Abstract
During the periparturient period there is a significant increase in the incidence of multiple metabolic and infectious diseases in dairy cows. Dairy cows are fed high-grain diets immediately after calving to support production of large amounts of milk. Mounting evidence indicates these types of diets are associated with the release of high amounts of endotoxins in the rumen fluid. If infected, the udder and uterus additionally become important sources of endotoxins during the postpartum period. There is increasing evidence that endotoxins translocate from rumen, uterus, or udder into the systemic circulation and trigger chronic low-grade inflammatory conditions associated with multiple diseases including fatty liver, mastitis, retained placenta, metritis, laminitis, displaced abomasum, milk fever, and downer cow syndrome. Interestingly, endotoxin-related diseases are triggered by a bacterial component and not by a specific bacterium. This makes prevention of these type of diseases different from classical infectious diseases. Prevention of translocation of endotoxins into the host systemic circulation needs to take priority and this could be achieved with a new approach: mucosal vaccination. In this review article, we discuss all the aforementioned issues in detail and also report some of our trials with regards to mucosal vaccination of periparturient dairy cows.
1. Introduction
Despite improvements to production and efficiency within the dairy industry, the high incidence of disease from 3 weeks prepartum to 3 weeks postpartum, termed the transition period, remains a major cause of high culling rates as well as significant economic losses for the producer [1]. During this period, dairy cows are under numerous physiological challenges including the nutritional and physical adaptation to lactation [1,2], immunosuppression [3] and increased mucosal exposure to immunogenic compounds, namely endotoxins [4,5,6]. It is at this time that metabolic disorders such as ruminal acidosis, fatty liver, ketosis, milk fever, laminitis, retained placenta (RP), displaced abomasum (DA), downer cow syndrome (DCS), and infectious diseases including mastitis and metritis, are most prevalent [7].
A strong link exists between the periparturient diet of dairy cows and the development of periparturient diseases, although recent evidence is shifting the view of how these factors are linked. Classically, most metabolic disorders are viewed as a result of negative energy balance (NEB) encountered at the end of parturition and especially at the onset of lactation, with each disorder due to imbalance of one metabolite, and increased infectious diseases are viewed to be the result of periparturient immunosuppression. These classical views are being challenged by new research indicating that the root of these disorders is the postpartum feeding of high-grain diets in support of high milk production [2,7,8].
The implementation of high-grain diets is associated with a rapid decrease in the pH of the rumen leading to significant changes in the microbial population. Ruminal acidosis is identified as depression of ruminal pH below physiological levels and is separated into acute and subacute acidosis (ARA and SARA, respectively) based on the respective critical pH thresholds of <5.0 and <5.6 [9]. The development of ruminal acidosis leads to shifts in the microbial population of the rumen towards starch-utilizing and lactic-acid-producing bacteria and away from lactic-acid-utilizing bacteria, ultimately leading to a dramatic increase in the concentration of free lipopolysaccharide [LPS; a component of Gram-negative bacteria (GNB)] in the rumen [5,7,10]. It is likely that the concentration of free lipoteichoic acid [LTA; a component of Gram-positive bacteria (GPB)] rises in the rumen fluid as a result of the microbial shift as well, although information regarding LTA concentration in the rumen, its translocation, and its fate in the host is not known.
Endotoxin is able to translocate across the mucosal tissues, including the gastrointestinal tract (GIT) [5], mammary gland [4] and uterus [6], into the systemic circulation. Multiple studies have implicated the role of bacterial endotoxins in the pathophysiology of not only ruminal acidosis but also inflammation and many periparturient diseases affecting both dairy and beef cattle [5,7,8]. Although it has been less extensively studied, LTA has also been implicated in inflammation and periparturient disease [7,11].
