Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers
Abstract
1. Introduction
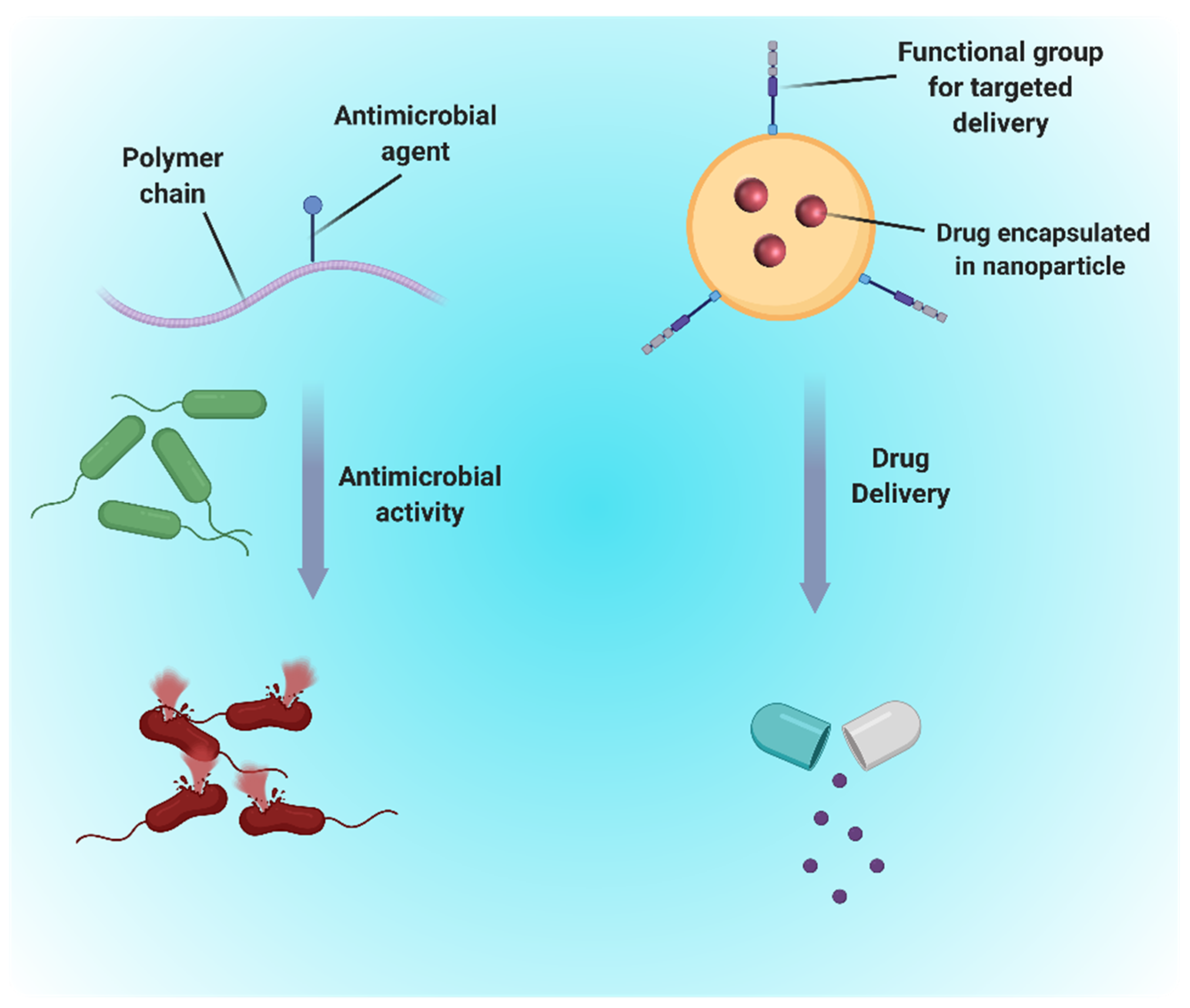
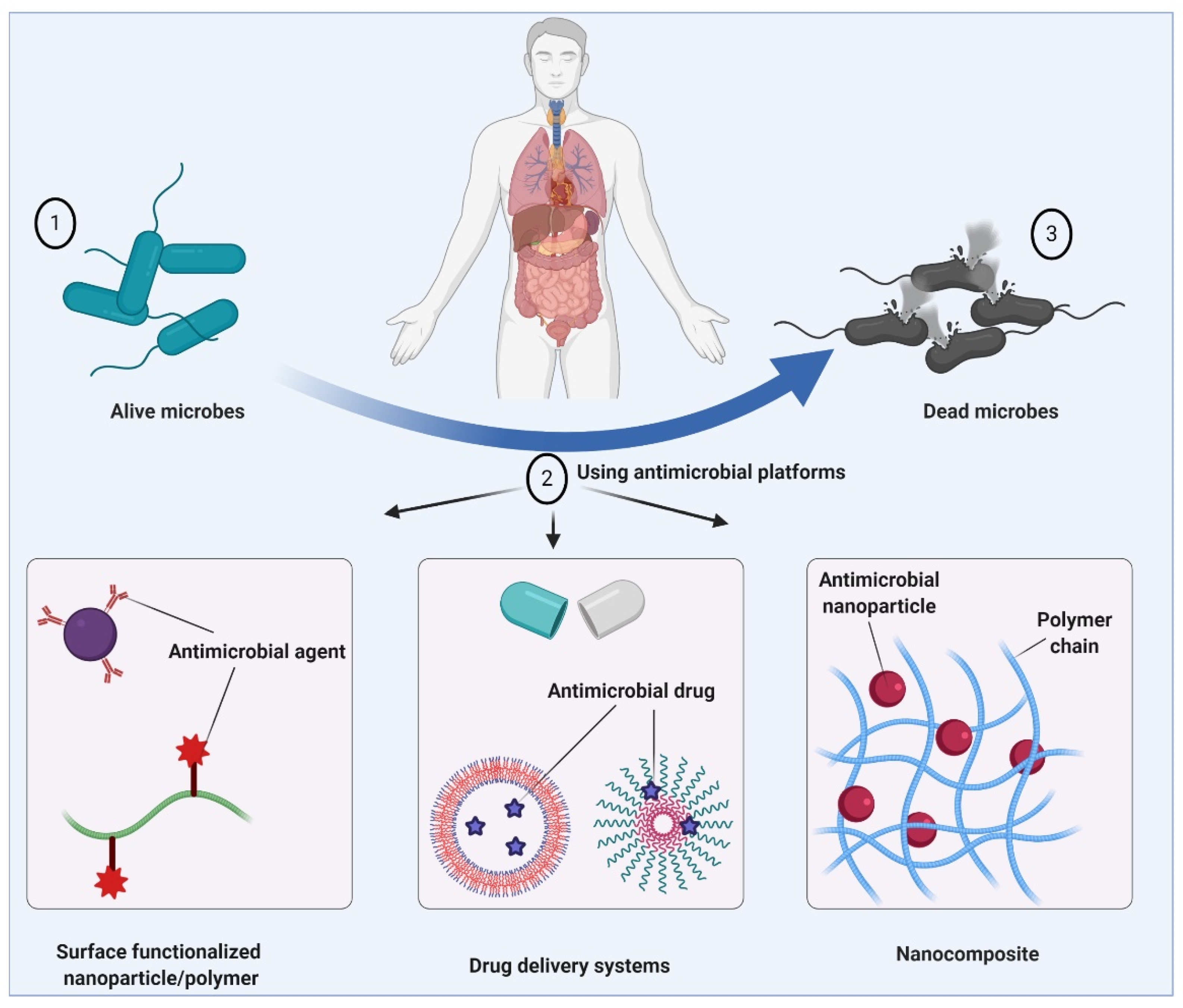
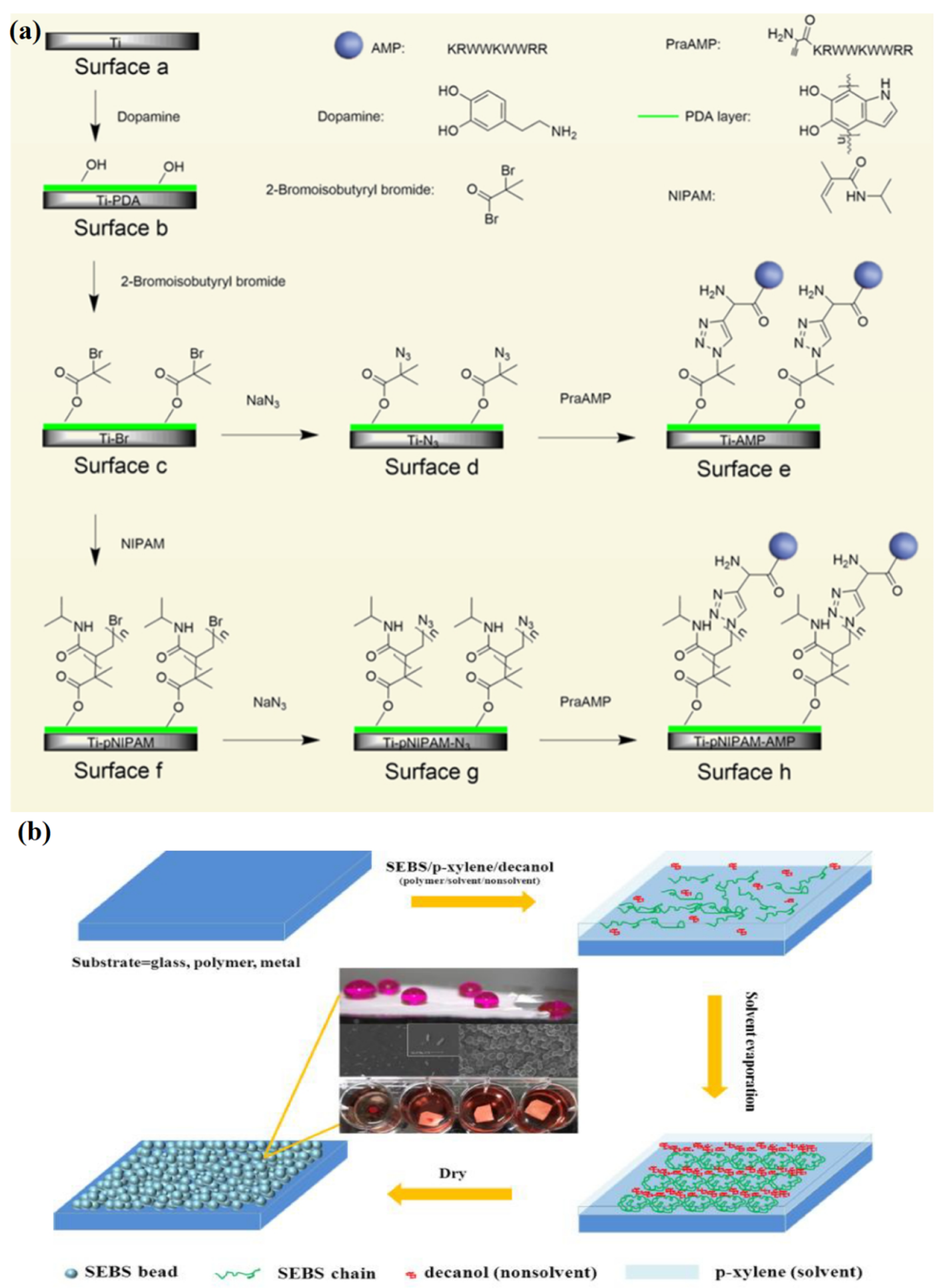
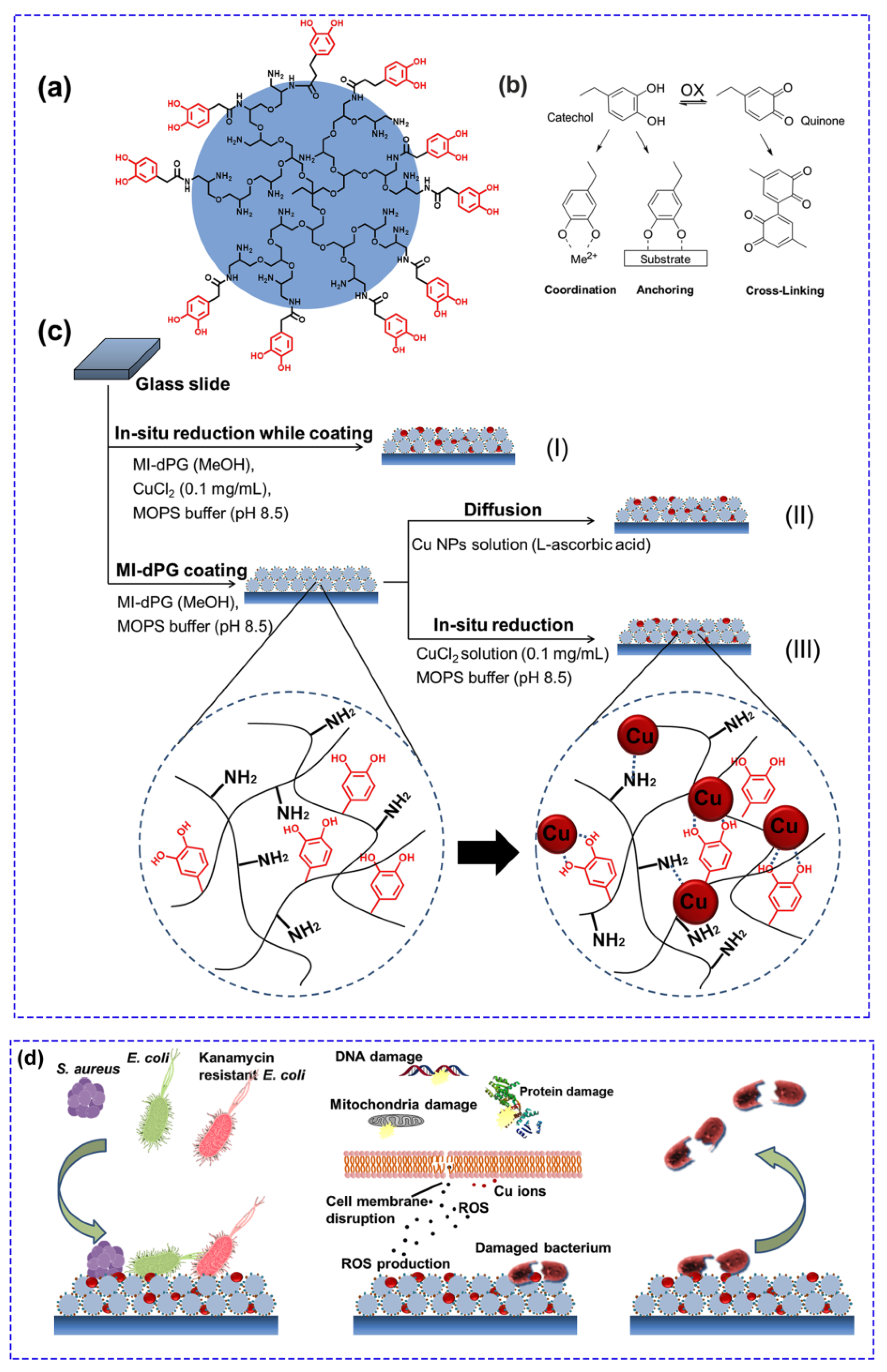
2. Antimicrobial Therapy
3. Drug Delivery
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Samareh Fekri, H.; Ranjbar, M.; Pardakhty, A. A Systematic Study of Cu Nanospheres Embedded in Non-ionic Surfactant-Based Vesicle: Photocatalytic Efficiency and In Vivo Imaging Study. J. Clust. Sci. 2019, 30, 561–570. [Google Scholar] [CrossRef]
- Leon-Garzon, A.R.; Dotelli, G.; Tommasini, M.; Bianchi, C.L.; Pirola, C.; Villa, A.; Lucotti, A.; Sacchi, B.; Barbieri, L. Experimental Characterization of Polymer Surfaces Subject to Corona Discharges in Controlled Atmospheres. Polymers 2019, 11, 1646. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Aidun, A. Conductive electrospun polyurethane-polyaniline scaffolds coated with poly (vinyl alcohol)-GPTMS under oxygen plasma surface modification. Mater. Today Commun. 2020, 22, 100752. [Google Scholar] [CrossRef]
- Yáñez-Pacios, A.J.; Martín-Martínez, J.M. Surface modification and adhesion of wood-plastic composite (WPC) treated with UV/ozone. Compos. Interfaces 2018, 25, 127–149. [Google Scholar] [CrossRef]
- Kehrer, M.; Duchoslav, J.; Hinterreiter, A.; Mehic, A.; Stehrer, T.; Stifter, D. Surface functionalization of polypropylene using a cold atmospheric pressure plasma jet with gas water mixtures. Surf. Coat. Technol. 2020, 384, 125170. [Google Scholar] [CrossRef]
- Kurusu, R.S.; Demarquette, N.R. Surface modification to control the water wettability of electrospun mats. Int. Mater. Rev. 2019, 64, 249–287. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A review of membrane wettability for the treatment of saline water deploying membrane distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I. Modification of the wettability of polymer surfaces using nanoparticles. Prog. Org. Coat. 2014, 77, 331–338. [Google Scholar] [CrossRef]
- Sham, M.L.; Li, J.; Ma, P.C.; Kim, J.-K. Cleaning and functionalization of polymer surfaces and nanoscale carbon fillers by UV/ozone treatment: A review. J. Compos. Mater. 2009, 43, 1537–1564. [Google Scholar] [CrossRef]
- Mozetič, M. Surface Modification to Improve Properties of Materials; MDPI: Basel, Switzerland, 2019. [Google Scholar]
- Jamaledin, R.; Yiu, C.K.Y.; Zare, E.N.; Niu, L.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in antimicrobial microneedle patches for combating infections. Adv. Mater. 2020, in press. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Makvandi, P.; Borzacchiello, A.; Tay, F.R.; Ashtari, B.; Padil, V.T.V. Antimicrobial gum bio-based nanocomposites and their industrial and biomedical applications. Chem. Commun. 2019, 55, 14871–14885. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Pollini, M.; Gallo, A.L.; Maffezzoli, A.; Esposito Corcione, C.; Montagna, F.; Paladini, F.; Jamaledin, R. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Polym. Adv. Technol. 2017, 29, 364–371. