Morton’s Extension on Hallux Rigidus Pathology
Abstract
1. Introduction
2. Methods
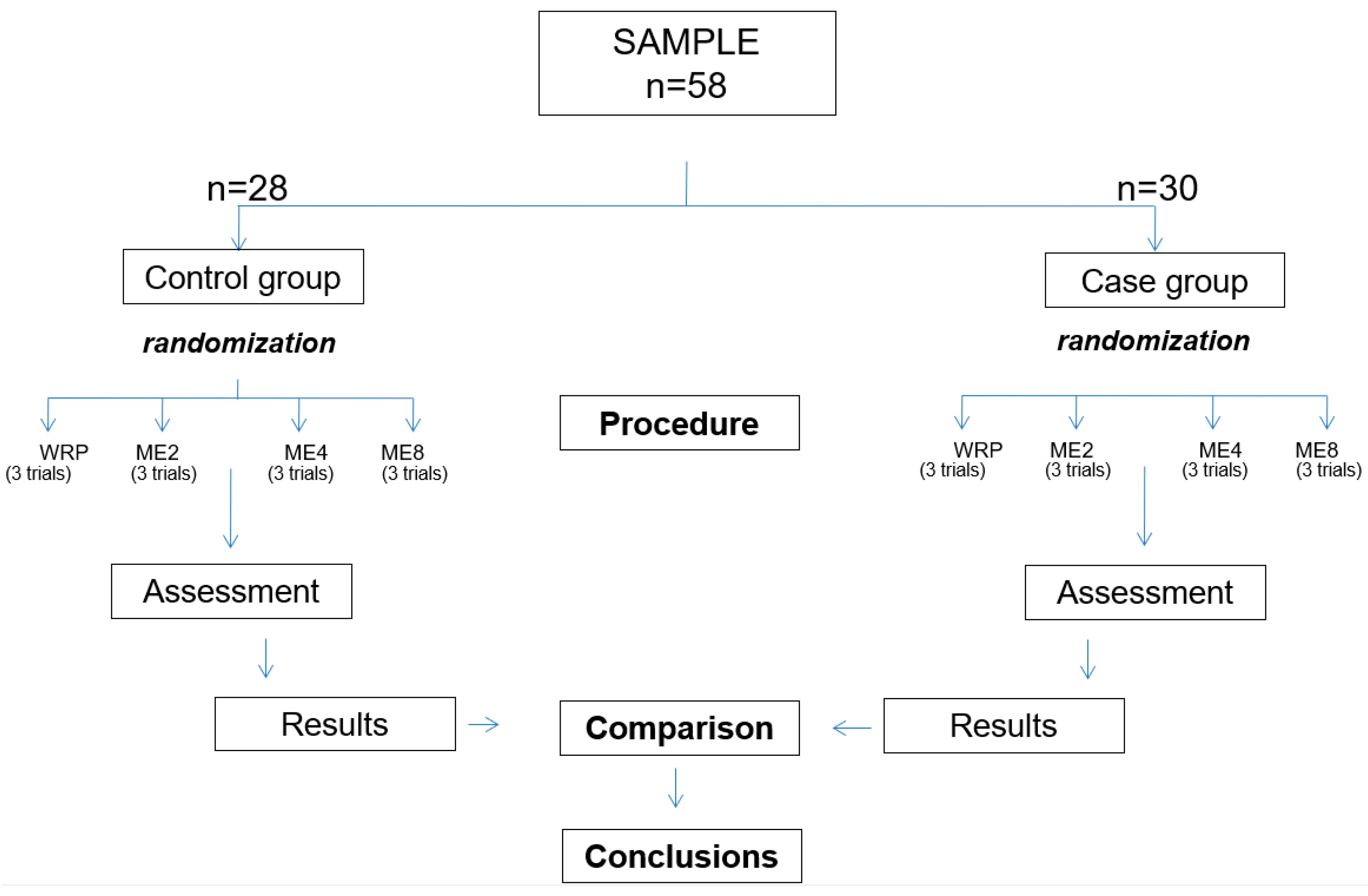
2.1. Study Design
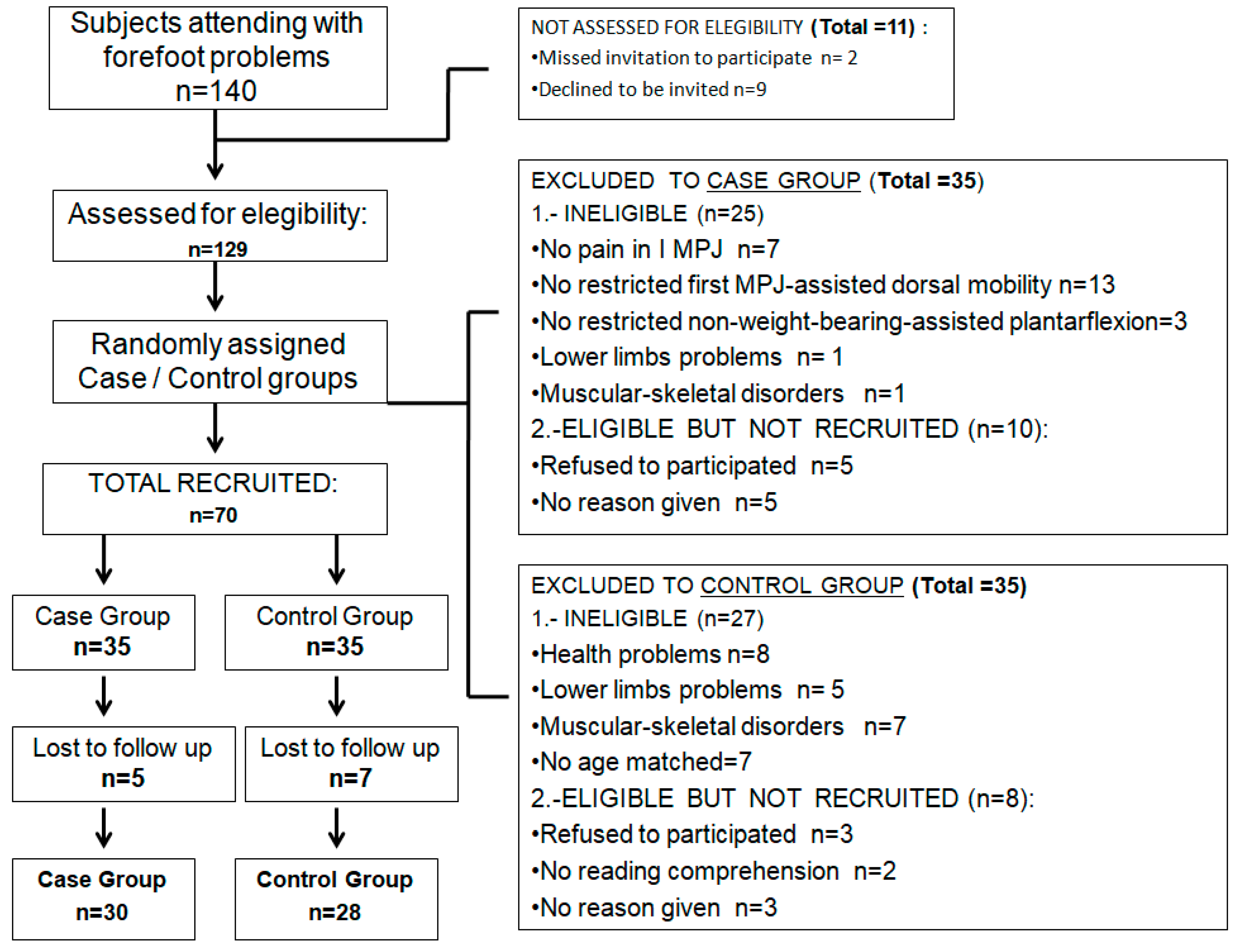
2.2. Participants
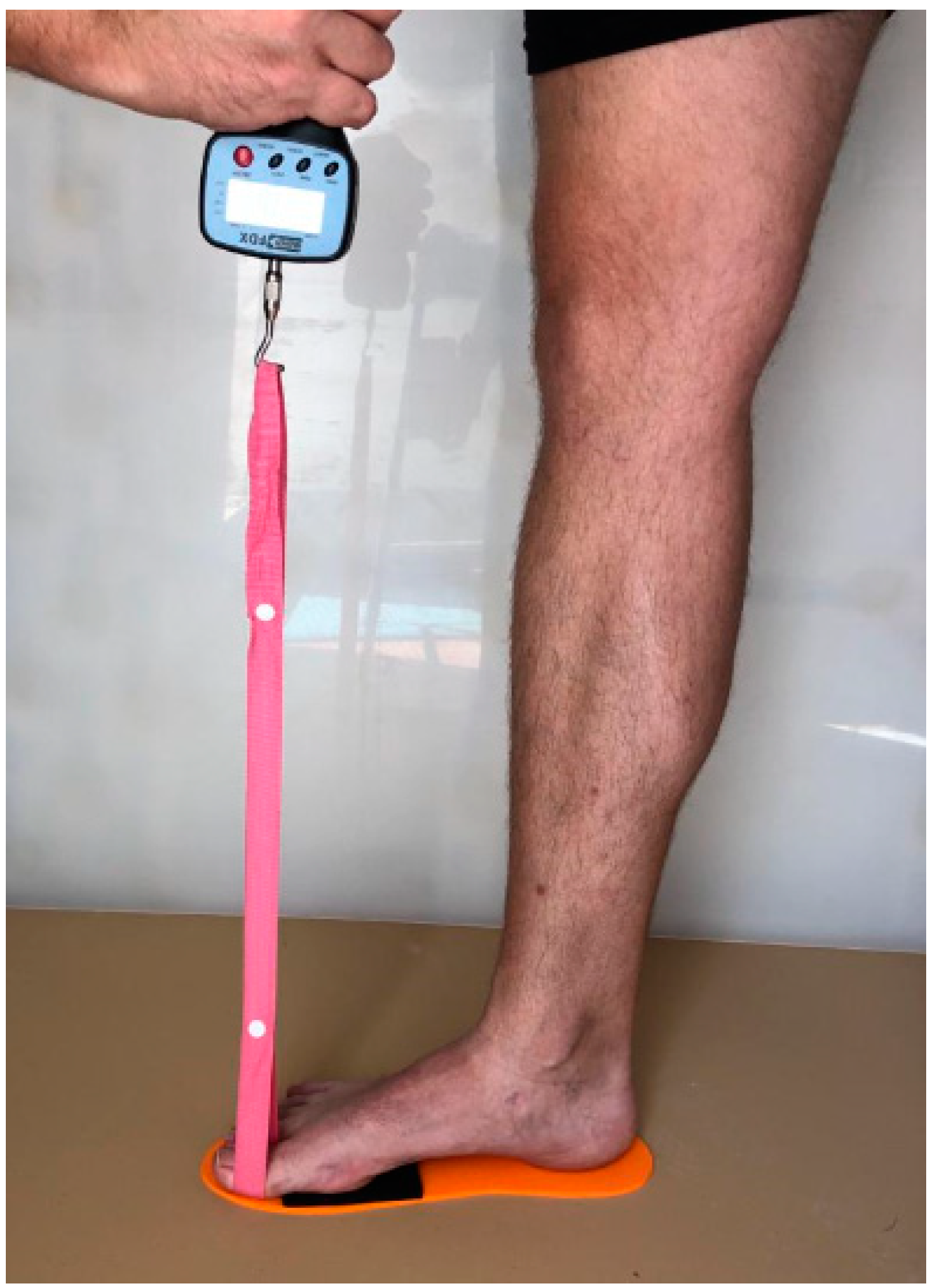
2.3. Measurement Procedures, Instruments, and Variables
2.4. Sample Size
2.5. Statistical Methods
3. Results
4. Discussion
Limitations
5. Conclusions
6. Clinical Relevance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stuck, R.M.; Moore, J.W.; Patwardhan, A.G.; Sartori, M. Forces under the hallux rigidus foot with surgical and orthotic intervention. J. Am. Podiatr. Med. Assoc. 1988, 78, 465–468. [Google Scholar] [PubMed]
- Cotterill, J.M. Stiffness of the Great Toe in Adolescents. Br. Med. J. 1887, 1, 1158. [Google Scholar] [CrossRef] [PubMed]
- Rzonca, E.; Levitz, S.; Lue, B. Hallux equinus. The stages of hallux limitus and hallux rigidus. J. Am. Podiatr. Med. Assoc. 1984, 74, 390–393. [Google Scholar] [CrossRef]
- Colò, G.; Fusini, F.; Samaila, E.M.; Rava, A.; Felli, L.; Alessio-Mazzola, M.; Magnan, B. The efficacy of shoe modifications and foot orthoses in treating patients with hallux rigidus: A comprehensive review of literature. Acta Biomed. 2020, 91, e2020016. [Google Scholar]
- Caravelli, S.; Mosca, M.; Massimi, S.; Pungetti, C.; Russo, A.; Fuiano, M.; Catanese, G.; Zaffagnini, S. A comprehensive and narrative review of historical aspects and management of low-grade hallux rigidus: Conservative and surgical possibilities. Musculoskelet. Surg. 2018, 102, 201–211. [Google Scholar] [CrossRef]
