Bioengineering Applied to Oral Implantology, a New Protocol: “Digital Guided Surgery”
Abstract
1. Introduction
1.1. Background
1.2. Aim
2. Materials and Methods
2.1. Prosthodontics and Dental Biomechanics
2.2. Dental Implants
- Reliability in terms of optimal biological response by the tissues, sustainable healing times, and adequate connection between the various elements (given the presence of a series of interconnected devices such as the intraosseous component that simulates the root of the dental elements, the supra-bone component that simulates the prosthetic posts as we know them for conventional fixed prosthetic preparations, and a component that simulates the morphology of the dental elements);
- Simplicity, which is a very important factor. The evolution of medical technology tends towards the optimization of implant shape and everything related to the possibility of replacing missing elements with dental implants. However, this must take place within a whole series of procedures and a reduction in the number of steps in currently time-consuming procedures that will then be easy to learn and require little surgical instrumentation;
- Versatility, which is the characteristic of being able to use implant devices interchangeably for several areas of the mouth as a connection method that can work well in many cases;
- Patient needs;
- Clinical case, because choices cannot be standardized butaimed at the individual patient;
- Experience of the operator.
2.3. Bioengineering in Dentistry
2.4. Digital Workflow in Dentistry
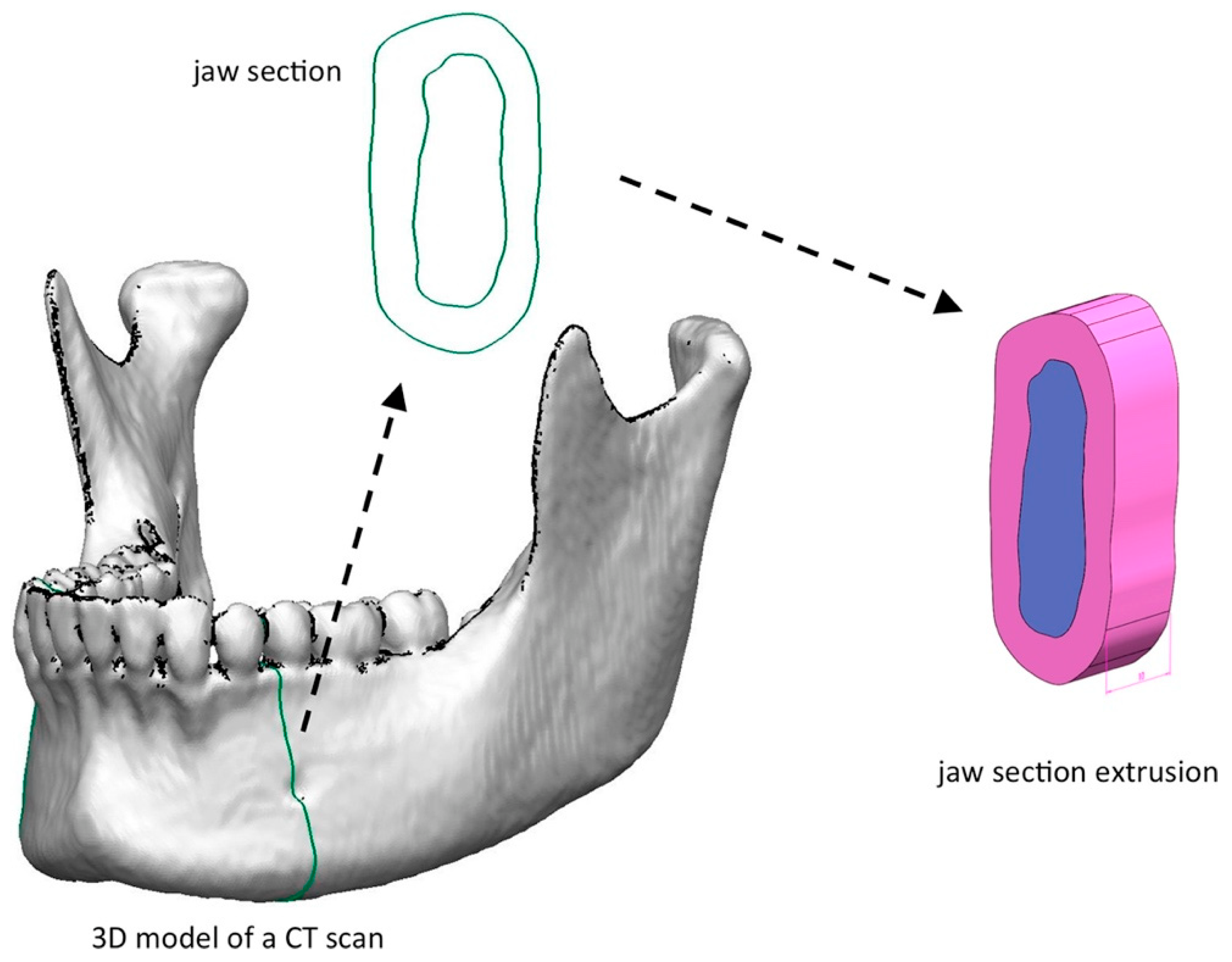
2.5. Finite Element Analysis
- Modelling: this phase is present in all engineering-type studies: we move from the physical system to a mathematical model, which abstracts some aspects of interest from the physical system, focusing attention on a few aggregate variables of interest and “filtering” the remaining ones. For example, when calculating the bending moment of a beam, interactions at the molecular level are not taken into account. The physical system of the complex is divided into subsystems. In the case in question, it is not necessary, or it can be