Survival of Selected Pathogenic Bacteria during PDO Pecorino Romano Cheese Ripening
Abstract
1. Introduction
2. Materials and Methods
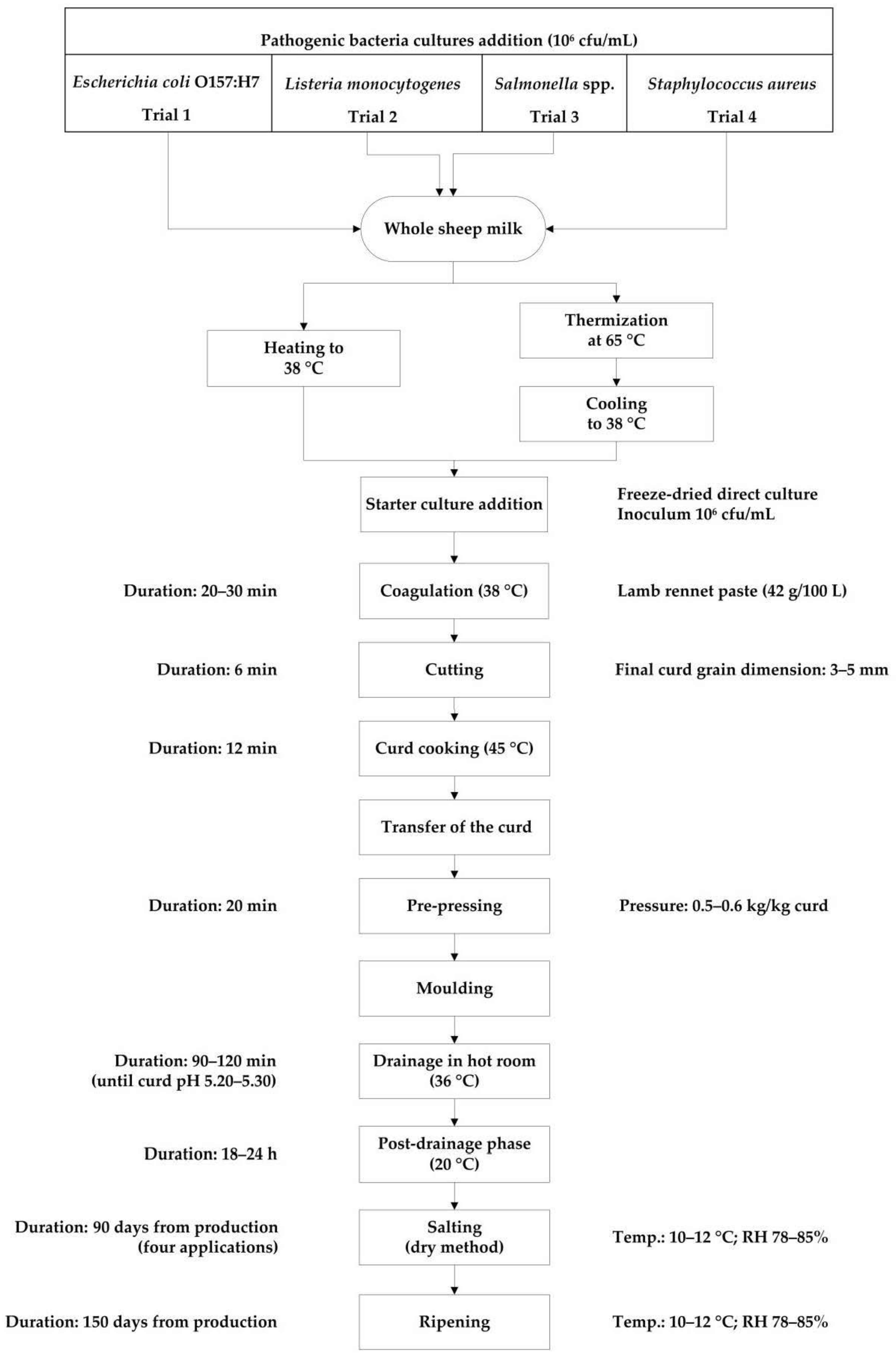
2.1. Experimental Design
2.2. Bacterial Strains and Inoculum Preparation
2.3. Cheese Production and Sampling
2.4. Physico-Chemical Analysis
2.5. Microbiological Analysis
- (a)
- Listeria monocytogenes. Detection and enumeration were performed according to ISO 11290-1:2017 and ISO 11290-2:2017, respectively [34,35]. For detection, samples were mixed with Fraser broth base (Oxoid, Thermo Fisher Scientific, Basingstoke, UK), homogenized 90 s in a Stomacher Lab Blender 400 (International PBI S.p.A., Milan, Italy), and incubated for 24 h at 30 °C (primary enrichment). Subsequently, 100 μL of primary enrichment were transferred to 10 mL of Fraser broth supplemented by Fraser selective supplement (Oxoid, Thermo Fisher Scientific, Basingstoke, UK), which were incubated for 24 h at 37 °C (secondary enrichment). From primary and secondary enrichments, aliquots of 100 μL were streaked onto selective differential medium plates: Agar Listeria according to Ottaviani and Agosti (ALOA, Biolife, Milan, Italy) and Listeria selective agar (Oxford formulation, Oxoid, Thermo Fisher Scientific, Basingstoke, UK) and incubated for up to 24–48 h at 37 °C. All isolates with typical L. monocytogenes characteristics were subjected to morphological and biochemical proofs as confirmatory tests.
- (b)
- Staphylococcus aureus. Detection and enumeration were performed according to ISO 6888-2:2004 [36]. Samples were weighed and mixed with buffered peptone water (BPW, Microbiol, Uta, Italy), ten-fold serial dilutions were prepared and aliquots of 100 μL were plated in duplicate on Baird Parker plates with Rabbit Plasma Fibrinogen supplement (RPF, Biolife, Milan, Italy) and incubated for 24–48 h at 37 °C.