- Dananberg, H.J. Gait style as an etiology to chronic postural pain. Part I. Functional hallux limitus. J. Am. Podiatr. Med. Assoc. 1993, 83, 433–441. [Google Scholar]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Coughlin, M.J.; Shurnas, P.S. Hallux Rigidus. J. Bone Joint. Surg. 2004, 86 Pt 2, 119–130. [Google Scholar] [CrossRef]
- Drago, J.J.; Oloff, L.; Jacobs, A.M. A comprehensive review of hallux limitus. J. Foot Surg. 1984, 23, 213–220. [Google Scholar]
- Shurnas, P.S. Hallux Rigidus: Etiology, Biomechanics, and Nonoperative Treatment. Foot Ankle Clin. 2009, 14, 1–8. [Google Scholar] [CrossRef]
- Gould, N. Hallux rigidus: Cheilotomy or implant? Foot Ankle 1981, 1, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.W.; Katchis, S.D.; Ayson, L.C. Outcomes in Hallux Rigidus Patients Treated Nonoperatively: A Long-Term Follow-Up Study. Foot Ankle Int. 2000, 21, 906–913. [Google Scholar] [CrossRef]
- Grady, J.F.; Axe, T.M.; Zager, E.J.; Sheldon, L.A. A Retrospective Analysis of 772 Patients with Hallux Limitus. J. Am. Podiatr. Med. Assoc. 2002, 92, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ebisui, J.M. The first ray axis and the first metatarsophalangeal joint: An anatomical and pathomechanical study. J. Am. Podiatr. Med. Assoc. 1968, 58, 160–168. [Google Scholar] [CrossRef]
- Kelso, S.F.; Richie, D.H.; Cohen, I.R.; Weed, J.H.; Root, M. Direction and range of motion of the first ray. J. Am. Podiatr. Med. Assoc. 1982, 72, 600–605. [Google Scholar] [CrossRef]
- Dananberg, H.J. Functional hallux limitus and its relationship to gait efficiency. J. Am. Podiatr. Med. Assoc. 1986, 76, 648–652. [Google Scholar] [CrossRef]
- Hicks, J. The mechanics of the foot. II. The plantar aponeurosis and the arch. J. Anat. 1954, 88, 25–30. [Google Scholar]
