Influence of Dry Period Length of Swedish Dairy Cows on the Proteome of Colostrum †
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals, Sampling
2.2. Milk Serum Preparation
2.3. Sample Preparation for LC-MS/MS Analysis
2.4. LC-MS/MS
2.5. Protein Identification and Quantification
2.6. Statistical Analyses
3. Results
3.1. Colostrum and Milk Composition
3.2. Proteome of Cows with a 4 or an 8 Week Dry Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuhn, M.T.; Hutchison, J.L.; Norman, H.D. Minimum days dry to maximize milk yield in subsequent lactation. Anim. Res. 2005, 54, 351–367. [Google Scholar] [CrossRef]
- van Knegsel, A.T.M.; Remmelink, G.J.; Jorjong, S.; Fievez, V.; Kemp, B. Effect of dry period length and dietary energy source on energy balance, milk yield, and milk composition of dairy cows. J. Dairy Sci. 2014, 97, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Rastani, R.R.; Grummer, R.R.; Bertics, S.J.; Gumen, A.; Wiltbank, M.C.; Mashek, D.G.; Schwab, M.C. Reducing dry period length to simplify feeding transition cows: Milk production, energy balance, and metabolic profiles. J. Dairy Sci. 2005, 88, 1004–1014. [Google Scholar] [CrossRef]
- Annen, E.L.; Collier, R.J.; McGuire, M.A.; Vicini, J.L.; Ballam, J.M.; Lormore, M.J. Effect of Modified Dry Period Lengths and Bovine Somatotropin on Yield and Composition of Milk from Dairy Cows. J. Dairy Sci. 2004, 87, 3746–3761. [Google Scholar] [CrossRef]
- Church, G.T.; Fox, L.K.; Gaskins, C.T.; Hancock, D.D.; Gay, J.M. The Effect of a Shortened Dry Period on Intramammary Infections During the Subsequent Lactation. J. Dairy Sci. 2008, 91, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Mayasari, N.; Reilingh, G.D.; Nieuwland, M.G.B.; Remmelink, G.J.; Parmentier, H.K.; Kemp, B.; van Knegsel, A.T.M. Effect of maternal dry period length on colostrum immunoglobulin content and on natural and specific antibody titers in calves. J. Dairy Sci. 2015, 98, 3969–3979. [Google Scholar] [CrossRef]
- Watters, R.D.; Wiltbank, M.C.; Guenther, J.N.; Brickner, A.E.; Rastani, R.R.; Fricke, P.M.; Grummer, R.R. Effect of dry period length on reproduction during the subsequent lactation. J. Dairy Sci. 2009, 92, 3081–3090. [Google Scholar] [CrossRef]
- de Vries, R.; van Knegsel, A.; Johansson, M.; Lindmark-Månsson, H.; van Hooijdonk, T.; Holtenius, K.; Hettinga, K. Effect of shortening or omitting the dry period of Holstein-Friesian cows on casein composition of milk. J. Dairy Sci. 2015, 98, 8678–8687. [Google Scholar] [CrossRef]
