RT2 defects [7].
The reduction in RD in the test group compared to the control group was statistically significant in only one RCT study. In this matter, Pilloni [21] reported p = 0.011. In both case series included in the present review, it was not possible to determine whether HA affected root coverage. Notwithstanding, the RD in Guldener et al.’s study [29] was comparable to the results obtained in the study by Sculean et al. [33] and Stähli et al. [47]. On the other hand, Lanzrein et al. [28] found higher RD values at follow-up compared to those observed by Górski et al. [48]. This could be related by different values of RD at baseline, with those of Lanzrein et al. [28] being higher than those reported by Górski et al. [48].
The flap design used in all selected articles unavoidably results in apical displacement [49]. Indeed, despite proper passivation, the flap repositioned more coronally is vulnerable to the tractive force of wound contraction and the activation of neighboring muscles [40,50] that tend to displace the flap apically. The mechanical and chemical properties of HA could reduce the severity of tensile strains tolerated by the flap and, consequently, a significant reduction in RD. In addition, the position of the gingival margin at the end of surgery seems to be an important factor in the final RD reduction. In this respect, suturing the gingival margin at least 2 mm coronally to the CEJ may promote complete root coverage [51]. Although the grade of flap passivation before suturing has not been quantified in the included studies, RD reduction values achieved with adjunctive HA in both RCTs and case series are in accordance with the literature [4,32,33].
Mucogingival surgery should be performed in selected patients adopting very strict inclusion criteria. The absence of bleeding on probing, no trauma and plaque control may promote a rapid healing and the maintenance of healthy PD values. The latter occurs either in new clinical attachments or wound healing with healthy junctional epithelium [52]. In this matter, no significant variation of PD values was observed across the included studies. Accordingly, as reported in the literature, root covering procedures of RT1 [7] recessions can provide significant RD reduction and CAL gain without altering PD [53]. It should be noted that CAL, PD and RD are directly related variables. In the included studies, modifications of CAL were connected to variations of RD, while PD values remained relatively constant. The remarkable results of CAL gain in Guldener et al.’s case series [29] could be explained by the use of an sCTG. There is consensus in the literature that the presence of an sCTG provides stability and reduces soft tissue contraction, improving clinical outcomes in terms of reduced RD and CAL gain [54].
Interestingly, Lanzrein et al. [28] observed lower CRC and MRC than the other included studies. In this regard, treatment of multiple gingival recessions may result in decreased CRC and MRC compared to single recession treatments. Anatomically and technically, multiple gingival recessions are more difficult to treat. Multiple defects need more challenging and time-consuming surgical techniques. Moreover, wound healing is more susceptible to complications due to a large avascular surface area, inadequate blood supply and/or poor tooth position [55]. The test groups in the included RCTs showed improved results compared to control groups in terms of CRC and MRC. However, the reported percentages are comparable with those in the literature. The success rate treating RT1 [7] defects is high, with a mean root coverage of 80.9% (50% to 97.3%) and total root coverage achieved in 46.6% (7.7% to 91.6%) of cases [53,56]. Future RCTs on the use of HA in the surgical treatment of recessions should be performed with a double-blind design, specifying and quantifying the factors that may influence root coverage. These include the position and tension of the flap [50,57], the dimension of the adjacent papillae [58] and the thickness of the flap [30,59], among others. This may reduce bias and make the advantages of HA application really quantifiable.
Also, HA composition and application methods should be assessed as they may have an additional influence on MRC and CRC. Different physical and chemical conformations of HA are available on the market. In fact, the native molecular design is subject to faster degradation than the cross-linked formulation which involves joining the HA chains using covalent bonds [60]. Two different conformations of HA were applied in the examined studies. Specifically, Pilloni [21], Guldener [29] and Lanzrein [28] used a formulation consisting of 1.6% cross-linked HA and 0.2% linear HA, while Kumar [22] employed 0.2% linear HA. It is possible that some structures and concentrations may have an inhibitory effect on cell proliferation and migration during wound healing. In this respect, an in vitro study investigated the relationship between the concentrations of HA solutions and the physicochemical properties and the biocompatibility of blended Cs–Gel–HA membranes. It was noted that only concentrations of HA in a certain range (0.01–0.1%) could enhance cell adhesion, migration and proliferation. When the concentration was above 0.1%, this formulation would reduce or even inhibit these effects [61].