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Chaker, A.; Boufi, S. Cationic nanofibrillar cellulose with high antibacterial properties. Carbohydr. Polym. 2015, 131, 224–232. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Y.; Jiang, X.; Huang, D.; Yan, Y. Chitosan derivatives with dual-antibacterial functional groups for antimicrobial finishing of cotton fabrics. Carbohydr. Polym. 2011, 85, 221–227. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, H.-M.; Yoon, J.H. Synthesis of a Quaternary Ammonium Derivative of Chitosan and Its Application to a Cotton Antimicrobial Finish. Text. Res. J. 2008, 68, 428–434. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2019, 107, 10195. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P. Antimicrobial Metal-Based Nanomaterials and Their Industrial and Biomedical Applications. In Engineered Antimicrobial Surfaces; Snigdha, S., Thomas, S., Radhakrishnan, E., Kalarikkal, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–134. [Google Scholar]
- Wang, C.; Makvandi, P.; Zare, E.N.; Tay, F.R.; Niu, L. Advances in antimicrobial organic and inorganic nanocompounds in biomedicine. Adv. Ther. 2020, in press. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, in press. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P.; Belliveau, P.P.; Nightingale, C.H.; Quintiliani, R.; Freeman, C.D. Implementation of a once-daily aminoglycoside program in a large community-teaching hospital. Hosp. Pharm. 1995, 30, 674–676. [Google Scholar]
- Zhan, J.; Wang, L.; Zhu, Y.; Gao, H.; Chen, Y.; Chen, J.; Jia, Y.; He, J.; Fang, Z.; Zhu, Y.; et al. Temperature-Controlled Reversible Exposure and Hiding of Antimicrobial Peptides on an Implant for Killing Bacteria at Room Temperature and Improving Biocompatibility in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 35830–35837. [Google Scholar] [CrossRef]
- Ye, W.; Shi, Q.; Hou, J.; Jin, J.; Fan, Q.; Wong, S.-C.; Xu, X.; Yin, J. Superhydrophobic coating of elastomer on different substrates using a liquid template to construct a biocompatible and antibacterial surface. J. Mater. Chem. B 2014, 2, 7186–7191. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghaemy, M.; Ghadiri, A.A.; Mohseni, M. Photocurable, Antimicrobial Quaternary Ammonium-modified Nanosilica. J. Dent. Res. 2015, 94, 1401–1407. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.P.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Coll. Surf. B Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef]
- Li, M.; Gao, L.; Schlaich, C.; Zhang, J.; Donskyi, I.S.; Yu, G.; Li, W.; Tu, Z.; Rolff, J.; Schwerdtle, T. Construction of functional coatings with durable and broad-spectrum antibacterial potential based on mussel-inspired dendritic polyglycerol and in situ-formed copper nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 35411–35418. [Google Scholar] [CrossRef]
- Pearson, H.A.; Urban, M.W. Simple click reactions on polymer surfaces leading to antimicrobial behavior. J. Mater. Chem. B 2014, 2, 2084–2087. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ho, P.-L.; Tsang, K.W.T.; Wang, L.; Xu, B. Using Biofunctional Magnetic Nanoparticles to Capture Vancomycin-Resistant Enterococci and Other Gram-Positive Bacteria at Ultralow Concentration. J. Am. Chem. Soc. 2003, 125, 15702–15703. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible Amoxicillin-Grafted Bacterial Cellulose Sponges for Wound Dressing: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef] [PubMed]
