Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies
Abstract
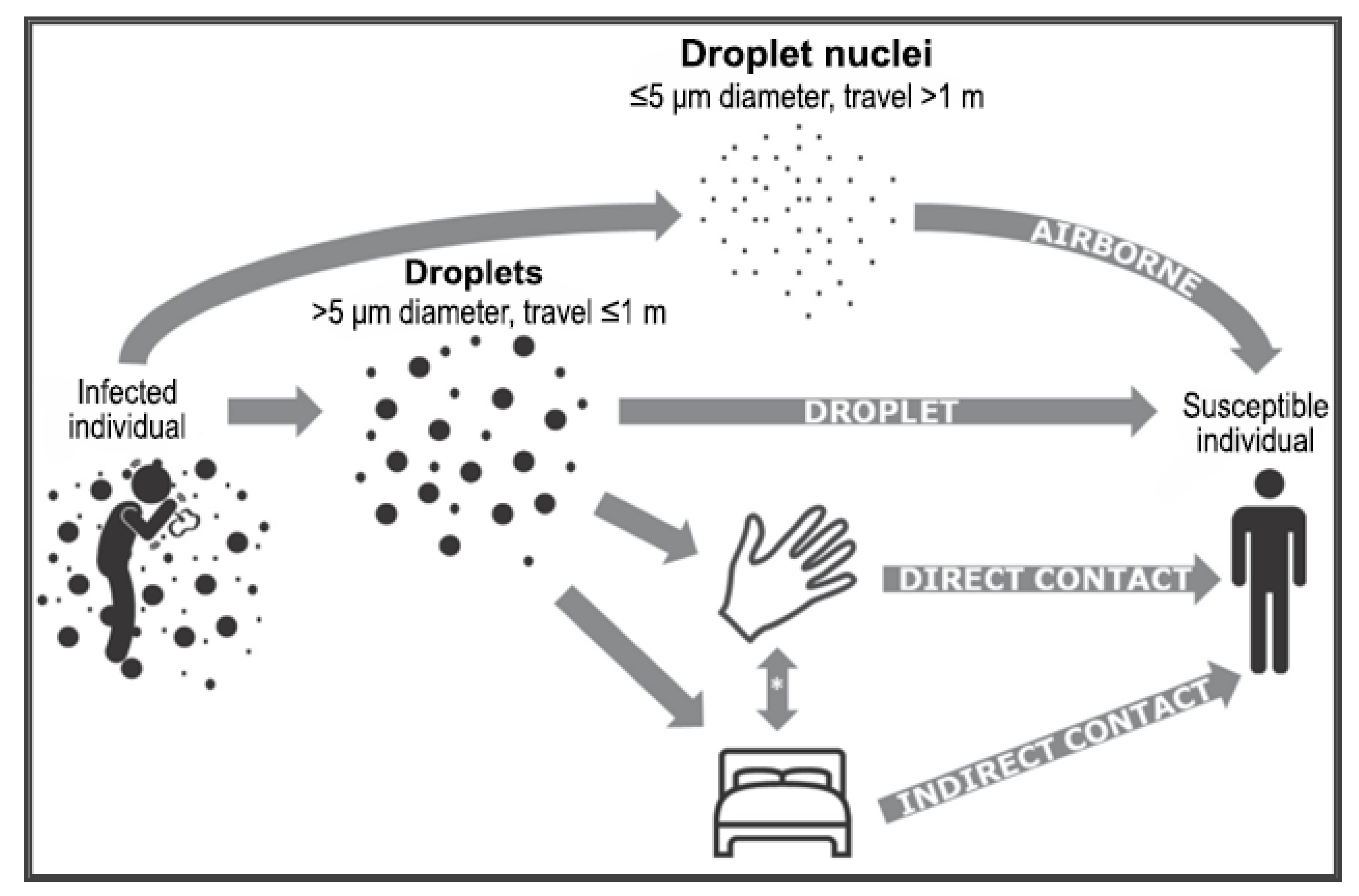
1. Introduction
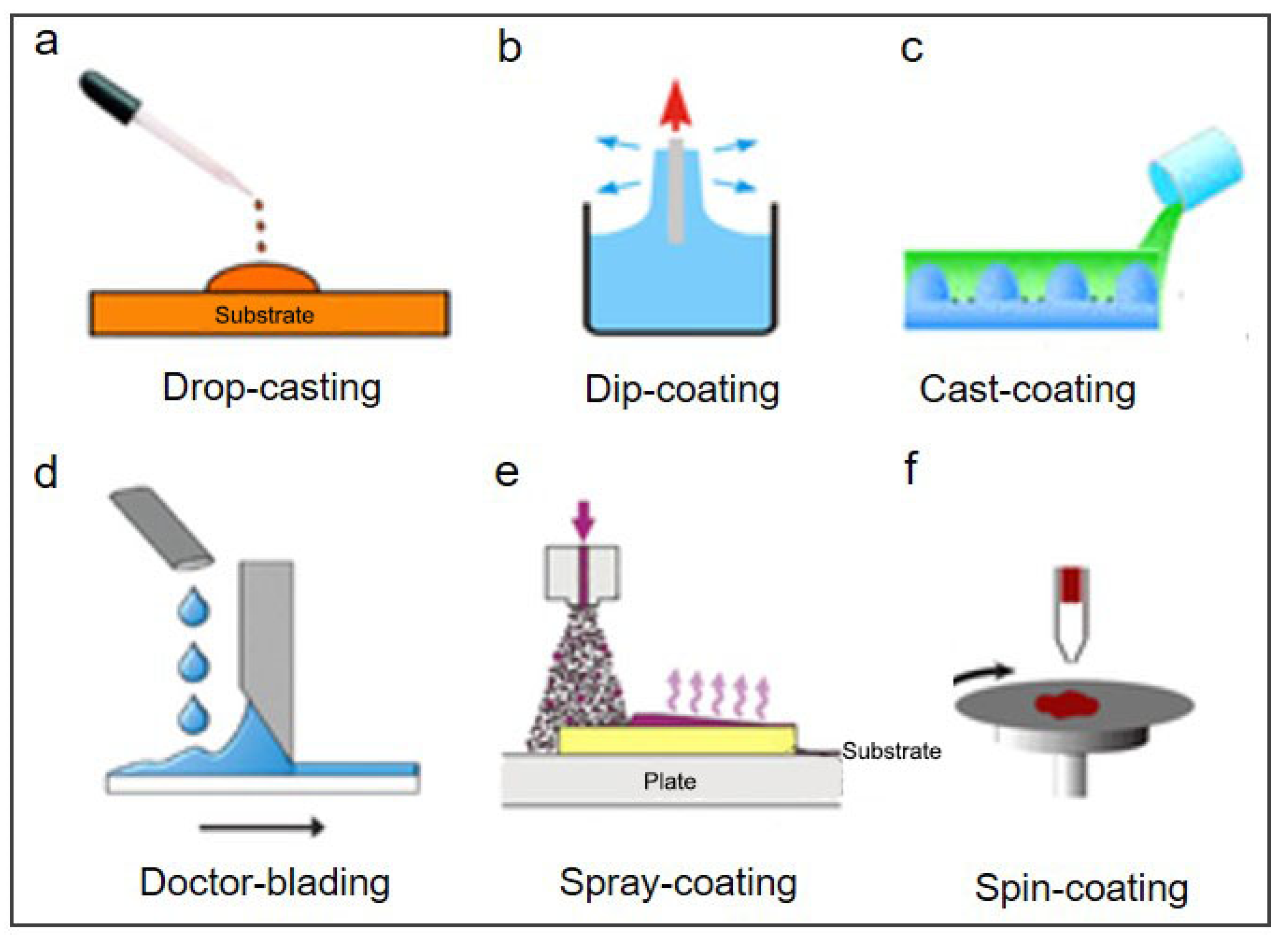
2. Coating Strategies
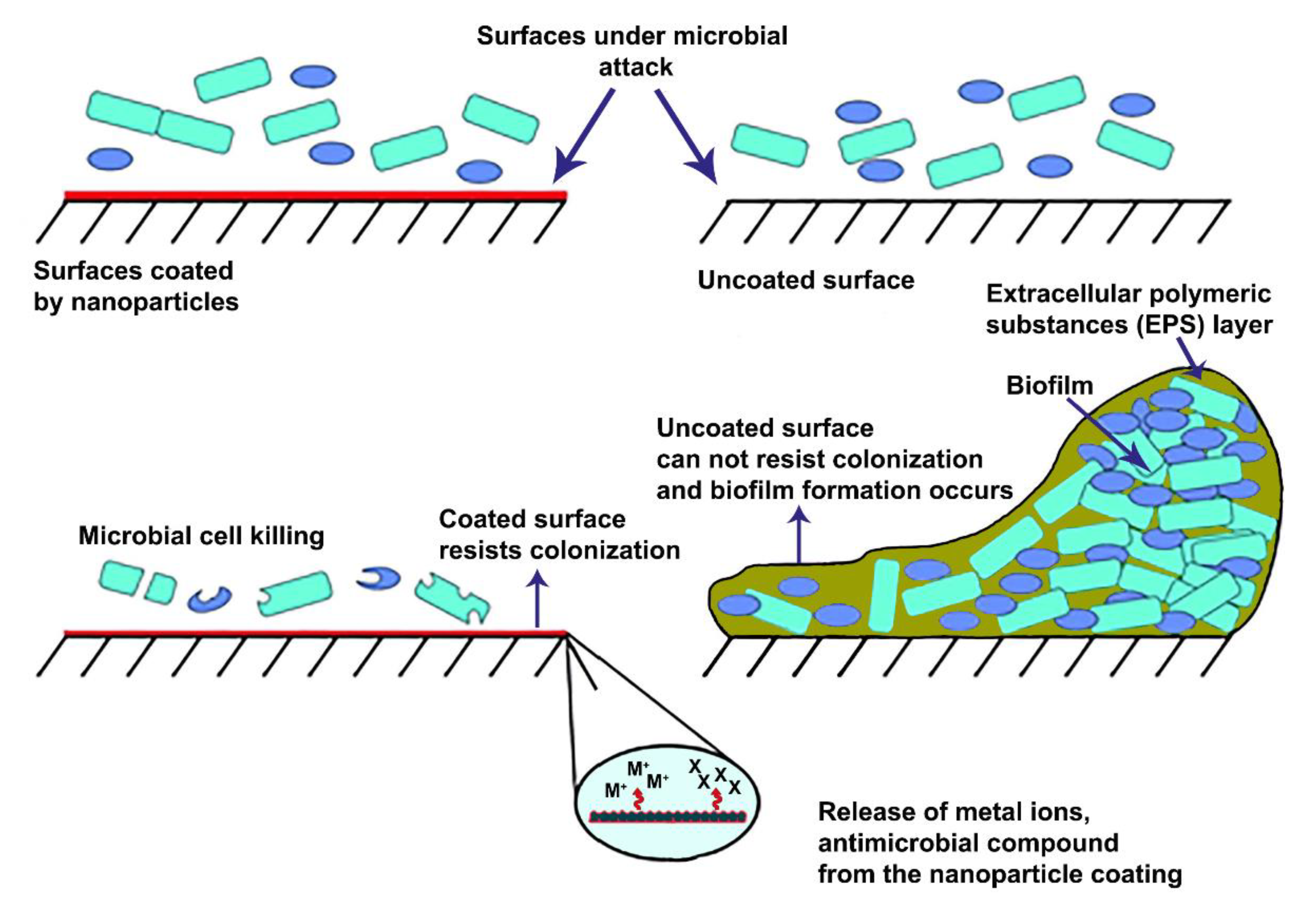
3. Coated Surfaces
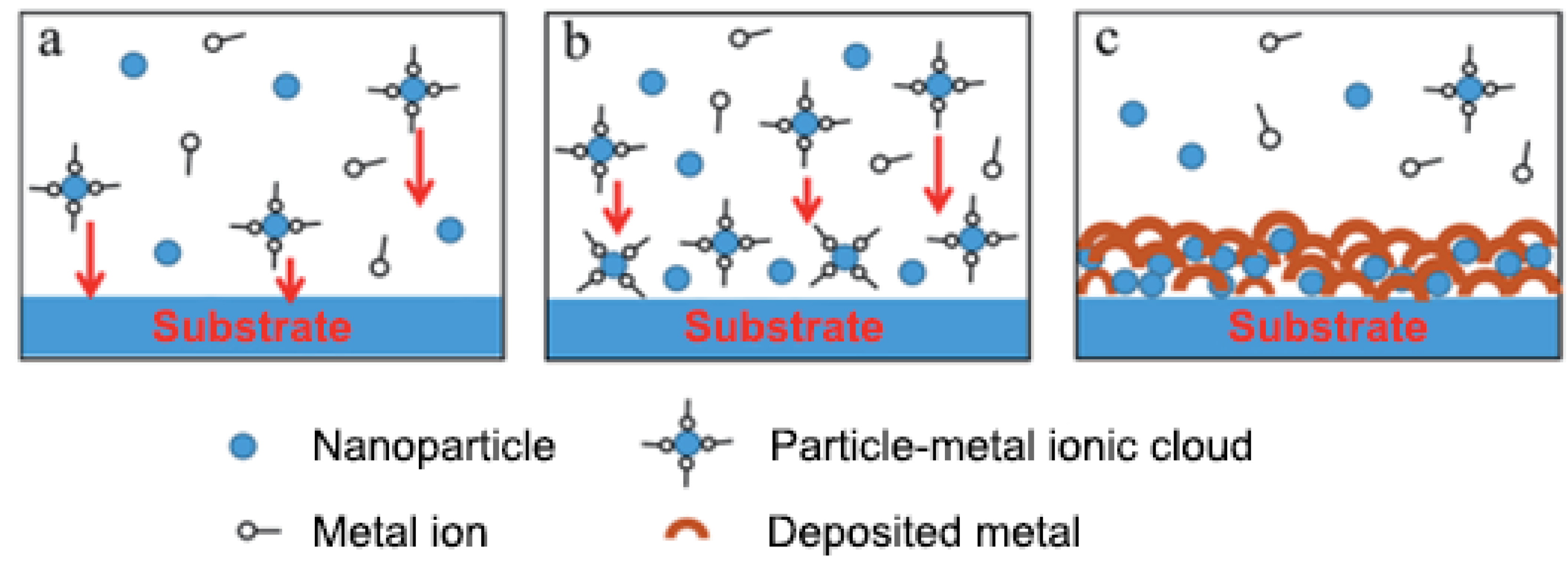
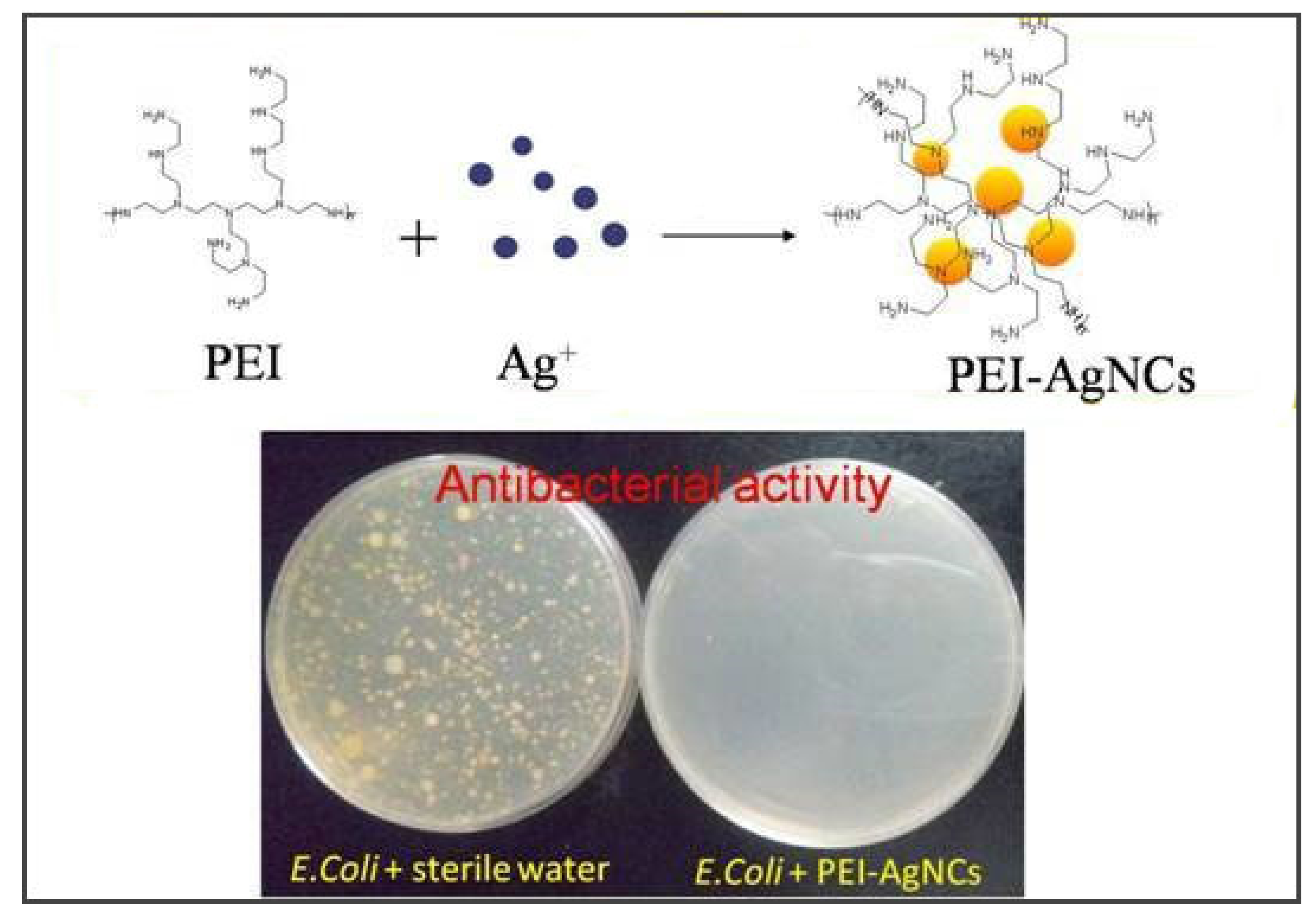
3.1. Metal-Based Nanomaterial Coatings
3.2. Polymer-Based Surfaces
3.2.1. Antimicrobial Agent Coupled Polymers
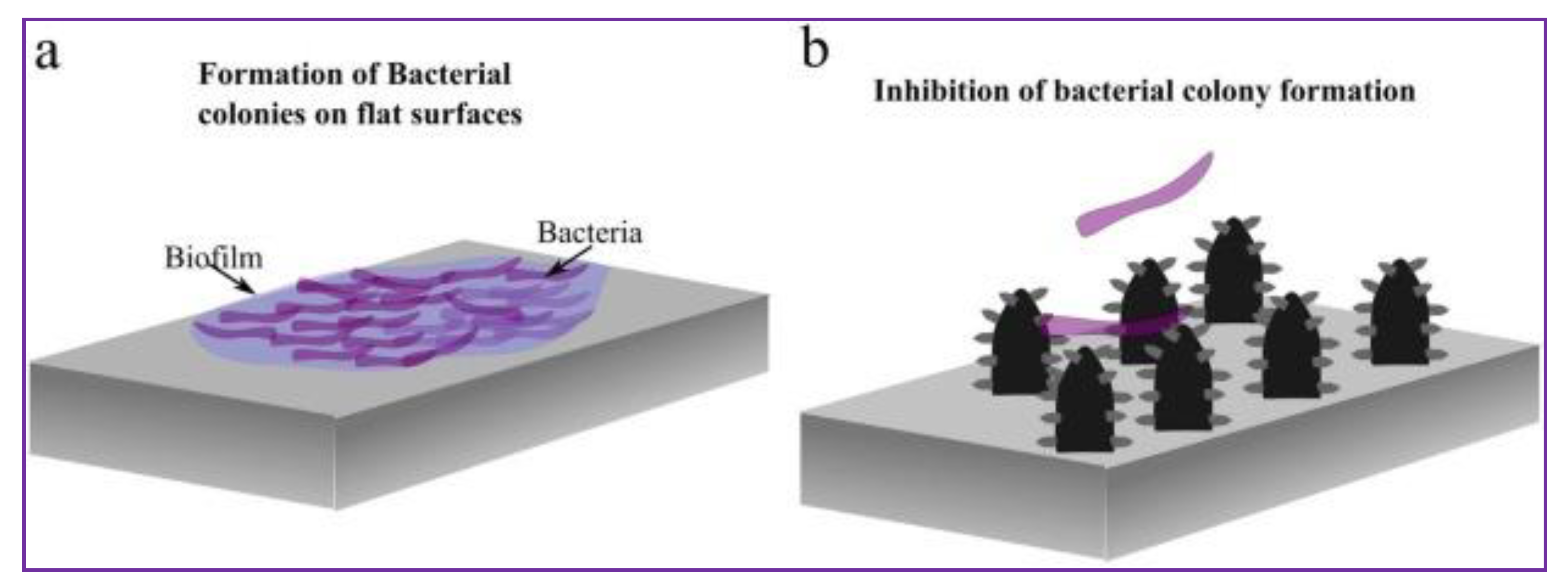
3.2.2. Cationic Polymers
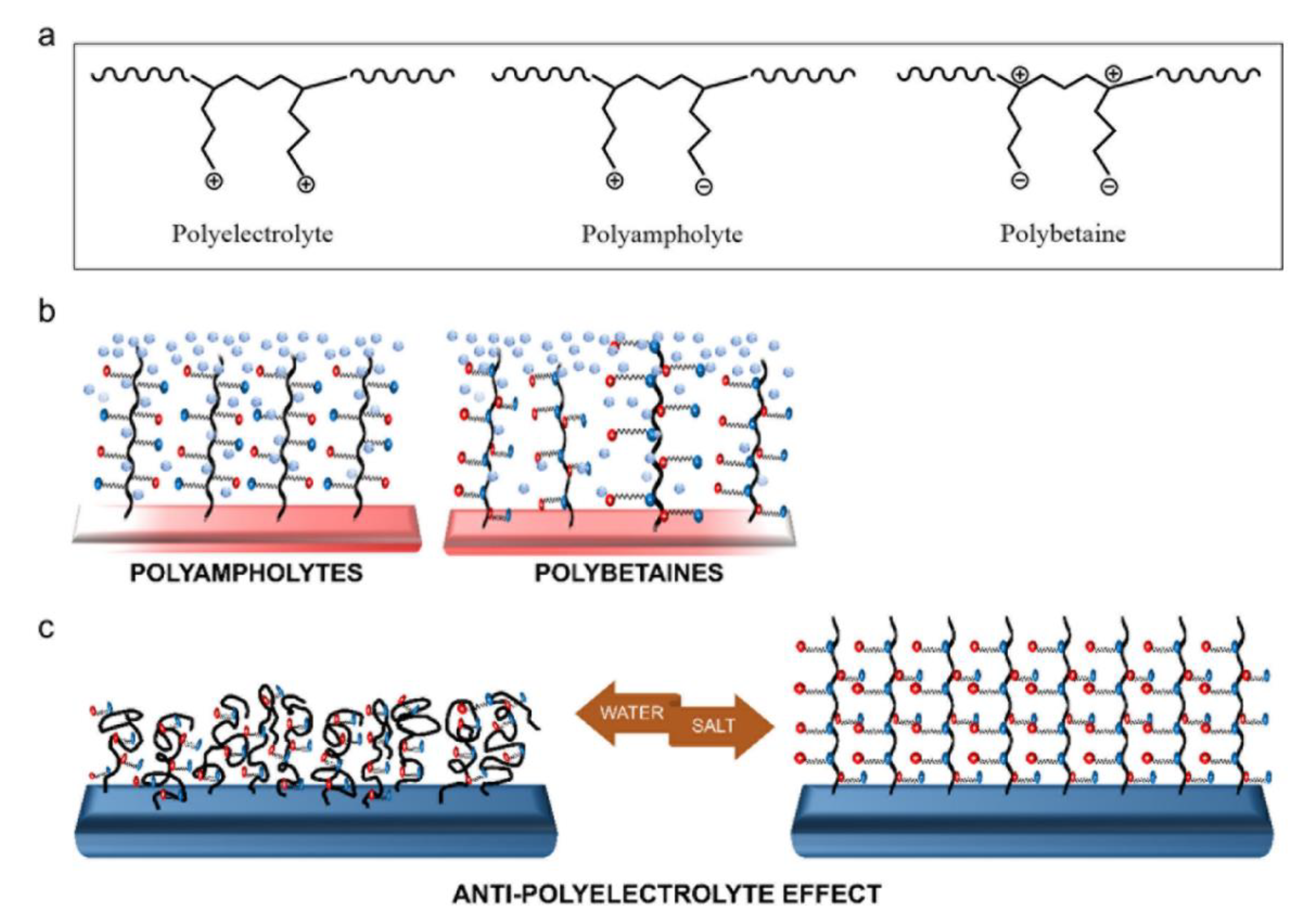
3.2.3. Polyzwitterions
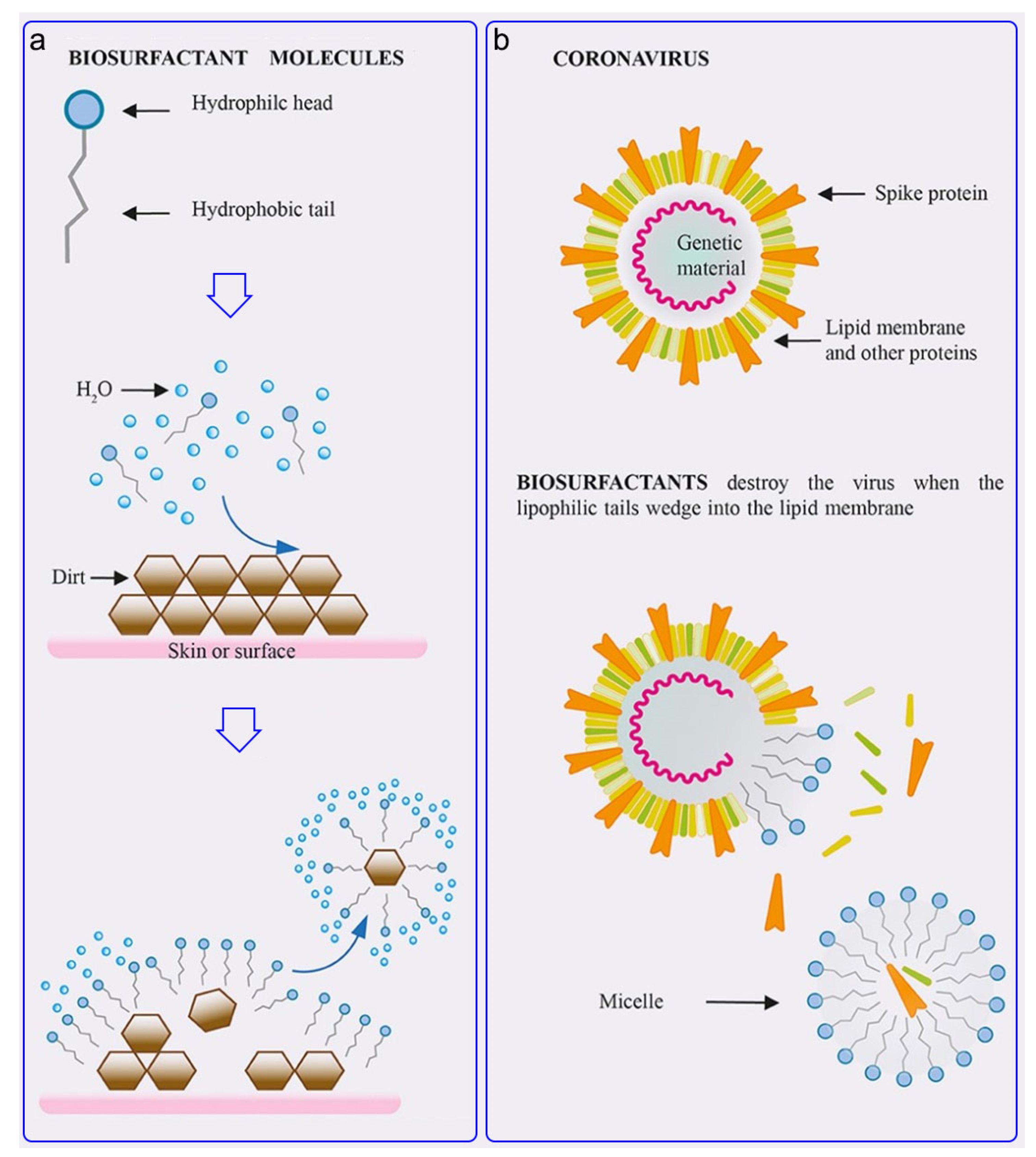
3.3. Surfactants
4. Modification of Surface Topography
4.1. Anti-Fouling Surface Structures
4.2. Fluorine-Containing Polymers
5. Current Status of Virus Inactivating Surfaces
6. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6. [Google Scholar] [CrossRef]
- Charnley, M.; Textor, M.; Acikgoz, C. Designed polymer structures with antifouling–antimicrobial properties. React. Funct. Polym. 2011, 71, 329–334. [Google Scholar] [CrossRef]
- Swartjes, J.J.T.M.; Sharma, P.K.; Kooten, T.G.v.; Mei, H.C.v.d.; Mahmoudi, M.; Busscher, H.J.; Rochford, E.T.J. Current Developments in Antimicrobial Surface Coatings for Biomedical Applications. Curr. Med. Chem. 2015, 22, 2116–2129. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Erkoc, P.; Yasa, I.C.; Ceylan, H.; Yasa, O.; Alapan, Y.; Sitti, M. Mobile Microrobots for Active Therapeutic Delivery. Adv. Ther. 2019, 2, 1800064. [Google Scholar] [CrossRef]
- Muhammad, W.; Zhai, Z.; Gao, C. Antiviral Activity of Nanomaterials against Coronaviruses. Macromol. Biosci. 2020, 20, 2000196. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Li, X.; Yu, H.; Che, C.; Luan, S.; Ren, Y.; Li, S.; Liu, P.; Yu, X.; et al. Multifunctional Antibacterial Materials Comprising Water Dispersible Random Copolymers Containing a Fluorinated Block and Their Application in Catheters. Acs Appl. Mater. Interfaces 2020, 12, 7617–7630. [Google Scholar] [CrossRef]
- Reina, G.; Peng, S.; Jacquemin, L.; Andrade, A.F.; Bianco, A. Hard Nanomaterials in Time of Viral Pandemics. Acs Nano 2020, 14, 9364–9388. [Google Scholar] [CrossRef]
- Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics. Materials 2020, 13, 4041. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Li, R.; Cui, L.; Chen, M.; Huang, Y. Nanomaterials for Airborne Virus Inactivation: A Short Review. Aerosol Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Jiang, C.-c.; Cao, Y.-k.; Xiao, G.-y.; Zhu, R.-f.; Lu, Y.-p. A review on the application of inorganic nanoparticles in chemical surface coatings on metallic substrates. RSC Adv. 2017, 7, 7531–7539. [Google Scholar] [CrossRef]
- Kausar, A. Polymer coating technology for high performance applications: Fundamentals and advances. J. Macromol. Sci. Part A 2018, 55, 440–448. [Google Scholar] [CrossRef]
- Habibi, M.H.; Parhizkar, J. Cobalt ferrite nano-composite coated on glass by Doctor Blade method for photo-catalytic degradation of an azo textile dye Reactive Red 4: XRD, FESEM and DRS investigations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Jurasin, D.D.; Curlin, M.; Capjak, I.; Crnkovic, T.; Lovric, M.; Babic, M.; Horak, D.; Vinkovic Vrcek, I.; Gajovic, S. Surface coating affects behavior of metallic nanoparticles in a biological environment. Beilstein J. Nanotechnol. 2016, 7, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.-y.; Zare, E.N.; Borzacchiello, A.; Niu, L.-n.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Felton, L.A. Characterization of coating systems. AAPS PharmSciTech 2007, 8, 258–266. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J. Scientific Importance of Water-Processable PEDOT–PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1121–1150. [Google Scholar] [CrossRef]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Erkoc, P. Sodium Borohydride and Essential Oils as Reducing Agents for the Chemically and Green Synthesis of Silver Nanoparticles: A Comparative Analysis. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Heinonen, S.; Nikkanen, J.P.; Laakso, J.; Raulio, M.; Priha, O.; Levänen, E. Bacterial growth on a superhydrophobic surface containing silver nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47, 012064. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Yang, M.; Wang, K.; Zhang, Y.; He, J. CuO Nanoparticles-Containing Highly Transparent and Superhydrophobic Coatings with Extremely Low Bacterial Adhesion and Excellent Bactericidal Property. Acs Appl. Mater. Interfaces 2018, 10, 25717–25725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, X.; Xiong, Z.; Huang, Q.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. Novel micro/nanostructured TiO2/ZnO coating with antibacterial capacity and cytocompatibility. Ceram. Int. 2018, 44, 9711–9719. [Google Scholar] [CrossRef]