- Andrée O’Hara, E.; Omazic, A.; Olsson, I.; Båge, R.; Emanuelson, U.; Holtenius, K. Effects of dry period length on milk production and energy balance in two cow breeds. Animal 2018, 12, 508–514. [Google Scholar] [CrossRef]
- Norgaard, J.V.; Theil, P.K.; Sorensen, M.T.; Sejrsen, K. Cellular mechanisms in regulating mammary cell turnover during lactation and dry period in dairy cows. J. Dairy Sci. 2008, 91, 2319–2327. [Google Scholar] [CrossRef]
- Capuco, A.V.; Akers, R.M.; Smith, J.J. Mammary Growth in Holstein Cows During the Dry Period: Quantification of Nucleic Acids and Histology. J. Dairy Sci. 1997, 80, 477–487. [Google Scholar] [CrossRef]
- Pezeshki, A.; Capuco, A.V.; De Spiegeleer, B.; Peelman, L.; Stevens, M.; Collier, R.J.; Burvenich, C. An integrated view on how the management of the dry period length of lactating cows could affect mammary biology and defence. J. Anim. Physiol. Anim. Nutr. 2010, 94, e7–e30. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.; Chen, J.; Kemp, B.; Van Knegsel, A.T.M. Review: Dry period length in dairy cows and consequences for metabolism and welfare and customised management strategies. Animal 2019, 13, S42–S51. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Barton, L.D.; Sanders, J.T.; Zhang, Q.A. Exploration of Bovine Milk Proteome in Colostral and Mature Whey Using an Ion-Exchange Approach. J. Proteome Res. 2011, 10, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Boeren, S.; Hageman, J.A.; Van Hooijdonk, T.; Vervoort, J.; Hettinga, K. Bovine milk proteome in the first 9 days: Protein interactions in maturation of the immune and digestive system of the newborn. PLoS ONE 2015, 10, e0116710. [Google Scholar] [CrossRef] [PubMed]
- Ibeagha-Awemu, E.M.; Ibeagha, A.E.; Messier, S.; Zhao, X. Proteomics, Genomics, and Pathway Analyses of Escherichia coli and Staphylococcus aureus Infected Milk Whey Reveal Molecular Pathways and Networks Involved in Mastitis. J. Proteome Res. 2010, 9, 4604–4619. [Google Scholar] [CrossRef]
- Danielsen, M.; Codrea, M.C.; Ingvartsen, K.L.; Friggens, N.C.; Bendixen, E.; Røntved, C.M. Quantitative milk proteomics–Host responses to lipopolysaccharide-mediated inflammation of bovine mammary gland. Proteomics 2010, 10, 2240–2249. [Google Scholar] [CrossRef]