assumed that it is a part belonging to a more complex system, for example, a ship or an aeroplane. The subsystem will then be divided into finite elements to which a mathematical model will be applied. Unlike the analytical treatments, it is sufficient that the chosen mathematical model is suitable for the simple geometries of finite elements. The choice of an element type in a software program is equivalent to an implicit choice of the mathematical model underlying it. The error that can lead to the use of a model must be evaluated with experimental tests, an operation that is generally expensive in terms of time and resources.
- Discretization: in a numerical simulation it is necessary to pass from an infinite number of degrees of freedom (condition proper to the “continuum”) to a finite number (situation proper to the grid). The discretization, in space or time, aims to obtain a discrete model characterized by a finite number of degrees of freedom. An error is inserted given by the discrepancy with the exact solution of the mathematical model. This error can be appropriately evaluated if there is a mathematical model suitable for the entire structure (and therefore preferable to use concerning FEA analysis), and in the absence of numerical calculation errors, this can be considered true using electronic calculators [23,24].
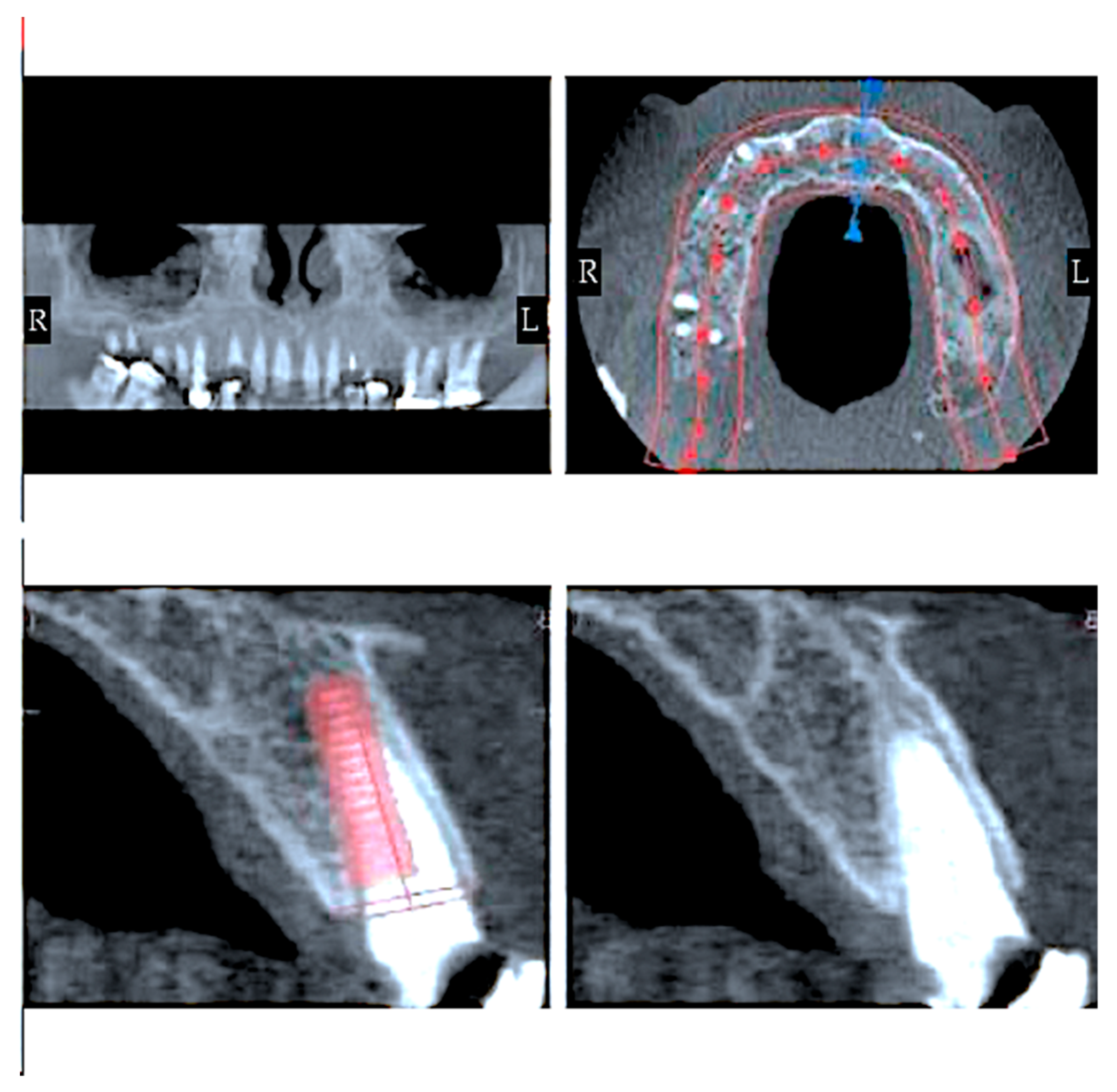
2.6. Finite Element Analysis and Dental Implants
2.7. Von Mises
2.8. Fracture Mechanics Analysis
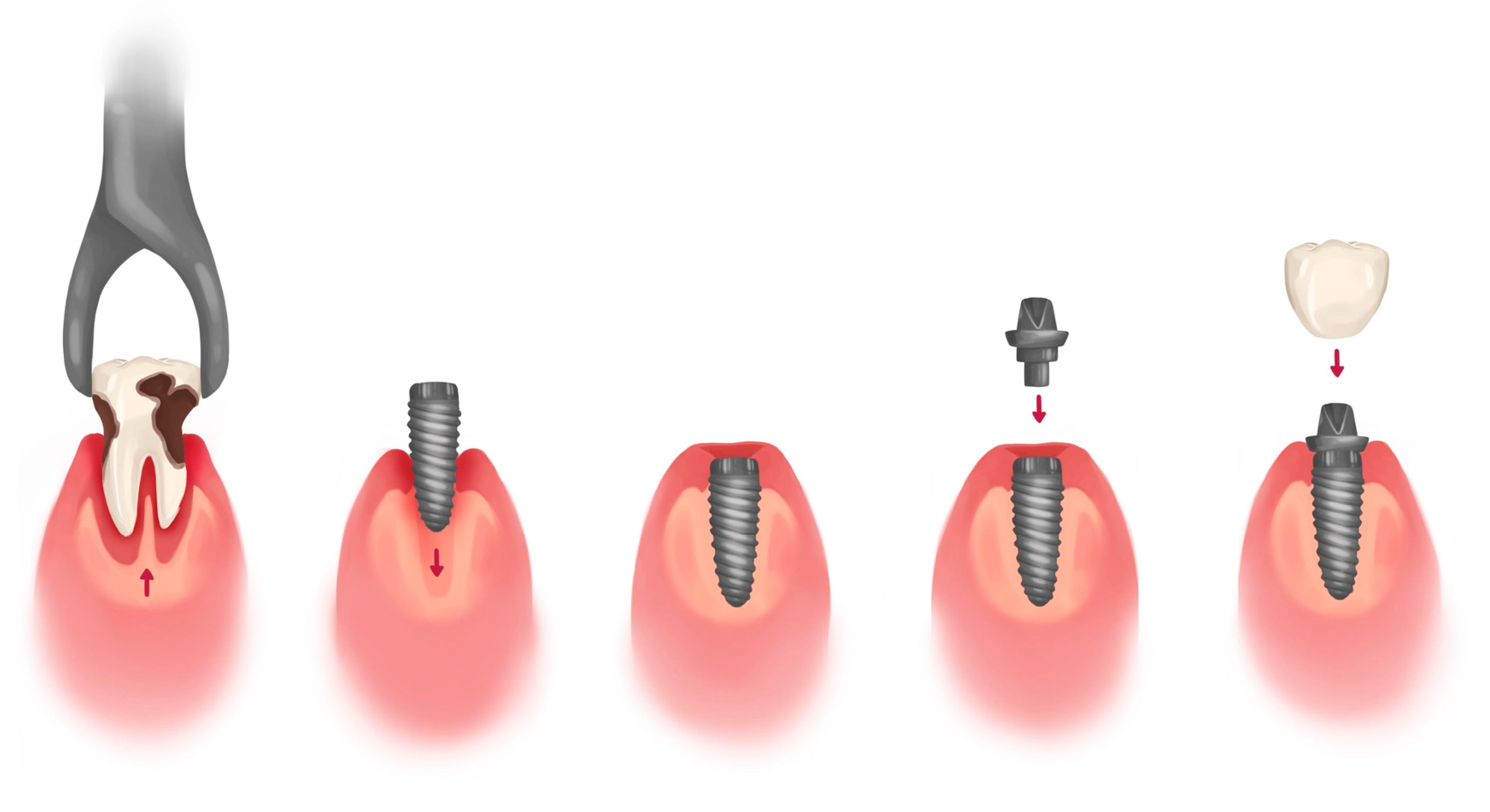
2.9. Oral Surgery, Guided Surgery and Dental Implant Surgery
- (1)
- A surgical period where, after osseointegration, the gum will not have to be re-incised to see the position of the implant, as is the case for transmucosal implants;
- (2)
- Two surgical stages, as is the case for fully submerged implants, in which the gum will have to be re-incised after osseointegration to see where the implant is and allow it to be connected to the supra-body prosthetic devices.
- Immediate post-extraction implants, in which the implant is inserted immediately after the extraction;
