Therapeutic Role of Nystatin Added to Tissue Conditioners for Treating Denture-Induced Stomatitis: A Systematic Review
Abstract
1. Introduction
2. Methodology
2.1. Focus Question
“What is the antifungal potential of “Nystatin” added to different TCs for the management of DIS?”
2.2. Literature Search
2.3. Eligibility Criteria
2.4. Data Extraction
- Publication year and authors details.
- Experimental group(s) reported by the study.
- TC used with nystatin.
- Main results of the study.
2.5. Quality Assessment
3. Results
3.1. Types of Study Designs
3.2. Types of TCs Used with Nystatin
3.3. Antifungal Potential of Nystatin When Added to TCs
3.4. Effect of Nystatin on the Properties of TCs
3.5. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shulman, J.; Rivera-Hidalgo, F.; Beach, M. Risk factors associated with denture stomatitis in the United States. J. Oral Pathol. Med. 2005, 34, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Pachava, K.R.; Nadendla, L.K.; Alluri, L.S.C.; Tahseen, H.; Sajja, N.P. Invitro antifungal evaluation of denture soft liner incorporated with tea tree oil: A new therapeutic approach towards denture stomatitis. J. Clin. Diagn. 2015, 9, ZC62–ZC64. [Google Scholar] [CrossRef] [PubMed]
- Kulak, Y.; Arikan, A. Aetiology of denture stomatitis. J. Marmara Univ. Dent. Fac. 1993, 1, 307–314. [Google Scholar]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; López-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Pereira-Cenci, T.; Del Bel Cury, A.A.; Crielaard, W.; Ten Cate, J.M. Development of Candida-associated denture stomatitis: New insights. J. Appl. Oral Sci. 2008, 16, 86–94. [Google Scholar] [CrossRef]
- Evren, B.A.; Uludamar, A.; Işeri, U.; Ozkan, Y.K. The association between socioeconomic status, oral hygiene practice, denture stomatitis and oral status in elderly people living different residential homes. Arch. Gerontol. Geriatr. 2011, 53, 252–257. [Google Scholar] [CrossRef]
- Shinozaki, S.; Moriyama, M.; Hayashida, J.N.; Tanaka, A.; Maehara, T.; Ieda, S.; Nakamura, S. Close association between oral Candida species and oral mucosal disorders in patients with xerostomia. Oral Dis. 2012, 18, 667–672. [Google Scholar] [CrossRef]
- Nadig, S.D.; Ashwathappa, D.T.; Manjunath, S.K.M.; Annaji, A.G.; Shivaprakash, P.K. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J. Oral Maxillofac. Pathol. 2017, 21, 316. [Google Scholar] [CrossRef]
- Emami, E.; De Grandmont, P.; Rompré, P.; Barbeau, J.; Pan, S.; Feine, J. Favoring trauma as an etiological factor in denture stomatitis. J. Dent. Res. 2008, 87, 440–444. [Google Scholar] [CrossRef]
- Lombardi, T.; Budtz-Jørgensen, E. Treatment of denture-induced stomatitis: A review. Eur. J. Prosthodont. Restor. Dent. 1993, 2, 17–22. [Google Scholar] [PubMed]
- Martins, K.V.; de Lacerda Gontijo, S.M. Treatment of denture stomatitis: Literature review. Revistas 2017, 74, 215–220. [Google Scholar] [CrossRef]
- Aoun, G.; Berberi, A. Prevalence of Chronic erythematous candidiasis in Lebanese denture wearers: A clinico-microbiological study. Mater. Sociomed. 2017, 29, 26. [Google Scholar] [CrossRef] [PubMed]
- Moosazadeh, M.; Akbari, M.; Tabrizi, R.; Ghorbani, A.; Golkari, A.; Banakar, M.; Sekhavati, E.; Kavari, S.H.; Lankarani, K.B. Denture stomatitis and Candida albicans in Iranian population: A systematic review and meta-analysis. J. Dent. 2016, 17, 283. [Google Scholar]
- Byrd, W.C.; Schwartz-Baxter, S.; Carlson, J.; Barros, S.; Offenbacher, S.; Bencharit, S. Role of salivary and candidal proteins in denture stomatitis: An exploratory proteomic analysis. Mol. Biosyst. 2014, 10, 2299–2304. [Google Scholar] [CrossRef]
