Evaluation of the Impact of Whey Edible Coatings with Bioprotective Cultures and Thyme Essential Oil Applied to Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Production
2.2. Cheese Production
2.3. Physicochemical Analysis
2.4. Microbiological Evaluation
2.5. Sensory Analysis
2.6. Statistical Analysis
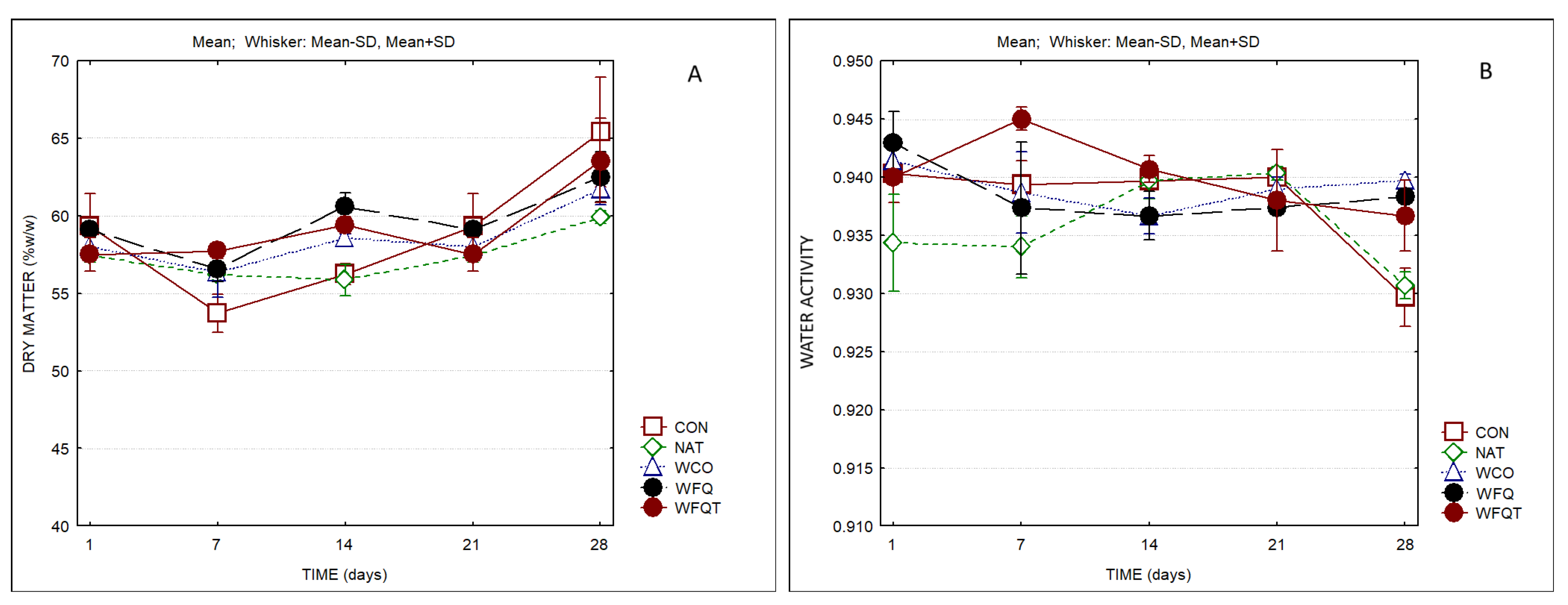
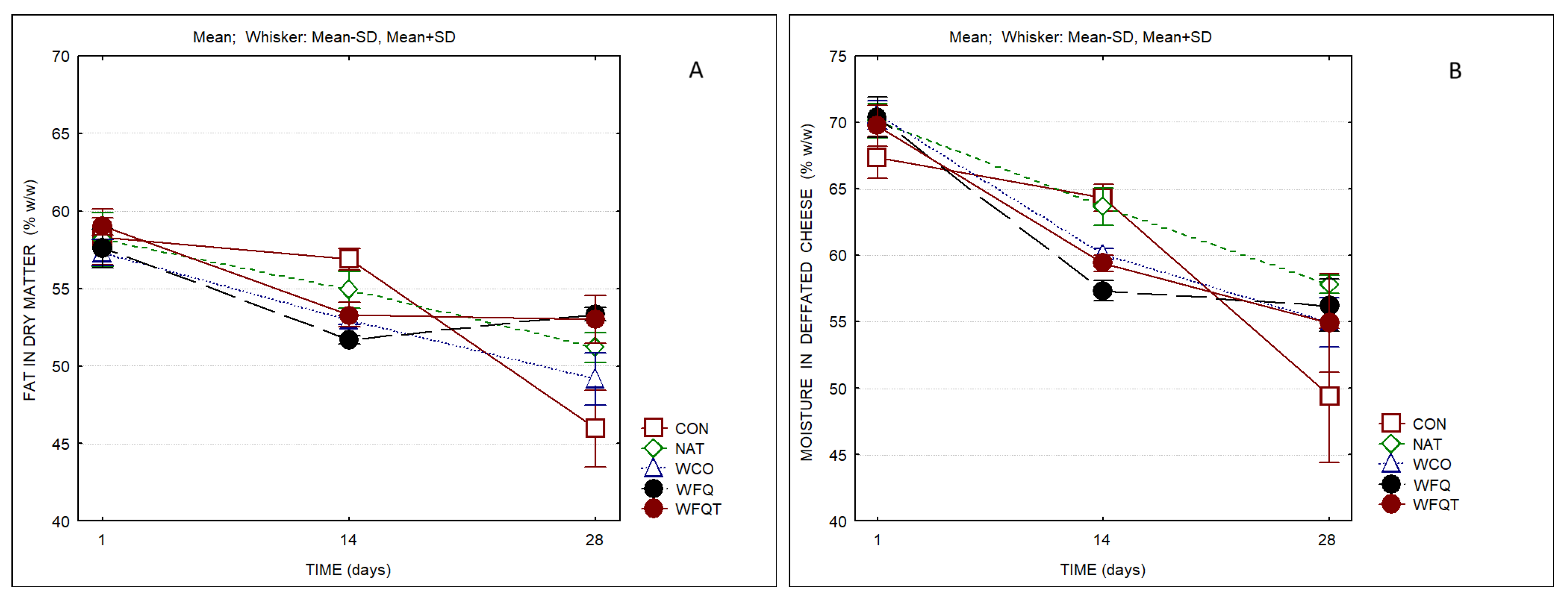
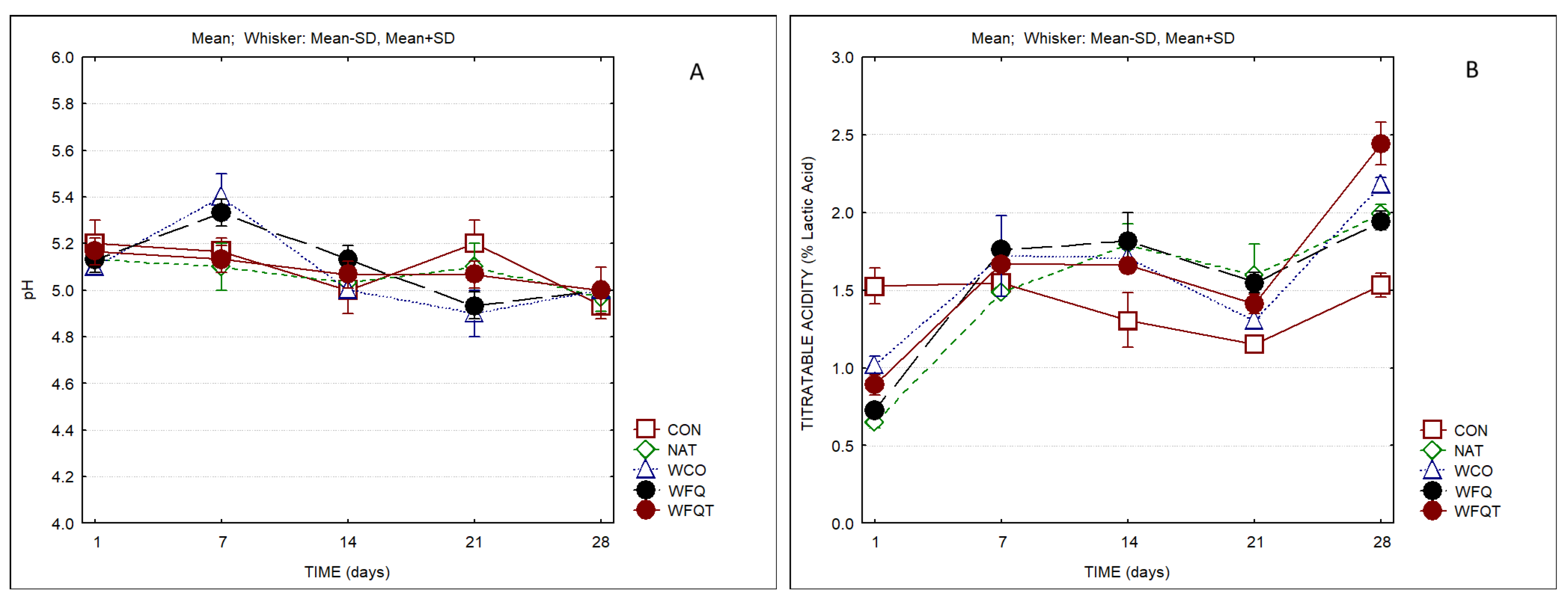
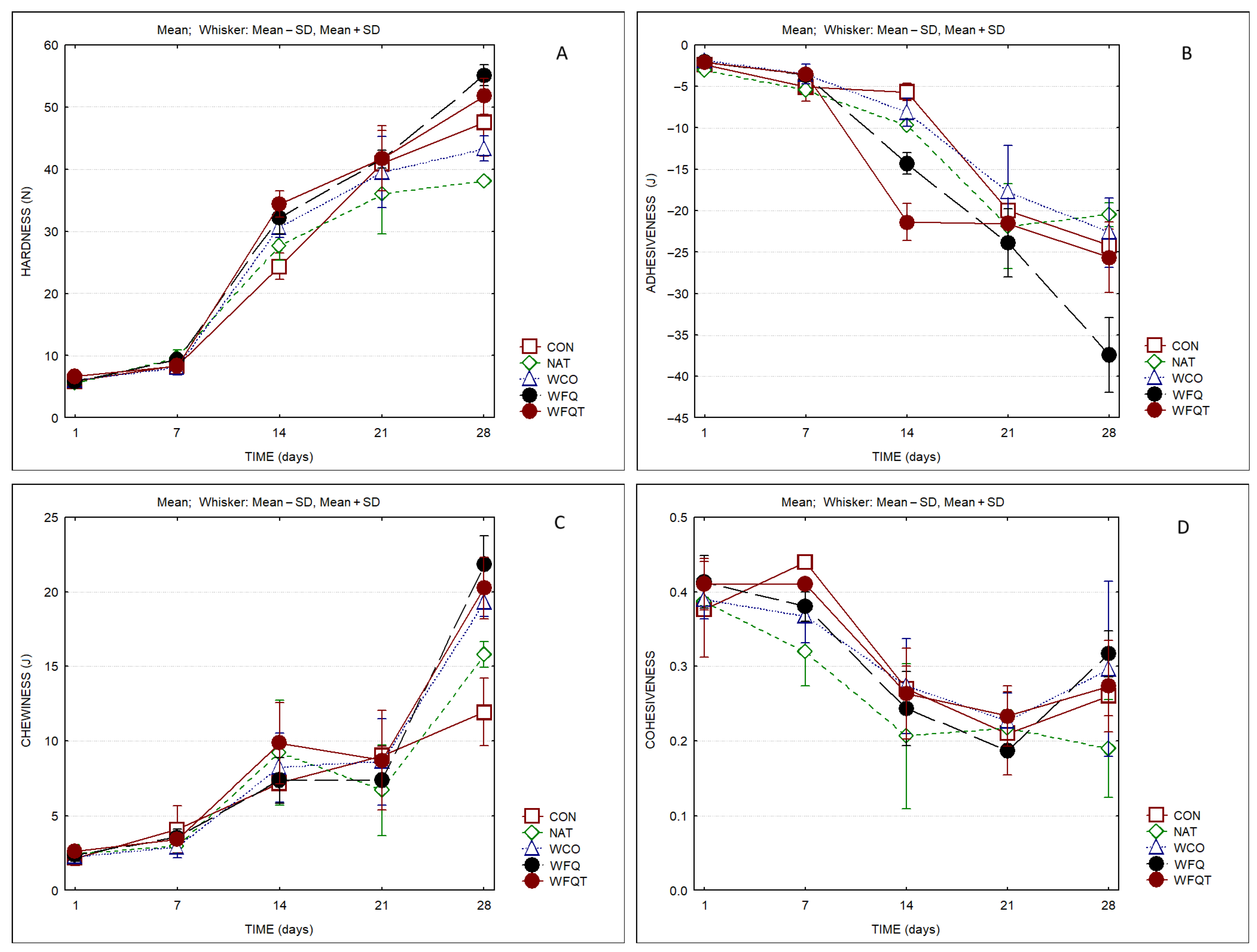
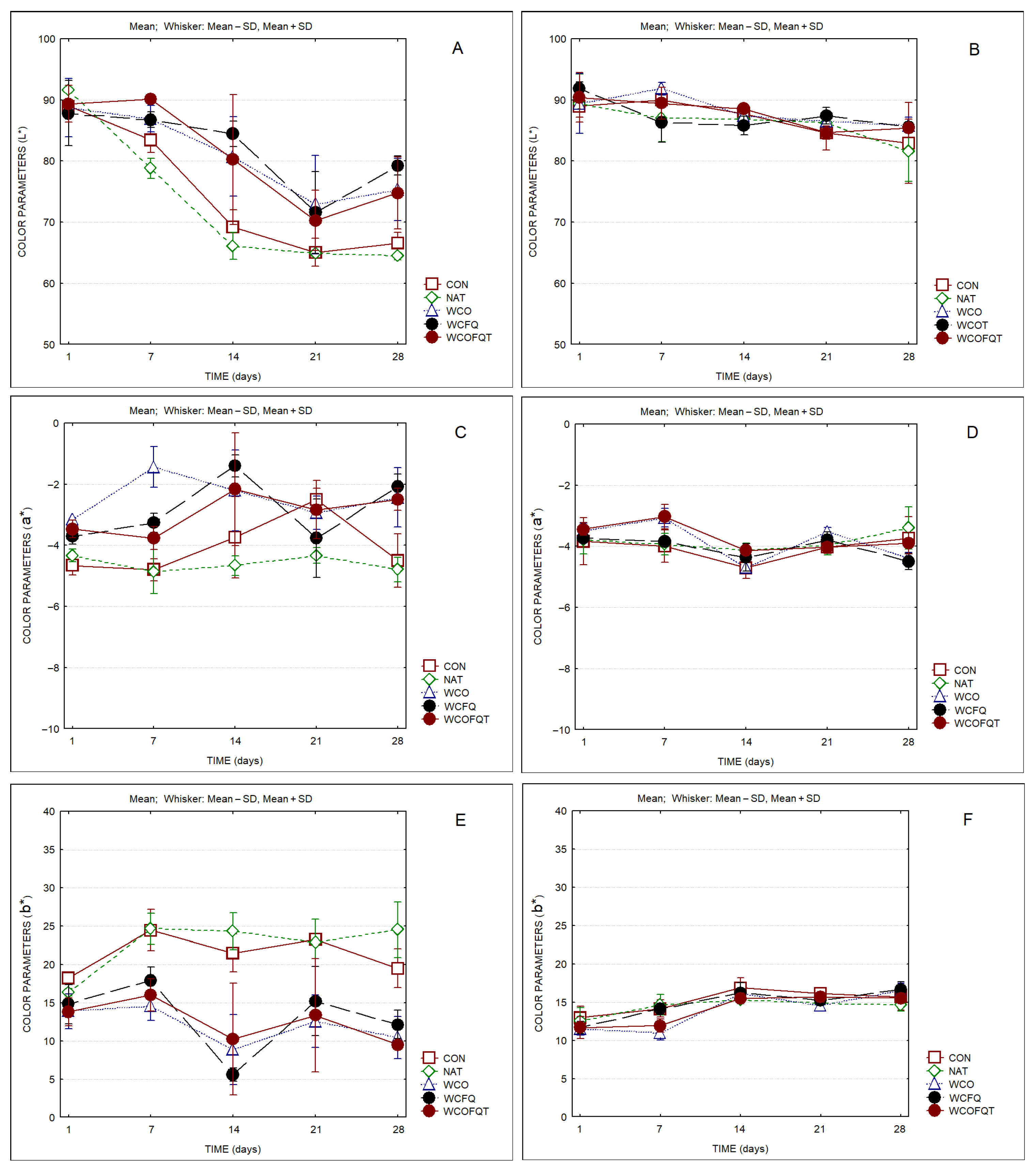
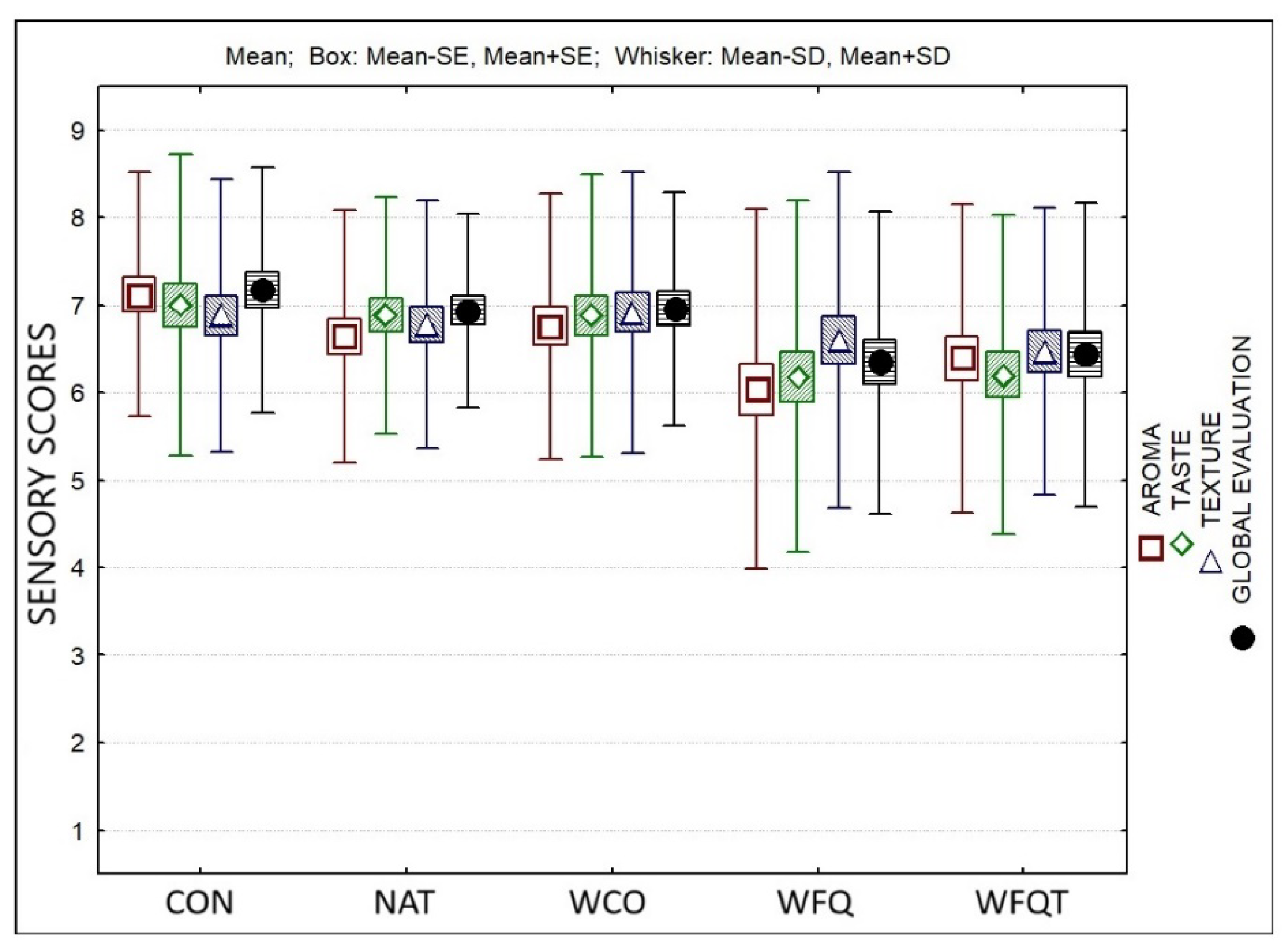
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, L.M.; Guimarães, J.T.; Pimentel, T.C.; Esmerino, E.A.; Freitas, M.Q.; Carvalho, C.W.P.; Cruz, A.G.; Silva, M.C. Edible whey protein films and coatings added with prebiotic ingredients. In Agri-Food Industry Strategies for Healthy Diets and Sustainability: New Challenges in Nutrition and Public Health; Barba, F.J., Kovačević, D.B., Eds.; Academic Press: New York, NY, USA, 2020; pp. 177–193. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of whey protein-based edible films and coatings in food industries: An updated overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey-protein-coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 2012, 562381. [Google Scholar] [CrossRef]
- Vasiliauskaite, A.; Mileriene, J.; Songisepp, E.; Rud, I.; Muizniece-Brasava, S.; Ciprovica, I.; Axelsson, L.; Lutter, L.; Aleksandrovas, E.; Tammsaar, E.; et al. Application of edible coating based on liquid acid whey protein concentrate with indigenous Lactobacillus helveticus for acid-curd cheese quality improvement. Foods 2022, 11, 3353. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Santos, A.C.; Leão, M.V.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Pintado, M.E.; Malcata, F.X. Antimicrobial activity of edible coatings prepared from whey protein isolate and formulated with various antimicrobial agents. Int. Dairy J. 2012, 25, 132–141. [Google Scholar] [CrossRef]