- (c)
- Escherichia coli O157:H7. The detection was performed according to ISO 16654:2001/A1:2017 and four successive stages were necessitated [37].
- (1)
- Enrichment of the test portion homogenized in modified tryptone soya broth containing novobiocin (mTSB + N, Biolife, Milan, Italy) with incubation at 41.5 °C for 6 h and subsequently for a further 12 h to 18 h.
- (2)
- Separation and concentration of microorganisms by means of immunomagnetic particles coated with antibodies to E. coli O157:H7.
- (3)
- Isolation by subculture of the immunomagnetic particles with adhering bacteria onto cefixime tellurite sorbitol MacConkey agar (CT-SMAC, Biolife, Milan, Italy) and sorbitol MacConkey agar (SMAC, Biolife, Milan, Italy) incubated at 37 °C for 24 h.
- (4)
- Confirmation of typical colonies.
- (d)
- Salmonella spp. The detection was performed according to ISO method 6579-1:2017 [38]. The method required the following successive stages. A pre-enrichment in buffered peptone water (BPW, Microbiol, Uta, Italy) at 37 °C for 24 h. A selective enrichment in Rappaport-Vassiliadis with soy broth (RVS, Oxoid, Basingstoke, UK) and Müller-Kauffmann tetrathionate-novobiocin broth (MKTTn, Microbiol, Uta, Italy) for 24 h at 41.5 and 37 °C, respectively. Aliquots of the selective broths were streaked onto two selective isolation agar media, xylose lysine deoxycholate agar (XLD, Microbiol, Uta, Italy) and Salmonella detection and identification agar (SMID, BioMérieux, Marcy L’Etoile, France). The agar plates were incubated at 37 °C for 24 h. Confirmation of suspect colonies was carried out by biochemical and serological testing.