- Noori, A.J.; Kareem, F.A. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon 2019, 5, e02568. [Google Scholar] [CrossRef]
- Piedade, A.P.; Pinho, A.C.; Branco, R.; Morais, P.V. Evaluation of antimicrobial activity of ZnO based nanocomposites for the coating of non-critical equipment in medical-care facilities. Appl. Surf. Sci. 2020, 513, 145818. [Google Scholar] [CrossRef]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small 2010, 6, 1044–1050. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Xu, T.; Wang, C.; Zhao, M.; Xiao, M.; Wang, H.; Deng, N.; Zhu, B. Delivery of VP1 siRNA to inhibit the EV71 virus using functionalized silver nanoparticles through ROS-mediated signaling pathways. RSC Adv. 2017, 7, 1453–1463. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Garza Treviño, E.N.; Singh, D.K. Use of silver nanoparticles increased inhibition of cell-associated HIV-1 infection by neutralizing antibodies developed against HIV-1 envelope proteins. J. Nanobiotechnol. 2011, 9, 38. [Google Scholar] [CrossRef]
- Yang, C.; Gao, S.; Dagnæs-Hansen, F.; Jakobsen, M.; Kjems, J. Impact of PEG Chain Length on the Physical Properties and Bioactivity of PEGylated Chitosan/siRNA Nanoparticles in Vitro and in Vivo. Acs Appl. Mater. Interfaces 2017, 9, 12203–12216. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Neu, M.; Germershaus, O.; Merkel, O.; Sitterberg, J.; Bakowsky, U.; Kissel, T. Influence of Polyethylene Glycol Chain Length on the Physicochemical and Biological Properties of Poly(ethylene imine)-graft-Poly(ethylene glycol) Block Copolymer/SiRNA Polyplexes. Bioconjug. Chem. 2006, 17, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Noorisafa, F.; Razmjou, A.; Emami, N.; Low, Z.-X.; Korayem, A.H.; Kajani, A.A. Surface modification of polyurethane via creating a biocompatible superhydrophilic nanostructured layer: Role of surface chemistry and structure. J. Exp. Nanosci. 2016, 11, 1087–1109. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23. [Google Scholar] [CrossRef]
- Nguyen, S.; Hiorth, M.; Rykke, M.; Smistad, G. Polymer coated liposomes for dental drug delivery—Interactions with parotid saliva and dental enamel. Eur. J. Pharm. Sci. 2013, 50, 78–85. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- James, B.; Ramakrishnan, R.; Aprem, A.S. Development of Environmentally Safe Biodegradable, Antibacterial Surgical Sutures Using Nanosilver Particles. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Reinbold, J.; Uhde, A.-K.; Müller, I.; Weindl, T.; Geis-Gerstorfer, J.; Schlensak, C.; Wendel, H.-P.; Krajewski, S. Preventing Surgical Site Infections Using a Natural, Biodegradable, Antibacterial Coating on Surgical Sutures. Moleculars 2017, 22, 1570. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(ethylene imine)s as antimicrobial agents with selective activity. Macromol. Biosci. 2012, 12, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Delaviz, Y.; Santerre, J.P.; Cvitkovitch, D.G. 11—Infection resistant biomaterials. In Biomaterials and Medical Device—Associated Infections; Barnes, L., Cooper, I.R., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 223–254. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Ramalho, P.; Silva, A.P.; Teixeira-Santos, R.; Pina-Vaz, C.; Rodrigues, A.G. Polyethyleneimine and polyethyleneimine-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med Microbiol. 2014, 63, 1167–1173. [Google Scholar] [CrossRef]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Antimicrobial Activity of Chitosan-Based Films Enriched with Green Tea Extracts on Murine Norovirus, Escherichia coli, and Listeria innocua. Int. J. Food Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ciejka, J.; Wolski, K.; Nowakowska, M.; Pyrc, K.; Szczubiałka, K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C 2017, 76, 735–742. [Google Scholar] [CrossRef]