- Watters, R.D.; Guenther, J.N.; Brickner, A.E.; Rastani, R.R.; Crump, P.M.; Clark, P.W.; Grummer, R.R. Effects of Dry Period Length on Milk Production and Health of Dairy Cattle. J. Dairy Sci. 2008, 91, 2595–2603. [Google Scholar] [CrossRef]
- Lu, J.; Boeren, S.; de Vries, S.C.; van Valenberg, H.J.F.; Vervoort, J.; Hettinga, K. Filter-aided sample preparation with dimethyl labeling to identify and quantify milk fat globule membrane proteins. J. Proteom. 2011, 75, 34–43. [Google Scholar] [CrossRef]
- Boersema, P.J.; Raijmakers, R.; Lemeer, S.; Mohammed, S.; Heck, A.J.R. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 2009, 4, 484–494. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/ (accessed on 1 March 2014).
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Boeren, S.; Smits, M.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K. Proteomic study on the stability of proteins in bovine, camel, and caprine milk sera after processing. Food Res. Int. 2016, 82, 104–111. [Google Scholar] [CrossRef]
- Alonso-Fauste, I.; Andrés, M.; Iturralde, M.; Lampreave, F.; Gallart, J.; Álava, M.A. Proteomic characterization by 2-DE in bovine serum and whey from healthy and mastitis affected farm animals. J. Proteom. 2012, 75, 3015–3030. [Google Scholar] [CrossRef] [PubMed]
- Kosciuczuk, E.M.; Lisowski, P.; Jarczak, J.; Krzyzewski, J.; Zwierzchowski, L.; Bagnicka, E. Expression patterns of beta-defensin and cathelicidin genes in parenchyma of bovine mammary gland infected with coagulase-positive or coagulase-negative Staphylococci. BMC Vet. Res. 2014, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- McCann, K.B.; Shiell, B.J.; Michalski, W.P.; Lee, A.; Wan, J.; Roginski, H.; Coventry, M.J. Isolation and characterisation of a novel antibacterial peptide from bovine alpha(s1)-casein. Int. Dairy J. 2006, 16, 316–323. [Google Scholar] [CrossRef]
- Smolenski, G.; Broadhurst, M.; Stelwagen, K.; Haigh, B.; Wheeler, T. Host defence related responses in bovine milk during an experimentally induced Streptococcus uberis infection. Proteome Sci. 2014, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Boeren, S.; Hageman, J.A.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K. Perspective on calf and mammary gland development through changes in the bovine milk proteome over a complete lactation. J. Dairy Sci. 2015, 98, 5362–5373. [Google Scholar] [CrossRef]