- Deferred post-extraction implants, in which the insertion of the implant takes place a few weeks after dental extraction when healing of the molal tissues has occurred;
- Delayed post-extraction implants, in which the insertion of the implant takes place 12–16 after weeks of dental extraction;
- Late post-extraction implants, in which the implant is inserted 3–4 months after extraction.
3. Results
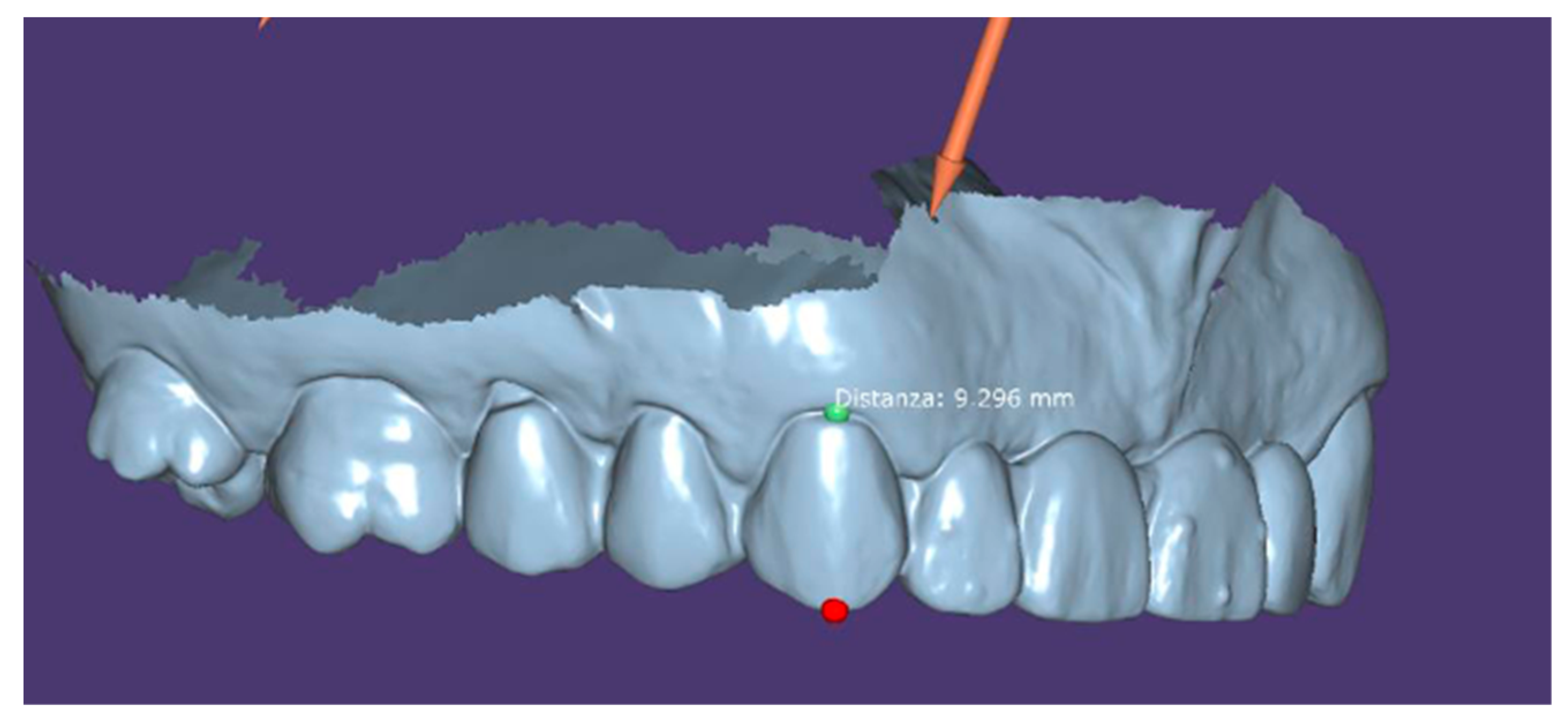
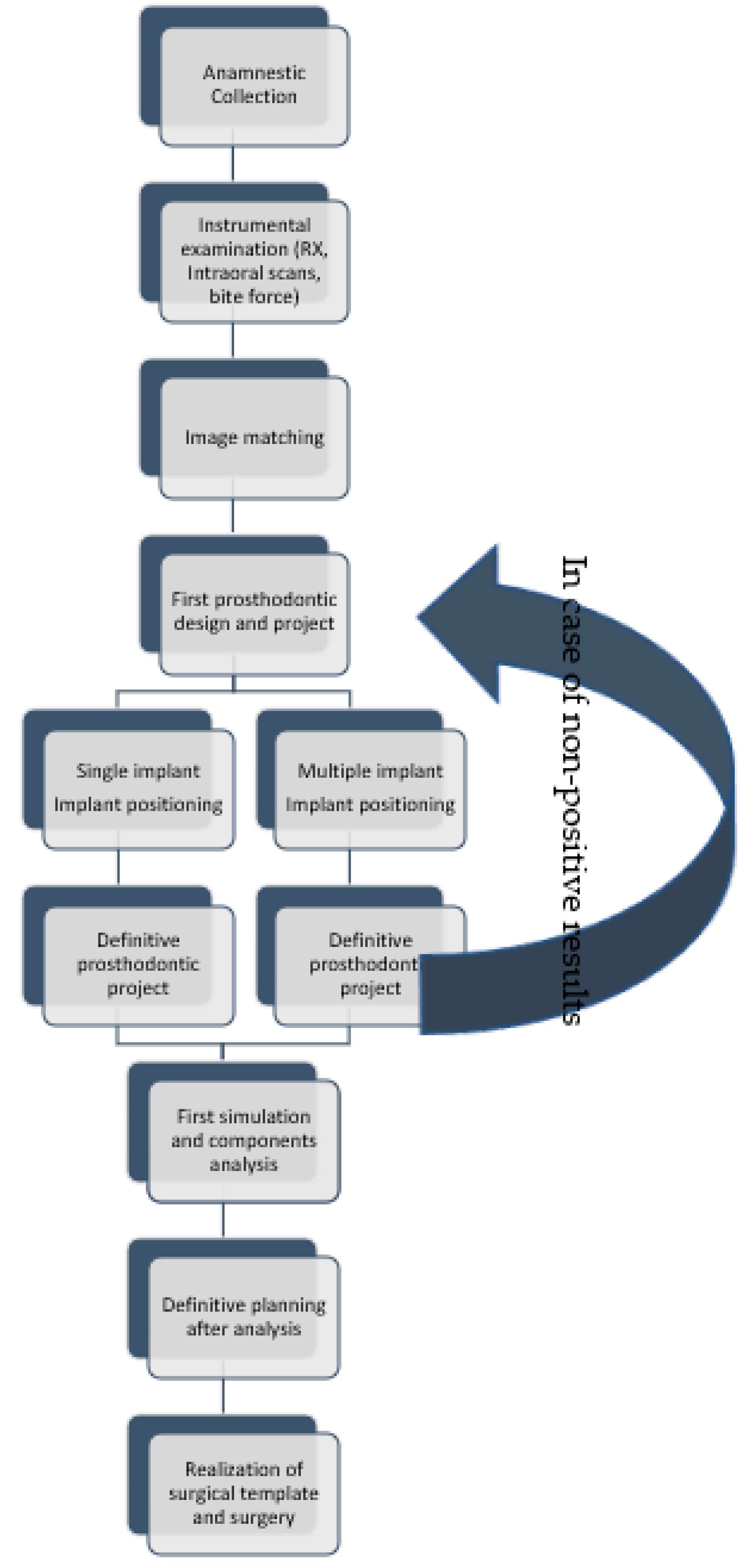
3.1. Digital-Assisted Pre-Guided Implant Surgery
- Anamnestic collection;
- Instrumental examinations (three-dimensional radiography, intraoral optical impression);
- Image matching and transformation from .dicom to .stl;
- The first design of a prosthodontic with an advantageous position from a biomechanical point of view:
- ○
- Implant positioning respecting anatomy as much as possible;
- ○
- Realization of definitive prosthetic design;
- First simulation:
- ○
- Analysis of force peaks and attenuation by moving dental implant;
- ○
- Definitive implant planning;
- Realization of the surgical guide;
- Surgical intervention (Figure 5).
