The Radical Pair Mechanism and Its Quantum Role in Plant Reactive Oxygen Species Production Under Hypomagnetic Fields
Abstract
1. Introduction
2. Sites of ROS Production in Plants
2.1. Photosynthesis Is a Major Source of ROS
2.2. Mitochondrial Respiration Provides Chemical Energy and Also Generates ROS
2.3. Peroxisomes Are Characterized by Oxidative Enzymes but Lack an RPM
2.4. In the Plasma Membrane, RBOH Generates ROS
3. The Quantum Biology of the Radical Pair Mechanism in ROS Production
4. Under Abiotic Stress, Reduced ROS Production Is a Quantum Signature
4.1. Direct Quantum Effects on Radical Pairs and ROS Production
4.2. Secondary Physiological Responses
4.3. The Role of Cryptochrome
5. Discriminating RPM from Alternative Quantum Mechanisms
5.1. Orientation and Resonance Protocols
5.2. Genetic and Mutational Dissection
5.3. Isotope-Sensitive Tests
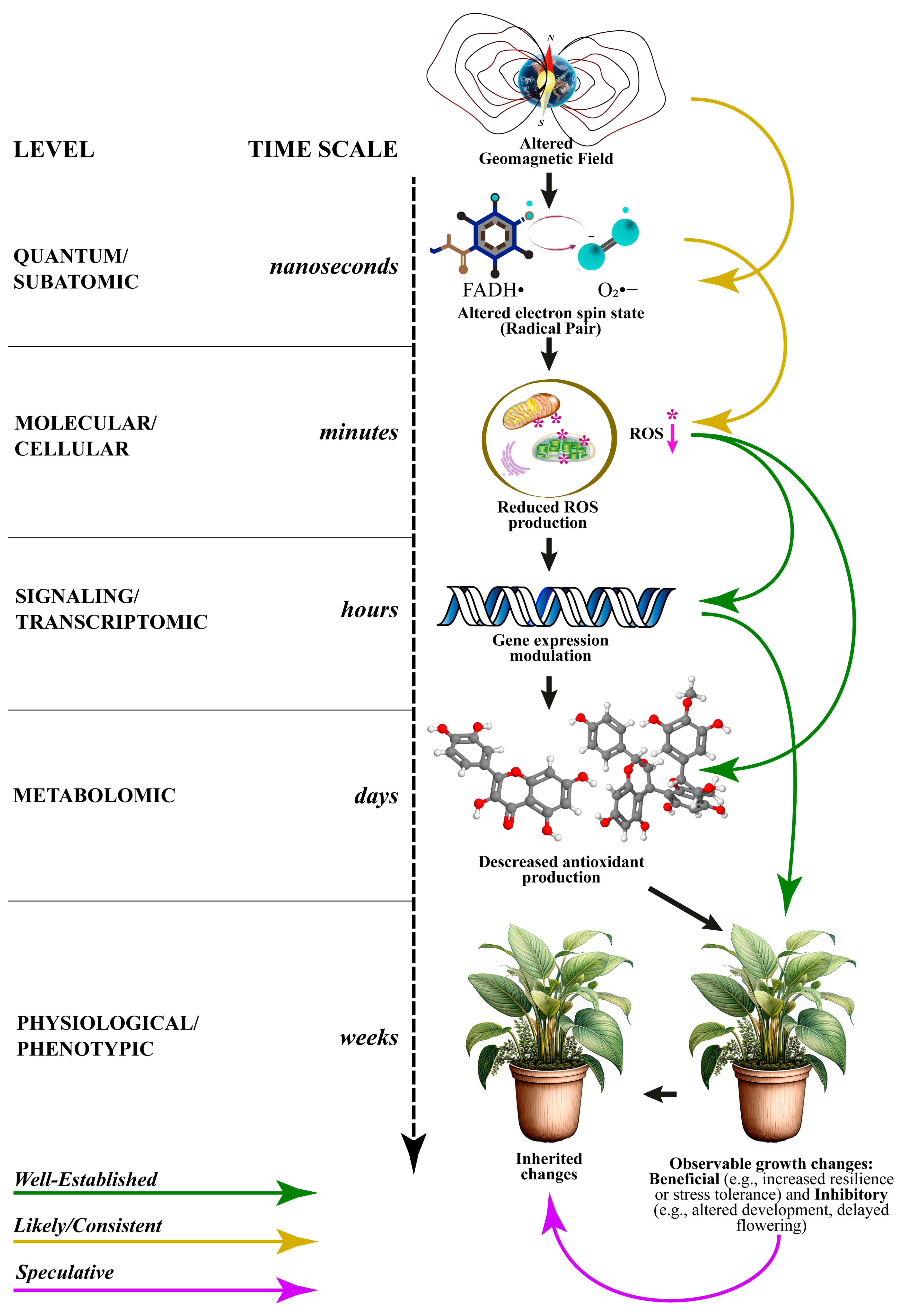
6. A Comprehensive Model for Quantum Effects on ROS Production
6.1. The Subatomic Level Involves a Quantum Trigger (Timescale Nanoseconds)
6.2. The Molecular/Cellular Level Is Where ROS Production Is Reduced (Timescale Minutes)
6.3. Genetic Regulation Occurs at the Signalling/Transcriptomic Level (Timescale Hours)
6.4. The Metabolomic Level Impacts on Antioxidant Regulation (Timescale Days)
6.5. The Physiolocial/Phenotypic Level Involves Growth and Development (Timescale Weeks)
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMFE | Anisotropic Magnetic Field Effects |
| APX | Ascorbate Peroxidase |
| CAT | Catalase |
| DEGs | Differentially Expressed Genes |
| ESR | Electron Spin Resonance |
| ETC | Electron Transport Chain |
| GMF | Geomagnetic Field |
| hMF | Hypomagnetic field |
| MF | Magnetic Field |
| NMR | Nuclear Magnetic Resonance |
| PSI | Photosystem I |
| PSII | Photosystem II |
| RBOH | Respiratory Burst Oxidase Homolog |
| ROS | Reactive Oxygen Species |
| RPM | Radical Pair Mechanism |
| SOD | Superoxide Dismutase |
References
- Lin, Y.; Marti, P.; Jackson, A. Invariance of dynamo action in an early-Earth model. Nature 2025, 644, 109–114. [Google Scholar] [CrossRef]
- Lyon, J. The solar wind-magnetosphere-ionosphere system. Science 2000, 288, 1987–1991. [Google Scholar] [CrossRef]
- Gill, J.; Taylor, B. Navigation by magnetic signatures in a realistic model of Earth’s magnetic field. Bioinspir. Biomim. 2024, 19, 036006. [Google Scholar] [CrossRef]
- Grob, R.; Mueller, V.; Gruebel, K.; Roessler, W.; Fleischmann, P. Importance of magnetic information for neuronal plasticity in desert ants. Proc. Natl. Acad. Sci. USA 2024, 121, e2320764121. [Google Scholar] [CrossRef]
- Grob, R.; Wegmann, J.; Rössler, W.; Fleischmann, P. Cataglyphis ants have a polarity-sensitive magnetic compass. Curr. Biol. 2024, 34, 5833–5838.e2. [Google Scholar] [CrossRef]
- Serna, J.; Alves, O.; Abreu, F.; Acosta-Avalos, D. Magnetite in the abdomen and antennae of Apis mellifera honeybees. J. Biol. Phys. 2024, 50, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, M.; Vodeneev, V. The role of signaling systems of plant in responding to key astrophysical factors: Increased ionizing radiation, near-null magnetic field and microgravity. Planta 2025, 261, 31. [Google Scholar] [CrossRef] [PubMed]
