The feeding value of a feedstuff is generally determined by its energy and nutrient content but also by the presence of substances potentially capable of compromising the health and performance of animals. Feedstuffs with different energy and nutrient concentrations are combined to form a daily ration which meets the energy and nutrient requirements of the animal. Thus, deficits of individual feedstuffs are balanced in diet formulation. Maize silage (MS) is characterized by a higher energy concentration while grass silage (GS) contains more crude protein, making both silages complementary in the formulation of dairy cow rations. While these aspects are commonly considered in practical dairy feeding, less attention is paid to the possible feed and food hygienic consequences of feeding these distinctly different silage types. These consequences result from the presence of silage-specific contaminants, such as mycotoxins, biogenic amines and Clostridia. Besides, varying physico-chemical properties need to be considered, such as starch resistance and protein solubility, giving rise to gastro-intestinal conditions favorable to the forced fecal shedding of harmful micro-organisms potentially contaminating milk and beef, such as Shiga toxin (stx)-forming E. coli.
4.1. Microbiological Evaluation of Silages and Contamination by Fungal and Other Metabolites
Contrary to feedstuffs stored under dry air conditions (moisture content ≤14%) such as cereal grains, silage is a high-moisture forage preserved by fermentation [
49]. While mycotoxin formation in stored feedstuffs is usually terminated or drastically reduced when the moisture content is ≤14%, it might continue under ensiling conditions whereby the pattern of mycotoxins differs from those “harvested” from the field due to different molds colonizing the ensiled crops [
49,
50].
Penicillium, Fusarium, Aspergillus,
Mucor,
Byssochlamys,
Absidia,
Arthrinium,
Geotrichum,
Monascus,
Scopulariopsis, and
Trichoderma belong to the most frequently detected fungal genera while species of
Fusarium, Alternaria,
Cladosporium,
Claviceps, and endophytic fungi are predominantly isolated prior to storage and ensiling [
49].
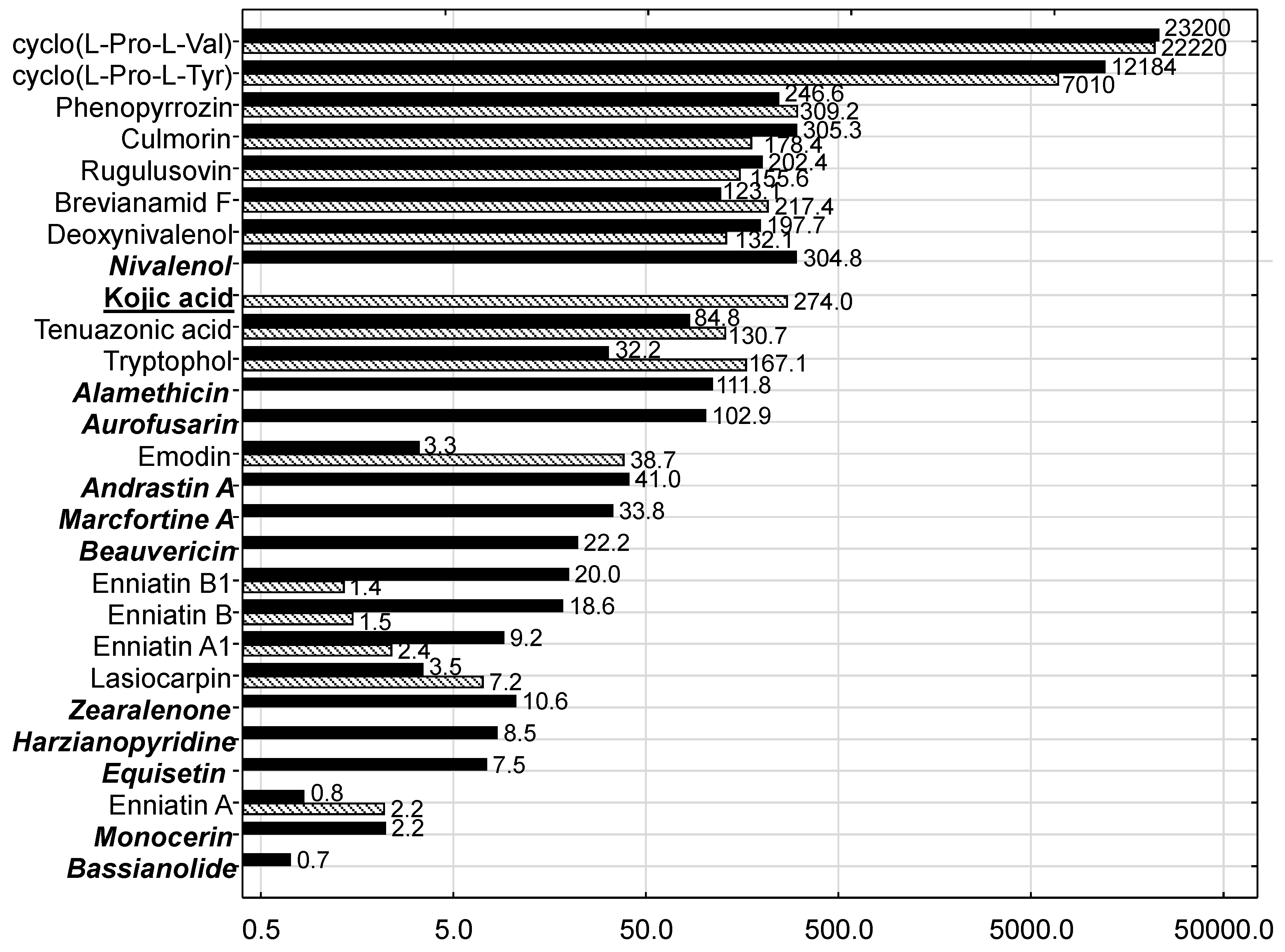
In the present study the silages were characterized microbiologically by an evaluation schema proposed by the Association of German Agricultural Analytic and Research Institutes (VDLUFA), which enables the condensation of the microbial condition of feedstuffs to a single quality class. Both product-specific and spoilage-indicating germs were in the range typically observed for MS and GS. The higher yeast content observed in MS might be due to higher temperatures in May and June in combination with the higher starch and sugar content of MS in comparison to GS. These higher yeast contents confirm the expectations that MS is at higher risk for yeast proliferation which is also reflected by the generally higher orientation value for this germ group. However, as the DMI of the cows fed MS-based diets was not compromised, an intake-depressing effect due to higher yeast contamination cannot be stated. Except for yeasts in MS, all other fungus-related germ groups were in the range normally observed for MS and GS. This might explain the non-detectability and the low levels of detectable mycotoxins typically formed in spoiled silage. Thus, common silage-born
Aspergillus toxins such as gliotoxin, fumitremorgins and fumigaclavines,
Penicillium toxins such as roquefortins and mycophenolic acid or
Monascus toxins such as monacolins remained undetectable in both silage types. This situation also agrees with the observation that MS and GS samples which were visibly molded also contained higher levels of roquefortins, monacolins, mycophenolic acid and further mycotoxins compared to normal-appearing silage samples characterized by a lower incidence of fungus infection and mycotoxin contamination [
2,
51,
52].