- Lyritis, G. Developmental disorders of the proximal epiphysis of the hallux. Skelet. Radiol. 1983, 10, 250–254. [Google Scholar] [CrossRef]
- Root, M.L.; Orien, W.P. Normal and Abnormal Function of the Foot; Corp, C.B., Ed.; Clinical Biomechanics Corp.: Los Angeles, CA, USA, 1977; Volume II. [Google Scholar]
- Lambrinudi, C. Metatarsus Primus Elevatus. Proc. R. Soc. Med. 1938, 31, 1273. [Google Scholar] [CrossRef]
- Ohara, K.; Tanaka, Y.; Taniguchi, A.; Kurokawa, H.; Kumai, T.; Yamada, H. Is metatarsus primus elevatus truly observed in hallux rigidus? Radiographic study using mapping methods. J. Orthop. Sci. 2018, 24, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Roukis, T.; Scherer, P.; Anderson, C. Position of the first ray and motion of the first metatarsophalangeal joint. J. Am. Podiatr. Med. Assoc. 1996, 86, 538–546. [Google Scholar] [CrossRef]
- Kim, H.K.; Mirjalili, S.A.; Fernandez, J. Gait kinetics, kinematics, spatiotemporal and foot plantar pressure alteration in response to long-distance running: Systematic review. Hum. Mov. Sci. 2018, 57, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Downey, M. Tarsal coalitions. A surgical classification. J. Am. Podiatr. Med. Assoc. 1991, 81, 187–197. [Google Scholar]
- Jack, E.A. Naviculo-cuneiform fusion in the treatment of flat foot. J. Bone Jt. Surg. 1953, 35-B, 75–82. [Google Scholar] [CrossRef]
- Heng, M.L.; Chua, Y.K.; Pek, H.K.; Krishnasamy, P.; Kong, P.W. A novel method of measuring passive quasi-stiffness in the first metatarsophalangeal joint. J. Foot Ankle Res. 2016, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P.; Bowen, C.; Ellis, R.; Rome, K.; Jackson, A.; Carroll, M. Ultrasound