- Parmagnani, A.; D’Alessandro, S.; Maffei, M. Iron-sulfur complex assembly: Potential players of magnetic induction in plants. Plant Sci. 2022, 325, 111483. [Google Scholar] [CrossRef] [PubMed]
- Thoradit, T.; Thongyoo, K.; Kamoltheptawin, K.; Tunprasert, L.; El-Esawi, M.; Aguida, B.; Jourdan, N.; Buddhachat, K.; Pooam, M. Cryptochrome and quantum biology: Unraveling the mysteries of plant magnetoreception. Front. Plant Sci. 2023, 14, 1266357. [Google Scholar] [CrossRef]
- Galván, I.; Hassasfar, A.; Adams, B.; Petruccione, F. Isotope effects on radical pair performance in cryptochrome: A new hypothesis for the evolution of animal migration. Bioessays 2024, 46, e2300152. [Google Scholar] [CrossRef]
- Paponov, I.A.; Fliegmann, J.; Narayana, R.; Maffei, M.E. Differential root and shoot magnetoresponses in Arabidopsis thaliana. Sci. Rep. 2021, 11, 9195. [Google Scholar] [CrossRef] [PubMed]
- Denjalli, I.; Knieper, M.; Uthoff, J.; Vogelsang, L.; Kumar, V.; Seidel, T.; Dietz, K. The centrality of redox regulation and sensing of reactive oxygen species in abiotic and biotic stress acclimatization. J. Exp. Bot. 2024, 75, 4494–4511. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Plant quantum biology: The quantum dimension of plant responses to stress. Plant Stress 2025, 17, 100930. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; Mannino, G.; Maffei, M.E. Transcriptomics and Metabolomics of Reactive Oxygen Species Modulation in Near-Null Magnetic Field-Induced Arabidopsis thaliana. Biomolecules 2022, 12, 1824. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.; Han, C.; Wang, S.; Bai, M.; Song, C. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Foyer, C.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Zhang, M.; Ming, Y.; Wang, H.-B.; Jin, H.-L. Strategies for adaptation to high light in plants. aBIOTECH 2024, 5, 381–393. [Google Scholar] [CrossRef]
- Mattila, H.; Mishra, S.; Tyystjärvi, T.; Tyystjärvi, E. Singlet oxygen production by photosystem II is caused by misses of the oxygen evolving complex. New Phytol. 2023, 237, 113–125. [Google Scholar] [CrossRef]
- Koppenol, W.H. A resurrection of the Haber–Weiss reaction. Nat. Commun. 2022, 13, 396. [Google Scholar] [CrossRef]
- Cohen-Hoch, D.; Chen, T.; Sharabi, L.; Dezorella, N.; Itkin, M.; Feiguelman, G.; Malitsky, S.; Fluhr, R. Osmotic stress in roots drives lipoxygenase-dependent plastid remodeling through singlet oxygen production. Plant Physiol. 2024, 197, kiae589. [Google Scholar] [CrossRef]