However, the signature of mycotoxins and other fungal metabolites was clearly different between MS and GS and points to different fungal infection patterns and timings of infection. The most prominent difference was attributable to Fusarium toxins in that MS was contaminated with more of these toxins compared to GS and in most cases at higher concentrations. These results suggest that the differences in mycotoxin patterns between MS and GS were due to pre-harvest contamination while post-harvest mycotoxin formation was of lower importance owing to good silaging conditions not favoring mold growth and consequently mycotoxin formation.
As well as DON, the
Fusarium toxin nivalenol also belongs to the group of trichothecene toxins and therefore shares some of the toxicological features of DON, such as immuno-toxicity. Lower and upper bound mean and maximum concentrations of 80, 174 and 1022 µg/kg fresh weight have been reported [
53] for MS. Recalculating the nivalenol concentration measured for MS to the same reference basis revealed a concentration of approximately 127 µg/kg fresh weight suggesting that the MS used in the present experiment was contaminated by nivalenol at background level. As no studies on the toxic effects of nivalenol on ruminants have been identified [
53], an interactive effect with DON cannot be excluded although ruminal de-epoxidation similar to DON might be assumed.
Beauvericin and enniatins are characterized by a low acute toxicity; no studies have been reported examining adverse effects of these mycotoxins on ruminants and European Food Safety Authority (EFSA) precluded an evaluation of the health risk from chronic exposure to beauvericin and enniatins [
54]. The mechanisms by which these compounds exert their cytotoxic effects appears to be related to their ionophoric activity that increases ion permeability in biological membranes [
55]. The in vitro proliferation of bovine granulosa cells was shown to be inhibited at 3 µM [
56]. As well as beauvericin and enniatins, bassianolides are cyclooligomer depsipeptides; the latter was shown to be produced by the fungus
Beauveria bassiana which also forms beauvericin. Bassianolides exert insecticidial activity [
57,
58]. The in vivo relevance of bassianolides with regard to effects on ruminal microbiota, ruminal metabolism and systemic effects needs to be elucidated.
Equisetin was isolated from
Fusarium equiseti and was described as one of the most potent
Fusarium metabolites exerting antibiotic properties, particularly against some Gram-positive bacteria including mycobacteria and some
Staphylococcus aureus strains [
59]. The relevance for the ruminant is unclear so far, although equisetin has been isolated in large amounts from cottonseed cake contaminated by multiple mycotoxins and associated with cattle mortality and feed refusal [
60].
Culmorin is considered as an “emerging mycotoxin”; it often co-occurs with DON [
61] which was also confirmed in the present experiment where its concentration was slightly higher than that of DON in both silage types, although in MS at a higher level than in GS. Comparative toxicity studies with pigs revealed that culmorin alone did not affect pigs [
62]. When combined with DON, the adverse effects were dominated by DON without any hint of interactive effects, thus supporting the notion of low toxicity of culmorin for pigs under the test conditions [
62]. Nothing is known about the effects of culmorin on cattle.
The toxic effects of aurofusarin have not yet been investigated for cattle, but for quails it adversely affects the antioxidative system, including that of the developing embryo [
63,
64].
The cyclic dipeptides cyclo(
l-pro-
l-tyr) and cyclo(
l-pro-
l-val) were the most prominent metabolites out of the 26 and 16 metabolites quantified in MS and GS, respectively, and accounted for approximately 95% in both silage types. Cyclic dipeptides are referred as to diketopiperazines (DKPs) and are formed by a broad spectrum of organisms including not only bacteria, fungi and porifera but also mammals. As a large substance group they are described to exert antibacterial, antifungal, antiviral, phytotoxic and cytotoxic effects. Amongst others, they are degraded compound-specifically by microbial and mammalian enzymes [
65,
66]. To the authors’ knowledge no information is available about the outcome, particularly on the ruminal metabolism, and the effects in cattle. As well as cyclo(
l-pro-
l-tyr) and cyclo(
l-pro-
l-val), a further DKP, cyclo-(
l-Trp-
l-Pro), also known as brevianamide F, was detected in both silages but at comparatively low concentrations.
In the present experiment, andrastin A and marcfortine A as
Penicillium metabolites were exclusively detected in MS. Their toxicological relevance for higher animals has not yet been examined [
2,
67]. Regarding their mode of action, andrastins were shown to act as protein farnesyltransferase inhibitors [
68,
69], a feature making them interesting for anticancer and antimalarial therapy [
70,
71] and supporting their biological activity. Cytotoxicity testing revealed that the 50% inhibitory concentration (IC
50) of andrastin A was not determinable in Caco-2 cells and therefore exceeded the maximum tested concentration of 50 µg/mL [
72]. Marcfortines belong to oxindole alkaloids and as nicotinic cholinergic antagonists they exert anthelmintic activities; they are also active at mammalian receptors in vitro [
73]. The relevance of these biological features for cattle remains to be clarified.