- Nakajima, K.; Itoh, F.; Nakamura, M.; Kawamura, A.; Yamazaki, T.; Kozakai, T.; Takusari, N.; Ishisaki, A. Short communication: Opposing effects of lactoferrin on the proliferation of fibroblasts and epithelial cells from bovine mammary gland. J. Dairy Sci. 2015, 98, 1069–1077. [Google Scholar] [CrossRef]
- 3Tang, L.; Wu, J.J.; Ma, Q.; Cui, T.; Andreopoulos, F.M.; Gil, J.; Valdes, J.; Davis, S.C.; Li, J. Human lactoferrin stimulates skin keratinocyte function and wound re-epithelialization. Br. J. Dermatol. 2010, 163, 38–47. [Google Scholar] [CrossRef]
- Engelmayer, J.; Blezinger, P.; Varadhachary, A. Talactoferrin stimulates wound healing with modulation of inflammation. J. Surg. Res. 2008, 149, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sodek, J.; da Silva, A.P.B.; Zohar, R. Osteopontin and mucosal protection. J. Dent. Res. 2006, 85, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Sando, L.; Pearson, R.; Gray, C.; Parker, P.; Hawken, R.; Thomson, P.C.; Meadows, J.R.S.; Kongsuwan, K.; Smith, S.; Tellam, R.L. Bovine Muc1 is a highly polymorphic gene encoding an extensively glycosylated mucin that binds bacteria. J. Dairy Sci. 2009, 92, 5276–5291. [Google Scholar] [CrossRef] [PubMed]
- Dowbenko, D.; Kikuta, A.; Fennie, C.; Gillett, N.; Lasky, L.A. Glycosylation-dependent cell-adhesion molecule-1 (GLYCAM-1) mucin is expressed by lactating mammary-gland epithelial-cells and is present in milk. J. Clin. Investig. 1993, 92, 952–960. [Google Scholar] [CrossRef]
- Hettinga, K.; van Valenberg, H.; de Vries, S.; Boeren, S.; van Hooijdonk, T.; van Arendonk, J.; Vervoort, J. The host defense proteome of human and bovine milk. PLoS ONE 2011, 6, e19433. [Google Scholar] [CrossRef] [PubMed]
- Tomazic, P.V.; Birner-Gruenberger, R.; Leitner, A.; Obrist, B.; Spoerk, S.; Lang-Loidolt, D. Nasal mucus proteomic changes reflect altered immune responses and epithelial permeability in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Nathan, B.P.; Gairhe, S.; Nwosu, I.; Clark, S.; Struble, R.G. Reconstitution of the olfactory epithelium following injury in ApoE-deficient mice. Exp. Neurol. 2010, 226, 40–46. [Google Scholar] [CrossRef]
- Arnold, L.; Perrin, H.; de Chanville, C.B.; Saclier, M.; Hermand, P.; Poupel, L.; Guyon, E.; Licata, F.; Carpentier, W.; Vilar, J.; et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nat. Commun. 2015, 6, 8972. [Google Scholar] [CrossRef]