- Bahramian, B.; Chrzanowski, W.; Kondyurin, A.; Thomas, N.; Dehghani, F. Fabrication of Antimicrobial Poly(propylene carbonate) Film by Plasma Surface Modification. Ind. Eng. Chem. Res. 2017, 56, 12578–12587. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Huang, C.-J. Functionalization of Polydopamine via the Aza-Michael Reaction for Antimicrobial Interfaces. Langmuir 2016, 32, 5019–5028. [Google Scholar] [CrossRef] [PubMed]
- Fadida, T.; Kroupitski, Y.; Peiper, U.M.; Bendikov, T.; Sela Saldinger, S.; Poverenov, E. Air-ozonolysis to generate contact active antimicrobial surfaces: Activation of polyethylene and polystyrene followed by covalent graft of quaternary ammonium salts. Coll. Surf. B Biointerfaces 2014, 122, 294–300. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Goddard, J.M. Development of antimicrobial stainless steel via surface modification with N-halamines: Characterization of surface chemistry and N-halamine chlorination. J. Appl. Polym. Sci. 2013, 127, 821–831. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Lin, T. Robust, self-healing superamphiphobic fabrics prepared by two-step coating of fluoro-containing polymer, fluoroalkyl silane, and modified silica nanoparticles. Adv. Funct. Mater. 2013, 23, 1664–1670. [Google Scholar] [CrossRef]
- Zhang, X.; Jeremic, D.; Kim, Y.; Street, J.; Shmulsky, R. Effects of surface functionalization of lignin on synthesis and properties of rigid bio-based polyurethanes foams. Polymers 2018, 10, 706. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A.J. Kraft lignin-based rigid polyurethane foam. J. Wood Chem. Technol. 2012, 32, 210–224. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Baylan, S.; Park, B.-W.; Richter, G.; Sitti, M. Hydrophobic pinning with copper nanowhiskers leads to bactericidal properties. PLoS ONE 2017, 12, e0175428. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimi, M.; Bevilacqua, C. Exogenous hyaluronic acid and wound healing: An updated vision. Panminerva Med. 2012, 54, 129–135. [Google Scholar] [PubMed]
- Wang, X.; Xiang, Q.; Cao, W.; Jin, F.; Peng, X.; Hu, B.; Xing, X. Fabrication of magnetic nanoparticles armed with quaternarized N-halamine polymers as recyclable antibacterial agents. J. Biomater. Sci. Polym. Ed. 2016, 27, 1909–1925. [Google Scholar] [CrossRef]
- Sankarganesh, M.; Jose, P.A.; Raja, J.D.; Kesavan, M.P.; Vadivel, M.; Rajesh, J.; Jeyamurugan, R.; Kumar, R.S.; Karthikeyan, S. New pyrimidine based ligand capped gold and platinum nano particles: Synthesis, characterization, antimicrobial, antioxidant, DNA interaction and in vitro anticancer activities. J. Photochem. Photobiol. B Biol. 2017, 176, 44–53. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Dual action antimicrobials: Nitric oxide release from quaternary ammonium-functionalized silica nanoparticles. Biomacromolecules 2012, 13, 3334–3342. [Google Scholar] [CrossRef]