Imaging Acquisition Procedures for Evaluating the First Metatarsophalangeal Joint: A Scoping Review. Ultrasound Med. Biol. 2021, 48, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Moisan, G.; McBride, S.; Isabelle, P.; Chicoine, D.; Walha, R. Intrarater and interrater reliability of the first metatarsophalangeal joint dorsiflexion resistance test. Musculoskelet. Care 2022, 14, 1–6. Available online: https://pubmed.ncbi.nlm.nih.gov/35833706/ (accessed on 7 February 2023). [CrossRef]
- Leow, Y.; Kong, P.W.; Liu, Y.; Pan, J.W.; Fong, D.T.; Chan, C.C.; Heng, M.L. Test-retest reliability of a clinical foot assessment device for measuring first metatarsophalangeal joint quasi-stiffness. Foot 2020, 45, 101742. [Google Scholar] [CrossRef]
- Curran, S.; Jones, A. Intrarater and interrater reliability of first metatarsophalangeal joint dorsiflexion: Goniometry versus visual estimation. J. Foot Ankle Res. 2010, 3, P5. [Google Scholar] [CrossRef]
- Mens, M.; Bouman, C.; Dobbe, J.; Bus, S.; Nieuwdorp, M.; Maas, M.; Wellenberg, R.; Streekstra, G. Metatarsophalangeal and interphalangeal joint angle measurements on weight-bearing CT images. Foot Ankle Surg. 2023, 23, S1268-7731. Available online: https://pubmed.ncbi.nlm.nih.gov/36641368/ (accessed on 9 February 2023). [CrossRef]
- Desmyttere, G.; Hajizadeh, M.; Bleau, J.; Begon, M. Effect of Foot Orthosis Design on Lower Limb Joint Kinematics and Kinetics during Walking in Flexible pes Planovalgus: A Systematic Review and Meta-Analysis. In Clinical Biomechanics; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 59, pp. 117–129. Available online: https://pubmed.ncbi.nlm.nih.gov/30227277/ (accessed on 20 May 2021).