Only 2 Aspergillus toxins were quantifiable in the present study. While phenopyrrozin was detectable both in MS and GS, kojic acid, which is also formed by other fungus genera, was solely found in GS. Phenopyrrozin was described as a radical scavenger and its IC50 against lipid peroxidation induced by Cr2K2O7 amounted to 73 µg/mL. The significance of this effect for ruminants needs to be examined.
Kojic acid is used in cosmetic products for skin whitening or depigmenting [
74]. Owing to its use in cosmetics, the toxicological properties of kojic acid are better characterized than those of phenopyrrozin. The no-observed-adverse-effect level (NOAEL) of 6 mg kojic acid/kg BW/day is based on histopathological findings and altered iodine uptake after oral administration in rats [
74]. Assuming a DM intake of 20 kg/cow/d, a BW of 600 kg and a (maximum) GS proportion of 80% of the TMR, the daily exposure of this cow would amount to approximately 0.008 mg/kg BW/d based on the measured kojic acid concentration of 274 µg/kg GS. The comparison of this exposure with the mentioned NOAEL suggests that the kojic acid level measured in GS was probably toxicologically not relevant.
Tryptophol and emodin were detectable in native grass [
75] and were also present in both silage types in the present study. Emodin, which is produced both by fungi and by plants, exerts immunosuppressive, anticancer, anti-inflammatory, anti-atherosclerotic and vasorelaxant effects, but hepatotoxicity, kidney toxicity and reproductive toxicity have also been reported particularly under chronical exposure scenarios [
76]. Tryptophol (3-indole-ethanol) is found in many microorganisms and plants and is also a secondary metabolite produced by
Candida albicans. It possesses a quorum-sensing ability and is cytotoxic, cytostatic, and genotoxic in lymphocytes [
77]. The relevance of the presence of emodin and tryptophol in feed for cattle remains to be clarified.
The fungus
Exserohilum turcicum, causing the northern corn leaf blight, has been described as a producer of monocerin which exerts phytotoxic effects [
78]. Interestingly, this metabolite was exclusively detected in MS in the present study. As well as
E. turcicum,
Alternaria spp. are known as monocerin producers [
79]. Monocerin effectively inhibited
Plasmodium falciparum with an IC
50 of 0.68 µM, but was not cytotoxic against various tumor cell lines [
80]. In contrast to monocerin, the
Alternaria toxin tenuazonic acid was quantified both in MS and GS suggesting that another fungus species was responsible. The viability of Caco-2 cells was significantly reduced after exposure to
Alternaria tenuissima extracts containing several
Alternaria toxins besides tenuaconic acid as a dominant toxin [
72].
The
Trichoderma metabolites alamethicin and harzianopyridine were exclusively detected in MS.
T. harzianum was described to synthesize harzianopyridine, a non-volatile antibiotic which has been demonstrated to inhibit a number of pathogens [
81]. Moreover, a crude methanolic extract from
T. harzianum was shown to depress the motility of boar sperm and cause plasma membrane lesions [
82]. Alamethicin produced by
T. viride was shown to inhibit DNA synthesis and the mitogen stimulation of bovine lymphocytes isolated from lymph nodes at 2 and 1 µM, respectively. These effects were ascribed to the pore-forming properties of this antibiotic. Whether this substance affects ruminal microbiota or if it is metabolized by rumen microbiota itself before being absorbed and potentially influences lymphocytes is not known to the authors’ knowledge.
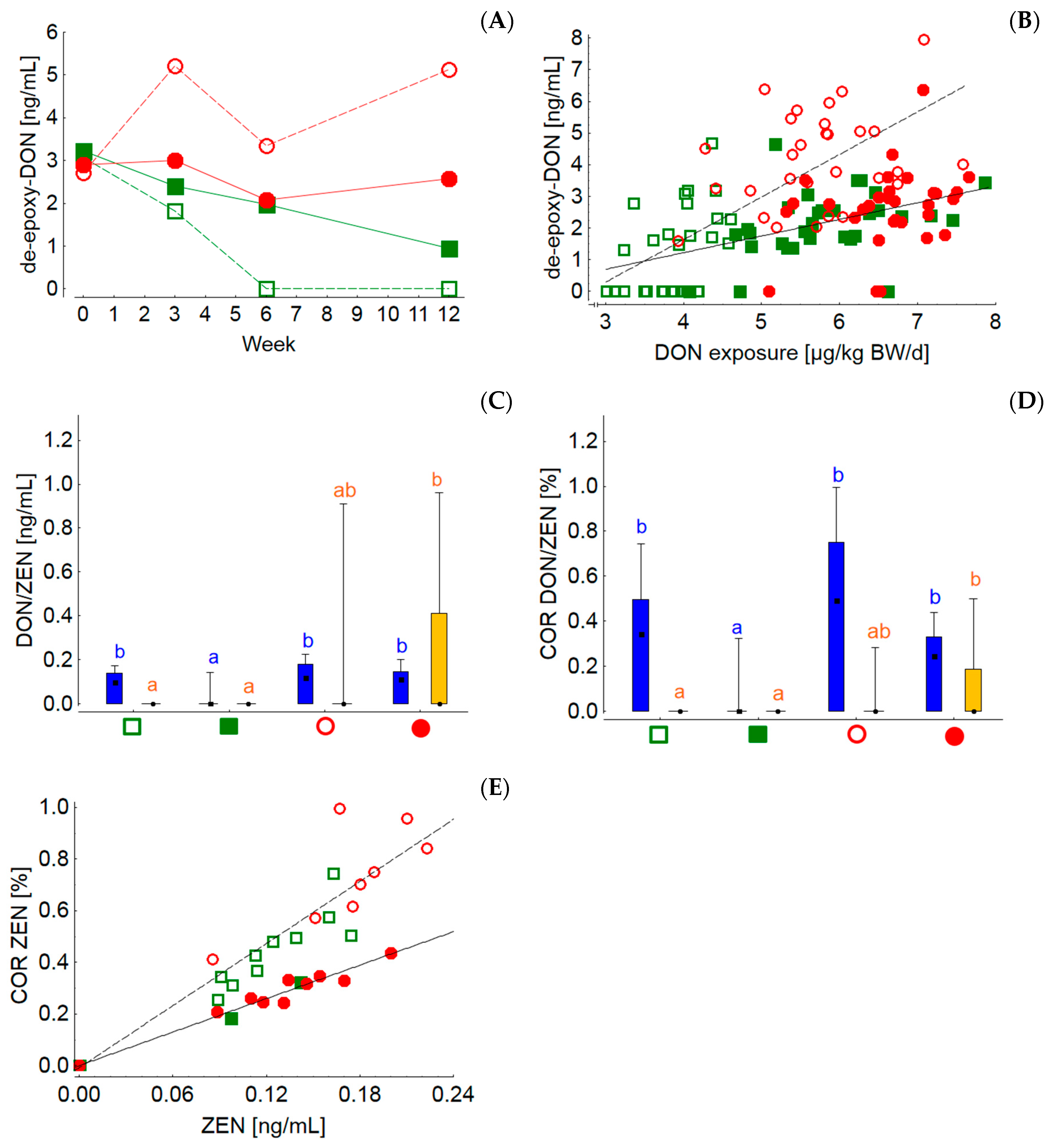
Compared to all the other mycotoxins and metabolites detected in the silages, only for DON and ZEN were sufficient data available for a risk evaluation for cattle by EFSA. Based on this EFSA evaluation the European Commission established guidance values for critical DON and ZEN concentrations in feed. For ruminating cattle 5 mg DON and 0.5 mg ZEN must not be exceeded per kg of the daily ration at a reference DM content of 88% in order to protect the animals from the adverse effects of these toxins [