- Darwazeh, A.M.-G.; Al-Refai, S.; Al-Mojaiwel, S. Isolation of Candida species from the oral cavity and fingertips of complete denture wearers. J. Prosthet. Dent. 2001, 86, 420–423. [Google Scholar] [CrossRef]
- Barbeau, J.; Séguin, J.; Goulet, J.P.; de Koninck, L.; Avon, S.L.; Lalonde, B.; Rompré, P.; Deslauriers, N. Reassessing the presence of Candida albicans in denture-related stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 51–59. [Google Scholar] [CrossRef]
- Silva, M.M.; de Oliveira Mima, E.G.; Colombo, A.L.; Sanitá, P.V.; Jorge, J.H.; Massucato, E.M.S.; Vergani, C.E. Comparison of denture microwave disinfection and conventional antifungal therapy in the treatment of denture stomatitis: A randomized clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 469–479. [Google Scholar] [CrossRef]
- Mima, E.; Vergani, C.E.; Machado, A.L.; Massucato, E.M.S.; Colombo, A.L.; Bagnato, V.S.; Pavarina, A.C. Comparison of Photodynamic Therapy versus conventional antifungal therapy for the treatment of denture stomatitis: A randomized clinical trial. Clin. Microbiol. Infect. 2012, 18, E380–E388. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Takamiya, A.S.; Feresin, L.P.; Gorup, L.F.; de Camargo, E.R.; Delbem, A.C.B.; Henriques, M.; Barbosa, D.B. Susceptibility of Candida albicans and Candida glabrata biofilms to silver nanoparticles in intermediate and mature development phases. J. Prosthodont. Res. 2015, 59, 42–48. [Google Scholar] [CrossRef]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martínez, F.; Aldape-Barrios, B.; Quindós, G.; Sánchez-Vargas, L.O. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral Patol. Oral Cir. Bucal 2005, 10, E27–E39. [Google Scholar] [PubMed]
- Hoshi, N.; Mori, H.; Taguchi, H.; Taniguchi, M.; Aoki, H.; Sawada, T.; Kawabata, M.; Kuwabara, A.; Oono, A.; Tanaka, K. Management of oral candidiasis in denture wearers. J. Prosthodont. Res. 2011, 55, 48–52. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2016, 24, 515–524. [Google Scholar] [CrossRef]
- Emami, E.; Kabawat, M.; Rompre, P.H.; Feine, J.S. Linking evidence to treatment for denture stomatitis: A meta-analysis of randomized controlled trials. J. Dent. 2014, 42, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Yarborough, A.; Cooper, L.; Duqum, I.; Mendonça, G.; McGraw, K.; Stoner, L. Evidence regarding the treatment of denture stomatitis. J. Prosthodont. 2016, 25, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Al-Shayyab, M.H.; Abu-Hammad, O.A.; Al-Omiri, M.K.; Dar-Odeh, N.S. Antifungal prescribing pattern and attitude towards the treatment of oral candidiasis among dentists in Jordan. Int. Dent. J. 2015, 65, 216–226. [Google Scholar] [CrossRef]
- Lyu, X.; Zhao, C.; Yan, Z.-M.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Des. Devel. Ther. 2016, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Martin-Mazuelos, E.; Aller, A.; Romero, M.; Armijo, A.R.; Gutierrez, M.; Bernal, S.; Montero, O. Response to fluconazole and itraconazole of Candida spp. in denture stomatitis. Mycoses 1997, 40, 283–289. [Google Scholar] [CrossRef]
- Falah-Tafti, A.; Jafari, A.A.; Lotfi-Kamran, M.H.; Fallahzadeh, H.; Hayan, R.S. A comparison of the efficacy of nystatin and fluconazole incorporated into tissue conditioner on the in vitro attachment and colonization of Candida albicans. Dent. Res. J. 2010, 7, 18–22. [Google Scholar]
- Alcântara, C.S.; de Macêdo, A.F.; Gurgel, B.C.; Jorge, J.H.; Neppelenbroek, K.H.; Urban, V.M. Peel bond strength of resilient liner modified by the addition of antimicrobial agents to denture base acrylic resin. J. Appl. Oral Sci. 2012, 20, 607–612. [Google Scholar] [CrossRef][Green Version]