In the included studies, a similar application method to that normally adopted for EMD was used [62,63]. The difference was that HA was placed on the roots of the treated elements without prior use of ethylenediaminetetraacetic (EDTA), which is conversely used as etching in the case of EMD. Thus, although both HA and EMD are employed with the aim of improving healing [39,64], the application methods may differ. EMD, in conjunction with EDTA, is a molecule that promotes the proliferation of cells involved in the regulation of bone remodeling and periodontal ligament regeneration [39]. On the other hand, HA, due to its biochemical properties, can reduce healing time and stabilize the wound. Consequently, according to a different mechanism of action, it remains unclear whether HA should be applied in direct contact with the root with or without mechanically treating the bio-inactive surface of the tooth. In all studies included in the present review, hyaluronan gel was applied on the root surface prior to suturing, but again, the appropriate timing of application has to be established. During the surgical procedure, external factors, such as physiological saline irrigations, may change the properties and effectiveness of HA. The biomolecule can be applied immediately before or even after suturing through infiltration inside the flap, to ensure permanence in the wound. Thus, future studies should investigate which formulation and application technique is better in order to optimize the performance of HA.
The follow-up period is another important variable. The studies included herein showed follow-ups ranging from 18 weeks to 30 months [21,22,28,29]. Although positive results were generally observed in the short term, longer follow-up periods are needed to monitor the evolution of the surgical outcome. For instance, Pini Prato et al. observed that gingival recessions treated with CAF presented apical displacement of the gingival margin after 5 years. Conversely, sites treated with CAF associated with sCTG showed an increased percentage of CRC due to creeping attachment at 5 years [65]. In the long term, it might also be useful to evaluate the root coverage esthetic score (RES). This parameter evaluates five variables for each recession at a minimum of six months following surgery, when tissues have achieved adequate stability and maturation for esthetic evaluations [66,67,68]. In the case report by Lanzrein et al. [28], RES was examined, providing a mean score of 7.9. However, since there was no control group, no objective conclusions can be drawn concerning the improvement in esthetic outcome when HA is used in gingival recession coverage procedures.
The limited number of included studies, the different types and formulations of HA used, and the lack of a well-structured and standardized protocol constitute important limitations of the present review. For this reason, although promising results have emerged, it is not possible to draw significant conclusions related to the application of HA in surgical gingival recession treatment. Furthermore, as the esthetic variable is one of the reasons why patients undergo treatment of gingival recessions, future studies should demonstrate whether there is a correlation between the use of HA and improved esthetic performances in mucogingival surgery. The clinical results of this molecule in dentistry should be confirmed by more RCTs in order to develop efficient and functional application protocols. It would be desirable to define the optimal chemical concentration, biomolecular structure, timing and application method of HA to achieve more predictable outcomes in mucogingival surgery.
5. Conclusions
HA seems to improve the clinical outcomes of gingival recession treatment in terms of RD, CAL and PD, but the magnitude of this effect is limited and confined to short-term follow-up periods. Furthermore, it is not possible to indicate which formulation, timing and application method may result in better clinical and esthetic outcomes.