3.2. Fi-Index Tool
4. Discussion
- Insufficient sterilization of the operating field;
- Overheating of the bone;
- The lack of primary stability at the time of its insertion;
- The occlusal overload of the implant screw.
- genetic predisposition for periodontitis
- the inaccurate crown which causes greater plaque accumulation around the implant
- lack of the contact point that determines food impaction, i.e., accumulation of food and plaque between the teeth
- presence of cement under the gum which is colonized by bacteria
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Diesendorf, M. The mystery of declining tooth decay. Nature 1986, 322, 125–129. [Google Scholar] [CrossRef]
- Fiorillo, L. Oral Health: The First Step to Well-Being. Medicina 2019, 55, 676. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Klussmann, L.; Wöstmann, B.; Schlenz, M.A. Accuracy of Digital and Conventional Full-Arch Impressions in Patients: An Update. J. Clin. Med. 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Uechi, J.; Konno, M.; Sasamoto, S.; Iijima, M.; Mizoguchi, I. Accuracy of digital models generated by conventional impression/plaster-model methods and intraoral scanning. Dent. Mater. J. 2018, 37, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, M.; Ceruso, F.M.; Muzzi, L.; Meloni, S.M.; Kim, Y.-J.; Gargari, M.; Martinolli, M. Effect of Simultaneous Immediate Implant Placement and Guided Bone Reconstruction with Ultra-Fine Titanium Mesh Membranes on Radiographic and Clinical Parameters after 18 Months of Loading. Materials 2019, 12, 1710. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Yang, R. Digital Design and Application of 3D Printed Surgical Guide for Long Screw Fixation of Condylar Sagittal Fracture. J. Craniofac. Surg. 2021, 32, e632–e634. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Ferrini, F.; Crespi, R.; Gastaldi, G.; Cappare, P. Digital impressions for fabrication of definitive "all-on-four" restorations. Implant Dent. 2015, 24, 125–129. [Google Scholar] [CrossRef]
- Kolata, G.B. The finite element method: A mathematical revival. Science 1974, 184, 887–889. [Google Scholar] [CrossRef]
- Makin, S. Searching for digital technology's effects on well-being. Nature 2018, 563, S138–S140. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H.; Lee, Y.M.; Chung, D.J.; Lee, J.I.; Hong, S.D. A biocompatibility study of a reinforced acrylic-based hybrid denture composite resin with polyhedraloligosilsesquioxane. J. Oral Rehabil. 2007, 34, 389–395. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Gangadhar, S.A.; Shah, I. Flexural strength of heat-polymerized polymethyl methacrylate denture resin reinforced with glass, aramid, or nylon fibers. J. Prosthet. Dent. 2001, 86, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, S.K.; Kim, S.C.; Okubo, C.; Lee, H.H. Investigation of the cytotoxicity of thermoplastic denture base resins. J. Adv. Prosthodont. 2017, 9, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Sher, J.; Kirkham-Ali, K.; Luo, J.D.; Miller, C.; Sharma, D. Dental Implant Placement in Patients With a History of Medications Related to Osteonecrosis of the Jaws: A Systematic Review. J. Oral Implantol. 2021, 47, 249–268. [Google Scholar] [CrossRef]
- Norcia, A.; Cicciù, M.; Matacena, G.; Bramanti, E. Dental implant positioning by using the root way. A predictable technique for postextractive surgery. Minerva. Stomatol. 2016, 65, 393–402. [Google Scholar]
- Camardella, L.T.; Breuning, H.; de Vasconcellos Vilella, O. Accuracy and reproducibility of measurements on plaster models and digital models created using an intraoral scanner. J. Orofac. Orthop. 2017, 78, 211–220. [Google Scholar] [CrossRef]
- Tallarico, M. Computerization and Digital Workflow in Medicine: Focus on Digital Dentistry. Materials 2020, 13, 2172. [Google Scholar] [CrossRef]