- Ramos, O.T.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Lopes-da-Silva, J.A.; Pintado, M.E.; Malcata, F.X. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy Sci. 2012, 95, 6282–6292. [Google Scholar] [CrossRef]
- Jalilzadeha, A.; Hesaria, J.; Peighambardousta, S.H.; Javidipourb, I. The effect of whey protein concentrate based edible coatings containing natamycin or lysozyme-xanthan conjugate on microbial properties of ultrafiltrated white cheese. J. Food Bioprocess Eng. 2020, 3, 168–177. [Google Scholar] [CrossRef]
- Siriwardana, J.; Wijesekara, I. Analysis of the effectiveness of an antimicrobial edible coating prepared from sweet whey base to improve the physicochemical. microbiological. and sensory attributes of swiss cheese. Adv. Agric. 2021, 2021, 5096574. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, H.; Tian, B.; Jiang, B.; Xu, J.; Li, D.; Feng, Z.; Liu, C. Novel edible coating with antioxidant and antimicrobial activities based on whey protein isolate nanofibrils and carvacrol and its application on fresh-cut cheese. Coatings 2019, 9, 583. [Google Scholar] [CrossRef]
- Pires, A.; Cobos, A.; Pereira, C.; Diaz, O. Edible films based on ovine second cheese whey with oregano essential oil. Appl. Sci. 2025, 15, 5325. [Google Scholar] [CrossRef]
- Pires, A.F.; Díaz, O.; Cobos, A.; Pereira, C.D. A review of recent developments in edible films and coatings-focus on whey-based materials. Foods 2024, 13, 2638. [Google Scholar] [CrossRef]
- Tamošaitis, A.; Jaruševičien, Ė.A.; Strykait, Ė.M.; Damašius. J. Analysis of antimicrobial whey protein-based biocomposites with lactic acid, tea tree (Melaleuca alternifolia) and garlic (Allium sativum) essential oils for Edam cheese coating. Int. J. Dairy Technol. 2022, 75, 611–618. [Google Scholar] [CrossRef]