83]. Based on the analyzed mycotoxin concentrations of silages and concentrate feeds and their proportions in the whole ration, the DON/ZEN concentrations of the rations grass-20, grass-60, maize-20 and maize-60 amounted to 144/1, 160/4, 196/10 and 187/8 µg/kg at 88% DM, respectively. Compared to the guidance values these contamination levels can be regarded as a low background contamination. Nevertheless, the concentrations of both toxins were higher both in MS and in the resulting MS-containing rations. Even at these low dietary DON concentrations, the differences between both silages could be followed in blood where the time-dependent group LSmeans of the de-DON concentrations clearly mirrored the differences in dietary DON concentration. Interestingly, when de-DON concentrations were plotted against individual DON exposures it became obvious that at similar DON exposures the de-DON levels in blood were higher when cows were fed rations with lower concentrate feed proportions (grass-20 and maize-20). These results indicate differences in the bioavailability of DON originating from either silages or concentrate feed. Although much higher DON exposures between approximately 90 and 200 µg/kg BW/d yielded no differences in the serum de-DON levels between cows fed rations with low or high concentrate feed portions, it also appeared that cows fed the diet with the lower concentrate feed portion transferred DON more efficiently to blood as de-DON [
84]. The observed differences in DON availability measured as de-DON concentrations in blood might be due to differences in the rumino-intestinal efficiency of liberating DON from silages and concentrate feed, to differences in absorption rates driven by diet-induced variable physico-chemical chyme conditions or to variable ruminal turnover rates. It has been shown that ZEN was degraded to beta-ZEL less efficiently when the DMI of cows increased, which is equivalent to a reduced ruminal retention time of ingesta (i.e., higher turnover rate) or, in other words, a reduced time not only for the fermentation of nutrients but also for metabolism for mycotoxins. However, as ruminal pH is largely influenced by the concentrate feed proportion and also by the level of DMI, pH effects on toxin metabolism have to be considered in the interpretation. Zearalenones are probably subject to pH-dependent reduction–oxidation steady states [
85]. Such effects might also be responsible for the more efficient transfer of ZEN to the milk of cows fed rations with low concentrate feed proportions irrespective of silage type. The total ZEN residue concentrations in milk were shown to increase in a dose-dependent manner, with maximum concentrations of 1.08 ng/mL corresponding to a dietary ZEN concentration of 581 µg/kg at 88% DM [
34]. In contrast, the dietary ZEN contamination level of the rations fed in the present experiment varied between 1–10 µg/kg at a DM content of 88% and reflected a typical practical situation. Under these conditions the ZEN residue levels in milk reached maximum concentrations of 0.2 ng/mL. Thus, the relative transfer from feed to milk (carry-over rate) appeared to be higher at low dietary background contamination and reached levels up to 1.0% compared to 0.75% at artificially fortified dietary ZEN levels. Model calculations revealed that the consumption of 1500 g milk/d by humans would contribute to 4% of the tolerable ZEN intake of 0.25 µg/kg BW per day when a median background ZEN concentration of 58 µg/kg feed was assumed [
86]. These calculations demonstrate that the milk contamination levels detected in the present experiment are of no concern with regard to food safety.
The presence of lasiocarpin in both silage types is considered to result from weed contamination as this substance is a secondary plant metabolite and belongs to the group of pyrrolizidine alkaloids (PA) mainly formed by plant families other than
Poaceae [
87,
88] although it has been recently shown that other types of PA are also synthesized by cool-season grasses [
89]. Lasiocarpin levels were not reported for 252 evaluated/analyzed forage and roughage samples although this PA is considered as one of the most toxic ones [
88].