- Rodrigues, S.B.; Franken, P.; Celeste, R.K.; Leitune, V.C.B.; Collares, F.M. CAD/CAM or conventional ceramic materials restorations longevity: A systematic review and meta-analysis. J. Prosthodont. Res. 2019, 63, 389–395. [Google Scholar] [CrossRef]
- Ferrini, F.; Sannino, G.; Chiola, C.; Capparé, P.; Gastaldi, G.; Gherlone, E.F. Influence of Intra-Oral Scanner (I.O.S.) on The Marginal Accuracy of CAD/CAM Single Crowns. Int. J. Environ. Res. Public Health 2019, 16, 544. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Arzukanyan, A.V.; Spagnuolo, G.; Cicciu, M. Dental Restorative Digital Workflow: Digital Smile Design from Aesthetic to Function. Dent. J. 2019, 7, 30. [Google Scholar] [CrossRef]
- Clough, R. Thoughts about the origin of the finite element method. Comput. Struct. 2001, 79, 2029–2030. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Arzukanyan, A.; Spagnuolo, G.; Campagna, P.; Cicciù, M. Application Of Bioengineering Devices For The Stress Evaluation In Dentistry: The Last 10 Years Fem Parametric Analysis Of Outcomes And Current Trends. Minerva Stomatol. 2020, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Cervino, G.; Milone, D.; Risitano, G. FEM investigation of the stress distribution over mandibular bone due to screwed overdenture positioned on dental implants. Materials 2018, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Cervino, G.; Milone, D.; Risitano, G. FEM analysis of dental implant-abutment interface overdenture components and parametric evaluation of Equator® and Locator® prosthodontics attachments. Materials 2019, 12, 592. [Google Scholar] [CrossRef]
- D’Amico, C.; Bocchieri, S.; Sambataro, S.; Surace, G.; Stumpo, C.; Fiorillo, L. Occlusal Load Considerations in Implant-Supported Fixed Restorations. Prosthesis 2020, 2, 252–265. [Google Scholar] [CrossRef]
- Yamanishi, Y.; Yamaguchi, S.; Imazato, S.; Nakano, T.; Yatani, H. Influences of implant neck design and implant–abutment joint type on peri-implant bone stress and abutment micromovement: Three-dimensional finite element analysis. Dent. Mater. 2012, 28, 1126–1133. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Evaluating dental zirconia. Dent. Mater. 2019, 35, 15–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef]
- Hanawa, T. Zirconia versus titanium in dentistry: A review. Dent. Mater. J. 2020, 39, 24–36. [Google Scholar] [CrossRef]
- Filardi, V. Stress shielding FE analysis on the temporomandibular joint. J. Orthop. 2020, 18, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; Cicciù, M.; D'Amico, C.; Mauceri, R.; Oteri, G.; Cervino, G. Finite Element Method and Von Mises Investigation on Bone Response to Dynamic Stress with a Novel Conical Dental Implant Connection. Biomed. Res. Int. 2020, 2020, 2976067. [Google Scholar] [CrossRef] [PubMed]
- Sabet, F.A.; Raeisi Najafi, A.; Hamed, E.; Jasiuk, I. Modelling of bone fracture and strength at different length scales: A review. Interface Focus 2016, 6, 20150055. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.R.; Arshi, A.R.; Eslami, M.R.; Fariborz, S.; Moeinzadeh, M.H. Micromechanics fracture in osteonal cortical bone: A study of the interactions between microcrack propagation, microstructure and the material properties. J. Biomech. 2007, 40, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Mischinski, S.; Ural, A. Finite Element Modeling of Microcrack Growth in Cortical Bone. J. Appl. Mech. 2011, 78, 041016. [Google Scholar] [CrossRef]
- Mischinski, S.; Ural, A. Interaction of microstructure and microcrack growth in cortical bone: A finite element study. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 81–94. [Google Scholar] [CrossRef]