- Couchman, J.R.; Woods, A. Structure and Biology of Pericellular Proteoglycans; Roberts, D.D., Mecham, R.P., Eds.; Academic Press Inc.: San Diego, CA, USA, 1993. [Google Scholar]
- Uno, K.; Hayashi, H.; Kuroki, M.; Uchida, H.; Yamauchi, Y.; Kuroki, M.; Oshima, K. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem. Biophys. Res. Commun. 2004, 315, 928–934. [Google Scholar] [CrossRef]
- 4Stein, T.; Salomonis, N.; Gusterson, B.A. Mammary gland involution as a multi-step process. J. Mammary Gland Biol. Neoplasia 2007, 12, 25–35. [Google Scholar] [CrossRef]
- Annen, E.L.; Stiening, C.M.; Crooker, B.A.; Fitzgerald, A.C.; Collier, R.J. Effect of continuous milking and prostaglandin E-2 on milk production and mammary epithelial cell turnover, ultrastructure, and gene expression. J. Anim. Sci. 2008, 86, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Tacoma, R.; Fields, J.; Ebenstein, D.B.; Lam, Y.W.; Greenwood, S.L. Characterization of the bovine milk proteome in early-lactation Holstein and Jersey breeds of dairy cows. J. Proteom. 2016, 130, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Vincent, D.; Ezernieks, V.; Elkins, A.; Nguyen, N.; Moate, P.J.; Cocks, B.G.; Rochfort, S. Milk bottom-up proteomics: Method optimisation. Front. Genet. 2016, 6, 360. [Google Scholar] [CrossRef] [PubMed]
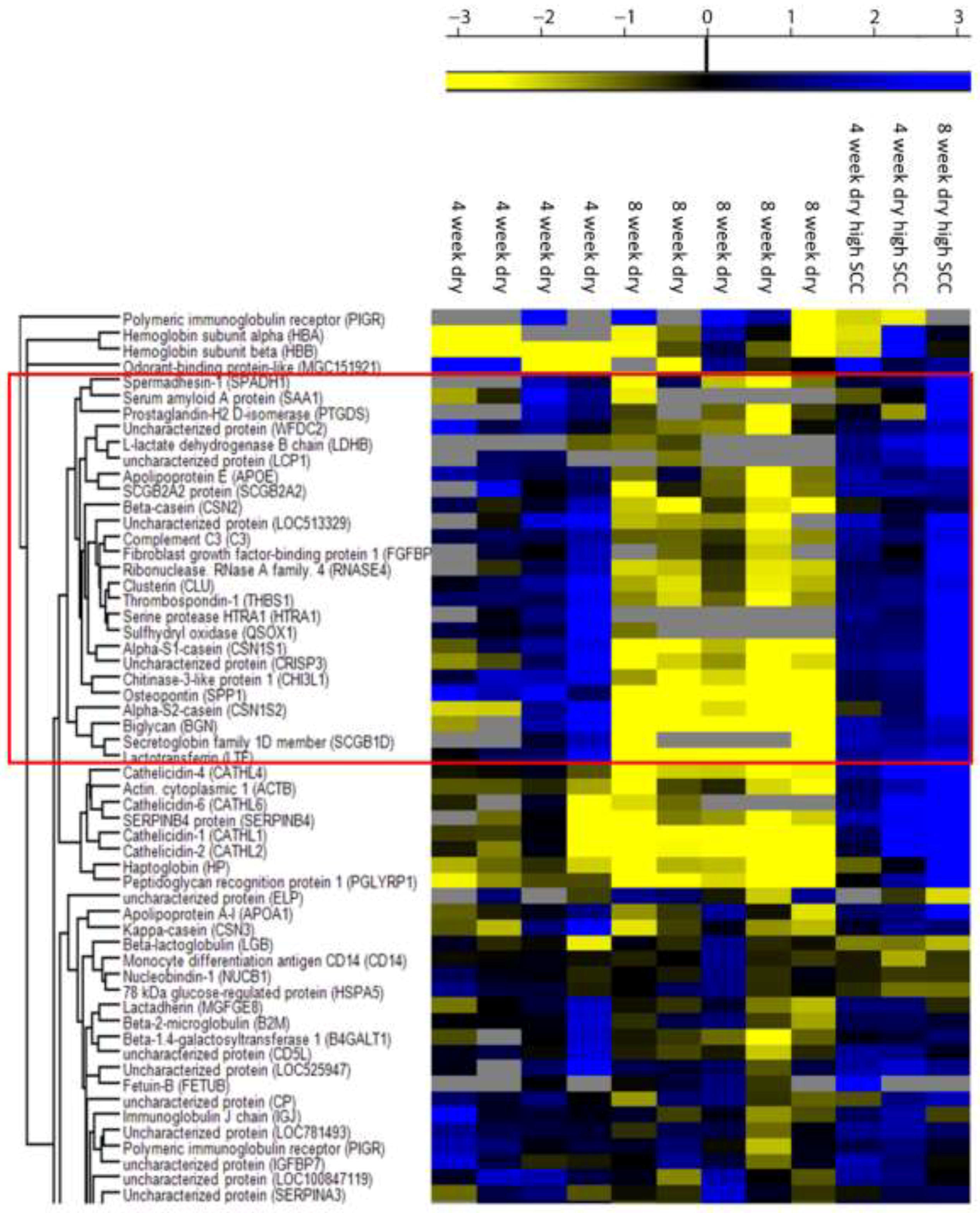
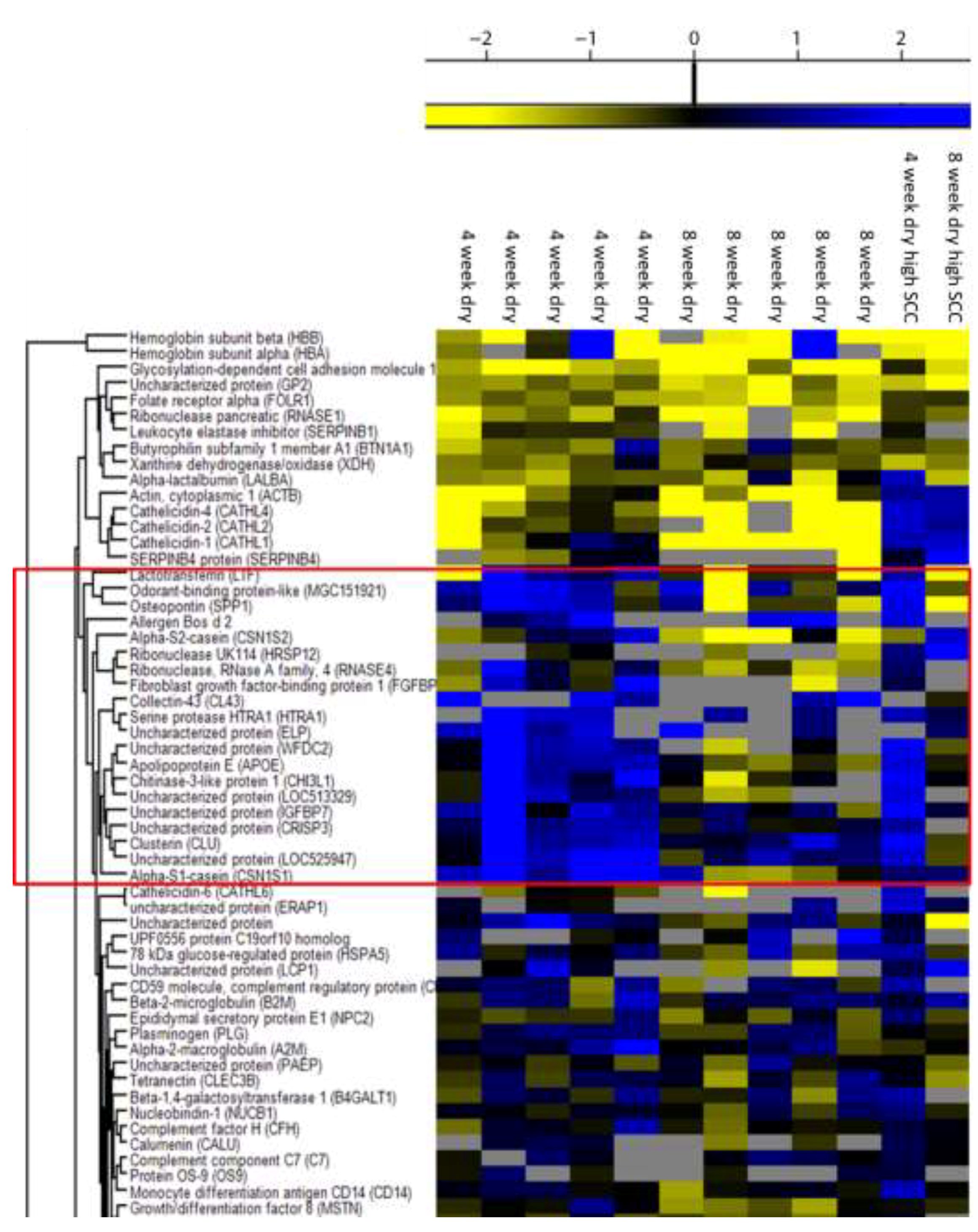

| Swedish Red | Swedish Holstein | ||||||
|---|---|---|---|---|---|---|---|
| 4 Week Dry N = 4 | 8 Week Dry N = 5 | SEM | 4 Week Dry N = 5 | 8 Week Dry N = 5 | SEM | ||
| Colostrum | Yield (kg/d) | 5.5 | 10.0 | 2.0 | 6.2 | 7.2 | 1.0 |
| Fat (%) | 5.5 | 5.8 | 1.1 | 5.9 | 10.7 | 2.3 | |
| Protein (%) | 15.1 | 12.6 | 1.4 | 15.5 * | 11.7 | 1.0 | |
| Lactose (%) | 5.3 | 5.3 | 0.2 | 5.2 * | 5.5 | 0.1 | |
| SCC (×103 cells/mL) 1 | 1838 | 1702 | 1020 | 4996 * | 1398 | 1221 | |
| Proteins quantified | 107 | 124 | 8.3 | 133 | 119 | 6.2 | |
| Transition milk | Yield (kg/d) | 15.9 | 23.8 | 3.0 | 26.5 | 28.5 | 4.1 |
| Fat (%) | 5.4 | 4.0 | 1.2 | 3.0 | 3.0 | 0.5 | |
| Protein (%) | 5.8 | 5.6 | 0.2 | 5.4 | 5.1 | 0.2 | |
| Lactose (%) | 6.4 | 6.5 | 0.1 | 6.7 | 6.6 | 0.1 | |
| SCC (×103 cells/mL) 1 | 1292 | 510 | 755 | 2524 | 997 | 1529 | |
| Proteins quantified | 127 | 123 | 3.9 | 143 * | 124 | 6.2 | |