- Batista, C.C.S.; Albuquerque, L.J.C.; de Araujo, I.; Albuquerque, B.L.; da Silva, F.D.; Giacomelli, F.C. Antimicrobial activity of nano-sized silver colloids stabilized by nitrogen-containing polymers: The key influence of the polymer capping. RSC Adv. 2018, 8, 10873–10882. [Google Scholar] [CrossRef]
- Wongpreecha, J.; Polpanich, D.; Suteewong, T.; Kaewsaneha, C.; Tangboriboonrat, P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 2018, 199, 641–648. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zu, Y.; Fu, Y.; Li, N.; Guo, N.; Liu, R.; Zhang, Y. Synthesis of polyethylenimine (PEI) functionalized silver nanoparticles by a hydrothermal method and their antibacterial activity study. Mater. Sci. Eng. C 2014, 42, 31–37. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.; Kim, J.; Jang, Y.; Shin, K.; Ha, E.; Ryu, S.; Kim, B.-G.; Wooh, S.; Char, K. Development of Multimodal Antibacterial Surfaces Using Porous Amine-Reactive Films Incorporating Lubricant and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 6550–6560. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Zodrow, K.R.; Genggeng, Q.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Surface functionalization of thin-film composite membranes with copper nanoparticles for antimicrobial surface properties. Environ. Sci. Technol. 2014, 48, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, Y.; Feng, X.; Wang, W.; Xu, F.; Zhang, L. Antibacterial surfaces through dopamine functionalization and silver nanoparticle immobilization. Mater. Chem. Phys. 2010, 121, 534–540. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2017, 79, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wan, P.; Zhai, Z.; Mao, Z.; Ouyang, Z.; Yu, D.; Sun, Q.; Tan, L.; Ren, L. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations. Biomaterials 2016, 106, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Battisti, M.; Vecchione, R.; Casale, C.; Pennacchio, F.A.; Lettera, V.; Jamaledin, R.; Profeta, M.; Di Natale, C.; Imparato, G.; Urciuolo, F. Non-invasive production of multi-compartmental biodegradable polymer microneedles for controlled intradermal drug release of labile molecules. Front. Bioeng. Biotechnol. 2019, 7, 296. [Google Scholar] [CrossRef]
- Jamaledin, R.; di Natale, C.; Onesto, V.; Taraghdari, Z.B.; Zare, E.N.; Makvandi, P.; Vecchione, R.; Netti, P.A. Progress in Microneedles-mediated Protein Delivery. J. Clin. Med. 2020, 2, 542. [Google Scholar] [CrossRef]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Cu, Y.; Saltzman, W.M. Controlled Surface Modification with Poly(ethylene)glycol Enhances Diffusion of PLGA Nanoparticles in Human Cervical Mucus. Mol. Pharm. 2009, 6, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Fong, P.M.; Lu, J.; Russell, K.S.; Booth, C.J.; Saltzman, W.M.; Fahmy, T.M. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 410–418. [Google Scholar] [CrossRef]
- Nafee, N.; Taetz, S.; Schneider, M.; Schaefer, U.F.; Lehr, C.M. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: Effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 173–183. [Google Scholar] [CrossRef]