- Hashem, M.I. Advances in soft denture liners: An update. J. Contemp. Dent. Pract. 2015, 16, 314–318. [Google Scholar] [PubMed]
- Sahebjamee, M.; Shabestari, S.B.; Asadi, G.; Neishabouri, K. Predisposing factors associated with denture induced stomatitis in complete denture wearers. J. Dent. 2019, 11, 35–39. [Google Scholar]
- Leite, D.P.; Piva, M.R.; Martins-Filho, P.R.S. Identification of Candida species in patients with denture stomatitis and evaluation of susceptibility to miconazole and photodynamic therapy. Rev. Odontol. UNESP 2015, 44, 12–17. [Google Scholar] [CrossRef]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777. [Google Scholar]
- Yamamoto, D.; Shinohara, Y.; Nagadome, H.; Terada, Y. Development of tissue conditioner capable of binding with anti-microbial protein lactoferrin. J. Prosthodont. Res. 2009, 53, 136–141. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; Ferguson, M.M. Delivery of antifungal agents to the oral cavity. Adv. Drug Deliv. Rev. 1994, 13, 161–179. [Google Scholar] [CrossRef]
- Akiba, N.; Hayakawa, I.; Keh, E.-S.; Watanabe, A. Antifungal effects of a tissue conditioner coating agent with TiO2 photocatalyst. J. Med. Dent. Sci. 2005, 52, 223–227. [Google Scholar]
- Kumar, N.; Kumari, A.; Priyadarshi, V.; Kumar, A.; Prasad, R.S.; Kumar, B. A comparative efficacy of Nystatin and Fluconazole incorporated into tissue conditioner as drug delivery method for Denture stomatitis. J. Adv. Med. Dent. Sci. Res. 2020, 8, 159–162. [Google Scholar]
- Webb, B.; Thomas, C.; Willcox, M.; Harty, D.; Knox, K. Candida-associated denture stomatitis. Aetiology and management: A review: Part1. Factors influencing distribution of candida species in the oral cavity. Aust. Dent. J. 1998, 43, 45–50. [Google Scholar] [CrossRef]
- Chow, C.; Matear, D.; Lawrence, H. Efficacy of antifungal agents in tissue conditioners in treating candidiasis. Gerodontology 1999, 16, 110–118. [Google Scholar] [CrossRef]
- Geerts, G.; Stuhlinger, M.; Basson, N. Effect of an antifungal denture liner on the saliva yeast count in patients with denture stomatitis: A pilot study. J. Oral Rehabil. 2008, 35, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Julian, J.; Sunil, S.; Baby, G.G. Efficacy of tissue conditioner acting as effective fungicidal drug delivery system-An invitro study. Oral Maxillofac. Pathol. J. 2010, 1, 10–15. [Google Scholar]
- Chopde, N.; Pharande, A.; Khade, M.N.; Khadtare, Y.R.; Shah, S.S.; Apratim, A. In vitro antifungal activity of two tissue conditioners combined with nystatin, miconazole and fluconazole against Candida albicans. J. Contemp. Dent. Pract. 2012, 13, 695–698. [Google Scholar] [PubMed]
- Fallah-Tafti, A.; Jafari, A.; Mirzaeiipoorm, L.; Ashoori, H. Stability and duration of antifungal effects of nystatin and fluconazole mixed with a tissue conditioner on colonization of Candida albicans (in vitro). Res. Dent. Sci. 2014, 11, 21–26. [Google Scholar]
- Bueno, M.; Urban, V.; Barbério, G.; Da Silva, W.; Porto, V.; Pinto, L.; Neppelenbroek, K. Effect of antimicrobial agents incorporated into resilient denture relines on the Candida albicans biofilm. Oral Dis. 2015, 21, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Barua, D.R.; Basavanna, J.M.; Varghese, R.K. Efficacy of neem extract and three antimicrobial agents incorporated into tissue conditioner in inhibiting the growth of C. albicans and S. mutans. J. Clin. Diagn. 2017, 11, ZC97–ZC101. [Google Scholar] [CrossRef]
- Hotta, J.; Garlet, G.P.; Cestari, T.M.; Lima, J.F.M.; Porto, V.C.; Urban, V.M.; Neppelenbroek, K.H. In vivo biocompatibility of an interim denture resilient liner containing antifungal drugs. J. Prosthet. Dent. 2019, 121, 135–142. [Google Scholar] [CrossRef]