4.2. Dietary Effects on Rumen, Hindgut and General Health and Performance
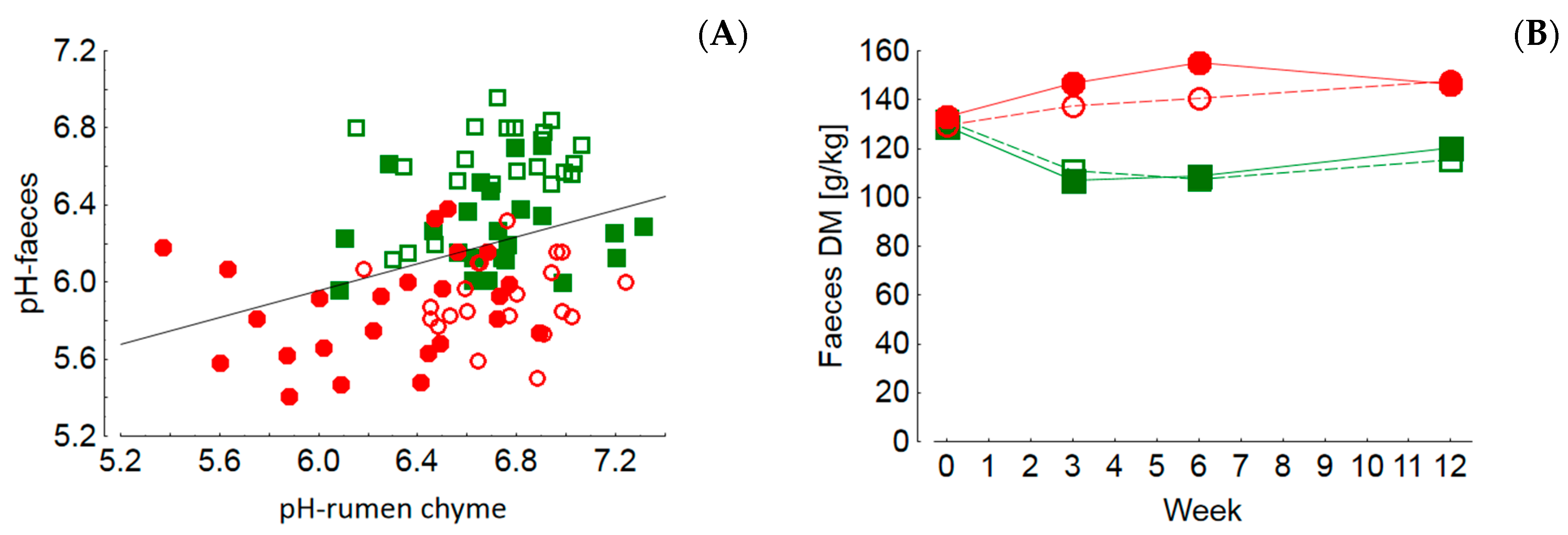
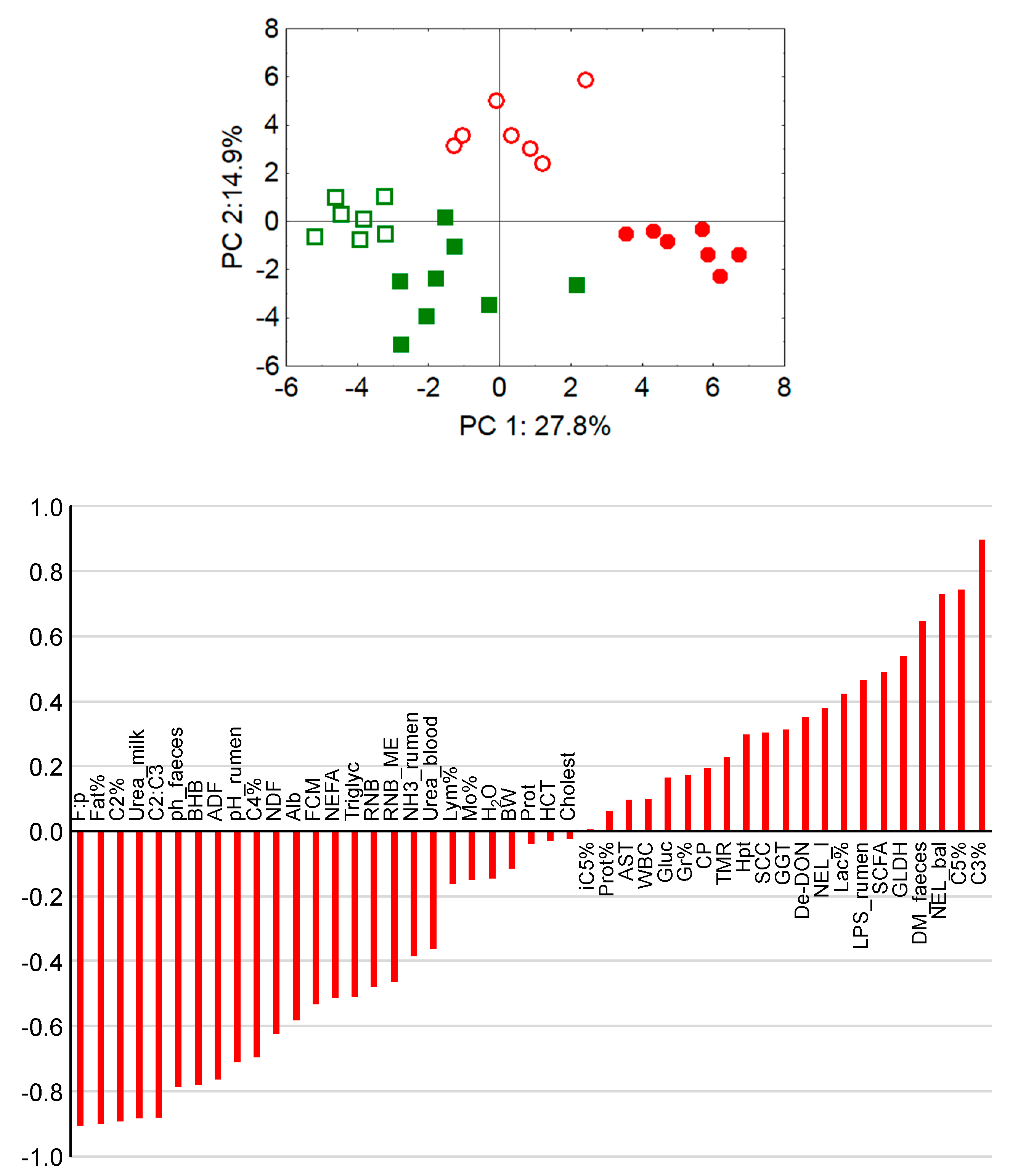
The pH values of rumen chyme and also of rectal feces were higher in cows fed diets with lower concentrate feed portions which also reflects differences in fermentation pattern and microbial communities. Low pH values of rumen chyme and rectal feces, as detected in cows fed rations with high concentrate feed portions, are indicators of rumen and hindgut acidosis, respectively [
90,
91,
92,
93], and suggest a significant ruminal bypass of starch reaching the hindgut [
90]. While for rumen pH various threshold values were defined to indicate subacute ruminal acidosis (SARA), e.g., lower than 5.6 on an average basis, the associations between pH decline and hindgut acidosis (HGA) are currently poorly defined [
93]. We used an oral rumen probe to collect rumen chyme, a procedure which is critically discussed due to possible saliva contamination and uncertainties with regard to a standardized collection site [
90,
91,
94]. Therefore, we can only assume that the pH values which were measured in the present study represented a mean value systematically adulterated by the method used. However, comparing the pH values between the experimental groups within the present study, we found clear associations with diet features, resulting in the lowest pH values in groups fed MS, particularly in combination with the high concentrate feed proportion. Looking at the individual data pairs relating rumen to rectal pH values, this conclusion is substantiated by a distinct cluster characterized by both low ruminal and fecal pH values in the maize-60 group. Although the mean ruminal pH value of 6.2 is not indicative in SARA, further parameters recorded in the experiment support the notion that cows of this group most probably suffered from SARA. A severe milk fat depression characterized by a milk fat content of 2.15% and a milk fat to milk protein ratio (FPR) of 0.66 was found in this group. An FPR of <1.0 is regarded as a reliable indicator for SARA, particularly in late-lactating cows [
90,