- Raghuwanshi, D.; Mishra, V.; Das, D.; Kaur, K.; Suresh, M.R. Dendritic Cell Targeted Chitosan Nanoparticles for Nasal DNA Immunization against SARS CoV Nucleocapsid Protein. Mol. Pharm. 2012, 9, 946–956. [Google Scholar] [CrossRef]
- Buzzacchera, I.; Vorobii, M.; Kostina, N.Y.; De Los Santos Pereira, A.; Riedel, T.; Bruns, M.; Ogieglo, W.; Möller, M.; Wilson, C.J.; Rodriguez-Emmenegger, C. Polymer Brush-Functionalized Chitosan Hydrogels as Antifouling Implant Coatings. Biomacromolecules 2017, 18, 1983–1992. [Google Scholar] [CrossRef]
- Kumar, A.M.; Suresh, B.; Das, S.; Obot, I.B.; Adesina, A.Y.; Ramakrishna, S. Promising bio-composites of polypyrrole and chitosan: Surface protective and in vitro biocompatibility performance on 316L SS implants. Carbohydr. Polym. 2017, 173, 121–130. [Google Scholar] [CrossRef]
- Yi, X.; He, J.; Wang, X.; Zhang, Y.; Tan, G.; Zhou, Z.; Chen, J.; Chen, D.; Wang, R.; Tian, W.; et al. Tunable Mechanical, Antibacterial, and Cytocompatible Hydrogels Based on a Functionalized Dual Network of Metal Coordination Bonds and Covalent Crosslinking. Acs Appl. Mater. Interfaces 2018, 10, 6190–6198. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.-N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. Acs Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef]
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Wu, Z.; Hong, Y. Combination of the Silver–Ethylene Interaction and 3D Printing To Develop Antibacterial Superporous Hydrogels for Wound Management. Acs Appl. Mater. Interfaces 2019, 11, 33734–33747. [Google Scholar] [CrossRef] [PubMed]
- Demircan, D.; Zhang, B. Facile synthesis of novel soluble cellulose-grafted hyperbranched polymers as potential natural antimicrobial materials. Carbohydr. Polym. 2017, 157, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A Nanocomposite Hydrogel with Potent and Broad-Spectrum Antibacterial Activity. Acs Appl. Mater. Interfaces 2018, 10, 15163–15173. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Haldar, J. Direct Synthesis of Dextran-Based Antibacterial Hydrogels for Extended Release of Biocides and Eradication of Topical Biofilms. Acs Appl. Mater. Interfaces 2017, 9, 15975–15985. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Bousserrhine, N.; Alphonse, V.; Carbonnier, B. From beta-cyclodextrin polyelectrolyte to layer-by-layer self-assembly microcapsules: From inhibition of bacterial growth to bactericidal effect. Food Hydrocoll. 2019, 95, 219–227. [Google Scholar] [CrossRef]
- Pan, Y.; Xue, Y.; Snow, J.; Xiao, H. Tailor-Made Antimicrobial/Antiviral Star Polymer via ATRP of Cyclodextrin and Guanidine-Based Macromonomer. Macromol. Chem. Phys. 2015, 216, 511–518. [Google Scholar] [CrossRef]
- Chiloeches, A.; Echeverría, C.; Cuervo-Rodríguez, R.; Plachà, D.; López-Fabal, F.; Fernández-García, M.; Muñoz-Bonilla, A. Adhesive antibacterial coatings based on copolymers bearing thiazolium cationic groups and catechol moieties as robust anchors. Prog. Org. Coat. 2019, 136, 105272. [Google Scholar] [CrossRef]
- Tu, Q.; Tian, C.; Ma, T.; Pang, L.; Wang, J. Click synthesis of quaternized poly(dimethylaminoethyl methacrylate) functionalized graphene oxide with improved antibacterial and antifouling ability. Colloids Surf. B Biointerfaces 2016, 141, 196–205. [Google Scholar] [CrossRef]
- Plachá, D.; Muñoz-Bonilla, A.; Škrlová, K.; Echeverria, C.; Chiloeches, A.; Petr, M.; Lafdi, K.; Fernández-García, M. Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials. Nanomaterials 2020, 10, 1218. [Google Scholar] [CrossRef]
- Fang, B.; Jiang, Y.; Nüsslein, K.; Rotello, V.M.; Santore, M.M. Antimicrobial surfaces containing cationic nanoparticles: How immobilized, clustered, and protruding cationic charge presentation affects killing activity and kinetics. Colloids Surf. B Biointerfaces 2015, 125, 255–263. [Google Scholar] [CrossRef]