Author Contributions
M.M.: visualization; F.E.S. and M.T.: formal analysis; P.P.P.: investigation and methodology; F.E.S. and M.T.: writing—original draft; P.P.P. and M.B.: writing—review and editing; C.M.: project administration and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Italian Ministry of Health—Current research IRCCS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S204–S213. [Google Scholar] [CrossRef]
- Patel, R.R.; Richards, P.S.; Inglehart, M.R. Periodontal health, quality of life, and smiling patterns—An exploration. J. Periodontol. 2008, 79, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Mounssif, I. Periodontal plastic surgery. Periodontology 2000 2015, 68, 333–368. [Google Scholar] [CrossRef] [PubMed]
- Meza Mauricio, J.; Furquim, C.P.; Bustillos-Torrez, W.; Soto-Peñaloza, D.; Peñarrocha-Oltra, D.; Retamal-Valdes, B.; Faveri, M. Does enamel matrix derivative application provide additional clinical benefits in the treatment of maxillary Miller class I and II gingival recession? A systematic review and meta-analysis. Clin. Oral. Investig. 2021, 25, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D., Jr. A classification of marginal tissue recession. Int. J. Periodontics Restor. Dent. 1985, 5, 8–13. [Google Scholar]
- Cairo, F.; Nieri, M.; Cincinelli, S.; Mervelt, J.; Pagliaro, U. The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: An explorative and reliability study. J. Clin. Periodontol. 2011, 38, 661–666. [Google Scholar] [CrossRef]
- McGuire, M.K.; Scheyer, E.T.; Schupbach, P. A Prospective, Case-Controlled Study Evaluating the Use of Enamel Matrix Derivative on Human Buccal Recession Defects: A Human Histologic Examination. J. Periodontol. 2016, 87, 645–653. [Google Scholar] [CrossRef]
- McGuire, M.K.; Cochran, D.L. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 2: Histological evaluation. J. Periodontol. 2003, 74, 1126–1135. [Google Scholar] [CrossRef]
- França-Grohmann, I.L.; Sangiorgio, J.P.M.; Bueno, M.R.; Casarin, R.C.V.; Silvério Ruiz, K.G.; Nociti, F.H., Jr.; Casati, M.Z.; Sallum, E.A. Treatment of dehiscence-type defects with collagen matrix and/or enamel matrix derivative: Histomorphometric study in minipigs. J. Periodontol. 2020, 91, 967–974. [Google Scholar] [CrossRef]
- Chambrone, L.; Pini Prato, G.P. Clinical insights about the evolution of root coverage procedures: The flap, the graft, and the surgery. J. Periodontol. 2019, 90, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.L.; Roberts, J.L.; Moseley, R.; Griffiths, P.C.; Thomas, D.W. Evaluation of the physical and biological properties of hyaluronan and hyaluronan fragments. Int. J. Pharm. 2011, 420, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Palmer, J.W. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. Faseb. J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic effects of hyaluronic acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R. Hyaluronic Acid: A boon in periodontal therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef]
- Prato, G.P.; Rotundo, R.; Magnani, C.; Soranzo, C.; Muzzi, L.; Cairo, F. An autologous cell hyaluronic acid graft technique for gingival augmentation: A case series. J. Periodontol. 2003, 74, 262–267. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral. Investig. 2019, 23, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Srinivas, M.; Pai, J.; Suragimath, G.; Prasad, K.; Polepalle, T. Efficacy of hyaluronic acid (hyaluronan) in root coverage procedures as an adjunct to coronally advanced flap in Millers Class I recession: A clinical study. J. Indian Soc. Periodontol. 2014, 18, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Stern, C.; Aromataris, E.; Lockwood, C.; Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Bateson, M. Systematic Reviews to Support Evidence-Based Medicine: How to Review and Apply Findings of Healthcare Research. Postgrad. Med. J. 2004, 80, 123. [Google Scholar]