- Souza, L.V.; Martins, E.; Botelho Moreira, I.M.F.; Carvalho, A.F. Strategies for the development of bioprotective cultures in food preservation. Int. J. Micr. 2022, 2022, 6264170. [Google Scholar] [CrossRef]
- Vignolo, G.; Fadda, S. Starter Cultures: Bioprotective Cultures. In Handbook of Fermented Meat and Poultry; Toldrá, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 147–157. [Google Scholar] [CrossRef]
- Bintsis, T.; Papademas, P. The application of protective cultures in cheese: A review. Fermentation 2024, 10, 117. [Google Scholar] [CrossRef]
- Zhao, Z.; Simpson, D.J.; Gänzle, M.G. Bioprotective lactobacilli in Crescenza and Gouda cheese models to inhibit fungal spoilage. Int. Dairy J. 2024, 152, 105883. [Google Scholar] [CrossRef]
- Makki, G.M.; Kozak, S.M.; Jencarelli, K.G.; Alcaine, S.D. Evaluation of the efficacy of commercial protective cultures to inhibit mold and yeast in cottage cheese. J. Dairy Sci. 2021, 104, 2709–2718. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.M.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C.C.G. Application of an alginate-based edible coating with bacteriocin-producing Lactococcus strains in fresh cheese preservation. LWT 2022, 153, 112486. [Google Scholar] [CrossRef]
- Meloni, M.P.; Piras, F.; Siddi, G.; Migoni, M.; Cabras, D.; Cuccu, M.; Nieddu, G.; McAuliffe, O.; De Santis, E.P.L.; Scarano, C. Effect of commercial and autochthonous bioprotective cultures for controlling Listeria monocytogenes contamination of Pecorino Sardo Dolce PDO cheese. Foods 2023, 12, 3797. [Google Scholar] [CrossRef]
- Spanu, C.; Scarano, C.; Piras, F.; Spanu, V.; Pala, C.; Casti, D.; Lamon, S.; Cossu, F.; Ibba, M.; Nieddu, G.; et al. Testing commercial biopreservative against spoilage microorganisms in MAP packed Ricotta fresca cheese. Food Microbiol. 2017, 66, 72–76. [Google Scholar] [CrossRef]