- Radnai, M.; Whiley, R.; Friel, T.; Wright, P.S. Effect of antifungal gels incorporated into a tissue conditioning material on the growth of Candida albicans. Gerodontology 2010, 27, 292–296. [Google Scholar] [CrossRef]
- de Souza, R.F.; Khiyani, M.F.; Chaves, C.A.; Feine, J.; Barbeau, J.; Fuentes, R.; Borie, E.; Crizostomo, L.C.; Silva-Lovato, C.H.; Rompre, P. Improving practice guidelines for the treatment of denture-related erythematous stomatitis: A study protocol for a randomized controlled trial. Trials 2017, 18, 211. [Google Scholar] [CrossRef]
- Bakhshi, M.; Taheri, J.B.; Shabestari, S.B.; Tanik, A.; Pahlevan, R. Comparison of therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatitis. Gerodontology 2012, 29, e680–e684. [Google Scholar] [CrossRef]
- Ibraheem, E.M.A.; Dehis, W.M. Effect of tissue conditioner combined with nystatin on growth of candida albicans in complete denture wearers. Egypt. Dent. J. 2016, 62, 393–398. [Google Scholar] [CrossRef]
- Schneid, T.R. An in vitro analysis of a sustained release system for the treatment of denture stomatitis. Spec. Care Dentist. 1992, 12, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Urban, V.M.; De Souza, R.F.; Arrais, C.A.G.; Borsato, K.T.; Vaz, L.G. Effect of the association of nystatin with a tissue conditioner on its ultimate tensile strength. J. Prosthodont. 2006, 15, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Urban, V.M.; Seó, R.S.; Giannini, M.; Arrais, C.A.G. Superficial distribution and identification of antifungal/antimicrobial agents on a modified tissue conditioner by SEM-EDS microanalysis: A preliminary study. J. Prosthodont. 2009, 18, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Urban, V.M.; Lima, T.F.; Bueno, M.G.; Giannini, M.; Filho, J.N.A.; de Almeida, A.L.P.; Neppelenbroek, K.H. Effect of the addition of antimicrobial agents on Shore A hardness and roughness of soft lining materials. J. Prosthodont. 2015, 24, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.F.; Maciel, J.G.; Arrais, C.A.; Porto, V.C.; Urban, V.M.; Neppelenbroek, K.H. Effect of incorporating antifungals on the water sorption and solubility of interim resilient liners for denture base relining. J. Prosthet. Dent. 2016, 115, 611–616. [Google Scholar] [CrossRef]
- Lima, J.F.M.; Maciel, J.G.; Hotta, J.; Vizoto, A.C.P.; Honório, H.M.; Urban, V.M.; Neppelenbroek, K.H. Porosity of temporary denture soft liners containing antifungal agents. J. Appl. Oral Sci. 2016, 24, 453–461. [Google Scholar] [CrossRef]
- Homsiang, W.; Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Effect of zinc oxide nanoparticles incorporated into tissue conditioner on antifungal, physical, and mechanical properties. Dent. Mater. J. 2020, 2020–2095. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. MetaArXiv 2020. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Br. Med. J. 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Douglas, W.H.; Clarke, D. Physical and mechanical properties of nystatin-containing denture liners. J. Prosthet. Dent. 1975, 34, 428–434. [Google Scholar] [CrossRef]
- Barkvoll, P.; Attramadal, A. Effect of nystatin and chlorhexidine digluconate on Candida albicans. Oral Surg. Oral Med. Oral Pathol. 1989, 67, 279–281. [Google Scholar] [CrossRef]
- El-Charkawi, H.; El-Said, E.; Safouh, H.; El-Raghi, N. Effect of addition antimicrobial agents to denture reliners. Egypt. Dent. J. 1994, 40, 785–790. [Google Scholar]