- Kirby, K.A. The medial heel skive technique. Improving pronation control in foot orthoses. J. Am. Podiatr. Med. Assoc. 1992, 82, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Calvo-Lobo, C.; Navarro-Flores, E.; Palomo-López, P.; Romero-Morales, C.; López-López, D. Reliability Study of Diagnostic Tests for Functional Hallux Limitus. Foot Ankle Int. 2020, 41, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Dananberg, H.J. The Kinetic Wedge. J. Am. Podiatr. Med. Assoc. 1988, 78, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.; Baumhauer, J. Hallux rigidus. EFORT Open Rev. 2017, 2, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Shereff, M.J.; Bejjani, F.J.; Kummer, F.J. Kinematics of the first metatarsophalangeal joint. J. Bone Jt. Surg. 1986, 68, 392–398. [Google Scholar] [CrossRef]
- Mann, R.A.; Coughlin, M.J.; Duvries, H.L. Hallux rigidus: A review of the literature and a method of treatment. Clin. Orthop. Relat. Res. 1979, 142, 57–63. [Google Scholar] [CrossRef]
- Redmond, A.C.; Crosbie, J.; Ouvrier, R.A. Development and validation of a novel rating system for scoring standing foot posture: The Foot Posture Index. Clin. Biomech. 2006, 21, 89–98. [Google Scholar] [CrossRef]
- Sarcevic, Z.Z.; Tepavcevic, A.P. Association between abductor hallucis abductory force and navicular drop index, a predictive correlational study. J. Pediatr. Orthop. B 2020, 30, 484–487. [Google Scholar] [CrossRef]
- Kelly-Martin, R.; Doughty, L.; Garkavi, M.; Wasserman, J.B. Reliability of modified adheremeter and digital pressure algometer in measuring normal abdominal tissue and C-section scars. J. Bodyw. Mov. Ther. 2018, 22, 972–979. [Google Scholar] [CrossRef]
- Portney, L.; Watkins, M. Foundations of Clinical Research: Applications to Practice. In Survey of Ophthalmology, 3rd ed.; Hall, P.P., Ed.; Prentice Hall Health: Upper Saddle River, NJ, USA, 2009; Volume 47, 598p. [Google Scholar]
- Park, C.H.; Chang, M.C. Forefoot disorders and conservative treatment. Yeungnam Univ. J. Med. 2019, 36, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kunnasegaran, R.; Thevendran, G. Hallux Rigidus Nonoperative Treatment and Orthotics. In Foot and Ankle Clinics; W.B. Saunders: Philadelphia, PA, USA, 2015; Volume 20, pp. 401–412. [Google Scholar]