- Remini, H.; Remini-Sahraoui, Y.; Benbara, T.; Sadoun, D. From farm to cheeseboard: Harnessing the biopreserving performance and enhancing safety of Lactococcus lactis KJ660075 in goat’s milk cheese. Int. Dairy J. 2024, 157, 105977. [Google Scholar] [CrossRef]
- Aljasir, S.F.; Gensler, C.; Sun, L.; D’Amico, D.J. The efficacy of individual and combined commercial protective cultures against Listeria monocytogenes, Salmonella O157 and non-O157 shiga toxin-producing Escherichia coli in growth medium and raw milk. Food Control 2020, 109, 106924. [Google Scholar] [CrossRef]
- Silva, B.N.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Meta-Regression models describing the effects of essential oils and added lactic acid bacteria on pathogen inactivation in cheese. Microb. Risk Anal. 2021, 18, 100131. [Google Scholar] [CrossRef]
- Bagheripoor, N.; Khoshgozaran-Abras, S.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M.; MollaKhalili, N.; Jazaeri, S. Application of active edible coatings to improve the shelf-life of cheese. Food Sci. Technol. Res. 2018, 24, 949–962. [Google Scholar] [CrossRef]
- Paidari, S.; Ahari, H.; Pasqualone, A.; Anvar, A.A.; Allah, Y.B.S.; Moradi, S. Bio-nanocomposites and their potential applications in physiochemical properties of cheese: An updated review. J. Food Meas. Charact. 2023, 17, 2595–2606. [Google Scholar] [CrossRef]
- Gochev, V.K.; Girova, T.D. Antimicrobial activity of various essential oils against spoilage and pathogenic microorganisms isolated from meat. Biotechnol. Biotechnol. Equip. 2009, 23, 900–904. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Ahmad Bhat, M.; Prabhakar, A.; Hussain Shalla, A.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F. Antibacterial and antifungal activities of essential oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons: Hoboken, NY, USA, 2010; pp. 255–306. [Google Scholar] [CrossRef]