- Truhlar, M.R.; Shay, K.; Sohnle, P. Use of a new assay technique for quantification of antifungal activity of nystatin incorporated in denture liners. J. Prosthet. Dent. 1994, 71, 517–524. [Google Scholar] [CrossRef]
- Blomgren, J.; Berggren, U.; Jontell, M. Fluconazole versus nystatin in the treatment of oral candidosis. Acta Odontol. Scand. 1998, 56, 202–205. [Google Scholar] [CrossRef]
- Ellepola, A.N.; Samaranayake, L. Adhesion of oral Candida albicans isolates to denture acrylic following limited exposure to antifungal agents. Arch. Oral Biol. 1998, 43, 999–1007. [Google Scholar] [CrossRef]
- AL-Dwairi, Z.N.; AL-Quran, F.A.; AL-Omari, O.Y. The effect of antifungal agents on surface properties of poly (methyl methacrylate) and its relation to adherence of Candida albicans. J. Prosthodont. Res. 2012, 56, 272–280. [Google Scholar] [CrossRef]
- Sanchez-Aliaga, A.; Pellissari, C.V.G.; Arrais, C.A.G.; Michel, M.D.; Neppelenbroek, K.H.; Urban, V.M. Peel bond strength of soft lining materials with antifungal to a denture base acrylic resin. Dent. Mater. J. 2016, 35, 194–203. [Google Scholar] [CrossRef]
- Atai, Z.; Atai, M.; Amini, J. In vivo study of antifungal effects of low-molecular-weight chitosan against Candida albicans. J. Oral Sci. 2017, 59, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.G.; de Sousa, E.J.B.; Hotta, J.; Porto, V.C.; Urban, V.M.; Neppelenbroek, K.H. Surface properties of temporary soft liners modified by minimum inhibitory concentrations of antifungals. Braz. Dent. J. 2017, 28, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Garaicoa, J.L.; Fischer, C.L.; Bates, A.M.; Holloway, J.; Avila-Ortiz, G.; Guthmiller, J.M.; Johnson, G.K.; Stanford, C.; Brogden, K.A. Promise of combining antifungal agents in denture adhesives to fight Candida species infections. J. Prosthodont. 2018, 27, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Neppelenbroek, K.H.; Lima, J.F.M.; Hotta, J.; Galitesi, L.L.; Almeida, A.L.P.F.; Urban, V.M. Effect of incorporation of antifungal agents on the ultimate tensile strength of temporary soft denture liners. J. Prosthodont. 2018, 27, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.C.; Boriollo, M.F. Amphotericin B, fluconazole, and nystatin as development inhibitors of Candida albicans biofilms on a dental prosthesis reline material: Analytical models in vitro. J. Prosthet. Dent. 2020. [Google Scholar] [CrossRef]
- Thomas, C.; Nutt, G. The in vitro fungicidal properties of Visco-gel, alone and combined with nystatin and amphotericin B. J. Oral Rehabil. 1978, 5, 167–172. [Google Scholar] [CrossRef]
- Kim, H.J.; Son, J.S.; Kwon, T.Y. Antifungal Effect of a Dental Tissue Conditioner Containing Nystatin-Loaded Alginate Microparticles. J. Nanosci. Nanotechnol. 2018, 18, 848–852. [Google Scholar] [CrossRef]
- Douglas, W.H.; Walker, D.M. Nystatin in denture liners--an alternative treatment of denture stomatitis. Br. Dent. J. 1973, 135, 55–59. [Google Scholar] [CrossRef]
- Bergendal, T.; Isacsson, G. Effect of nystatin in the treatment of denture stomatitis. Scand. J. Dent. Res. 1980, 88, 446–454. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alsahhaf, A.; Alofi, R.S.; Al-Aali, K.A.; Abduljabbar, T.; Vohra, F. Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: A randomized clinical study. Photodiagn. Photodyn. Ther. 2019, 28, 98–101. [Google Scholar] [CrossRef]
- Afroozi, B.; Zomorodian, K.; Lavaee, F.; Shahrabadi, Z.Z.; Mardani, M. Comparison of the efficacy of indocyanine green-mediated photodynamic therapy and nystatin therapy in treatment of denture stomatitis. Photodiagn. Photodyn. Ther. 2019, 27, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Gonoudi, E.; Rezai, M.; Farrokhnia, T.; Goudarzi, M.; Sima, A. Comparison of Antifungal Efficacy of Zataria multiflora and Nystatin for Treatment of Denture Stomatitis: A Randomized Clinical Trial. J. Dent. 2020. [Google Scholar] [CrossRef]