95]. Also, the lower urea concentration in milk and blood and the higher molar proportion of ruminal propionate along with the higher blood glucose level and the distinctly higher ruminal propionate proportion and LPS concentration support the conclusion that SARA was present in the maize-60 group. Further blood biochemical and hematological traits have been described to be unresponsive to the presence of SARA, which was also the case in the present experiment. For example, the liver lesion-indicating enzymes GLDH, GGT and ASAT were not only increased in the maize-60 group but also in the grass-60 group, compared to the groups fed the diets with the low concentrate feed proportion. Therefore, the treatment-related effects on the liver were most probably not related to SARA but rather to concentrate feed portion-induced effects such as DMI which was generally stimulated due to higher dietary energy contents. The increased nutrient flow needed finally to be processed by the liver. Thus, the increased peripheral activities of hepatocellular lesion-indicating enzymes might just reflect a generally increased quantitative hepatic metabolic load.
Based on the discussed parameters it might be concluded that marked differences in the energy supply induce more pronounced physiological adaptations than the presence of SARA, as detected in the maize-60 group. Acute ruminal acidosis but also SARA and hindgut acidosis have been associated with compromised epithelial barriers, facilitating LPS transfer to the liver and eventually to the systemic circulation, thus giving rise to the induction of an acute phase reaction and systemic inflammation in ruminants [
17,
18,
19,
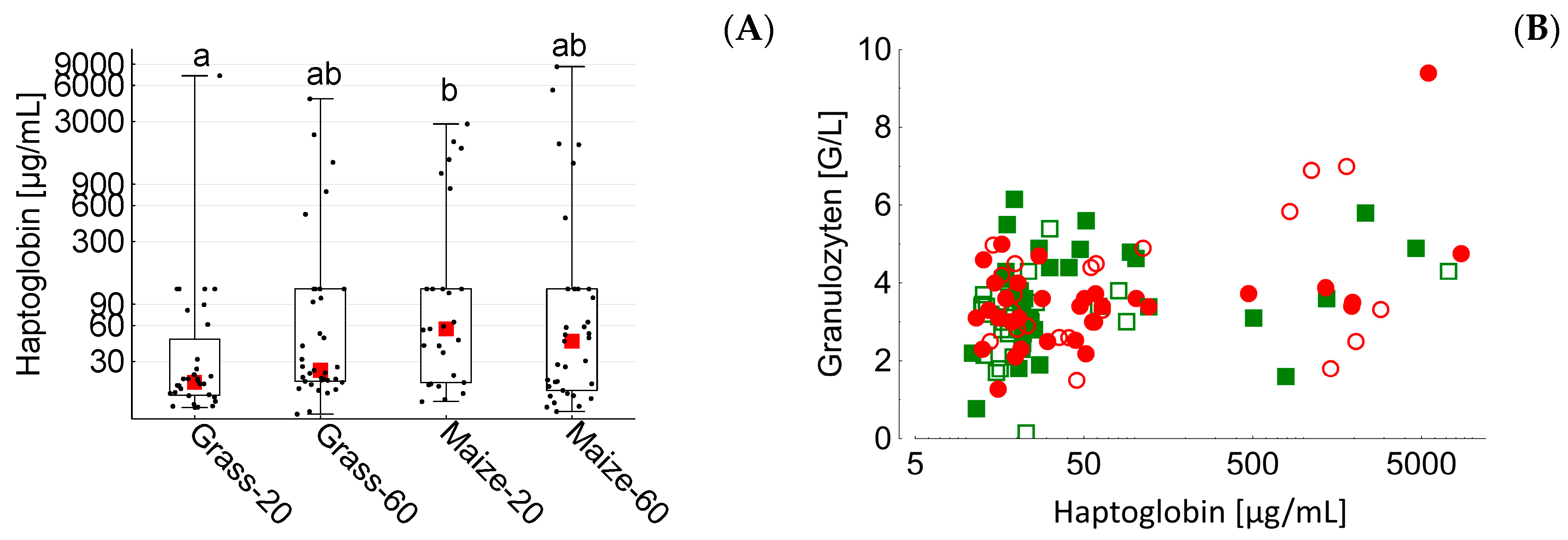
20]. Haptoglobin is regarded as one of the main bovine acute phase proteins [
96]; consequently, it is a possible indicator molecule for the presence and severity of SARA [
97]. However, in the present experiment the distinctly higher ruminal LPS concentration and the presence of SARA in the maize-60 group did not result in distinctly higher peripheral haptoglobin levels compared to all other groups. Total blood granulocytes increased (weakly) linearly with haptoglobin levels, which can be interpreted as indicative of the progression of a pro-inflammatory situation.