- Viswanath, K.; Varakumar, P.; Pamuru, R.; Basha, S.; Mehta, S.; Rao, A. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; Caverzan, A.; Rauber, R.; de Souza, F.E.; Margis-Pinheiro, M.; Galina, A. Succinate dehydrogenase (mitochondrial complex II) is a source of reactive oxygen species in plants and regulates development and stress responses. New Phytol. 2015, 208, 776–789. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Armas, A.M.; Balparda, M.; Terenzi, A.; Busi, M.V.; Pagani, M.A.; Gomez-Casati, D.F. Iron-Sulfur Cluster Complex Assembly in the Mitochondria of Arabidopsis thaliana. Plants 2020, 9, 1171. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Wang, X.; Jiang, C.; Zhao, Y.; Lu, M.; Zhang, J. Peroxisome-Mediated Reactive Oxygen Species Signals Modulate Programmed Cell Death in Plants. Int. J. Mol. Sci. 2022, 23, 10087. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Zhang, X.; Liu, D.; Cheng, Y.; Shen, F. Role of plant respiratory burst oxidase homologs in stress responses. Free Radic. Res. 2018, 52, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, T. Entrainment and coherence in biology. Int. J. Yoga 2015, 8, 1–2. [Google Scholar] [CrossRef]
- Finkler, A.; Dasari, D. Quantum Sensing and Control of Spin-State Dynamics in the Radical-Pair Mechanism. Phys. Rev. Appl. 2021, 15, 034066. [Google Scholar] [CrossRef]
- Vishwamittar. Quantum Tunnelling—What, Where and Whatnot. Resonance 2025, 30, 59–75. [Google Scholar] [CrossRef]
- Mostajabi Sarhangi, S.; Matyushov, D.V. Electron Tunneling in Biology: When Does it Matter? ACS Omega 2023, 8, 27355–27365. [Google Scholar] [CrossRef] [PubMed]
- Abdallat, M.; Qaswal, A.B.; Eftaiha, M.; Qamar, A.R.; Alnajjar, Q.; Sallam, R.; Kollab, L.; Masa’deh, M.; Amayreh, A.; Mihyar, H.; et al. A mathematical modeling of the mitochondrial proton leak via quantum tunneling. AIMS Biophys. 2024, 11, 189–233. [Google Scholar] [CrossRef]
- Nunn, A.V.W.; Guy, G.W.; Bell, J.D. The quantum mitochondrion and optimal health. Biochem. Soc. Trans. 2016, 44, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Parmagnani, A.S.; Maffei, M.E. Reduction of the geomagnetic field to hypomagnetic field modulates tomato (Solanum lycopersicum L. cv Microtom) gene expression and metabolomics during plant development. J. Plant Physiol. 2025, 306, 154453. [Google Scholar] [CrossRef]
- Li, H.; Fang, Y.; Huang, J. Reactive oxygen species mediate bioeffects of static magnetic field via impairment of long-chain fatty acid degradation in Escherichia coli. Front. Microbiol. 2025, 16, 1586233. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; Betterle, N.; Mannino, G.; D’Alessandro, S.; Nocito, F.F.; Ljumovic, K.; Vigani, G.; Ballottari, M.; Maffei, M.E. The Geomagnetic Field (GMF) Is Required for Lima Bean Photosynthesis and Reactive Oxygen Species Production. Int. J. Mol. Sci. 2023, 24, 2896. [Google Scholar] [CrossRef]
- Wu, W.; Guo, X.; Dai, C.; Zhou, Z.; Sun, H.; Zhong, Y.; Sheng, H.; Zhang, C.; Yao, J. Magnetically Boosted Generation of Intracellular Reactive Oxygen Species toward Magneto-Photodynamic Therapy. J. Phys. Chem. B 2022, 126, 1895–1903. [Google Scholar] [CrossRef]
- Rishabh, R.; Zadeh-Haghighi, H.; Salahub, D.; Simon, C. Radical pairs may explain reactive oxygen species-mediated effects of hypomagnetic field on neurogenesis. PLoS Comput. Biol. 2022, 18, e1010198. [Google Scholar] [CrossRef]