| Protein | Gene | Colostrum | Transition Milk | ||
|---|---|---|---|---|---|
| SR (N = 4 + 5) | SH (N = 5 + 5) | SR (N = 4 + 5) | SH (N = 5 + 5) | ||
| Tissue regeneration | |||||
| Apolipoprotein E | APOE | 3.4 * | 4.0 | 2.9 | 1.7 |
| Biglycan | BGN | 57.7 * | ND | 3.5 | ND |
| Thrombospondin-1 | THBS1 | 7.9 * | ND | 1.3 | ND |
| Chitinase-3-like protein 1 | CHI3L1 | 17.7 * | 6.1 | 3.2 * | 0.7 |
| Clusterin | CLU | 8.9 * | 2.0 | 6.4 * | 1.0 |
| Host-defence | |||||
| Cathelicidin-2 | CATHL2 | 6.0 * | 7.8 | 5.1 | 2.0 |
| Cathelicidin-4 | CATHL4 | 5.1 * | 5.1 | 5.6 | 2.3 |
| Peptidoglycan recognition protein 1 | PGLYRP1 | 3.4 * | 1.6 | 3.3 | 4.1 |
| Ribonuclease. RNase A family 4 | RNASE4 | 6.0 * | 3.7 | 1.1 | 1.0 |
| Mucosal protection | |||||
| Glycosylation-dependent cell adhesion molecule 1 | GLYCAM1 | 4.0 * | 1.3 | 2.0 * | 3.1 |
| Lactotransferrin | LTF | 39.1 * | 12.9 | 13.5 * | 2.1 |
| Osteopontin | SPP1 | 33.7 * | 9.6 | 4.1 * | 1.8 |
| Uncharacterized protein | WFDC2 | 7.7 * | 5.8 | 8.7 * | 2.8 |
| Transport | |||||
| Alpha-S1-casein | CSN1S1 | 18.5 * | 4.8 | 3.8 * | 1.2 |
| Beta-casein | CSN2 | 6.2 * | 1.4 | 5.2 * | 1.1 |
| Complement system | |||||
| Complement C3 | C3 | 4.3 * | 1.7 | 2.0 * | 1.0 |
| Uncharacterized protein | CFI | 3.4 * | 1.6 | 2.0 * | 1.1 |
| Uncharacterized protein | LOC513329 | 15.3 * | 10.1 | 3.2 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vries, R.; Boeren, S.; Holtenius, K.; Vervoort, J.; Lindmark-Månsson, H.; Hettinga, K. Influence of Dry Period Length of Swedish Dairy Cows on the Proteome of Colostrum. Dairy 2020, 1, 313-325. https://doi.org/10.3390/dairy1030021
de Vries R, Boeren S, Holtenius K, Vervoort J, Lindmark-Månsson H, Hettinga K. Influence of Dry Period Length of Swedish Dairy Cows on the Proteome of Colostrum. Dairy. 2020; 1(3):313-325. https://doi.org/10.3390/dairy1030021
Chicago/Turabian Stylede Vries, Ruben, Sjef Boeren, Kjell Holtenius, Jacques Vervoort, Helena Lindmark-Månsson, and Kasper Hettinga. 2020. "Influence of Dry Period Length of Swedish Dairy Cows on the Proteome of Colostrum" Dairy 1, no. 3: 313-325. https://doi.org/10.3390/dairy1030021
APA Stylede Vries, R., Boeren, S., Holtenius, K., Vervoort, J., Lindmark-Månsson, H., & Hettinga, K. (2020). Influence of Dry Period Length of Swedish Dairy Cows on the Proteome of Colostrum. Dairy, 1(3), 313-325. https://doi.org/10.3390/dairy1030021