- Fahrullah; Ervandi, M.; Rosyidi, D. Characterization and antimicrobial activity of whey edible film composite enriched with clove essential oil. Trop. Animal Sci. J. 2021, 44, 369–376. [Google Scholar] [CrossRef]
- Çakmak, H.; Özselek, Y.; Turan, O.Y.; Fıratlıgil, E.; Karbancioğlu-Güler, F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Seydim, A.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of whey protein edible films containing plant essential oils on microbial inactivation of sliced Kasar cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Tarhan, Ö.; Şen, R. Heat-denatured and alcalase-hydrolyzed protein films/coatings containing marjoram essential oil and thyme extract. Food Biosci. 2022, 45, 101466. [Google Scholar] [CrossRef]
- Antonino, C.; Difonzo, G.; Faccia, M.; Caponio, F. Effect of edible coatings and films enriched with plant extracts and essential oils on the preservation of animal-derived foods. J. Food Sci. 2024, 89, 748–772. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.D.; Varytskaya, H.; Łydzińska, O.; Szkolnicka, K.; Gomes, D.; Pires, A. Effect of sheep’s whey edible coatings with a bioprotective culture, kombucha tea or oregano essential oil on cheese characteristics. Foods 2024, 13, 4132. [Google Scholar] [CrossRef] [PubMed]
- NP 3544; Queijos e Queijos Fundidos. Determinação do Resíduo Seco e do Resíduo Seco Isento de Matéria Gorda. Direção Geral da Qualidade: Lisbon, Portugal, 1987. (In Portuguese)
- NP 2105; Queijos. Determinação do Teor de Matéria Gorda. Técnica de Van Gulick. Processo Corrente. Direção Geral da Qualidade: Lisbon, Portugal, 1983. (In Portuguese)
- NP 1598; Queijos. Definição. Classificação. Acondicionamento e Marcação. Direção Geral da Qualidade: Lisbon, Portugal, 1983. (In Portuguese)