- Mayol, L.; Biondi, M.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Borzacchiello, A. Amphiphilic hyaluronic acid derivatives toward the design of micelles for the sustained delivery of hydrophobic drugs. Carbohydr. Polym. 2014, 102, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Yang, J.A.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic acid-gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Fekri, H.S.; Ranjbar, M.; Noudeh, G.D.; Ziasistani, N. Green synthesis of strontium nanoparticles self-assembled in the presence of carboxymethyl cellulose: An in vivo imaging study. Luminescence 2019, 34, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, S.; Ashrafizadeh, M.; Deng, S.; Azarian, M.; Abdoli, A.; Motavaf, M.; Poormoghadam, D.; Khanbabaei, H.; Ghasemipour Afshar, E.; Mandegary, A. Autophagy modulators: Mechanistic aspects and drug delivery systems. Biomolecules 2019, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, W.; Zhang, H.; Zhang, T.T.; Chen, Z.; Yuan, S.; Peng, P.; Xiao, M.; Xu, L. Photothermally triggered cytosolic drug delivery of glucose functionalized polydopamine nanoparticles in response to tumor microenvironment for the GLUT1-targeting chemo-phototherapy. J. Control. Release 2020, 317, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef]
- Liao, S.-C.; Ting, C.-W.; Chiang, W.-H. Functionalized polymeric nanogels with pH-sensitive benzoic-imine cross-linkages designed as vehicles for indocyanine green delivery. J. Coll. Interface Sci. 2020, 561, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Smyth, P.; Gibson, T.J.; Irvine, G.; Black, G.; Lavery, D.; Semsarilar, M.; Scott, C.J.; Themistou, E. pH-Responsive benzaldehyde-functionalized PEG-based polymeric nanoparticles for drug delivery: Effect of preparation method on morphology, dye encapsulation and attachment. Eur. Polym. J. 2020, 124, 109471. [Google Scholar] [CrossRef]
- Wu, W.; Chen, M.; Luo, T.; Fan, Y.; Zhang, J.; Zhang, Y.; Zhang, Q.; Sapin-Minet, A.; Gaucher, C.; Xia, X. ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater. 2020, 103, 259–271. [Google Scholar] [CrossRef]
- Peng, H.; Huang, X.; Melle, A.; Karperien, M.; Pich, A. Redox-responsive degradable prodrug nanogels for intracellular drug delivery by crosslinking of amine-functionalized poly (N-vinylpyrrolidone) copolymers. J. Coll. Interface Sci. 2019, 540, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Lin, X.; Si, T.; He, Q. Gold-nanoshell-functionalized polymer nanoswimmer for photomechanical poration of single-cell membrane. J. Am. Chem. Soc. 2019, 141, 6601–6608. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Bagherifard, M.; Doroudian, M. Synthesis of micelles based on chitosan functionalized with gold nanorods as a light sensitive drug delivery vehicle. Int. J. Biol. Macromol. 2020, 149, 809–818. [Google Scholar] [CrossRef]
- Lin, A.; Giuliano, C.J.; Palladino, A.; John, K.M.; Abramowicz, C.; Yuan, M.L.; Sausville, E.L.; Lukow, D.A.; Liu, L.; Chait, A.R. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 2019, 11, eaaw8412. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Kotla, N.G.; Afshar, E.G.; Samarghandian, S.; Mandegary, A.; Pardakhty, A.; Mohammadinejad, R.; Sethi, G. Nanoparticles targeting STATs in cancer therapy. Cells 2019, 8, 1158. [Google Scholar] [CrossRef] [PubMed]