2.6. Statistical Analysis
3. Results and Discussion
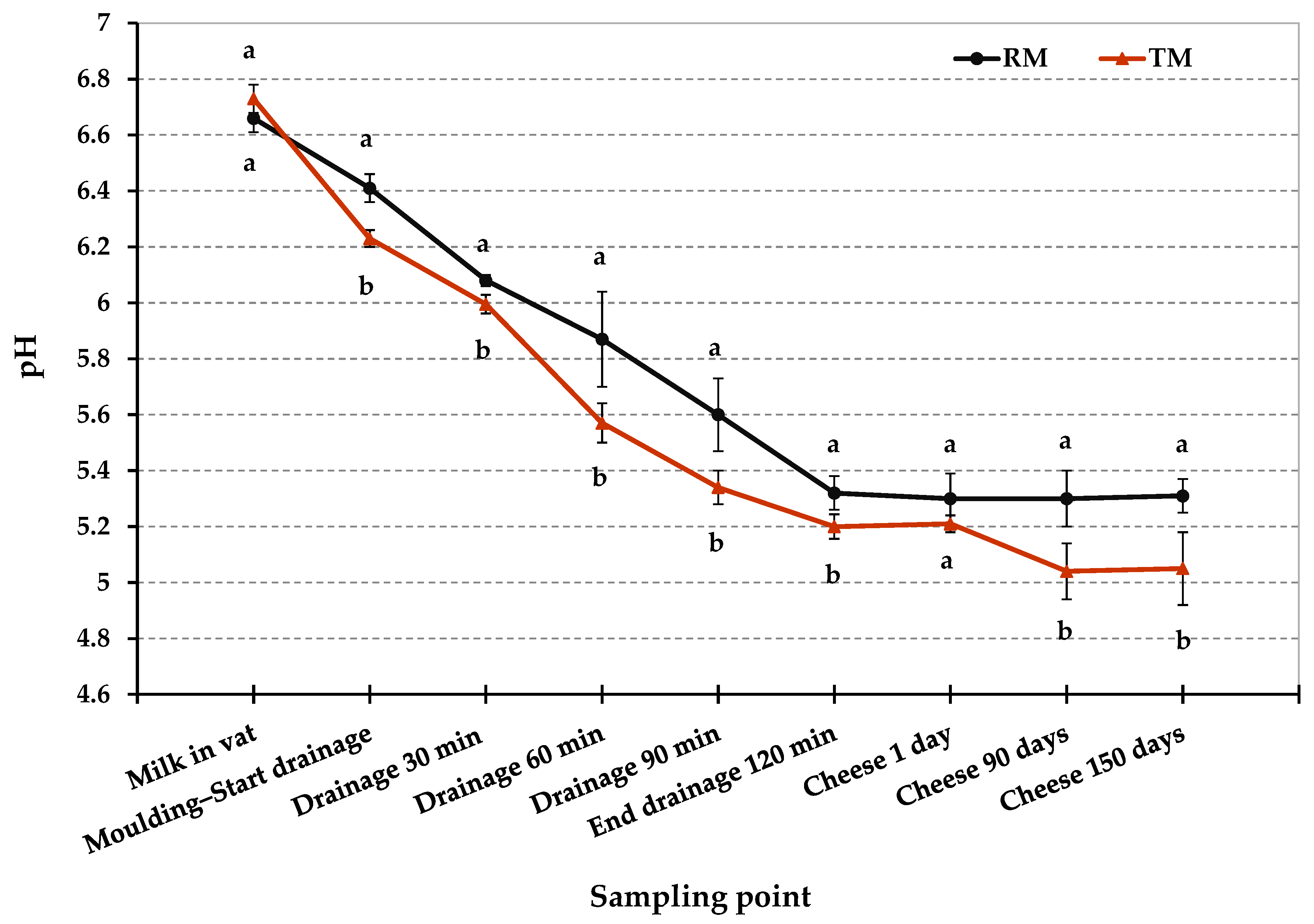
3.1. Physico-Chemical Properties of Cheese
3.2. Pathogenic Bacteria Counts in RM Cheese-Making Trials
3.3. Pathogenic Bacteria Counts in TM Cheese-Making Trials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colonna, A.; Durham, C.; Meunier-Goddik, L. Factors affecting consumers’ preferences for and purchasing decisions regarding pasteurized and raw milk specialty cheeses. J. Dairy Sci. 2011, 94, 5217–5226. [Google Scholar] [CrossRef] [PubMed]
- Montel, M.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Lee, S.; Choi, K.-H. Microbial benefits and risks of raw milk cheese. Food Control 2016, 63, 201–215. [Google Scholar] [CrossRef]
- Kousta, M.; Mataragas, M.; Skandamis, P.; Drosinos, E.H. Prevalence and sources of cheese contamination with pathogens at farm and processing levels. Food Control 2010, 21, 805–815. [Google Scholar] [CrossRef]
- Verraes, C.; Vlaemynck, G.; Van Weyenberg, S.; De Zutter, L.; Daube, G.; Sindic, M.; Uyttendaelec, M.; Herman, L. A review of the microbiological hazards of dairy products made from raw milk. Int. Dairy J. 2015, 50, 32–44. [Google Scholar] [CrossRef]
- Langer, A.J.; Ayers, T.; Grass, J.; Lynch, M.; Angulo, F.J.; Mahon, B.E. Nonpasteurized Dairy Products, Disease Outbreaks, and State Laws—United States, 1993–2006. Emerg. Infect. Dis. 2012, 18, 385–391. [Google Scholar] [CrossRef]
- Costard, S.; Espejo, L.; Groenendaal, H.; Zagmutt, F.J. Outbreak-related disease burden associated with consumption of unpasteurized cow’s milk and cheese, United States, 2009–2014. Emerg. Infect. Dis. 2017, 23, 957–964. [Google Scholar] [CrossRef]
- Rocourt, J. Foodborne diseases: Foodborne diseases and vulnerable groups. In Encyclopedia of Food Safety, 1st ed.; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; Volume 1, pp. 323–331. ISBN 978-0-12-378613-5. [Google Scholar]
- Ioanna, F.; Quaglia, N.C.; Storelli, M.M.; Castiglia, D.; Goffredo, E.; Storelli, A.; De Rosa, M.; Normanno, G.; Jambrenghi, A.C.; Dambrosio, A. Survival of Escherichia coli O157:H7 during the manufacture and ripening of Cacioricotta goat cheese. Food Microbiol. 2018, 70, 200–205. [Google Scholar] [CrossRef]
- Thakur, M.; Asrani, R.K.; Patial, V. Listeria monocytogenes: A Food-Borne Pathogen. In Foodborne Diseases, 1st ed.; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; Volume 15, pp. 157–192. ISBN 978-0-12-811444-5. [Google Scholar]