- Alkekhia, D.; Shukla, A. Influence of poly-l-lysine molecular weight on antibacterial efficacy in polymer multilayer films. J. Biomed Mater. Res. A 2019, 107, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, B.; Li, X.; Wu, Z.; He, Y.; Song, P.; Wang, R. Antimicrobial cationic acrylate-based hybrid coatings against microorganism contamination. Prog. Org. Coat. 2020, 142, 105576. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Goddard, J.M. Self-healing antimicrobial polymer coating with efficacy in the presence of organic matter. Appl. Surf. Sci. 2016, 378, 479–488. [Google Scholar] [CrossRef]
- Gokkaya, D.; Topuzogullari, M.; Arasoglu, T.; Trabzonlu, K.; Ozmen, M.M.; Abdurrahmanoğlu, S. Antibacterial properties of cationic copolymers as a function of pendant alkyl chain length and degree of quaternization. Polym. Int. 2020. [Google Scholar] [CrossRef]
- Özçelik, H.; Vrana, N.E.; Gudima, A.; Riabov, V.; Gratchev, A.; Haikel, Y.; Metz-Boutigue, M.H.; Carradò, A.; Faerber, J.; Roland, T.; et al. Harnessing the multifunctionality in nature: A bioactive agent release system with self-antimicrobial and immunomodulatory properties. Adv. Healthc. Mater. 2015, 4, 2026–2036. [Google Scholar] [CrossRef]
- Khalil, H.; Chen, T.; Riffon, R.; Wang, R.; Wang, Z. Synergy between polyethylenimine and different families of antibiotics against a resistant clinical isolate of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 1635–1641. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Zarebkohan, A.; Annabi, N.; Akbarzadeh, A.; Salehi, R. Significant role of cationic polymers in drug delivery systems. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1872–1891. [Google Scholar] [CrossRef]
- Babu, R.J.; Annaji, M.; Alsaqr, A.; Arnold, R.D. Chapter 15—Animal-Based Materials in the Formulation of Nanocarriers for Anticancer Therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Kesharwani, P., Paknikar, K.M., Gajbhiye, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 319–341. [Google Scholar] [CrossRef]
- Mehvar, R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J. Control. Release 2000, 69, 1–25. [Google Scholar] [CrossRef]
- Sajna, K.V.; Gottumukkala, L.D.; Sukumaran, R.K.; Pandey, A. Chapter 18—White Biotechnology in Cosmetics. In Industrial Biorefineries White Biotechnology; Pandey, A., Höfer, R., Taherzadeh, M., Nampoothiri, K.M., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 607–652. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef]
- Cryan, S.A.; Holohan, A.; Donohue, R.; Darcy, R.; O’Driscoll, C.M. Cell transfection with polycationic cyclodextrin vectors. Eur. J. Pharm. Sci. 2004, 21, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, L.; Gan, W.; Zhou, J.; Zhang, L. Self-assembled micelles based on hydrophobically modified quaternized cellulose for drug delivery. Colloids Surf. B Biointerfaces 2011, 83, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Reineke, T.M.; Davis, M.E. 9.26—Nucleic Acid Delivery via Polymer Vehicles. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 497–527. [Google Scholar] [CrossRef]
- Shi, C.; He, Y.; Feng, X.; Fu, D. ε-Polylysine and next-generation dendrigraft poly-L-lysine: Chemistry, activity, and applications in biopharmaceuticals. J. Biomater. Sci. Polym. Ed. 2015, 26, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, F.; Guo, J.; Ren, P.; Tian, Z.; Bai, J.; Hua, J. Polymeric micelles with aggregation-induced emission based on microbial ε-polylysine for doxorubicin delivery. Eur. Polym. J. 2020, 122, 109355. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Nam, J.P.; Nah, J.W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Gupta, K.C.; Jabrail, F.H. Effect of molecular weight and degree of deacetylation on controlled release of isoniazid from chitosan microspheres. Polym. Adv. Technol. 2008, 19, 432–441. [Google Scholar] [CrossRef]
- Hussain, M.R.; Iman, M.; Maji, T.K. Determination of Degree of Deacetylation of Chitosan and Their effect on the Release Behavior of Essential Oil from Chitosan and Chitosan- Gelatin Complex Microcapsules. Int. J. Adv. Eng. Appl. 2013, 2, 4–12. [Google Scholar]