- Rambarran, K.K. Reduce Relative Plantar Pressure—Search Results—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=Rambarran+KK%2C+reduce+relative+plantar+pressure (accessed on 10 February 2023).
- Kirby, K.A. Subtalar Joint Axis Location and Rotational Equilibrium Theory of Foot Function. J. Am. Podiatr. Med. Assoc. 2001, 91, 465–487. [Google Scholar] [CrossRef] [PubMed]
- van Gheluwe, B.; Dananberg, H.J.; Hagman, F.; Vanstaen, K. Effects of hallux limitus on plantar foot pressure and foot kinematics during walking. J. Am. Podiatr. Med. Assoc. 2006, 96, 428–436. [Google Scholar] [CrossRef]
- Horton, G.A.; Park, Y.-W.; Myerson, M.S. Role of Metatarsus Primus Elevatus in the Pathogenesis of Hallux Rigidus. Foot Ankle Int. 1999, 20, 777–780. [Google Scholar] [CrossRef]
- Fuller, E.A. Center of pressure and its theoretical relationship to foot pathology. J. Am. Podiatr. Med. Assoc. 1999, 89, 278–291. [Google Scholar] [CrossRef]
- Huerta, J.P.; Moreno, J.M.R.; Kirby, K.A. Static Response of Maximally Pronated and Nonmaximally Pronated Feet to Frontal Plane Wedging of Foot Orthoses. J. Am. Podiatr. Med. Assoc. 2009, 99, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.; Blanch, P.; Chapman, A.R.; McPoil, T.G.; Vicenzino, B. Foot orthoses and gait: A systematic review and meta-analysis of literature pertaining to potential mechanisms. Br. J. Sport. Med. 2009, 44, 1035–1046. [Google Scholar] [CrossRef]
- Nigg, B.M. The Role of Impact Forces and Foot Pronation: A New Paradigm. Clin. J. Sport Med. 2001, 11, 2–9. [Google Scholar] [CrossRef]
- Gatt, A.; Mifsud, T.; Chockalingam, N. Severity of pronation and classification of first metatarsophalangeal joint dorsiflexion increases the validity of the Hubscher Manoeuvre for the diagnosis of functional hallux limitus. Foot 2014, 24, 62–65. [Google Scholar] [CrossRef]
- Halstead, J.; Redmond, A.C. Weight-Bearing Passive Dorsiflexion of the Hallux in Standing Is Not Related to Hallux Dorsiflexion During Walking. J. Orthop. Sport. Phys. Ther. 2006, 36, 550–556. [Google Scholar] [CrossRef]