- Al-Ardah, A.J.; AlHelal, A.; Proussaefs, P.; AlBader, B.; Al Humaidan, A.A.; Lozada, J. Managing Titanium Mesh Exposure With Partial Removal of the Exposed Site: A Case Series Study. J. Oral. Implant. 2017, 43, 482–490. [Google Scholar] [CrossRef]
- Nandanwar, J.; Bhongade, M.; Puri, S.; Dhadse, P.; Datir, M.; Kasatwar, A. Comparison of effectiveness of hyaluronic acid in combination with polylactic acid/polyglycolic acid membrane and subepithelial connective tissue graft for the treatment of multiple gingival recession defects in human: A clinical study. J. Datta Meghe Inst. Med. Sci. Univ. 2018, 13, 48–53. [Google Scholar] [CrossRef]
- Lanzrein, C.; Guldener, K.; Imber, J.C.; Katsaros, C.; Stähli, A.; Sculean, A. Treatment of multiple adjacent recessions with the modified coronally advanced tunnel or laterally closed tunnel in conjunction with cross-linked hyaluronic acid and subepithelial connective tissue graft: A report of 15 cases. Quintessence Int. 2020, 51, 710–719. [Google Scholar] [CrossRef]
- Guldener, K.; Lanzrein, C.; Eliezer, M.; Katsaros, C.; Stähli, A.; Sculean, A. Treatment of single mandibular recessions with the modified coronally advanced tunnel or laterally closed tunnel, hyaluronic acid, and subepithelial connective tissue graft: A report of 12 cases. Quintessence Int. 2020, 51, 456–463. [Google Scholar] [CrossRef]
- Baldi, C.; Pini-Prato, G.; Pagliaro, U.; Nieri, M.; Saletta, D.; Muzzi, L.; Cortellini, P. Coronally advanced flap procedure for root coverage. Is flap thickness a relevant predictor to achieve root coverage? A 19-case series. J. Periodontol. 1999, 70, 1077–1084. [Google Scholar] [CrossRef]
- Zucchelli, G.; Stefanini, M.; Ganz, S.; Mazzotti, C.; Mounssif, I.; Marzadori, M. Coronally Advanced Flap with Different Designs in the Treatment of Gingival Recession: A Comparative Controlled Randomized Clinical Trial. Int. J. Periodontics Restor. Dent. 2016, 36, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Cosgarea, R.; Stähli, A.; Katsaros, C.; Arweiler, N.B.; Brecx, M.; Deppe, H. The modified coronally advanced tunnel combined with an enamel matrix derivative and subepithelial connective tissue graft for the treatment of isolated mandibular Miller Class I and II gingival recessions: A report of 16 cases. Quintessence Int. 2014, 45, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Allen, E.P. The Laterally Closed Tunnel for the Treatment of Deep Isolated Mandibular Recessions: Surgical Technique and a Report of 24 Cases. Int. J. Periodontics Restor. Dent. 2018, 38, 479–487. [Google Scholar] [CrossRef]
- West, D.C.; Hampson, I.N.; Arnold, F.; Kumar, S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985, 228, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.F.; Kakkar, V.V.; Goodwin, C.A.; O’Regan, M. Inhibition of fibrinolytic activity by hyaluronan and its alcohol ester derivatives. Thromb. Res. 1995, 78, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Simonelli, A.; Pramstraller, M.; Guarnelli, M.E.; Fabbri, C.; Maietti, E.; Farina, R. Clinical efficacy of a chlorhexidine-based mouthrinse containing hyaluronic acid and an antidiscoloration system in patients undergoing flap surgery: A triple-blind, parallel-arm, randomized controlled trial. Int. J. Dent. Hyg. 2018, 16, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Vänttinen, E.; Viljanto, J. Tensile strength of new connective tissue formed in pretreated viscose cellulose implants. Ann. Med. Exp. Biol. Fenn. 1965, 43, 257–259. [Google Scholar]