- Zhang, H.; Li, Y.; Zhang, X.; Liu, B.; Zhao, H.; Chen, D. Directly determining the molecular weight of chitosan with atomic force microscopy. Front. Nanosci. Nanotech. 2016, 2, 123–127. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cui, F.; Shi, K.; Wang, J.; Niu, M.; Ma, R. The effect of chitosan molecular weight on the characteristics of spray-dried methotrexate-loaded chitosan microspheres for nasal administration. Drug Dev. Ind. Pharm. 2009, 35, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials (Basel) 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Costa, F.; Sousa, D.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Hoogeterp, J.J.; Mattie, H.; Krul, A.M.; Terporten, P.; van Furth, R. Comparison of in vivo and in vitro activities of antibiotics with different modes of action against a tolerant and a non-tolerant Staphylococcus aureus strain. Scand. J. Infect. Dis. 1989, 21, 95–101. [Google Scholar] [CrossRef]
- Fantin, B.; Leggett, J.; Ebert, S.; Craig, W.A. Correlation between in vitro and in vivo activity of antimicrobial agents against gram-negative bacilli in a murine infection model. Antimicrob. Agents Chemother. 1991, 35, 1413–1422. [Google Scholar] [CrossRef]
- Liu, H.; Kim, Y.; Mello, K.; Lovaasen, J.; Shah, A.; Rice, N.; Yim, J.H.; Pappas, D.; Klibanov, A.M. Aerosol-assisted plasma deposition of hydrophobic polycations makes surfaces highly antimicrobial. Appl. Biochem. Biotechnol. 2014, 172, 1254–1264. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Q.; Yang, T.; Cao, J.; Lin, Q.; Yuan, Z.; Li, L. Polyethyleneimine Capped Silver Nanoclusters as Efficient Antibacterial Agents. Int. J. Environ. Res. Public Health 2016, 13, 334. [Google Scholar] [CrossRef]
- Sovadinova, I.; Palermo, E.F.; Huang, R.; Thoma, L.M.; Kuroda, K. Mechanism of Polymer-Induced Hemolysis: Nanosized Pore Formation and Osmotic Lysis. Biomacromolecules 2011, 12, 260–268. [Google Scholar] [CrossRef]
- Singh, M.; Tarannum, N. Polyzwitterions. In Engineering of Biomaterials for Drug Delivery Systems; Parambath, A., Ed.; Elsevier Inc.: Cambridge, UK, 2018; pp. 69–101. [Google Scholar] [CrossRef]
- Mangal, U.; Kwon, J.-S.; Choi, S.-H. Bio-Interactive Zwitterionic Dental Biomaterials for Improving Biofilm Resistance: Characteristics and Applications. Int. J. Mol. Sci. 2020, 21, 9087. [Google Scholar] [CrossRef]
- Paschke, S.; Lienkamp, K. Polyzwitterions: From Surface Properties and Bioactivity Profiles to Biomedical Applications. Acs Appl. Polym. Mater. 2020, 2, 129–151. [Google Scholar] [CrossRef]
- Liu, P.; Xu, G.; Pranantyo, D.; Xu, L.Q.; Neoh, K.G.; Kang, E.T. PH-Sensitive Zwitterionic Polymer as an Antimicrobial Agent with Effective Bacterial Targeting. Acs Biomater. Sci. Eng. 2018, 4, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Fang, Y.L.; Hsieh, P.S.; Li, C.C.; Dai, N.T.; Huang, C.J. Non-sticky and antimicrobial zwitterionic nanocomposite dressings for infected chronic wounds. Biomater. Sci. 2017, 5, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, M.; Eickenscheidt, A.; Guevara-Solarte, D.L.; Widyaya, V.T.; Marx, F.; Al-Ahmad, A.; Lienkamp, K. A Simultaneously Antimicrobial, Protein-Repellent, and Cell-Compatible Polyzwitterion Network. Biomacromolecules 2017, 18, 1373–1386. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Goda, T.; Matsumoto, A.; Takeuchi, H.; Yamaoka, S.; Miyahara, Y. Gold Nanoparticles with Ligand/Zwitterion Hybrid Layer for Individual Counting of Influenza A H1N1 Subtype Using Resistive Pulse Sensing. Langmuir 2019, 35, 1798–1806. [Google Scholar] [CrossRef]
- Sun, Z.; Ostrikov, K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020, 25, e00203. [Google Scholar] [CrossRef]
- Porter, M.R. Handbook of Surfactants, 1st ed.; Springer US: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of Surfactants and Polymers on Stability and Antibacterial Activity of Silver Nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Fages, E.; Pascual, J.; Fenollar, O.; García-Sanoguera, D.; Balart, R. Study of antibacterial properties of polypropylene filled with surfactant-coated silver nanoparticles. Polym. Eng. Sci. 2011, 51, 804–811. [Google Scholar] [CrossRef]
- Gîfu, I.C.; Maxim, M.E.; Cinteza, L.O.; Popa, M.; Aricov, L.; Leontieș, A.R.; Anastasescu, M.; Anghel, D.-F.; Ianchis, R.; Ninciuleanu, C.M.; et al. Antimicrobial Activities of Hydrophobically Modified Poly(Acrylate) Films and Their Complexes with Different Chain Length Cationic Surfactants. Coatings 2019, 9, 244. [Google Scholar] [CrossRef]