- Arqués, J.L.; Rodríguez, E.; Langa, S.; Landete, J.M.; Medina, M. Antimicrobial Activity of Lactic Acid Bacteria in Dairy Products and Gut: Effect on Pathogens. BioMed Res. Int. 2015, 2015, 584183. [Google Scholar] [CrossRef]
- Melero, B.; Stessl, B.; Manso, B.; Wagner, M.; Esteban-Carbonero, Ó.J.; Hernández, M.; Rovira, J.; Rodriguez-Lázaro, D. Listeria monocytogenes colonization in a newly established dairy processing facility. Int. J. Food Microbiol. 2019, 289, 64–71. [Google Scholar] [CrossRef]
- Castro, R.D.; Pedroso, S.H.S.P.; Sandes, S.H.C.; Silva, G.O.; Luiz, K.C.M.; Dias, R.S.; Filho, R.A.T.; Figueiredo, H.C.P.; Santos, S.G.; Nunes, A.C.; et al. Virulence factors and antimicrobial resistance of Staphylococcus aureus isolated from the production process of Minas artisanal cheese from the region of Campo das Vertentes, Brazil. J. Dairy Sci. 2020, 103, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Lahou, E.; Uyttendaele, M. Growth potential of Listeria monocytogenes in soft, semi-soft and semi-hard artisanal cheeses after post-processing contamination in deli retail establishments. Food Control 2017, 76, 13–23. [Google Scholar] [CrossRef]
- Castañeda-Ruelas, G.M.; Soto-Beltrán, M.; Chaidez, C. Detecting Sources of Staphylococcus aureus in One Small-Scale Cheese Plant in Northwestern Mexico. J. Food Saf. 2016, 37, e12290. [Google Scholar] [CrossRef]
- Normanno, G.; Spinelli, E.; Caruso, M.; Fraccalvieri, R.; Capozzi, L.; Barlaam, A.; Parisi, A. Occurrence and characteristics of methicillin-resistant Staphylococcus aureus (MRSA) in buffalo bulk tank milk and the farm workers in Italy. Food Microbiol. 2020, 91, 103509. [Google Scholar]
- D’Amico, D.J.; Donelly, C.W. Growth and Survival of Microbial Pathogens in Cheese. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; Volume 1, pp. 573–594. ISBN 978-0-12-417012-4. [Google Scholar]
- Alvarez-Ordóñez, A.; Broussolle, V.; Colin, P.; Nguyen-The, C.; Prieto, M. The adaptive response of bacterial foodborne pathogens in the environment, host and food: Implications for food safety. Int. J. Food Microbiol. 2015, 213, 99–109. [Google Scholar] [CrossRef]
- Melo, J.; Andrew, P.W.; Faleiro, M.L. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res. Int. 2015, 67, 75–90. [Google Scholar] [CrossRef]