- Omran, S.M.; Dastjerdi, M.R.; Zuashkiani, M.; Moqarabzadeh, V.; Taghizadeh-Armaki, M. In vitro antifungal susceptibility of candida species isolated from Iranian patients with denture stomatitis. BioMed Res. Int. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nairn, R. Nystatin and amphotericin B in the treatment of denture-related candidiasis. Oral Surg. Oral Med. Oral Pathol. 1975, 40, 68–75. [Google Scholar] [CrossRef]
- Bergman, B.; Carlsson, G.E.; Ericson, S. Effect of differences in habitual use of complete dentures on underlying tissues. Eur. J. Oral Sci. 1971, 79, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Jørgenskn, E.; Bertram, U. Denture stomatitis: II. The effect of antifungal and prosthetic treatment. Acta Odontol. Scand. 1970, 28, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Pindborg, J.J. Atlas of Diseases of the Oral Mucosa, 2nd ed.; Munksgaard: Copenhagen, Denmark, 1980. [Google Scholar]
- Woods, R.A. Nystatin-resistant mutants of yeast: Alterations in sterol content. J. Bacteriol. 1971, 108, 69–73. [Google Scholar] [CrossRef]
- Bard, M. Biochemical and genetic aspects of nystatin resistance in Saccharomyces cerevisiae. J. Bacteriol. 1972, 111, 649–657. [Google Scholar] [CrossRef]
- Mohamadi, J.; Motaghi, M. Anti-fungal resistance in candida isolated from oral and diaper rash candidiasis in neonates. Bioinformation 2014, 10, 667. [Google Scholar] [CrossRef]
- Iqbal, Z.; Zafar, M. Role of antifungal medicaments added to tissue conditioners: A systematic review. J. Prosthodont. Res. 2016, 60, 231–239. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in nanotechnology for restorative dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef] [PubMed]
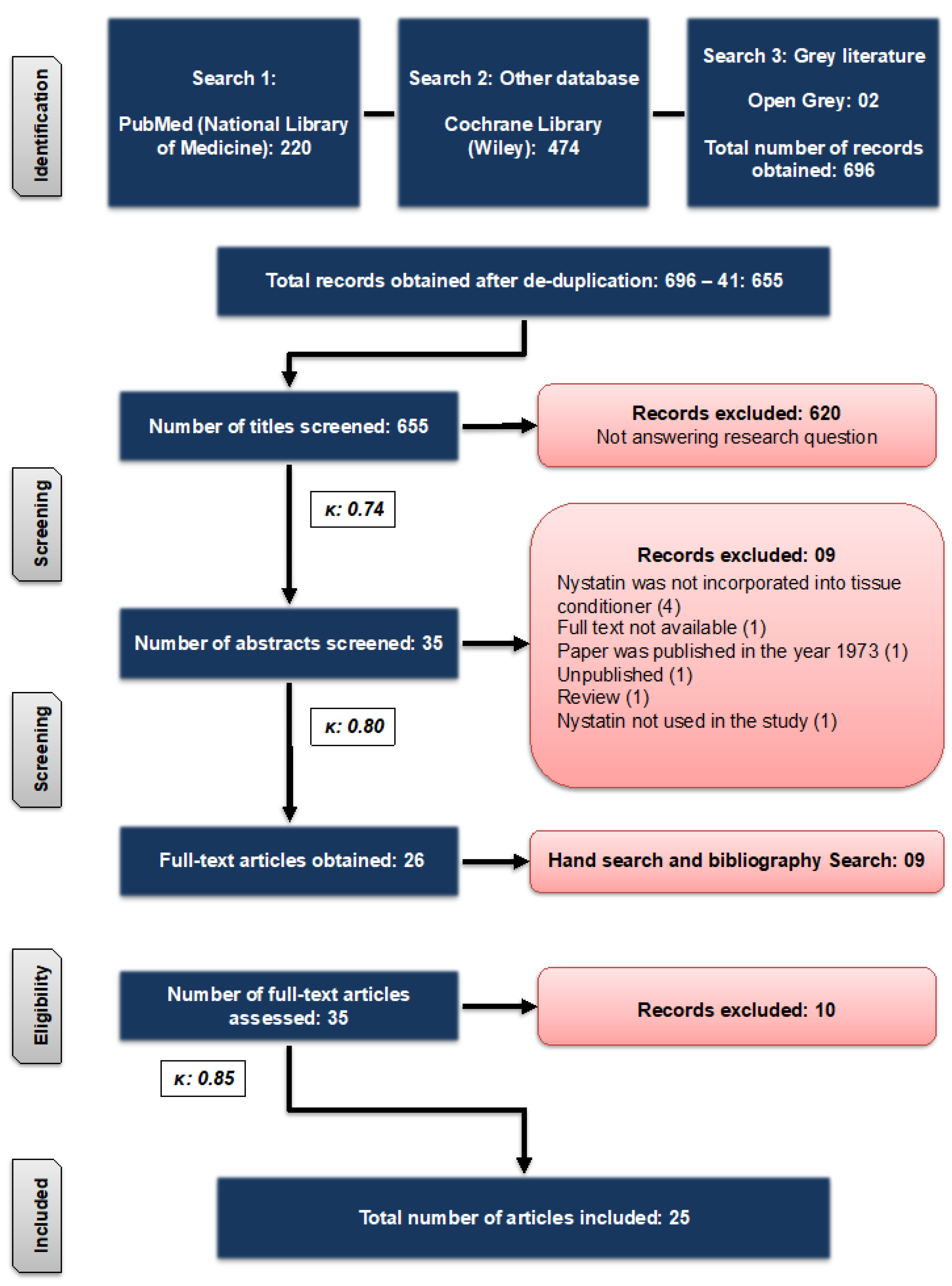
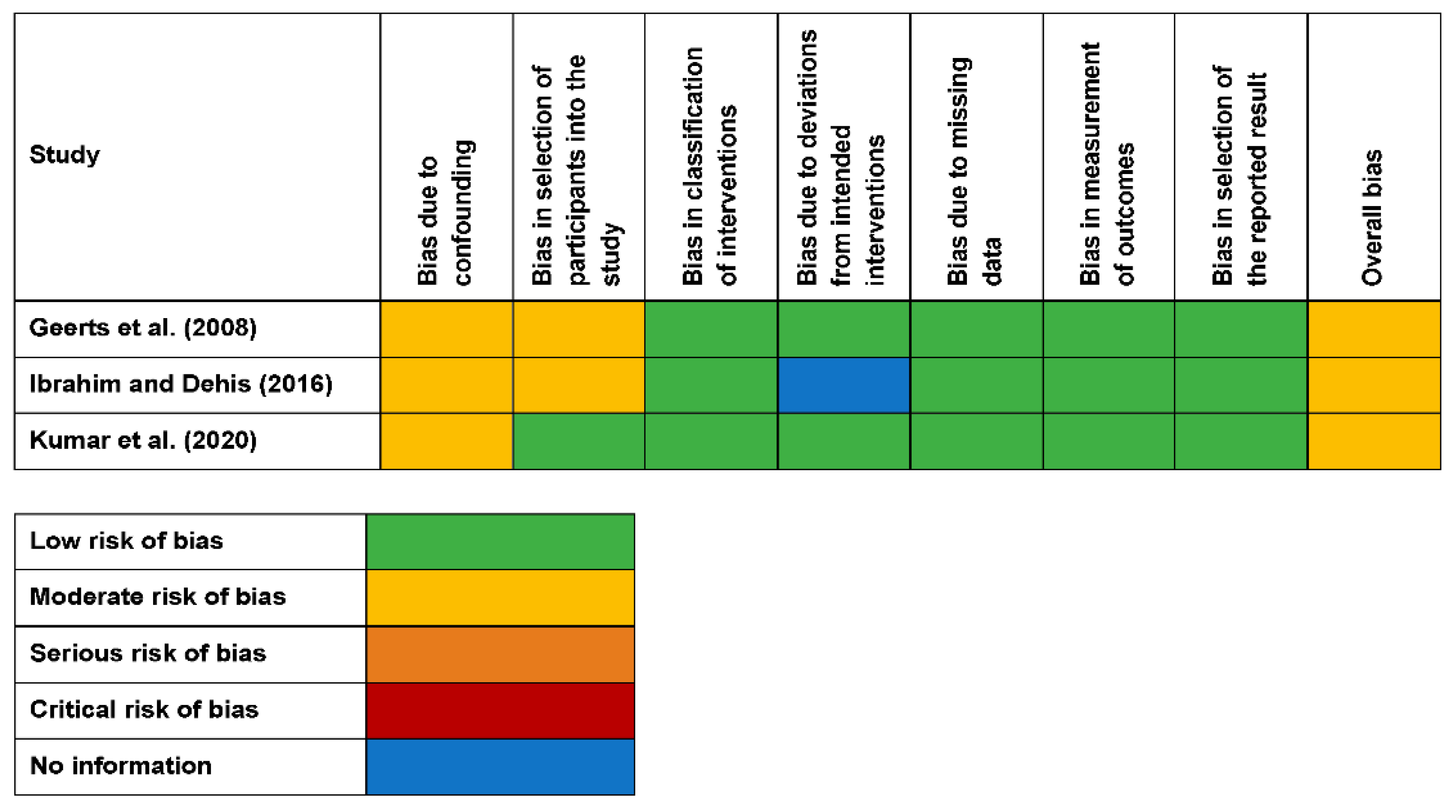
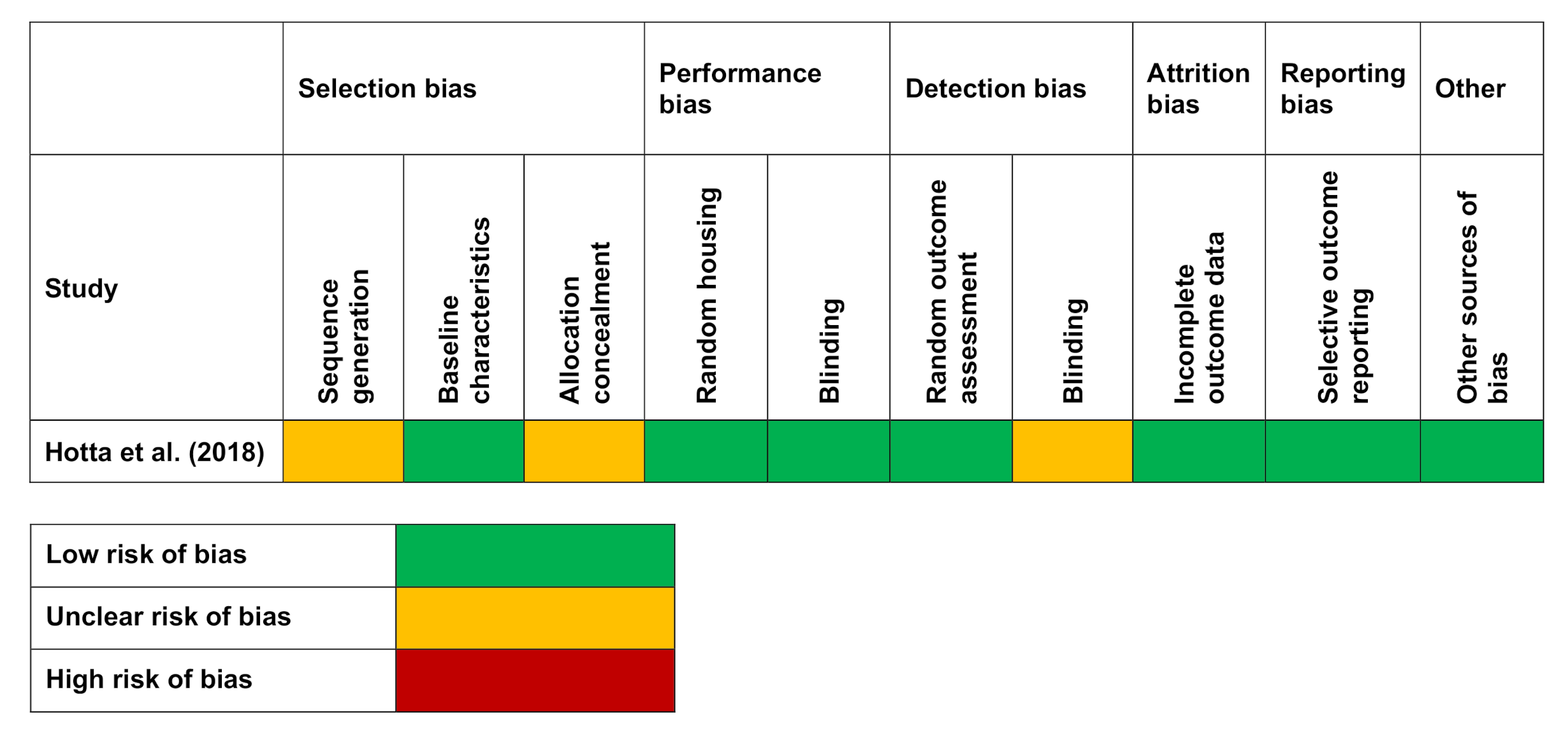
| Reason for Exclusion | References |
|---|---|
| Nystatin was not incorporated into tissue conditioner | [30,64,68,69,73] |
| Nystatin was used as a mouth rinse | [67,68,69,70,71] |
| Full text not available | [65] |
| Nystatin not used in the study | [48] |
| Nystatin–alginate composite was used | [77] |
| Study | Experimental Group(s) | TC and Nystatin | Key Outcomes |
|---|---|---|---|
| In-vitro studies | |||
| Douglas and Clarke (1975) [63] | Nystatin + Coe comfort Control | Coe comfort | Improved physical and mechanical properties of the denture liners by the addition of nystatin. |
| Thomas and Nutt (1978) [76] | Nystatin + Viscogel Amphotericin B + Viscogel Control | Viscogel | Nystatin demonstrated more effective outcomes in comparison to amphotericin B and control. |
| Schneid (1992) [52] | Nystatin + Lynal C/Temporary Reliner Chlorhexidine + Lynal TC/Temporary Reliner Clotrimazole + Lynal TC/Temporary Reliner Fluconazole + Lynal TC/Temporary Reliner Control | Lynal TC Temporary Reliner | Nystatin exhibited the growth inhibition of C. albicans and release from the TC matrix, which was either total, dose-related, or related to the incubation period prior to the inoculation. |
| Truhlar et al. (1994) [66] | Nystatin + Lynal/Viscogel Control | Lynal Viscogel | Incorporating a dose of nystatin into the reline material permitted release of the agent over 2 weeks’ time, warranting a high long-term dosage. Insignificant antifungal activity was showed by the control group. |