- Bračič, M.; Pérez, L.; Martinez-Pardo, R.I.; Kogej, K.; Hribernik, S.; Šauperl, O.; Fras Zemljič, L. A novel synergistic formulation between a cationic surfactant from lysine and hyaluronic acid as an antimicrobial coating for advanced cellulose materials. Cellulose 2014, 21, 2647–2663. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Elmanama, A.A.; Amara, N.; Qodih, F.S.; Selmane, M.; Chehimi, M.M. The efficacy of surfactants in stabilizing coating of nano-structured CuO particles onto the surface of cotton fibers and their antimicrobial activity. Mater. Chem. Phys. 2018, 215, 221–228. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef]
- Lin, S.-C. Biosurfactants: Recent advances. J. Chem. Technol. Biotechnol. 1996, 66, 109–120. [Google Scholar] [CrossRef]
- Janek, T.; Łukaszewicz, M.; Krasowska, A. Antiadhesive activity of the biosurfactant pseudofactin II secreted by the Arctic bacterium Pseudomonas fluorescensBD5. BMC Microbiol. 2012, 12, 24. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and -negative bacteria. FEMS Microbiol. Lett. 2016, 363, fnv224. [Google Scholar] [CrossRef]
- Borsanyiova, M.; Patil, A.; Mukherji, R.; Prabhune, A.; Bopegamage, S. Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol. 2016, 61, 85–89. [Google Scholar] [CrossRef]
- Smith, M.L.; Gandolfi, S.; Coshall, P.M.; Rahman, P.K.S.M. Biosurfactants: A Covid-19 Perspective. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Çelik, P.A.; Manga, E.B.; Çabuk, A.; Banat, I.M. Biosurfactants’ Potential Role in Combating COVID-19 and Similar Future Microbial Threats. Appl. Sci. 2021, 11, 334. [Google Scholar] [CrossRef]
- Zin Ivan, M.; Pokhmurskii Vasyl, I.; Korniy Sergiy, A.; Karpenko Olena, V.; Lyon Stuart, B.; Khlopyk Olha, P.; Tymus Mariana, B. Corrosion inhibition of aluminium alloy by rhamnolipid biosurfactant derived from pseudomonas sp. PS-17. Anti-Corros. Methods Mater. 2018, 65, 517–527. [Google Scholar] [CrossRef]
- Parthipan, P.; Sabarinathan, D.; Angaiah, S.; Rajasekar, A. Glycolipid biosurfactant as an eco-friendly microbial inhibitor for the corrosion of carbon steel in vulnerable corrosive bacterial strains. J. Mol. Liq. 2018, 261, 473–479. [Google Scholar] [CrossRef]
- Płaza, G.; Achal, V. Biosurfactants: Eco-Friendly and Innovative Biocides against Biocorrosion. Int. J. Mol. Sci. 2020, 21, 2152. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Lucera, A.; Costa, C.; Conte, A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Glinel, K.; Jonas, A.M.; Jouenne, T.; Leprince, J.; Galas, L.; Huck, W.T.S. Antibacterial and Antifouling Polymer Brushes Incorporating Antimicrobial Peptide. Bioconjug. Chem. 2009, 20, 71–77. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Larsson, L.; Granhag, L. Towards an absolute scale for adhesion strength of ship hull microfouling. Biofouling 2019, 35, 244–258. [Google Scholar] [CrossRef]
- Jalil, S.A.; Akram, M.; Bhat, J.A.; Hayes, J.J.; Singh, S.C.; ElKabbash, M.; Guo, C. Creating superhydrophobic and antibacterial surfaces on gold by femtosecond laser pulses. Appl. Surf. Sci. 2020, 506, 144952. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Milazzo, M.; Gallone, G.; Marcello, E.; Mariniello, M.D.; Bruschini, L.; Roy, I.; Danti, S. Biodegradable Polymeric Micro/Nano-Structures with Intrinsic Antifouling/Antimicrobial Properties: Relevance in Damaged Skin and Other Biomedical Applications. J. Funct. Biomater. 2020, 11, 60. [Google Scholar] [CrossRef]
- Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Schumacher, J.F.; Wilkerson, W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered antifouling microtopographies--correlating wettability with cell attachment. Biofouling 2006, 22, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Anatoliy, Y.V.; Chunlei, G. Femtosecond Laser Surface Structuring of Biocompatible Metals. SPIE: Bellingham, WA, USA, 2009. [Google Scholar] [CrossRef]
- Ko, S.H.; Choi, Y.; Hwang, D.J.; Grigoropoulos, C.P.; Chung, J.; Poulikakos, D. Nanosecond laser ablation of gold nanoparticle films. Appl. Phys. Lett. 2006, 89, 141126. [Google Scholar] [CrossRef]