- Ye, B.; He, S.; Zhou, X.; Cui, Y.; Zhou, M.; Shi, X. Response to Acid Adaptation in Salmonella enterica Serovar Enteritidis. J. Food Sci. 2019, 84, 599–605. [Google Scholar] [CrossRef]
- Bellio, A.; Bianchi, D.M.; Vitale, N.; Vernetti, L.; Gallina, S.; Decastelli, L. Behavior of Escherichia coli O157:H7 during the manufacture and ripening of Fontina Protected Designation of Origin cheese. J. Dairy Sci. 2018, 101, 4962–4970. [Google Scholar] [CrossRef]
- Dalzini, E.; Cosciani-Cunico, E.; Monastero, P.; Bernini, V.; Neviani, E.; Bellio, A.; Decastelli, L.; Losio, M.N.; Daminelli, P.; Varisco, G. Listeria monocytogenes in Gorgonzola cheese: Study of the behaviour throughout the process and growth prediction during shelf life. Int. J. Food Microbiol. 2017, 262, 71–79. [Google Scholar] [CrossRef]
- Peng, S.; Hoffmann, W.; Bockelmann, W.; Hummerjohann, J.; Stephan, R.; Hammer, P. Fate of Shiga toxin-producing and generic Escherichia coli during production and ripening of semihard raw milk cheese. J. Dairy Sci. 2013, 96, 815–823. [Google Scholar] [CrossRef]
- D’Amico, D.J.; Druart, M.J.; Donnelly, C.W. Behavior of Escherichia coli O157:H7 during the manufacture and aging of Gouda and stirred-curd cheddar cheeses manufactured from raw milk. J. Food Prot. 2010, 73, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.J.; Druart, M.J.; Donnelly, C.W. Comparing the Behavior of Multidrug-Resistant and Pansusceptible Salmonella during the Production and Aging of a Gouda Cheese Manufactured from Raw Milk. J. Food Prot. 2014, 77, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Chatelard-Chauvin, C.; Pelissier, F.; Hulin, S.; Montel, M.C. 2015. Behaviour of Listeria monocytogenes in raw milk Cantal type cheeses during cheese making, ripening and storage in different packaging conditions. Food Control 2015, 54, 53–65. [Google Scholar] [CrossRef]
- Consorzio per la Tutela del Formaggio Pecorino Romano. Specification and Regulations. Available online: https://www.pecorinoromano.com/en/pecorino-romano/specification-and-regulations (accessed on 27 May 2020).
- Addis, M.; Fiori, M.; Riu, G.; Pes, M.; Salvatore, E.; Pirisi, A. Physico-chemical and nutritional characteristics of PDO Pecorino Romano cheese: Seasonal variation. Small Rumin. Res. 2015, 126, 73–79. [Google Scholar] [CrossRef]
- CLAL. Italy: Export Pecorino e Fiore Sardo. 2020. Available online: https://www.clal.it/?section=impexpistat&cod=04069063&mov=E (accessed on 16 June 2020).