| Chow et al. (1999) [40] | Nystatin + Coe soft/Viscogel/Fitt Fluconazole + Core soft/Viscogel/Fitt Itraconazole + Coe soft/Viscogel/Fitt Control | Coe Soft Viscogel Fitt | Nystatin was found to be effectual as compared to the control group; however, in comparison with the other antifungals, it was the least efficient. |
| Urban et al. (2006) [53] | Nystatin + Dure conditioner Control | Dura conditioner | At greater concentrations and after 24 h, the tensile strength of the TC was considerably improved by the addition of nystatin. |
| Urban et al. (2009) [54] | Nystatin + Softone Chlorhexidine diacetate + Softone Ketoconazole + Softone Miconazole + Softone Control | Softone | Nystatin specimens exhibited particles with irregular shapes and sizes consistently distributed. The modified TC demonstrated differences in the particle distribution and nystatin size added to the plasticised matrix. |
| Falah-Tafti et al. (2010) [29] | Nystatin + Acrosoft Ketoconazole + Acrosoft Control | Acrosoft | TC added with nystatin can totally impede the adhesion, as well as colonisation, of C. albicans. |
| Julian et al. (2010) [42] | Nystatin + Viscogel Ketoconazole + Viscogel Clotrimazole + Viscogel | Viscogel | The TC can be used as an effective delivery system for nystatin. It also revealed the acrylic matrix efficiency for dispersion of the drug into the surrounding medium at constant therapeutic levels. |
| Chopde et al. (2012) [43] | Nystatin + Viscogel/GC soft Miconazole + Viscogel/GC soft Fluconazole + Viscogel/GC soft | Viscogel GC Soft | Nystatin displayed an improved inhibition of C. albicans compared to the control group, but in comparison with the other antifungals, it was the least efficient. |
| Fallah-Tafti et al. (2014) [44] | Nystatin + GC soft Fluconazole + GC soft | GC Soft | Nystatin showed greater inhibitory effects than fluconazole; nevertheless, the stability of nystatin added to TC was not adequate, and the antifungal effects were comprehended only for 3 days. |
| Urban et al. (2015) [55] | Nystatin + Trusoft/Softone Miconazole + Trusoft/Softone Ketoconazole + Trusoft/Softone Itraconazole + Trusoft/Softone Chlorhexidine diacetate + Trusoft/Softone Control | Trusoft Softone | Nystatin showed an increase in the hardness of soft materials with time. |
| Bueno et al. (2015) [45] | Nystatin + Trusoft/Softone Miconazole + Trusoft/Softone Ketoconazole + Trusoft/Softone Itraconazole + Trusoft/Softone Chlorhexidine diacetate+ Trusoft/Softone; Control | Trusoft Softone | Nystatin incorporation into the resilient materials hindered fungal growth during 2 weeks; however, with a lower MIC in comparison to other antifungals. |
| Lima et al. (2016a) [56] | Nystatin +Trusoft/Softone Chlorhexidine diacetate + Trusoft/Softone Ketoconazole + Trusoft/Softone Control | Trusoft Softone | Nystatin addition at the MIC in TC did not affect water sorption the following 2 weeks. The solubility of the two interim materials was unaltered by the addition of nystatin for up to 2 weeks. |
| Lima et al. (2016b) [57] | Nystatin + Trusoft/Softone Chlorhexidine diacetate + Trusoft/Softone Ketoconazole + Trusoft/Softone Control | Trusoft Softone | Nystatin addition in Trusoft at the MIC caused no harmful effects of the porosity at different periods of water immersion at 2 weeks. Following 2 weeks, no detrimental effect was noted for the TC porosity modified by nystatin at the MIC. |
| Sánchez-Aliaga et al. (2016) [70] | Nystatin + Trusoft/Softone Miconazole + Trusoft/Softone Ketoconazole + Trusoft/Softone Itraconazole + Trusoft/Softone Chlorhexidine diacetate+ Trusoft/Softone; Control | Trusoft Softone | Nystatin incorporation did not result in values below those recommended for the peel bond strength after 1 and 2 weeks of evaluation. |