- AOAC. 920.124. Acidity for Cheese. Titrimetric Method. In Official Methods of Analysis of Association of Official Analytical Chemists, 17th ed.; AOAC: Rockville, MD, USA, 2002. [Google Scholar]
- A Simple Review of CIEΔE* (Color Difference) Equations. Available online: https://techkonusa.com/a-simple-review-of-cie-de-color-difference-equations/ (accessed on 28 March 2025).
- ISO 7889:2003|IDF 117: 2003; Yogurt—Enumeration of Characteristic Microorganisms—Colony-Count Technique at 37 °C. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 6611:2004|IDF 94: 2004; Milk and Milk Products—Enumeration of Colony-Forming Units of Yeasts and/or Molds—Colony-Count Technique at 25 °C. International Organization for Standardization: Geneva, Switzerland, 2004.
- Stone, H.; Sidel, J. Sensory Evaluation Practices, 3rd ed.; Food Science and Technology; Academic Press: New York, NY, USA, 2004; pp. 247–277. [Google Scholar]
- Ye, J.; Ma, D.; Qin, W.; Liu, Y. Physical and antibacterial properties of sodium alginate—Sodium carboxymethylcellulose films containing Lactococcus lactis. Molecules 2018, 23, 2645. [Google Scholar] [CrossRef]
- Vasiliauskaite, A.; Mileriene, J.; Kasparaviciene, B.; Aleksandrovas, E.; Songisepp, E.; Rud, I.; Axelsson, L.; Muizniece-Brasava, S.; Ciprovica, I.; Paskevicius, A.; et al. Screening for antifungal indigenous lactobacilli strains isolated from local fermented milk for developing bioprotective fermentates and coatings based on acid whey protein concentrate for fresh cheese quality maintenance. Microorganisms 2023, 11, 557. [Google Scholar] [CrossRef]
- Henriques, M.; Santos, G.; Rodrigues, A.; Gomes, D.; Pereira, C.D.; Gil, M. Replacement of conventional cheese coatings by natural whey protein edible coatings with antimicrobial activity. J. Hyg. Eng. Des. 2013, 3, 34–47. [Google Scholar]