- Meunier-Goddik, L.; Waite-Cusic, J. Consumers Acceptance of Raw Milk and its Products. In Raw Milk, 1st ed.; Nero, L.A., De Carvalho, A.F., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 311–350. ISBN 978-0-12-810530-6. [Google Scholar]
- Brooks, J.C.; Martinez, B.; Stratton, J.; Bianchini, A.; Krokstrom, R.; Hutkins, R. Survey of raw milk cheeses for microbiological quality and prevalence of foodborne pathogens. Food Microbiol. 2012, 31, 154–158. [Google Scholar] [CrossRef]
- ISO 5534:2004. Cheese and Processed Cheese—Determination of the Total Solids content; International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 5943:2006. Cheese and Processed Cheese products—Determination of Chloride Content—Potentiometric Titration Method; International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 11290-1:2017. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 1: Detection Method; International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 11290-2:2017. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 2: Enumeration Method; International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6888-2:2004. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Technique Using Rabbit Plasma Fibrinogen Agar Medium; International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 16654:2001/A1:2017. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Escherichia Coli O157—Amendment 1: Annex B: Result of Interlaboratory Studies; International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6579-1:2017. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella Spp.; International Organization for Standardization: Geneva, Switzerland, 2017.
- Xiong, L.; Li, C.; Boeren, S.; Vervoort, J.; Hettinga, K. Effect of heat treatment on bacteriostatic activity and protein profile of bovine whey proteins. Food Res. Int. 2020, 127, 108688. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Fox, P.F.; Ciocia, F. Metabolism of Residual Lactose and of Lactate and Citrate. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; Mc Sweeney, P., Fox, P., Cotter, P., Everett, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 411–421. ISBN 978-0-12-417012-4. [Google Scholar]
- McSweeney, P.L.H. Biochemistry of cheese ripening: Introduction and overview. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P., Fox, P., Cotter, P., Everett, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 379–387. ISBN 978-0-12-417012-4. [Google Scholar]
- Addis, M.; Piredda, G.; Pes, M.; Di Salvo, R.; Scintu, M.F.; Pirisi, A. Effect of the use of three different lamb paste rennets on lipolysis of the PDO Pecorino Romano Cheese. Int. Dairy J. 2005, 15, 563–569. [Google Scholar] [CrossRef]
- Peng, S.; Tasara, T.; Hummerjohann, J.; Stephan, R. An overview of molecular stress response mechanisms in Escherichia coli contributing to survival of Shiga toxin-producing Escherichia coli during raw milk cheese production. J. Food Prot. 2011, 74, 849–864. [Google Scholar] [CrossRef]
- Modi, R.; Hirvi, Y.; Hill, A.; Griffiths, M.W. Effect of phage on survival of Salmonella Enteritidis during manufacture and storage of cheddar cheese made from raw and pasteurized milk. J. Food Protect. 2001, 64, 927–933. [Google Scholar] [CrossRef]
- Pexara, A.; Solomakos, N.; Sergelidis, D.; Govaris, A. Fate of enterotoxigenic Staphylococcus aureus and staphylococcal enterotoxins in Feta and Galotyri cheeses. J. Dairy Res. 2012, 79, 405–413. [Google Scholar] [CrossRef]
- Delbes, C.; Alomar, J.; Chougui, N.; Martin, J.F.; Montel, M.C. Staphylococcus aureus growth and enterotoxin production during the manufacture of uncooked, semihard cheese from cows’ raw milk. J. Food Prot. 2006, 69, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Farrokh, C.; Jordan, K.; Auvray, F.; Glass, K.; Oppegaard, H.; Raynaud, S.; Thevenot, D.; Condron, R.; De Reu, K.; Govaris, A.; et al. Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food Microbiol. 2013, 162, 190–212. [Google Scholar] [CrossRef]
- Calicioglu, M.; Dikici, A. Survival and Acid Adaptation Ability of Salmonella during Processing and Ripening of Savak Tulumi Cheese. J. Anim. Vet. Adv. 2009, 8, 1124–1130. [Google Scholar]
- Papadopoulou, C.; Maipa, V.; Dimitriou, D.; Pappas, C.; Voutsinas, L.; Malatou, H. Behavior of Salmonella enteritidis During the Manufacture, Ripening, and Storage of Feta Cheese Made from Unpasteurized Ewe’s Milk. J. Food Prot. 1993, 56, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wusimanjiang, P.; Ozturk, M.; Ayhan, Z.; Mehmetoglu, A.Ç. Effect of salt concentration on acid- and salt-adapted Escherichia coli O157:H7 and Listeria monocytogenes in recombined nonfat cast cheese. J. Food Process Pres. 2019, 43, e14208. [Google Scholar] [CrossRef]
- Consolidated Text: Commission Regulation (EC) No 2073/2005 on Microbiological Criteria for Food Stuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02005R2073-20200308 (accessed on 30 October 2020).