- Grebing, B.R.; Coughlin, M.J. The Effect of Ankle Position on the Exam for First Ray Mobility. Foot Ankle Int. 2004, 25, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Durrant, M.N.; Siepert, K.K. Role of soft tissue structures as an etiology of hallux limitus. J. Am. Podiatr. Med. Assoc. 1993, 83, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Maceira, E.; Monteagudo, M. Functional Hallux Rigidus and the Achilles-Calcaneus-Plantar System. Foot Ankle Clin. 2014, 19, 669–699. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Romero-Morales, C.; Gómez-Carrión, Á.; De-La-Cruz-Torres, B.; Zaragoza-García, I.; Anttila, P.; Kantola, M.; Ortuño-Soriano, I. Effects of Novel Inverted Rocker Orthoses for First Metatarsophalangeal Joint on Gastrocnemius Muscle Electromyographic Activity during Running: A Cross-Sectional Pilot Study. Sensors 2020, 20, 3205. [Google Scholar] [CrossRef]
- Munuera, P.V.; Domínguez, G.; Palomo, I.C.; Lafuente, G. Effects of rearfoot-controlling orthotic treatment on dorsiflexion of the hallux in feet with abnormal subtalar pronation: A preliminary report. J. Am. Podiatr. Med. Assoc. 2006, 96, 283–289. [Google Scholar] [CrossRef]
- Becerro de Bengoa Vallejo, R.; Gomez, R.S.; Losa Iglesias, M.E. Clinical improvement in functional hallux limitus using a cut-out orthosis. Prosthet. Orthot. Int. 2016, 40, 215–223. [Google Scholar] [CrossRef]
- Reina, M.; Lafuente, G.; Munuera, P.V. Effect of custom-made foot orthoses in female hallux valgus after one-year follow up. Prosthet. Orthot. Int. 2012, 37, 113–119. [Google Scholar] [CrossRef]
- Sung, P.S.; Zipple, J.T.; Andraka, J.M.; Danial, P. The kinetic and kinematic stability measures in healthy adult subjects with and without flat foot. Foot 2017, 30, 21–26. [Google Scholar] [CrossRef]

| Total Population n = 58 | CASES GROUP HR Participants n = 30 | CONTROL GROUP Healthy Participants n = 28 | ||
|---|---|---|---|---|
| Variable | Mean ± SD (95% CI) | Mean ± SD (95% CI) | Mean ± SD (95% CI) | p-Value |
| Age (years) | 40.62 ± 1.12 (40.98–40.33) | 42.53 ± 5.72 (44.57–40.48) | 38.57 ± 1.12 (38.98–38.15) | 0.9 |
| Weight (kg) | 67.44 ± 9.98 (70–64.87) | 66.6 ± 9.37 (69.95–63.24) | 68.35 ± 10.7 (72.31–64.38) | <0.001 |
| Height (cm) | 167.77 ± 10.01 (170.34–165.19) | 167.53 ± 7.72 (170.29–164.76) | 164 ± 12.14 (168.49–159.5) | <0.001 |
| Foot Size (Es) | 40.2 ± 1.9 (40.50–39.89) | 38.2 ± 2.10 (38.95–37.44) | 40.3 ± 0.35 (40.42–40.17) | <0.001 |
| BMI (kg/m2) | 21.48 ± 1.47 (21.85–21.1) | 20.2 ± 1.74 (20.82–19.57) | 22.95 ± 2.58 (23.90–21.99) | <0.001 |
| CASES GROUP n = 30 | CONTROL GROUP n = 28 | ||||