- Freschauf, L.R.; McLane, J.; Sharma, H.; Khine, M. Shrink-Induced Superhydrophobic and Antibacterial Surfaces in Consumer Plastics. PLoS ONE 2012, 7, e40987. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Mehrjou, B.; Tang, K.; Wang, H.; Huo, K.; Qasim, A.M.; Wang, G.; Chu, P.K. Dimensional-dependent antibacterial behavior on bioactive micro/nano polyetheretherketone (PEEK) arrays. Chem. Eng. J. 2020, 392, 123736. [Google Scholar] [CrossRef]
- Pegalajar-Jurado, A.; Easton, C.D.; Crawford, R.J.; McArthur, S.L. Fabrication of a platform to isolate the influences of surface nanotopography from chemistry on bacterial attachment and growth. Biointerphases 2015, 10, 011002. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, X.; Chen, C.; Hu, J.; Zhou, C.; Cai, X.; Wang, W.; Zheng, C.; Zhang, P.; Cheng, J.; et al. Durably Antibacterial and Bacterially Antiadhesive Cotton Fabrics Coated by Cationic Fluorinated Polymers. Acs Appl. Mater. Interfaces 2018, 10, 6124–6136. [Google Scholar] [CrossRef]
- de Groot, R.J.; Baker, S.C.; Baric, R.S.; Brown, C.S.; Drosten, C.; Enjuanes, L.; Fouchier, R.A.M.; Galiano, M.; Gorbalenya, A.E.; Memish, Z.A.; et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J. Virol. 2013, 87, 7790–7792. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, H.V. Pandemic Preparedness and Response — Lessons from the H1N1 Influenza of 2009. N. Engl. J. Med. 2014, 370, 1335–1342. [Google Scholar] [CrossRef]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Lentz, T.L. The recognition event between virus and host cell receptor: A target for antiviral agents. J. Gen. Virol. 1990, 71, 751–766. [Google Scholar] [CrossRef]
- Lai, M.Y.Y.; Cheng, P.K.C.; Lim, W.W.L. Survival of Severe Acute Respiratory Syndrome Coronavirus. Clin. Infect. Dis. 2005, 41, e67–e71. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, C.R.; Seale, H.; Dung, T.C.; Hien, N.T.; Nga, P.T.; Chughtai, A.A.; Rahman, B.; Dwyer, D.E.; Wang, Q. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open 2015, 5, e006577. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jayaraman, S. From containment to harm reduction from SARS-CoV-2: A fabric mask for enhanced effectiveness, comfort, and compliance. J. Text. Inst. 2020, 1–15. [Google Scholar] [CrossRef]
- Kawabata, N.; Yamazaki, K.; Otake, T.; Oishi, I.; Minekawa, Y. Removal of pathogenic human viruses by insoluble pyridinium-type resin. Epidemiol. Infect. 1990, 105, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H. Antibacterial/Antiviral Property and Mechanism of Dual-Functional Quaternized Pyridinium-type Copolymer. Polymers 2015, 7, 2290–2303. [Google Scholar] [CrossRef]
- Rao, G.; Brastad, K.S.; Zhang, Q.; Robinson, R.; He, Z.; Li, Y. Enhanced disinfection of Escherichia coli and bacteriophage MS2 in water using a copper and silver loaded titanium dioxide nanowire membrane. Front. Environ. Sci. Eng. 2016, 10, 11. [Google Scholar] [CrossRef]
- Katz, E.; Margalith, E. Inhibition of vaccinia virus maturation by zinc chloride. Antimicrob. Agents Chemother. 1981, 19, 213–217. [Google Scholar] [CrossRef]
- Haldar, J.; An, D.; Álvarez de Cienfuegos, L.; Chen, J.; Klibanov, A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17667–17671. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Patil, A.; Raza, B.G.; Reurink, D.; van den Hengel, S.K.; Rutjes, S.A.; de Roda Husman, A.M.; Roesink, H.D.W.; de Vos, W.M. Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J. Membr. Sci. 2019, 570–571, 494–503. [Google Scholar] [CrossRef]
- Ahirwar, H.; Zhou, Y.; Mahapatra, C.; Ramakrishna, S.; Kumar, P.; Nanda, H.S. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings 2020, 10, 264. [Google Scholar] [CrossRef]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.M.; Cortes, A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev. Med. Devices 2020, 17, 477–481. [Google Scholar] [CrossRef] [PubMed]

| Type | Polymer | Application | Species | References |
|---|---|---|---|---|
| Natural | Chitosan | 1 Edible films 2 Virus purification 3 Plasmid DNA-loaded biotinylated chitosan nanoparticles for severe acute respiratory syndrome coronavirus (SARS-CoV) immunization | 1 Murine norovirus, Listeria innocua and Escherichia coli (E. coli) K12 2 Human coronavirus NL63 (HCoV-NL63), human coronavirus OC43 (HCoV-OC43) and mouse hepatitis virus (MHV) 3 SARS-CoV | 1 Amankwaah et al. [45] 2 Ciejka et al. [46] 3 Raghuwanshi et al. [47] |