- Fiorillo, A.; Parmagnani, A.S.; Visconti, S.; Mannino, G.; Camoni, L.; Maffei, M.E. 14-3-3 Proteins and the Plasma Membrane H+-ATPase Are Involved in Maize (Zea mays) Magnetic Induction. Plants 2023, 12, 2887. [Google Scholar] [CrossRef] [PubMed]
- Usselman, R.; Chavarriaga, C.; Castello, P.; Procopio, M.; Ritz, T.; Dratz, E.; Singel, D.; Martino, C. The Quantum Biology of Reactive Oxygen Species Partitioning Impacts Cellular Bioenergetics. Sci. Rep. 2016, 6, 38543. [Google Scholar] [CrossRef]
- Shine, M.; Guruprasad, K. Impact of pre-sowing magnetic field exposure of seeds to stationary magnetic field on growth, reactive oxygen species and photosynthesis of maize under field conditions. Acta Physiol. Plant. 2012, 34, 255–265. [Google Scholar] [CrossRef]
- Austvold, C.; Keable, S.; Procopio, M.; Usselman, R. Quantitative measurements of reactive oxygen species partitioning in electron transfer flavoenzyme magnetic field sensing. Front. Physiol. 2024, 15, 1348395. [Google Scholar] [CrossRef]
- Kataria, S.; Rastogi, A.; Bele, A.; Jain, M. Role of nitric oxide and reactive oxygen species in static magnetic field pre-treatment induced tolerance to ambient UV-B stress in soybean. Physiol. Mol. Biol. Plants 2020, 26, 931–945. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M.; Rastogi, A.; Brestic, M. Static magnetic field treatment enhanced photosynthetic performance in soybean under supplemental ultraviolet-B radiation. Photosynth. Res. 2021, 150, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Hajnorouzi, A.; Vaezzadeh, M.; Ghanati, F.; Jamnezhad, H.; Nahidian, B. Growth promotion and a decrease of oxidative stress in maize seedlings by a combination of geomagnetic and weak electromagnetic fields. J. Plant Physiol. 2011, 168, 1123–1128. [Google Scholar] [CrossRef]
- Pooam, M.; El-Esawi, M.; Aguida, B.; Ahmad, M. Arabidopsis cryptochrome and Quantum Biology: New insights for plant science and crop improvement. J. Plant Biochem. Biotechnol. 2020, 29, 636–651. [Google Scholar] [CrossRef]
- Aguida, B.; Babo, J.; Baouz, S.; Jourdan, N.; Procopio, M.; El-Esawi, M.; Engle, D.; Mills, S.; Wenkel, S.; Huck, A.; et al. ‘Seeing’ the electromagnetic spectrum: Spotlight on the cryptochrome photocycle. Front. Plant Sci. 2024, 15, 1340304. [Google Scholar] [CrossRef] [PubMed]
- Pooam, M.; Arthaut, L.; Burdick, D.; Link, J.; Martino, C.; Ahmad, M. Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2019, 249, 319–332. [Google Scholar] [CrossRef]
- Agliassa, C.; Maffei, M.E. Reduction of geomagnetic field (GMF) to near null magnetic field (NNMF) affects some Arabidopsis thaliana clock genes amplitude in a light independent manner. J. Plant Physiol. 2019, 232, 23–26. [Google Scholar] [CrossRef]
- Agliassa, C.; Narayana, R.; Christie, J.M.; Maffei, M.E. Geomagnetic field impacts on cryptochrome and phytochrome signaling. J. Photochem. Photobiol. B—Biol. 2018, 185, 32–40. [Google Scholar] [CrossRef]
- Luo, J. On the anisotropic weak magnetic field effect in radical-pair reactions. J. Chem. Phys. 2023, 158, 234302. [Google Scholar] [CrossRef]