Lower hindgut pH values as observed in the MS-fed groups are probably based on an increased flow of starch to the large intestine due to resistance to ruminal degradation and/or to limitations on small intestinal starch digestion and glucose absorption [
5,
6,
7]. Therefore, the lowered pH values result from forced hindgut starch fermentation and from the associated microbial adaptations. It has been shown that the fecal microbial phyla
Firmicutes and
Bacteriodetes decrease and increase with the fecal starch content of cattle, respectively, while
Proteobacteria remain unrelated to starch content [
13].
Enterobacteriaceae are a large family within the phylum
Proteobacteria and comprise members relevant to human health such as
E.
coli which were shown to be increased in the colonic region with increasing grain and consequently starch content in feed [
15,
16]. Shiga toxin-producing
E.
coli (STEC), which are also referred as to verocytotoxigenic
E.
coli (VTEC), are a pathotype of enteric
E.
coli that also include the enterohaemorrhagic
E.
coli (EHEC) category [
98]. Shiga toxins (Stx), and more specifically Stx1 and Stx2, are virulence factors of the EHEC subgroup associated with human infections eventually resulting in severe clinical outcomes such as hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS). Cattle are regarded as the most important animal EHEC reservoir and human infection source since 65 out of 373 STEC serotypes isolated from cattle feces or hides were detected in human HUS patients [
99]. Control measures are required to assure the safety of beef and dairy products [
98,
99,
100]. The present results clearly demonstrate the effects of diet composition on total fecal
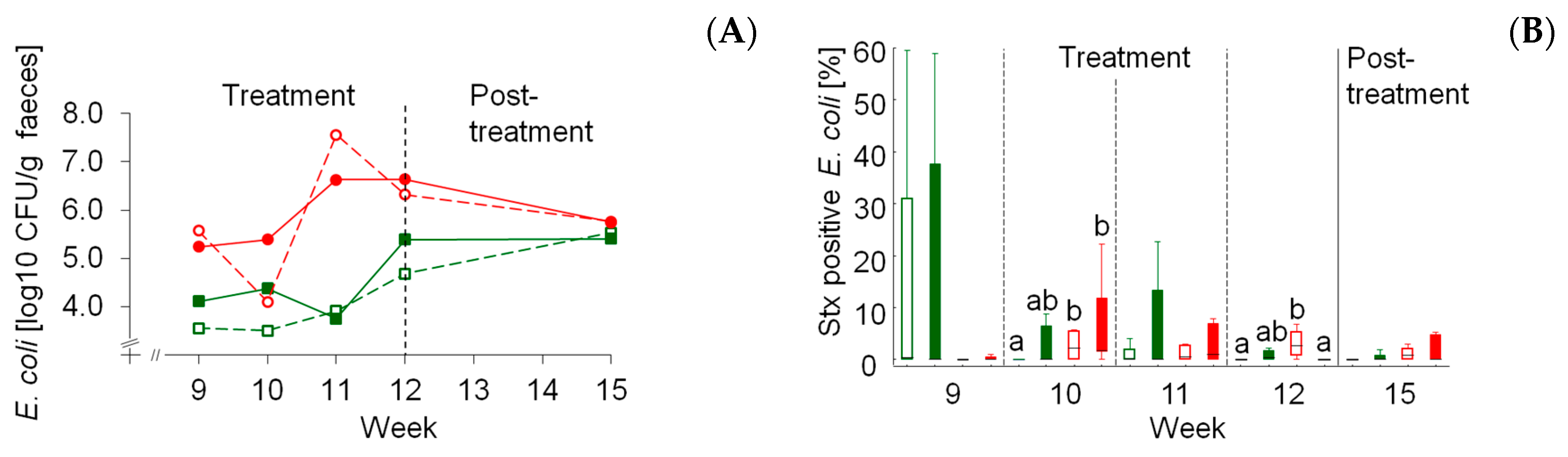
E.
coli counts and that the starch source was the dominating factor rather than the dietary concentrate feed proportion. Both MS-based rations increased
E.
coli counts similarly irrespective of concentrate feed portion. The main influence of diet composition on total fecal
E.
coli became obvious by comparable counts after switching all four experimental groups to a similar post-treatment diet consisting of a moderate portion of MS. The prevalence of
stx-positive
E.
coli dependent on maize starch was less clear but also suggested a trend for higher proportions of total
E.
coli. The risk for human infections is further increased by the fact that
E.
coli resistant to low pH, and thereby capable of escaping the low pH of the human stomach, are triggered by low colonic pH in cows which in turn is due to an enhanced inflow of undigested starch [
15].