- Pilloni, A.; Bernard, G.W. The effect of hyaluronan on mouse intramembranous osteogenesis in vitro. Cell Tissue Res. 1998, 294, 323–333. [Google Scholar] [CrossRef]
- Pilloni, A.; Rimondini, L.; De Luca, M.; Bernard, G.W. Effect of hyaluronan on calcification-nodule formation from human periodontal ligament cell culture. J. Appl. Biomater. Biomech. 2003, 1, 84–90. [Google Scholar]
- Wikesjö, U.M.; Selvig, K.A. Periodontal wound healing and regeneration. Periodontology 2000 1999, 19, 21–39. [Google Scholar] [CrossRef]
- Johannsen, A.; Tellefsen, M.; Wikesjö, U.; Johannsen, G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J. Periodontol. 2009, 80, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Pomowski, R.; Kundt, G.; Göcke, R. Treatment of gingivitis with hyaluronan. J. Clin. Periodontol. 2003, 30, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ficho, A.C.; de Souza Faloni, A.P.; Pennisi, P.R.C.; Borges, L.G.F.; de Macedo Bernadino, Í.; Paranhos, L.R.; Queiroz, T.P.; Santos, P.L. Is interdental papilla filling using hyaluronic acid a stable approach to treat black triangles? A systematic review. J. Esthet. Restor. Dent. 2021, 33, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Cantore, S.; Capodiferro, S.; Grassi, F.R. Esterified hyaluronic acid and autologous bone in the surgical correction of the infra-bone defects. Int. J. Med. Sci. 2009, 6, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kediege, S.D.; Gupta, A.; Jain, K. Evaluation of gengigel® application in the management of furcation with coronally advanced flap through surgical re-entry-a split mouth clinical study. J. Clin. Diagn. Res. 2017, 11, ZC27–ZC32. [Google Scholar] [CrossRef]
- Ibraheem, W.; Jedaiba, W.H.; Alnami, A.M.; Hussain Baiti, L.A.; Ali Manqari, S.M.; Bhati, A.; Almarghlani, A.; Assaggaf, M. Efficacy of hyaluronic acid gel and spray in healing of extraction wound: A randomized controlled study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3444–3449. [Google Scholar] [CrossRef]
- Stähli, A.; Duong, H.Y.; Imber, J.C.; Roccuzzo, A.; Salvi, G.E.; Katsaros, C.; Ramseier, C.A.; Sculean, A. Recession coverage using the modified coronally advanced tunnel and connective tissue graft with or without enamel matrix derivative: 5-year results of a randomised clinical trial. Clin. Oral. Investig. 2022, 27, 105–113. [Google Scholar] [CrossRef]
- Górski, B.; Szerszeń, M.; Kaczyński, T. Effect of 24% EDTA root conditioning on the outcome of modified coronally advanced tunnel technique with subepithelial connective tissue graft for the treatment of multiple gingival recessions: A randomized clinical trial. Clin. Oral. Investig. 2022, 26, 1761–1772. [Google Scholar] [CrossRef]
- Rasperini, G.; Acunzo, R.; Limiroli, E. Decision Making in Gingival Recession Treatment: Scientific Evidence and Clinical Experience. Clin. Adv. Periodontics 2011, 1, 41–52. [Google Scholar] [CrossRef]
- Pini Prato, G.; Pagliaro, U.; Baldi, C.; Nieri, M.; Saletta, D.; Cairo, F.; Cortellini, P. Coronally advanced flap procedure for root coverage. Flap with tension versus flap without tension: A randomized controlled clinical study. J. Periodontol. 2000, 71, 188–201. [Google Scholar] [CrossRef]
- Pini-Prato, G.; Baldi, C.; Pagliaro, U.; Nieri, M.; Saletta, D.; Rotundo, R.; Cortellini, P. Coronally advanced flap procedure for root coverage. Treatment of root surface: Root planning versus polishing. J. Periodontol. 1999, 70, 1064–1076. [Google Scholar] [CrossRef]