- Timmel, C.R.; Cintolesi, F.; Brocklehurst, B.; Hore, P.J. Model calculations of magnetic field effects on the recombination reactions of radicals with anisotropic hyperfine interactions. Chem. Phys. Lett. 2001, 334, 387–395. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J.; et al. A magnetic protein biocompass. Nat. Mater. 2016, 15, 217–226. [Google Scholar] [CrossRef]
- Stass, D.V.; Woodward, J.R.; Timmel, C.R.; Hore, P.J.; McLauchlan, K.A. Radiofrequency magnetic field effects on chemical reaction yields. Chem. Phys. Lett. 2000, 329, 15–22. [Google Scholar] [CrossRef]
- Usselman, R.; Hill, I.; Singel, D.; Martino, C. Spin Biochemistry Modulates Reactive Oxygen Species (ROS) Production by Radio Frequency Magnetic Fields. PLoS ONE 2014, 9, e93065. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free Radic. Biol. Med. 2018, 122, 52–64. [Google Scholar] [CrossRef]
- Mazzoccoli, G. Chronobiology Meets Quantum Biology: A New Paradigm Overlooking the Horizon? Front. Physiol. 2022, 13, 892582. [Google Scholar] [CrossRef]
- Bläsing, O.E.; Gibon, Y.; Günther, M.; Höhne, M.; Morcuende, R.; Osuna, D.; Thimm, O.; Usadel, B.; Scheible, W.R.; Stitt, M. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 2005, 17, 3257–3281. [Google Scholar] [CrossRef]
- Farré, E.M.; Weise, S.E. The interactions between the circadian clock and primary metabolism. Curr. Opin. Plant Biol. 2012, 15, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic Pathway of Natural Antioxidants, Antioxidant Enzymes and ROS Providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Agliassa, C.; Narayana, R.; Bertea, C.M.; Rodgers, C.T.; Maffei, M.E. Reduction of the geomagnetic field delays Arabidopsis thaliana flowering time through downregulation of flowering-related genes. Bioelectromagnetics 2018, 39, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Bertea, C.M.; Narayana, R.; Agliassa, C.; Rodgers, C.T.; Maffei, M.E. Geomagnetic Field (Gmf) and Plant Evolution: Investigating the Effects of Gmf Reversal on Arabidopsis thaliana Development and Gene Expression. J. Vis. Exp. 2015, 105, e53286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maffei, M.E. The Radical Pair Mechanism and Its Quantum Role in Plant Reactive Oxygen Species Production Under Hypomagnetic Fields. Quantum Rep. 2025, 7, 52. https://doi.org/10.3390/quantum7040052
Maffei ME. The Radical Pair Mechanism and Its Quantum Role in Plant Reactive Oxygen Species Production Under Hypomagnetic Fields. Quantum Reports. 2025; 7(4):52. https://doi.org/10.3390/quantum7040052
Chicago/Turabian StyleMaffei, Massimo E. 2025. "The Radical Pair Mechanism and Its Quantum Role in Plant Reactive Oxygen Species Production Under Hypomagnetic Fields" Quantum Reports 7, no. 4: 52. https://doi.org/10.3390/quantum7040052
APA StyleMaffei, M. E. (2025). The Radical Pair Mechanism and Its Quantum Role in Plant Reactive Oxygen Species Production Under Hypomagnetic Fields. Quantum Reports, 7(4), 52. https://doi.org/10.3390/quantum7040052