- Samarehfekri, H.; Ranjbar, M.; Pardakhty, A.; Amanatfard, A. Systematic Study of NaF Nanoparticles in Micelles loaded on Polylactic Acid Nanoscaffolds: In Vitro Efficient Delivery. J. Clust. Sci. 2020, 31, 453–461. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Chanphai, P.; Thomas, T.J.; Tajmir-Riahi, H.A. Application and biomolecular study of functionalized folic acid-dendrimer nanoparticles in drug delivery. J. Biomol. Struct. Dyn. 2020, 1–19. [Google Scholar] [CrossRef]
- Ilhami, F.B.; Huang, S.-Y.; Chen, J.-K.; Kao, C.-Y.; Cheng, C.-C. Multifunctional adenine-functionalized supramolecular micelles for highly selective and effective cancer chemotherapy. Polym. Chem. 2020, 11, 849–856. [Google Scholar] [CrossRef]
- Munzar, J.D.; Ng, A.; Juncker, D. Duplexed aptamers: History, design, theory, and application to biosensing. Chem. Soc. Rev. 2019, 48, 1390–1419. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Hu, X.; Shanmugam, S.; Chelliah, R.; Sekar, P.; Oh, D.-H.; Vijayakumar, S.; Kathiresan, K.; Wang, M.-H. Enhanced cancer therapy with pH-dependent and aptamer functionalized doxorubicin loaded polymeric (poly D, L-lactic-co-glycolic acid) nanoparticles. Arch. Biochem. Biophys. 2019, 671, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.-M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Sirois, A.R.; Vazquez Cegla, A.J.; Jumai’an, E.; Murata, N.; Buck, M.E.; Moore, S.J. Protein-Polymer Conjugates Synthesized Using Water-Soluble Azlactone-Functionalized Polymers Enable Receptor-Specific Cellular Uptake toward Targeted Drug Delivery. Bioconj. Chem. 2019, 30, 1220–1231. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, Y.; Wang, L.; Bu, P.; Sharkey, C.C.; Wu, Q.; Wun, B.; Roy, S.; Shen, X.; King, M.R. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials 2016, 76, 52–65. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Fekri, H.S.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Therapeutic and biological activities of berberine: The involvement of Nrf2 signaling pathway. J. Cell. Biochem. 2020, 121, 1575–1585. [Google Scholar] [CrossRef]
- Taraghdari, Z.B.; Imani, R.; Mohabatpour, F. A Review on Bioengineering Approaches to Insulin Delivery: A Pharmaceutical and Engineering Perspective. Macromol. Biosci. 2019, 19, 1800458. [Google Scholar] [CrossRef]
- Khafagy, E.-S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef]
- Fan, W.; Xia, D.; Zhu, Q.; Li, X.; He, S.; Zhu, C.; Guo, S.; Hovgaard, L.; Yang, M.; Gan, Y. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials 2018, 151, 13–23. [Google Scholar] [CrossRef]
- Singh, A.V.; Jahnke, T.; Xiao, Y.; Wang, S.; Yu, Y.; David, H.; Richter, G.; Laux, P.; Luch, A.; Srivastava, A. Peptide-induced biomineralization of tin oxide (SnO2) nanoparticles for antibacterial applications. J. Nanosci. Nanotechnol. 2019, 19, 5674–5686. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vermerris, W. Antimicrobial nanomaterials derived from natural products—A review. Materials 2016, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Yoo, J.; Jang, Y. Engineering Approaches to Create Antibacterial Surfaces on Biomedical Implants and Devices. In Racing for the Surface; Springer: Berlin/Heidelberg, Germany, 2020; pp. 313–340. [Google Scholar]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent advances in antimicrobial polymers: A mini-review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric nanocomposites and nanocoatings for food packaging: A review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Anh, L.H.T.; Phuong, P.T.T.; Van, N.T.T.; Tri, N.; Minh, N.V.; Ha, H.K.P. Preparation of Ag/Zn2TiO4 and its antibacterial activity on enamel tile. Chem. Pap. 2019, 73, 1019–1026. [Google Scholar] [CrossRef]
- Le, T.T.; Nguyen, T.V.; Nguyen, T.A.; Nguyen, T.T.H.; Thai, H.; Dinh, D.A.; Nguyen, T.M. Thermal, mechanical and antibacterial properties of water-based acrylic Polymer/SiO2-Ag nanocomposite coating. Mater. Chem. Phys. 2019, 232, 362–366. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Patel, P.; Hanini, A.; Shah, A.; Patel, D.; Patel, S.; Bhatt, P.; Pathak, Y. V Surface Modification of Nanoparticles for Targeted Drug Delivery. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 19–31. [Google Scholar]
- Lahkar, S.; Das, M.K. Brain-Targeted Drug Delivery with Surface-Modified Nanoparticles. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 277–310. [Google Scholar]
- Zhou, W.; Qiao, Z.; Zare, E.N.; Huang, J.; Zheng, X.; Sun, X.; Shao, M.; Wang, H.; Wang, X.; Chen, D.; et al. 4D-Printed Dynamic Materials in Biomedical Applications: Chemistry, Challenges, and Their Future Perspectives in the Clinical Sector. J. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Vogus, D.R.; Krishnan, V.; Mitragotri, S. A review on engineering polymer drug conjugates to improve combination chemotherapy. Curr. Opin. Coll. Interface Sci. 2017, 31, 75–85. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Nanocarrier mediated combination drug delivery for chemotherapy–A review. J. Drug Deliv. Sci. Technol. 2017, 39, 362–371. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.U. Current and Future Aspects of Smart Nanotheranostic Agents in Cancer Therapeutics. In Nanotheranostics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 213–227. [Google Scholar]

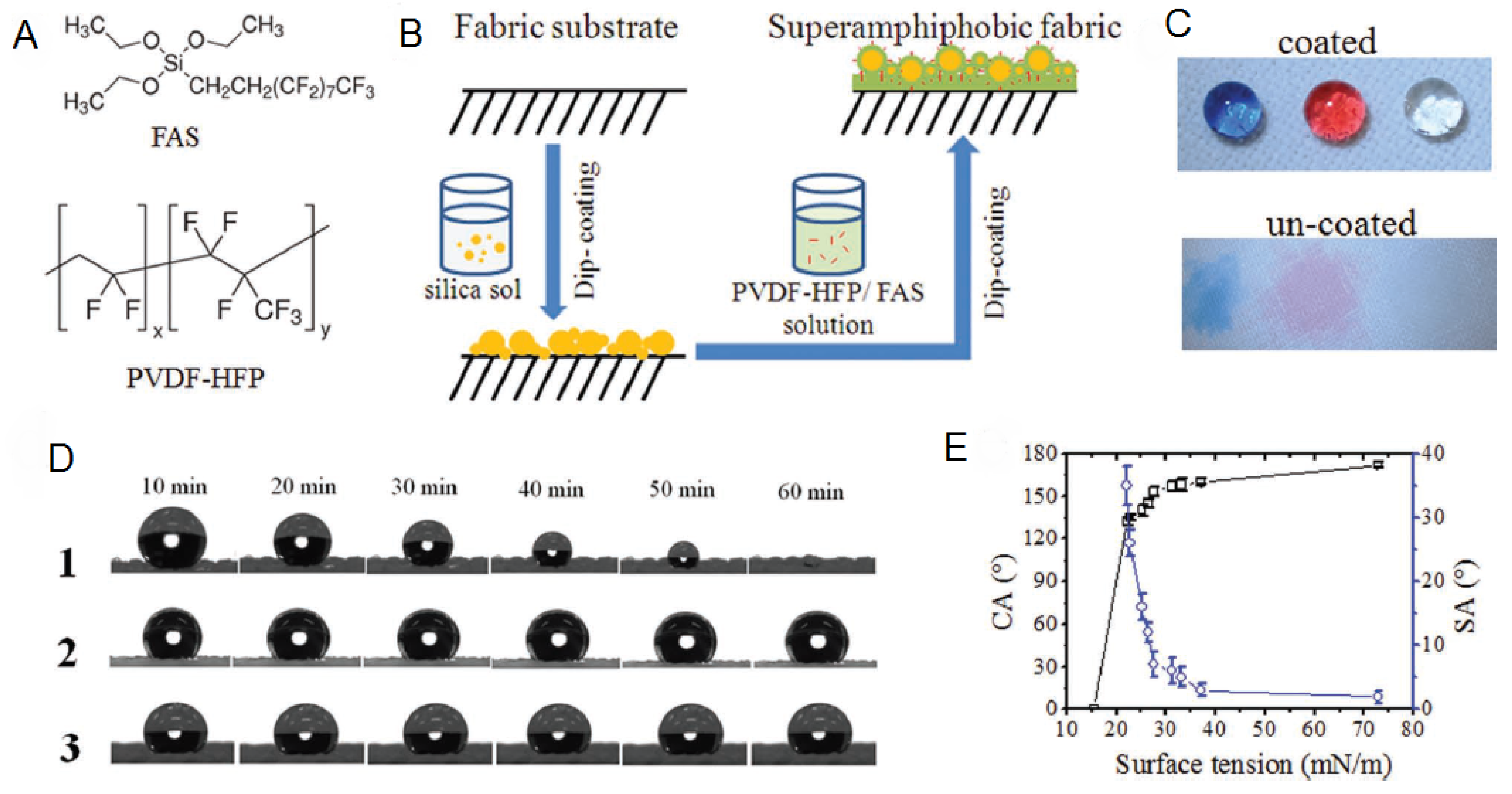
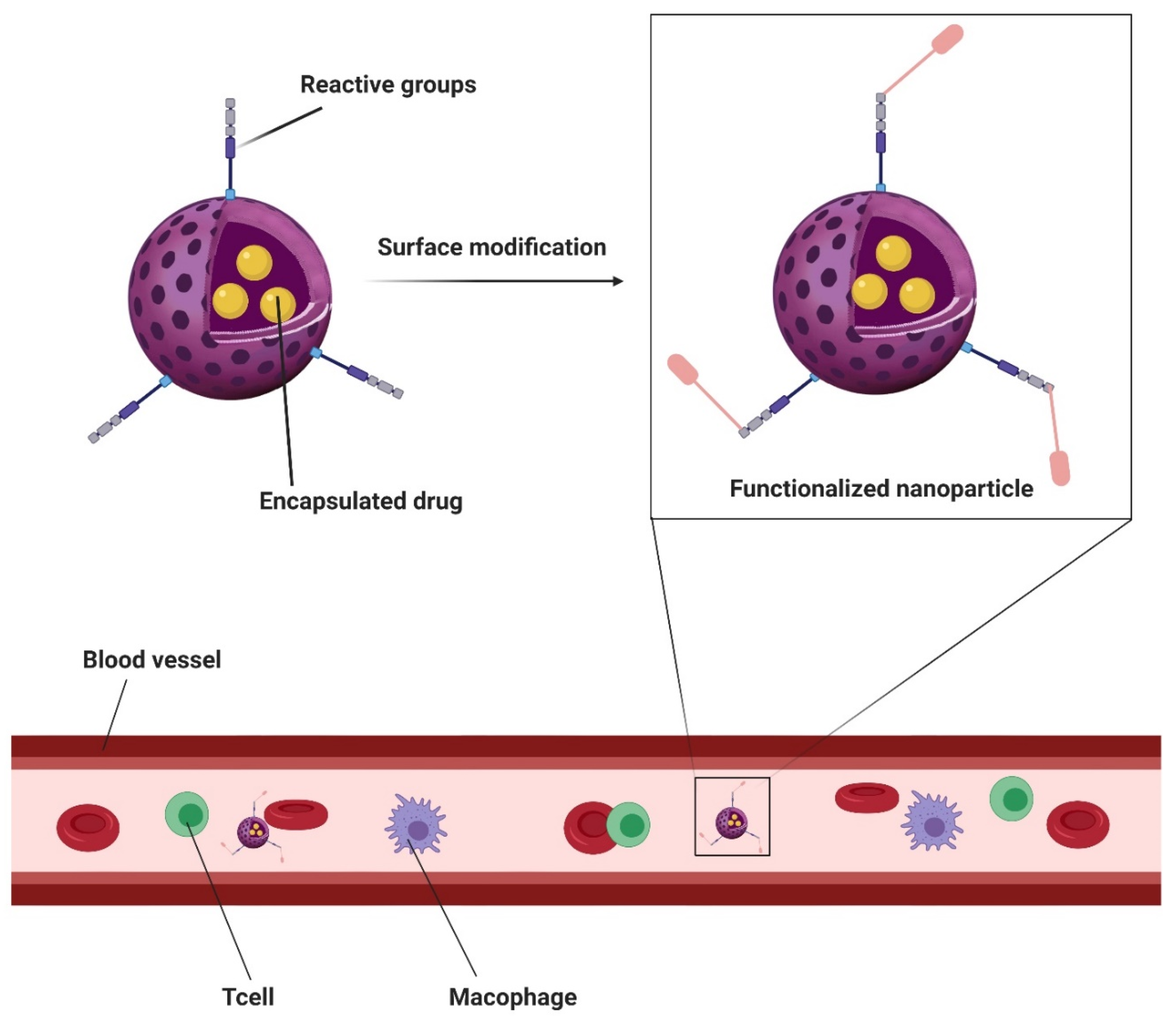

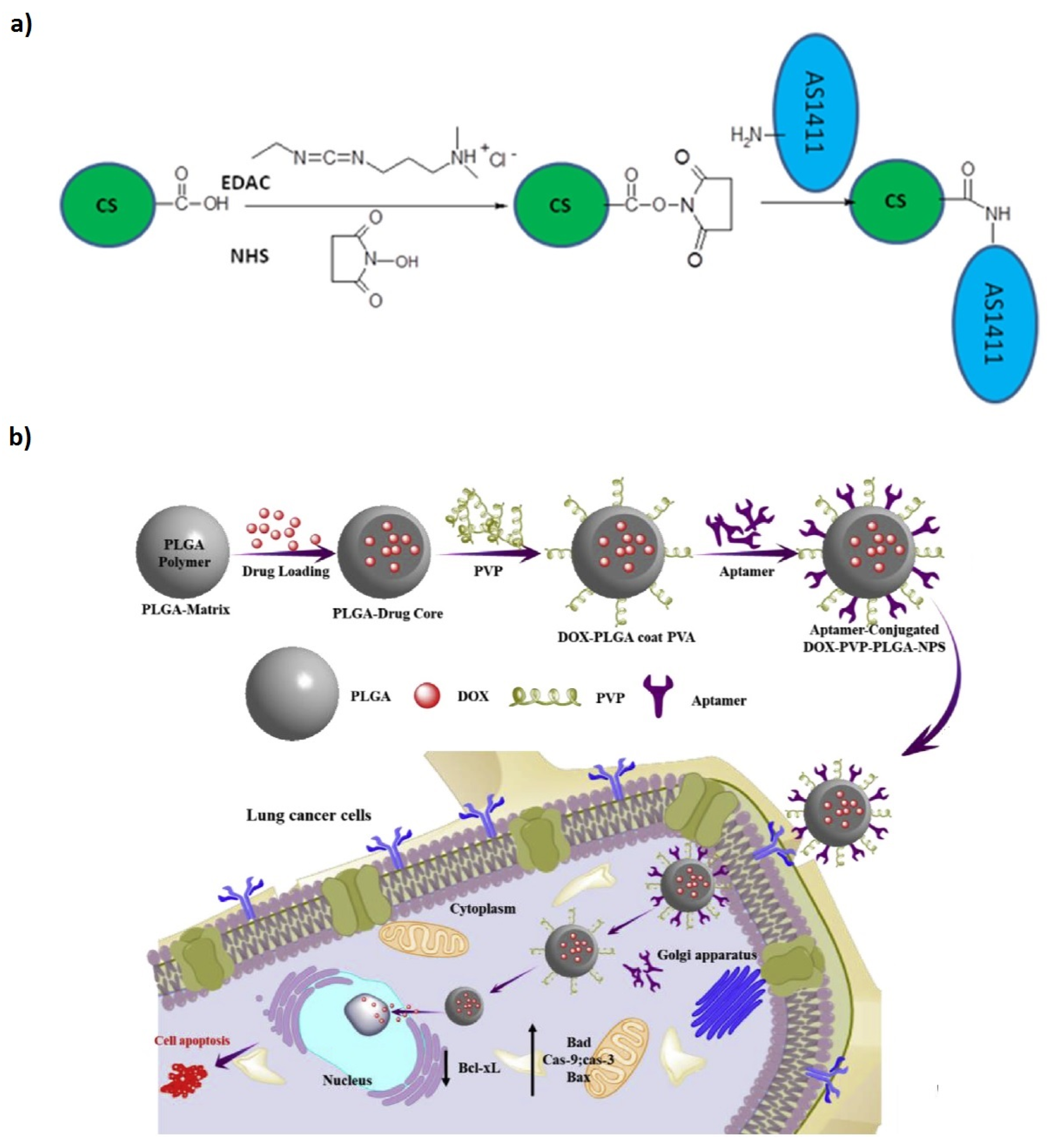

| Platforms/Materials | Antimicrobial Agent | Strategy | Application | Remarks | Ref |
|---|---|---|---|---|---|
| Hyaluronic acid hydrogel | Quaternary ammonium compounds | Contact killing | Wound healing | The hydrogel showed antibacterial activity against bacteria | [45] |
| Fe3O4 | Quaternarized N-halamine polymers | Contact killing | Water purification systems and household sanitation | Effective antimicrobial impact against Staphylococcus aureus and Escherichia coli (gram-negative) | [46] |
| Titanium dioxide core-shell nanoparticles | N-halamine | Drug release (Cisplatin) | Anticancer | The nanoparticles showed effective antimicrobial activities against S. aureus and E. coli | [47] |
| Silica nanoparticles with nitric oxide (NO) release capabilities | Quaternary ammonium compounds | Drug release (Nitric oxide) | Infection therapy | Very high bactericidal efficacy against S. aureus and P. aeruginosa | [48] |