| Chitosan-based coatings functionalized with methacrylate-based polymer brushes | Implantable sensor | Antifouling, leukocytes, and platelet rich plasma | Buzzacchera et al. [48] | |
| Polypyrrole/chitosan composites | Surface protective and in vitro biocompatible 316L stainless steel implant coating | MG-63 human osteoblast cell | Kumar et al. [49] | |
| Gelatin | 1 Antibacterial agent in the form of hydrogel 2 Injectable hydrogel wound dressing | E. coli and S. aureus 1 S. aureus, E. coli, MRSA 2 | 1 Yi et al. [50] 2 Zheng et al. [51] | |
| Gelatin/chlorhexidine acetate (CHA) | Self-healing hydrogel for wound healing | S. aureus, E. coli | Chen et al. [52] | |
| Cellulose | 1 Superporous hydrogel dressing 2 Natural antimicrobial material | 1S. aureus, E. coli 2E. coli, S. aureus, Proteus microbilis, Proteus vulgari, P. aeruginosa, Enterobacter aerogenes, Bacillus thuringiensis, Salmonella enterica serotype typhmurium, Streptococcus mutans | 1 Wu et al. [53] 2 Demircan et al. [54] | |
| Dextran | 1 Antibacterial hydrogel 2 Hydrogels for biocide release | 1S. aureus, S. Epidermidis, E. coli, P. Aeruginosa 2S. aureus, E. coli, methicillin-resistant S. aureus (MRSA) | 1 Dai et al. [55] 2 Hoque et al. [56] | |
| Cyclodextrin | 1 Polyelectrolyte to microcapsules with antibacterial effect 2 Antibacterial and antiviral agent | 1S. aureus, E. Coli 2E. coli and adenovirus (ADV) | 1 Belbekhouche et al. [57] 2 Pan et al. [58] | |
| Synthetic | 2-(4-methylthiazol-5-yl) ethyl methacrylate (MTA) and N-(3,4-dihydroxyphenethyl) methacrylamide (DOMA) copolymers | Adhesive bacterial coating | E. coli and S. aureus | Chiloeches et al. [59] |
| Poly(dimethylaminoethylmethacrylate)functionalizedgraphene oxide (GO–QPDMAEMA) | Antibacterial and antifouling effects | E. coli and S. aureus | Tu et al. [60] | |
| N-(3,4-dihydroxyphenethyl) methacrylamide (DOMA) and 2-(4-methylthiazol-5-yl) ethyl methacrylate (MTA) quaternized with methyl iodide | Antibacterial effects | S. aureus, S. Epidermidis, E. coli and P. aeruginosa | Plachá et al. [61] | |
| PEG brush surfaces- PLL coils composites and cationically functionalized gold nanoparticles | Antibacterial surface including cationic nanoparticles | S. aureus | Fang et al. [62] | |
| Poly-L-lysine (PLL) and hyaluronic acid (HA) denoted PLL30, PLL90, and PLL400 were used | Antibacterial coating | S. aureus, MRSA, P. aeruginosa and E. coli | Alkekhia et al. [63] | |
| Cationic acrylate-based copolymers (PAMs) by 3-(methacryloylamino) propyltrimethyl ammonium chloride (MPAC) and acrylates (BA, MMA) | Copolymers and their films were sued as surface coatings | E. coli and S.aureus | Wang et al. [64] | |
| Multilayers of Polyethylenimine (PEI) and styrene maleic anhydride copolymer (SMA) | Coating on polypyrrole (PP)-based substrates | E. coli | Bastarrachea et al. [65] | |
| Quaternized poly(4-vinylpyridine-co-N-vinylpyrrolidone) (P(4VP-co-NVP)) copolymers | Antibacterial activity test | E. coli and S. aureus | Gokkaya et al. [66] | |
| Poly-arginine (PAR) | Multifunctional coating polyelectrolyte layers | S. aureus | Özçelik et al. [67] | |
| PEI | 1 Bactericidal agent 2 Antimicrobial agent | 1Pseudomonas strains 2 S. aureus and E. Coli | 1 Khalil et al. [68] 2 Gibney et al. [42] | |
| PEI and PEI-based nanoparticles | Antibacterial agent on polyurethane based medical catheters | S. epidermidis, Acinetobacter baumannii (A. baumannii), S. aureus and Candida albicans | Azevedo et al. [44] | |
| For multiple examples of a polymer, corresponding study, application, and species are shown as superscript numbers. | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis 2021, 3, 25-52. https://doi.org/10.3390/prosthesis3010005
Erkoc P, Ulucan-Karnak F. Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis. 2021; 3(1):25-52. https://doi.org/10.3390/prosthesis3010005
Chicago/Turabian StyleErkoc, Pelin, and Fulden Ulucan-Karnak. 2021. "Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies" Prosthesis 3, no. 1: 25-52. https://doi.org/10.3390/prosthesis3010005
APA StyleErkoc, P., & Ulucan-Karnak, F. (2021). Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis, 3(1), 25-52. https://doi.org/10.3390/prosthesis3010005