- Makary, C.; Menhall, A.; Zammarie, C.; Lombardi, T.; Lee, S.Y.; Stacchi, C.; Park, K.B. Primary Stability Optimization by Using Fixtures with Different Thread Depth According To Bone Density: A Clinical Prospective Study on Early Loaded Implants. Materials 2019, 12, 2398. [Google Scholar] [CrossRef]
- Gluckman, H.; Salama, M.; Du Toit, J. Partial Extraction Therapies (PET) Part 2: Procedures and Technical Aspects. Int. J. Periodontics Restor. Dent. 2017, 37, 377–385. [Google Scholar] [CrossRef]
- Macedo, J.P.; Pereira, J.; Faria, J.; Pereira, C.A.; Alves, J.L.; Henriques, B.; Souza, J.C.M.; López-López, J. Finite element analysis of stress extent at peri-implant bone surrounding external hexagon or Morse taper implants. J. Mech. Behav. Biomed. Mater. 2017, 71, 441–447. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff's Law and bone's structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [CrossRef]
- Fiorillo, L. Fi-Index: A New Method to Evaluate Authors Hirsch-Index Reliability. Publ. Res. Q. 2022, 38, 465–474. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cicciù, M. The Use of Fi-Index Tool to Assess Per-manuscript Self-citations. Publ. Res. Q. 2022, 38, 684–692. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Terranova, A.; Risitano, G.; Raffaele, M.; Cucinotta, F.; Santonocito, D.; Fiorillo, L. Prosthetic and Mechanical Parameters of the Facial Bone under the Load of Different Dental Implant Shapes: A Parametric Study. Prosthesis 2019, 1, 41–53. [Google Scholar] [CrossRef]
- Michailidis, N.; Karabinas, G.; Tsouknidas, A.; Maliaris, G.; Tsipas, D.; Koidis, P. A FEM based endosteal implant simulation to determine the effect of peri-implant bone resorption on stress induced implant failure. Biomed. Mater. Eng. 2013, 23, 317–327. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y. Artificial intelligence (AI) models show potential in recognizing the dental implant type, predicting implant success, and optimizing implant design. J. Evid. -Based Dent. Pract. 2023, 101836. [Google Scholar] [CrossRef]
- Revilla-León, M.; Gómez-Polo, M.; Vyas, S.; Barmak, B.A.; Galluci, G.O.; Att, W.; Krishnamurthy, V.R. Artificial intelligence applications in implant dentistry: A systematic review. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Roy, S.; Dey, S.; Khutia, N.; Roy Chowdhury, A.; Datta, S. Design of patient specific dental implant using FE analysis and computational intelligence techniques. Appl. Soft Comput. 2018, 65, 272–279. [Google Scholar] [CrossRef]
- Al-Sarem, M.; Al-Asali, M.; Alqutaibi, A.Y.; Saeed, F. Enhanced Tooth Region Detection Using Pretrained Deep Learning Models. Int. J. Environ. Res. Public Health 2022, 19, 15414. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Aboalrejal, A.N. Artificial intelligence (AI) as an aid in restorative dentistry is promising, but still a work in progress. J. Evid. -Based Dent. Pract. 2023, 101837. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorillo, L.; Meto, A.; Cicciù, M. Bioengineering Applied to Oral Implantology, a New Protocol: “Digital Guided Surgery”. Prosthesis 2023, 5, 234-250. https://doi.org/10.3390/prosthesis5010018
Fiorillo L, Meto A, Cicciù M. Bioengineering Applied to Oral Implantology, a New Protocol: “Digital Guided Surgery”. Prosthesis. 2023; 5(1):234-250. https://doi.org/10.3390/prosthesis5010018
Chicago/Turabian StyleFiorillo, Luca, Agron Meto, and Marco Cicciù. 2023. "Bioengineering Applied to Oral Implantology, a New Protocol: “Digital Guided Surgery”" Prosthesis 5, no. 1: 234-250. https://doi.org/10.3390/prosthesis5010018
APA StyleFiorillo, L., Meto, A., & Cicciù, M. (2023). Bioengineering Applied to Oral Implantology, a New Protocol: “Digital Guided Surgery”. Prosthesis, 5(1), 234-250. https://doi.org/10.3390/prosthesis5010018