- Al-Nabulsi, A.A.; Osaili, T.M.; AbuNaser, R.A.; Olaimat, A.N.; Ayyash, M.; Al-Holy, M.A.; Kadora, K.M.; Holley, R.A. Factors affecting the viability of Staphylococcus aureus and production of enterotoxin during processing and storage of white-brined cheese. J. Dairy Sci. 2020, 103, 6869–6881. [Google Scholar] [CrossRef]
- Mohammadi, K.; Hanifian, S. Growth and enterotoxin production of Staphylococcus aureus in Iranian ultra-filtered white cheese. Int. J. Dairy Technol. 2015, 68, 111–117. [Google Scholar] [CrossRef]
- Valero, A.; Pérez-Rodríguez, F.; Carrasco, E.; Fuentes-Alventosa, J.M.; García-Gimeno, R.; Zurera, G. Modelling the growth boundaries of Staphylococcus aureus: Effect of temperature, pH and water activity. Int. J. Food Microbiol. 2009, 133, 186–194. [Google Scholar] [CrossRef]
- Ahmed, A.A.-H.; Maharik, N.M.S.; Valero, A.; Kamal, S.M. Incidence of enterotoxigenic Staphylococcus aureus in milk and Egyptian artisanal dairy products. Food Control 2019, 104, 20–27. [Google Scholar] [CrossRef]
- Viçosa, G.N.; Botelho, C.V.; Botta, C.; Bertolino, M.; de Carvalho, A.F.; Nero, L.A.; Cocolin, L. Impact of co-cultivation with Enterococcus faecalis over growth, enterotoxin production and gene expression of Staphylococcus aureus in broth and fresh cheeses. Int. J. Food Microbiol. 2019, 308, 108291. [Google Scholar] [CrossRef]
- Rukke, E.O.; Sørhaug, T.; Stepaniak, L. Heat Treatment of Milk|Thermization of Milk. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 693–698. ISBN 978-0-12-374407-4. [Google Scholar]
- Gabriel, A.A.; Bayaga, C.L.T.; Magallanes, E.A.; Aba, R.P.M.; Tanguilig, K.M.N. Fates of pathogenic bacteria in time-temperature-abused and Holder-pasteurized human donor-, infant formula-, and full cream cow’s milk. Food Microbiol. 2020, 89, 103450. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.E.; Smythe, B.W.; Crawford, R.A.; Oakley, E.; Hathaway, S.C.; Shepherd, J.M. Pasteurization of milk: The heat inactivation kinetics of milk-borne dairy pathogens under commercial-type conditions of turbulent flow. J. Dairy Sci. 2012, 95, 20–35. [Google Scholar] [CrossRef] [PubMed]
| Microorganism | Strain a | Collection b |
|---|---|---|
| Listeria monocytogenes | ATCC 15313 | ATCC 1 |
| ATCC 19114 | ATCC 1 | |
| ATCC 9525 | ATCC 1 | |
| ATCC 153/3 | ATCC 1 | |
| 2 | IZSLER 2 | |
| 90 | IZSLER 2 | |
| V7 | IZSLER 2 | |
| Staphylococcus aureus | ATCC 14458 | ATCC 1 |
| ATCC 25923 | ATCC 1 | |
| 401 | IZS 2 | |
| 466 | IZS 2 | |
| 64494 | IZS 2 | |
| Salmonella spp. | Typhimurium ATCC 6994 | ATCC 1 |