- Lang, N.P.; Lindhe, J. Clinical Periodontology and Implant Dentistry; 2 Volume Set; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Chambrone, L.; Tatakis, D.N. Periodontal soft tissue root coverage procedures: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S8–S51. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Nieri, M.; Pagliaro, U. Efficacy of periodontal plastic surgery procedures in the treatment of localized facial gingival recessions. A systematic review. J. Clin. Periodontol. 2014, 41 (Suppl. 15), S44–S62. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Lima, L.A.; Pustiglioni, F.E.; Chambrone, L.A. Systematic review of periodontal plastic surgery in the treatment of multiple recession-type defects. J. Can. Dent. Assoc. 2009, 75, 203a–203f. [Google Scholar]
- Chambrone, L.; Sukekava, F.; Araújo, M.G.; Pustiglioni, F.E.; Chambrone, L.A.; Lima, L.A. Root-coverage procedures for the treatment of localized recession-type defects: A Cochrane systematic review. J. Periodontol. 2010, 81, 452–478. [Google Scholar] [CrossRef] [PubMed]
- Pini Prato, G.P.; Baldi, C.; Nieri, M.; Franseschi, D.; Cortellini, P.; Clauser, C.; Rotundo, R.; Muzzi, L. Coronally advanced flap: The post-surgical position of the gingival margin is an important factor for achieving complete root coverage. J. Periodontol. 2005, 76, 713–722. [Google Scholar] [CrossRef]
- Saletta, D.; Pini Prato, G.; Pagliaro, U.; Baldi, C.; Mauri, M.; Nieri, M. Coronally advanced flap procedure: Is the interdental papilla a prognostic factor for root coverage? J. Periodontol. 2001, 72, 760–766. [Google Scholar] [CrossRef]
- Hwang, D.; Wang, H.L. Flap thickness as a predictor of root coverage: A systematic review. J. Periodontol. 2006, 77, 1625–1634. [Google Scholar] [CrossRef]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: Hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef]
- Liu, H.; Yin, Y.; Yao, K.; Ma, D.; Cui, L.; Cao, Y. Influence of the concentrations of hyaluronic acid on the properties and biocompatibility of Cs-Gel-HA membranes. Biomaterials 2004, 25, 3523–3530. [Google Scholar] [CrossRef]
- Castellanos, A.; de la Rosa, M.; de la Garza, M.; Caffesse, R.G. Enamel matrix derivative and coronal flaps to cover marginal tissue recessions. J. Periodontol. 2006, 77, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.T.; de Menezes, C.C.; Kahn, S.; Fischer, R.G.; da Silva Figueredo, C.M.; Fernandes, G.V.d.O. Gingival recession treatment with enamel matrix derivative associated with coronally advanced flap and subepithelial connective tissue graft: A split-mouth randomized controlled clinical trial with molecular evaluation. Clin. Oral. Investig. 2022, 26, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Caluseru, O.M.; Guillemette, V.; Zhang, Y.; Gemperli, A.C.; Chandad, F.; Sculean, A. Influence of enamel matrix derivative on cells at different maturation stages of differentiation. PLoS ONE 2013, 8, e71008. [Google Scholar] [CrossRef]
- Pini-Prato, G.P.; Cairo, F.; Nieri, M.; Franceschi, D.; Rotundo, R.; Cortellini, P. Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: A split-mouth study with a 5-year follow-up. J. Clin. Periodontol. 2010, 37, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Rotundo, R.; Miller, P.D.; Pini Prato, G.P. Root coverage esthetic score: A system to evaluate the esthetic outcome of the treatment of gingival recession through evaluation of clinical cases. J. Periodontol. 2009, 80, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, R.; Nieri, M.; Mori, M.; Clauser, C.; Prato, G.P. Aesthetic perception after root coverage procedure. J. Clin. Periodontol. 2008, 35, 705–712. [Google Scholar] [CrossRef]
- Cairo, F.; Barootchi, S.; Tavelli, L.; Barbato, L.; Wang, H.L.; Rasperini, G.; Graziani, F.; Tonetti, M. Aesthetic-And patient-related outcomes following root coverage procedures: A systematic review and network meta-analysis. J. Clin. Periodontol. 2020, 47, 1403–1415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).