|---|---|---|---|---|---|
| Thickness ME Variable | Mean (kgf) ± SD (95% CI) | ICC 95% IC (Li-Ls) | Mean (kgf) ± SD (95% CI) | ICC 95% IC (Li-Ls) | p-Value |
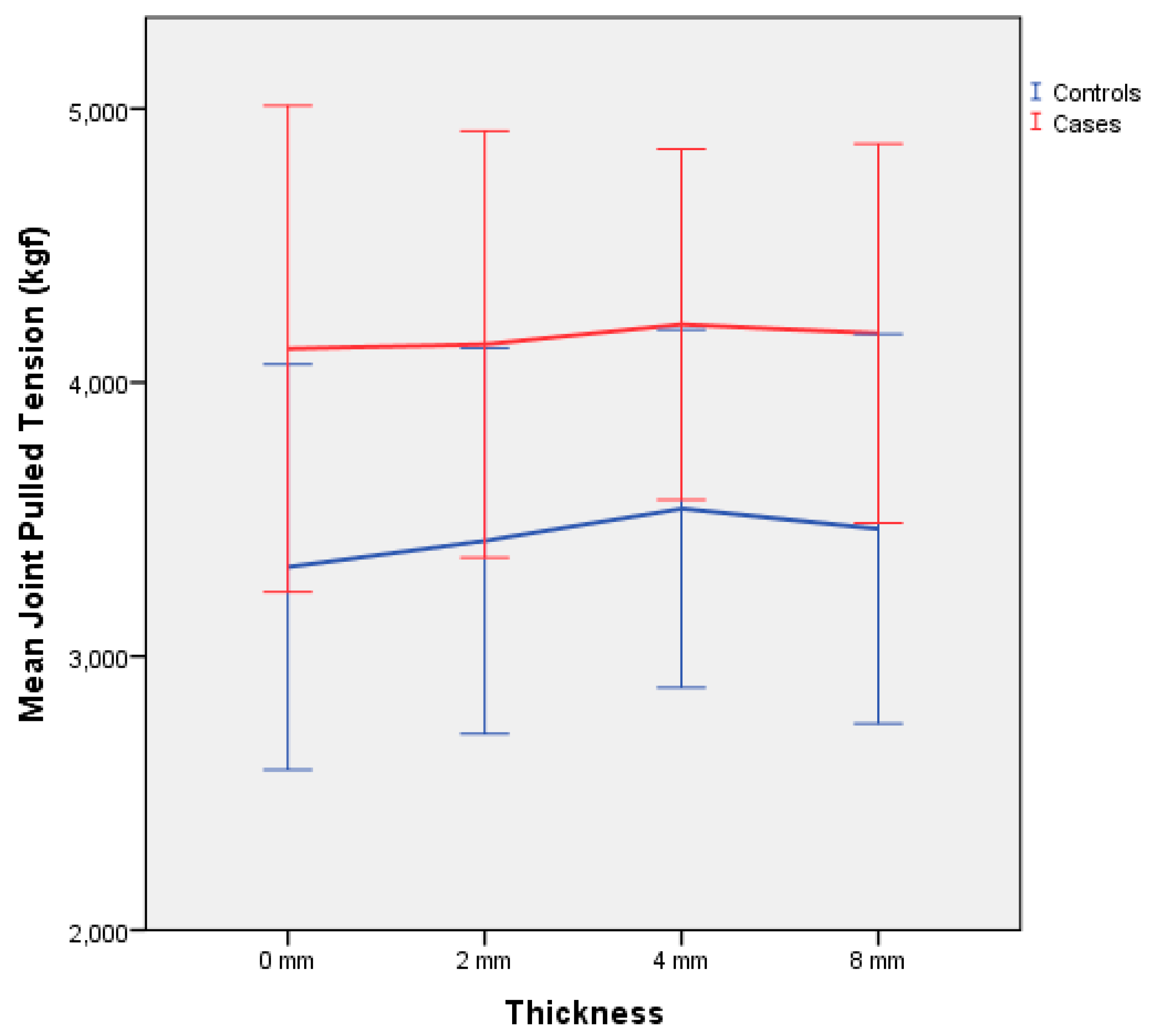
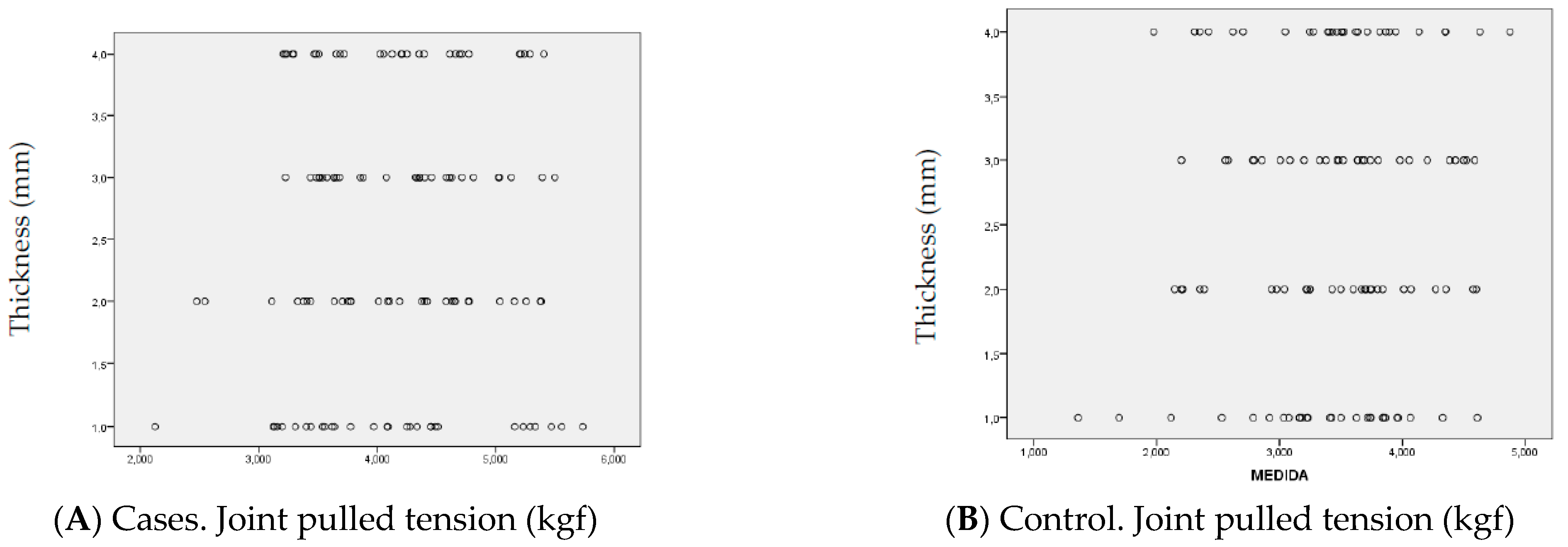
| I MPJ WRP | 4.122 ± 0.162 (3.79–4.45) | 0.989 (0.98–0.994) | 3.325 ± 0.139 (3.03–3.61) | 0.971 (0.948–0.98) | <0.001 |
| ME 2 mm | 4.139 ± 0.142 (3.84–4.43) | 0.97 (0.94–0.985) | 3.421 ± 0.133 (3.14–3.69) | 0.963 (0.928–0.982) | <0.001 |
| ME 4 mm | 4.211 ± 0.116 (3.97–4.45) | 0.969 (0.943–0.984) | 3.538 ± 0.123 (3.28–3.79) | 0.94 (0.88–0.97) | <0.001 |
| ME 8 mm | 4.179 ± 0.126 (3.92–4.43) | 0.972 (0.939–0.987) | 3.465 ± 0.134 (3.18–3.74) | 0.971 (0.94–0.986) | <0.001 |
| WRP vs. ME2 p-value | 1 | - | 0.956 | - | - |
| WRP vs. ME4 p-value | 0.969 | - | 0.669 | - | - |
| WRP vs. ME8 p-value | 0.992 | - | 0.879 | - | - |
| ME2 vs. ME4 p-value | 0.983 | - | 0.924 | - | - |
| ME2 vs. ME8 p-value | 0.997 | - | 0.996 | - | - |
| ME4 vs. ME8 p-value | 0.998 | - | 0.98 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gómez, R.; López-Alcorocho, J.M.; Núñez-Fernández, A.; González Fernández, M.L.; Martínez-Sebastián, C.; Ortuño-Soriano, I.; Zaragoza-García, I.; Gómez-Carrión, Á. Morton’s Extension on Hallux Rigidus Pathology. Prosthesis 2023, 5, 251-263. https://doi.org/10.3390/prosthesis5010019
Sánchez-Gómez R, López-Alcorocho JM, Núñez-Fernández A, González Fernández ML, Martínez-Sebastián C, Ortuño-Soriano I, Zaragoza-García I, Gómez-Carrión Á. Morton’s Extension on Hallux Rigidus Pathology. Prosthesis. 2023; 5(1):251-263. https://doi.org/10.3390/prosthesis5010019
Chicago/Turabian StyleSánchez-Gómez, Rubén, Juan Manuel López-Alcorocho, Almudena Núñez-Fernández, María Luz González Fernández, Carlos Martínez-Sebastián, Ismael Ortuño-Soriano, Ignacio Zaragoza-García, and Álvaro Gómez-Carrión. 2023. "Morton’s Extension on Hallux Rigidus Pathology" Prosthesis 5, no. 1: 251-263. https://doi.org/10.3390/prosthesis5010019
APA StyleSánchez-Gómez, R., López-Alcorocho, J. M., Núñez-Fernández, A., González Fernández, M. L., Martínez-Sebastián, C., Ortuño-Soriano, I., Zaragoza-García, I., & Gómez-Carrión, Á. (2023). Morton’s Extension on Hallux Rigidus Pathology. Prosthesis, 5(1), 251-263. https://doi.org/10.3390/prosthesis5010019