- Pires, A.; Pietruszka, H.; Bożek, A.; Szkolnicka, K.; Gomes, D.; Díaz, O.; Cobos, A.; Pereira, C. Sheep’s second cheese whey edible coatings with oregano and clary sage essential oils used as sustainable packaging material in cheese. Foods 2024, 13, 674. [Google Scholar] [CrossRef]
- Shi, C.; Maktabdar, M. Lactic acid bacteria as biopreservation against spoilage molds in dairy products—A Review. Front. Microbiol. 2022, 12, 819684. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef]
- Guimarães, A.; Ramos, Ó.; Cerqueira, M.; Venâncio, A.; Abrunhosa, L. Active whey protein edible films and coatings incorporating Lactobacillus buchneri for Penicillium nordicum control in cheese. Food Bioprocess Technol. 2020, 13, 1074–1086. [Google Scholar] [CrossRef]
- Ceylan, H.G.; Atasoy, A.F. Optimization and characterization of prebiotic concentration of edible films containing Bifidobacterium animalis subsp. lactis BB-12® and its application to block type processed cheese. Int. Dairy J. 2022, 134, 105443. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Emergence of cheese packaging by edible coatings for enhancing its shelf-life. J. Food Meas. Charact. 2024, 18, 5265–5280. [Google Scholar] [CrossRef]
- Do Nascimento, D.L.; De Moraes, A.A.B.; Da Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; De Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]


| SAMPLE | DM % | MDC % | FAT % | FDM % | ASH % |
|---|---|---|---|---|---|
| CON | 65.39 ± 2.89 a | 49.44 ± 4.13 a | 30.00 ± 0.00 a | 45.97 ± 2.02 a | 3.49 ± 0.53 a |
| NAT | 59.91 ± 0.34 a | 57.82 ± 0.57 b | 30.67 ± 0.50 a | 51.19 ± 0.79 b | 3.70 ± 0.14 a |
| WCO | 61.72 ± 0.81 a | 54.96 ± 1.52 ab | 30.33 ± 0.47 a | 49.16 ± 1.39 b | 3.05 ± 0.10 a |
| WFQ | 62.50 ± 1.32 a | 56.23 ± 1.59 ab | 33.33 ± 0.40 b | 53.34 ± 0.37 c | 3.10 ± 0.08 a |
| WFQT | 63.57 ± 2.22 a | 54.90 ± 3.02 ab | 33.67 ± 0.45 b | 53.00 ± 1.27 c | 3.33 ± 0.09 a |
| RIND | CON | NAT | WCO | WFQ | WFQT |
|---|---|---|---|---|---|