| Polyethyleneimine (PEI), polyvinylpyrrolidone (PVP) and poly (2-vinyl pyridine)-b-poly(ethylene oxide) (PEO-b-P2VP) | silver colloids | Contact killing | Antibacterial and antifungal | The materials exhibited high biocides against fungus and bacteria | [49] |
| Chitosan | Silver nanoparticles | Contact killing | Infection therapy | Very high antimicrobial impact against E. coli and S. aureus | [50] |
| Polyethylenimine (PEI) | Silver nanoparticles | Contact killing | Infection therapy | PEI-Ag nanoparticles showed effective antibacterial activity | [51] |
| Porous amine-reactive (PAR) polymer films from poly(pentafluorophenyl acrylate) (PPFPA) | Porous amine-reactive films incorporating lubricant and silver nanoparticles | Contact killing | Infection therapy | This film showed a multimodal anti-biofouling surface | [52] |
| Thin-film composite of polyamide reverse osmosis irreversible | Copper nanoparticles | Contact killing | RO desalination | The functionalized membrane exhibited significant Antibacterial activity for three different model bacterial strains. | [53] |
| Dopamine functionalized polyimide films | Silver nanoparticles | Contact killing | Antibacterial | Surface-silvered polymer film showed the antibacterial activity using Escherichia coli (E. coli). | [54] |
| Poly(lactic-co-glycolic acid)/ZnO nanorods/Ag NPs Hybrid coating on Ti | Silver nanoparticles and ZnO nanowire | Contact killing | Antibacterial | This platform exhibited high potential for biomedical application with excellent biocompatibility and self-antibacterial activity. | [55] |
| Biodegradable Mg-Cu alloy film | Mg-Cu alloy | Contact killing | Orthopedic infections | The results indicated the potential utility of this biofilm in treatment of orthopedic infections | [56] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delfi, M.; Ghomi, M.; Zarrabi, A.; Mohammadinejad, R.; Taraghdari, Z.B.; Ashrafizadeh, M.; Zare, E.N.; Agarwal, T.; Padil, V.V.T.; Mokhtari, B.; et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 117-139. https://doi.org/10.3390/prosthesis2020012
Delfi M, Ghomi M, Zarrabi A, Mohammadinejad R, Taraghdari ZB, Ashrafizadeh M, Zare EN, Agarwal T, Padil VVT, Mokhtari B, et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis. 2020; 2(2):117-139. https://doi.org/10.3390/prosthesis2020012
Chicago/Turabian StyleDelfi, Masoud, Matineh Ghomi, Ali Zarrabi, Reza Mohammadinejad, Zahra Baghban Taraghdari, Milad Ashrafizadeh, Ehsan Nazarzadeh Zare, Tarun Agarwal, Vinod V. T. Padil, Babak Mokhtari, and et al. 2020. "Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers" Prosthesis 2, no. 2: 117-139. https://doi.org/10.3390/prosthesis2020012
APA StyleDelfi, M., Ghomi, M., Zarrabi, A., Mohammadinejad, R., Taraghdari, Z. B., Ashrafizadeh, M., Zare, E. N., Agarwal, T., Padil, V. V. T., Mokhtari, B., Rossi, F., Perale, G., Sillanpaa, M., Borzacchiello, A., Kumar Maiti, T., & Makvandi, P. (2020). Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis, 2(2), 117-139. https://doi.org/10.3390/prosthesis2020012