| Barua et al. (2017) [46] | Nystatin + Viscogel Neem leaf extract + Viscogel Ketoconazole + Viscogel Chlorhexidine diacetate + Viscogel Control group | Viscogel | Nystatin was favourably efficient in inhibiting the growth of C. albicans. |
| Bueno et al. (2017) [72] | Nystatin + Trusoft/Softone Miconazole + Trusoft/Softone Ketoconazole + Trusoft/Softone Itraconazole + Trusoft/Softone Chlorhexidine diacetate + Trusoft/Softone Control | Trusoft Softone | Nystatin MIC in both the TC determined no unfavourable effects for roughness up to 2 weeks. |
| Neppelenbroek et al. (2018) [74] | Nystatin + Trusoft/Softone Miconazole + Trusoft/Softone Ketoconazole + Trusoft/Softone Itraconazole + Trusoft/Softone Chlorhexidine diacetate + Trusoft/Softone Control | Trusoft Softone | The MIC of nystatin for C. albicans caused no harmful effects on the tensile strength and elongation percentage of the temporary soft denture liners up to 2 weeks. |
| Homsiang et al. (2020) [58] | Nystatin + GC soft ZnOnps + GC soft Control | GC Soft | Nystatin provided a suitable antifungal effect up to 2 weeks, with no adverse effects on the TC penetration depth and tensile bond strength. |
| Bassi et al. (2020) [75] | Four preconditioning systems: Foetal bovine serum, artificial saliva, artificial saliva + foetal bovine serum and phosphate-buffered saline Nystatin + Silagum-Comfort Fluconazole + Silagum-Comfort Amphotericin B + Silagum-Comfort | Silagum-Comfort | Nystatin showed more effective results in reducing C. albicans biofilm metabolic activity dependent on time and its concentrations. |
| Clinical studies | |||
| Geerts et al. (2008) [41] | Nystatin + Viscogel | Viscogel | Nystatin containing short-term denture liner considerably reduced the salivary yeast count of individuals with DIS. |
| Ibraheem and Dehis (2016) [51] | Nystatin + Viscogel Control | Viscogel | Nystatin mixed with TC demonstrated no noticeable effect on C. albicans inhibition. |
| Kumar et al. (2020) [38] | Nystatin + TC Fluconazole+ TC | NR | Nystatin was effective against C. albicans and can be an effective treatment compared to fluconazole in DIS. |
| Animal study | |||
| Hotta et al. (2019) [47] | Nystatin + Trusoft Chlorhexidine diacetate + Trusoft Ketoconazole + Trusoft Control | Trusoft | Incorporation of nystatin MIC to TC did not prompt histopathological variations in the rat palatal mucosa, signifying the in vivo biocompatibility of the DIS treatment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, M.S.; Alnazzawi, A.; Habib, S.R.; Lone, M.A.; Zafar, M.S. Therapeutic Role of Nystatin Added to Tissue Conditioners for Treating Denture-Induced Stomatitis: A Systematic Review. Prosthesis 2021, 3, 61-74. https://doi.org/10.3390/prosthesis3010007
Shaikh MS, Alnazzawi A, Habib SR, Lone MA, Zafar MS. Therapeutic Role of Nystatin Added to Tissue Conditioners for Treating Denture-Induced Stomatitis: A Systematic Review. Prosthesis. 2021; 3(1):61-74. https://doi.org/10.3390/prosthesis3010007
Chicago/Turabian StyleShaikh, Muhammad Saad, Ahmad Alnazzawi, Syed Rashid Habib, Mohid Abrar Lone, and Muhammad Sohail Zafar. 2021. "Therapeutic Role of Nystatin Added to Tissue Conditioners for Treating Denture-Induced Stomatitis: A Systematic Review" Prosthesis 3, no. 1: 61-74. https://doi.org/10.3390/prosthesis3010007
APA StyleShaikh, M. S., Alnazzawi, A., Habib, S. R., Lone, M. A., & Zafar, M. S. (2021). Therapeutic Role of Nystatin Added to Tissue Conditioners for Treating Denture-Induced Stomatitis: A Systematic Review. Prosthesis, 3(1), 61-74. https://doi.org/10.3390/prosthesis3010007