| Enteritidis 670 | IZSLER 2 | |
| Escherichia coli O157:H7 | ATCC 43984 | ATCC 1 |
| 47 | IZLER 2 | |
| 719 | IZLER 2 |
| Ripening Time | Day 1 | Day 90 | Day 150 | Significance | |||||
|---|---|---|---|---|---|---|---|---|---|
| Thermal Treatment | RM | TM | RM | TM | RM | TM | T | R | T × R |
| Parameters | |||||||||
| pH | 5.3 ± 0.1 a | 5.21 ± 0.03 a | 5.3 ± 0.1 a | 5.0 ± 0.1 b | 5.3 ± 0.1 a | 5.0 ± 0.1 b | ** | ** | ** |
| Moisture (%) | 42 ± 1 a | 42 ± 1 a | 32 ± 1 bc | 32.5 ± 0.3 b | 32 ± 2 bc | 31.2 ± 0.4 c | NS | *** | * |
| Aw | 0.970 ± 0.005 b | 0.983 ± 0.002 a | 0.89 ± 0.01 c | 0.880 ± 0.005 cd | 0.880 ± 0.02 cd | 0.873 ± 0.004 d | NS | *** | *** |
| NaCl/DM (%) | 0.15 ± 0.01 c | 0.21 ± 0.01 c | 6.3 ± 0.2 b | 7.2 ± 0.3 a | 6.4 ± 0.2 b | 7.3 ± 0.2 a | *** | *** | *** |
| Pathogens | Raw Milk | Thermized Milk | Significance |
|---|---|---|---|
| E. coli O157:H7 | 6.1 ± 0.1 a | 2.6 ± 0.5 b | *** |
| Salmonella spp. | 6.5 ± 0.4 a | 3.3 ± 0.4 b | *** |
| L. monocytogenes | 6.01 ± 0.02 a | 3.6 ± 0.3 b | *** |
| S. aureus | 6.5 ± 0.1 a | 3.7 ± 0.5 b | *** |
| Ripening Time | Day 1 | Day 90 | Day 150 | Significance | |||||
|---|---|---|---|---|---|---|---|---|---|
| Thermal Treatment | RM | TM | RM | TM | RM | TM | T | R | T × R |
| Pathogens | |||||||||
| E. coli O157:H7 | 3.5 ± 0.1 a | 0 b | 0 b | 0 b | 0 b | 0 b | *** | *** | *** |
| Salmonella spp. | 2.3 ± 0.3 a | 0 b | 0 b | 0 b | 0 b | 0 b | *** | *** | *** |
| L. monocytogenes | 2.01 ± 0.02 a | 0 b | 0 b | 0 b | 0 b | 0 b | *** | *** | *** |
| S. aureus | 5.9 ± 0.3 a | <1 b | <1 b | <1 b | <1 b | <1 b | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, G.; Melillo, R.; Pes, M.; Addis, M.; Fadda, A.; Pirisi, A. Survival of Selected Pathogenic Bacteria during PDO Pecorino Romano Cheese Ripening. Dairy 2020, 1, 297-312. https://doi.org/10.3390/dairy1030020
Lai G, Melillo R, Pes M, Addis M, Fadda A, Pirisi A. Survival of Selected Pathogenic Bacteria during PDO Pecorino Romano Cheese Ripening. Dairy. 2020; 1(3):297-312. https://doi.org/10.3390/dairy1030020
Chicago/Turabian StyleLai, Giacomo, Rita Melillo, Massimo Pes, Margherita Addis, Antonio Fadda, and Antonio Pirisi. 2020. "Survival of Selected Pathogenic Bacteria during PDO Pecorino Romano Cheese Ripening" Dairy 1, no. 3: 297-312. https://doi.org/10.3390/dairy1030020
APA StyleLai, G., Melillo, R., Pes, M., Addis, M., Fadda, A., & Pirisi, A. (2020). Survival of Selected Pathogenic Bacteria during PDO Pecorino Romano Cheese Ripening. Dairy, 1(3), 297-312. https://doi.org/10.3390/dairy1030020