Besides these food safety aspects, the MS-driven rise in hindgut
E.
coli counts might also correlate with higher LPS concentrations, increasing the probability of transepithelial transfer and the discussed consequences for systemic inflammation and the health of the cow. Although the relative abundance of
Firmicutes decreased with fecal starch content in cattle [
13], individual genera of this phylum, such as
Clostridium sensu stricto 1, were shown to be increased after feeding rations with high grain portions in the colon of sheep [
17]. Moreover, besides
Clostridium sensu stricto 1,
Prevotella, unclassified
Erysipelotrichaceae and
Roseburia were also high positively correlated with the ovine colonic mRNA expression of the pro-inflammatory cytokines tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL-1β), suggesting a role in the pathogenesis of hindgut epithelial inflammation and damage with consequences for LPS translocation. This conclusion is underpinned by the strong negative correlation between colonic pH and the TNFα and IL-1β mRNA expression [
17]. Besides the fact that undigested starch-triggered hindgut proliferation of
Clostridium spp. is generally seen as critical with regard to the epithelial integrity and possible development of enteric diseases [
17],
C.
botulinum especially gained particular attention as putatively causative for the development of so-called visceral botulism [
101], a poorly defined designation for a chronically ill cow. In view of the diet-triggered starch flow to the large intestine that might stimulate passaging
C.
botulinum to proliferate and to produce toxins causative for botulism, we were interested in whether high concentrate feed portions would increase the presence of BoNT genes in rectal feces, particularly when combined with GS which was repeatedly reported as a carrier for
C.
botulinum [
8,
9] gained from soil particles attached to the silage. Furthermore, it was shown that proteolytic strains of
C.
botulinum types A and B were capable of toxin production with grass as a substrate [
102]. Further in vitro studies by these authors employing varying water activities and pH values suggested that
C.
botulinum might proliferate and synthesize toxins in wilted GS. However, in the present experiment none of the tested toxin genes could be detected in either fecal sample, making it clear that both feed materials and gut content were putatively free of
C.
botulinum carrying the investigated toxin genes. Chronic illness of cows has also been observed after feeding GS with tP contents of less than 40% [
103,
104] when the method according to Barnstein is used. Lower tP contents mainly result from a higher extent of proteolysis and are accompanied by increasing proportions of products of proteolysis such as biogenic amines, GABA and ammonia, which are collectively termed as non-protein nitrogen (NPN) compounds. In the present experiment the tP content was determined by two methods which yielded distinctly different results, although the negligible differences between MS and GS remained comparable. Taking the tP results according to Barnstein, the requested tP content of more than 50% [
104] was clearly met in the present experiment. However, it needs to be stressed that tP contents are highly variable and additionally also dependent on sample preparation, such as the drying method (oven vs. freeze-drying) and oven drying temperature [
105]. Although tP determination is easy to perform, the results provide only an indirect measure of the degree of proteolysis. Moreover, the composition of the proteolysis products remains unknown, which hampers the interpretation of the biological effects. In view of biogenic amines and GABA being low molecular weight substances (covered by crude protein fraction A: NPN) with potent biological activity, it should not be neglected that variations in dietary intake might disturb the steady state between their synthesis and exogenous supply, degradation and elimination with consequences for local and effective concentrations [
106]. Given their pharmacological potential, excessive intake of these substances might result in many toxicological effects mainly concerning the nervous and blood circulatory systems [
11,
106,
107]. Variations in exogenous supply might result from the excessive proteolysis of silages with poor quality [
12] but also from ruminal synthesis driven by high concentrate feed portions [
108]. It was shown that the SARA challenge does not only increase the ruminal formation of histamine but also its concentration in the circulation. Increased histamine concentration in the microcirculation of the claw region has been discussed as pathogenetically relevant for the development of bovine laminitis [
109]. Although we did not monitor lameness systematically in the present experiment, possible subclinical effects, particularly in the maize-60 group, which was clearly affected by SARA, cannot be excluded. Moreover, recently it has been demonstrated in vitro that histamine is capable of inducing an inflammatory response of rumen epithelium mediated by the NF-κB pathway [
110]. Hence, histamine could be a contributing factor for SARA-associated rumen epithelial lesions, LPS transfer and systemic inflammation as discussed above. Besides, at higher concentrations up to 40 g amines/kg DM, a depression in DM intake has been concluded from a literature survey [
12]. On the other hand, increasing doses of rumen-protected GABA stimulated DMI at doses between 0.3 and 0.91 g/d to the same extent. These doses corresponded to the estimated effective doses available at the duodenum. When related to DMI these doses corresponded to concentrations between 0.01 and 0.04 g duodenal available GABA/kg DM. Compared to the calculated GABA concentrations of grass-20 (3.1 g/kg DM), grass-60 (1.5 g/kg DM), maize-20 (2.1 g/kg DM) and maize-60 (1.1 g/kg DM) rations fed in the present experiment, these levels appear to be small but might suggest that most of the GABA present in feed in an unprotected form might be effectively degraded by rumen microbiota. On the other hand, it could also be concluded that even small amounts of GABA escaping rumen degradation might effectively influence DMI regulation and other metabolic processes. For the current study, treatment effects on DMI in combination with the calculated GABA contents of the rations did not suggest any effect of GABA.
Besides the potential adverse effects of biogenic amines, the generally higher nitrogen turnover of cows fed GS-based rations might have an impact on health and production traits. Ingested nitrogenous compounds are utilized to produce ammonia and microbial protein by rumen microbiota when the energy supply is sufficient for the energy-consuming process of protein synthesis. In cases of an energy deficit, excess ammonia is either absorbed and metabolized to urea by the liver or delivered to the small and large intestines when the rumen ammonia absorption capacity is exceeded. The nitrogen excess in GS-fed cows relative to MS-fed ones is reflected in the higher RNB, higher blood and milk urea levels and the lower DM content of the feces. The latter was discussed to be the result of an increased ammonia flow to the hindgut giving rise to epithelial irritation and consecutive stimulated motility, ultimately leading to less dewatered feces [
8]. Higher concentrate feed portions decreased the urea content in milk and concomitantly increased the protein content which underlines the dependency of microbial and finally milk protein synthesis from the energy supply. This effect occurred at a much lower level in the MS-fed groups, and the absolute milk urea contents in these groups indicated a nitrogen deficiency. It needs to be stressed that the discussed aspects on nitrogen turnover are rather comparative; in absolute terms only the milk composition of the grass-20 group indicated an optimum nitrogen and energy supply.