| 1st vs. 7th | 14.1 | 1.3 | 1.9 | 10.4 | 3.9 |
| 7th vs. 14th | 20.1 | 10.2 | 2.0 | 3.4 | 14.7 |
| 14th vs. 21th | 9.0 | 16.3 | 2.5 | 2.4 | 10.7 |
| 21st vs. 28th | 2.9 | 9.2 | 1.8 | 4.1 | 5.4 |
| PASTE | CON | NAT | WCO | WFQ | WFQT |
| 1st vs. 7th | 1.6 | 3.2 | 2.5 | 6.2 | 1.1 |
| 7th vs. 14th | 3.6 | 0.7 | 7.0 | 2.2 | 3.8 |
| 14th vs. 21th | 3.3 | 0.8 | 2.2 | 2.0 | 3.9 |
| 21st vs. 28th | 1.7 | 4.7 | 2.3 | 2.4 | 0.8 |
| CHEESES | RIND | PASTE |
|---|---|---|
| CON vs. NAT | 81.5 | 1.8 |
| CON vs. WCO | 124.7 | 3.1 |
| CON vs. WFQ | 96.8 | 2.8 |
| CON vs. WFQT | 101.1 | 2.4 |
| NAT vs. WCO | 114.2 | 4.8 |
| NAT vs. WFQ | 102.9 | 4.5 |
| NAT vs. WFQT | 94.1 | 4.0 |
| WCO vs. WFQ | 34.5 | 0.4 |
| WCO vs. WFQT | 24.0 | 1.2 |
| WFQ vs. WFQT | 21.1 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, C.D.; Hodowaniec, K.; Kucz, K.; Szkolnicka, K.; Gomes, D.; Pires, A. Evaluation of the Impact of Whey Edible Coatings with Bioprotective Cultures and Thyme Essential Oil Applied to Cheese. Dairy 2025, 6, 56. https://doi.org/10.3390/dairy6050056
Pereira CD, Hodowaniec K, Kucz K, Szkolnicka K, Gomes D, Pires A. Evaluation of the Impact of Whey Edible Coatings with Bioprotective Cultures and Thyme Essential Oil Applied to Cheese. Dairy. 2025; 6(5):56. https://doi.org/10.3390/dairy6050056
Chicago/Turabian StylePereira, Carlos Dias, Klaudia Hodowaniec, Karolina Kucz, Katarzyna Szkolnicka, David Gomes, and Arona Pires. 2025. "Evaluation of the Impact of Whey Edible Coatings with Bioprotective Cultures and Thyme Essential Oil Applied to Cheese" Dairy 6, no. 5: 56. https://doi.org/10.3390/dairy6050056
APA StylePereira, C. D., Hodowaniec, K., Kucz, K., Szkolnicka, K., Gomes, D., & Pires, A. (2025). Evaluation of the Impact of Whey Edible Coatings with Bioprotective Cultures and Thyme Essential Oil Applied to Cheese. Dairy, 6(5), 56. https://doi.org/10.3390/dairy6050056