Given that the major sources of bacterial endotoxins are mucosal sites, it is important to boost the mucosal immune system in order to prevent translocation of these compounds. Conventional vaccines are typically administered subcutaneously or intravenously, which poorly stimulate the mucosal immune system, while mucosal application can not only increase immune exclusion at the mucosa through stimulation of secretory immunoglobulin A (sIgA) production but also stimulate the systemic immune system as well [12,13]. A pilot study conducted by our team showed that oral administration of increasing doses of LPS with a flat dose of LTA to periparturient dairy cows stimulated innate and humoral immune responses [13]. Additional studies by our team utilizing LPS alone also observed the stimulation of humoral immune responses during oral administration [13,14] as well as changes to the concentration of plasma metabolites and minerals in periparturient dairy cows during oral or oronasal administration [15,16]. While previous studies have demonstrated promise in using mucosal vaccination of dairy cows with LPS and LTA to reduce periparturient disease there has been no investigation of the benefit to combining both mucosal and parenteral vaccination [13,14,15,16].
2. High-Grain Diets, Rumen Microbiota, and Periparturient Disease
The function of the bovine rumen is to facilitate the digestion of dietary fiber through a symbiotic relationship with the inhabiting microbes. The rumen serves as an environment for these microbes to survive and they, in return, can digest dietary fiber and subsequently provide the host with necessary nutrients, such as protein and vitamins, they would otherwise be unable to utilize. Ruminants naturally consume fiber-rich diets with very little starch content; however, within the dairy industry they are provided feedstuffs containing large quantities of non-fiber carbohydrates (NFC) to support higher milk production [2,17]. While the practice of high-grain feeding has been shown to improve milk production, it also causes a shift in the microbial population away from cellulose-digesting species and towards starch-digesting species, the latter being mostly composed of GNB [9]. It is well documented that a consequence of this shift in the population is the release of free lipopolysaccharide (LPS) into the rumen [18,19]. Currently, little research has been done to evaluate the concentration and fate of LTA in the rumen although it is likely to be released into the rumen as well during the microbial shift as the rumen is rich in both Gram-negative and Gram-positive bacterial species.
2.1. Structure and Function of Endotoxins
Lipopolysaccharide and LTA are the main components of bacterial cell wall membranes of GNB and GPB species, respectively, and are vital to their stability and function [20]. These molecules are known as endotoxins and while they are not harmful when embedded in the cell membrane, once released they are highly immunostimulatory and largely responsible for the signs of bacterial infection [21]. Endotoxins are released in minute amounts when bacteria grow. They are released in larger amounts when bacteria disintegrate or die, for various reasons. GNB also release LPS as part of outer membrane vesicles (OMV). These vesicles have different functions including secretion of important proteins in colonization of the host, for delivery of autolysins in a competitive environment, for bacterial survival to kill other competitors, or nutrient acquisition (for more details read [22]).
2.1.1. Structure of Lipopolysaccharide
Lipopolysaccharide is an amphiphilic molecule expressed by all GNB and is necessary for the structure and function of their outer membrane. All forms of LPS possess the same general structure consisting of three regions: The O-polysaccharide chain, the core polysaccharide, and lipid A (Figure 1). The O-polysaccharide, also referred to as the O-chain, is the outermost region of LPS and its structure is highly variable, consisting of one to eight glycosyl residues. The variation within this area is a result of differences in sequence, sugars, substitution, linkage and ring form utilized within the repeating oligosaccharide subunit. In being the outermost area, the O-chain is also the main target for host antibody responses and is commonly referred to as the O-antigen. The high variability in this region allows for specificity of the host antibody response against different bacterial strains and is also used in the serological typing of GNB isolates [23,24].
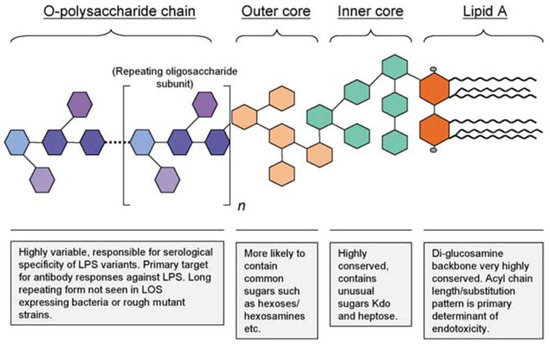
Figure 1.
The general structure of Gram-negative bacterial lipopolysaccharide [23].
The core polysaccharide consists of the inner and outer core, with the latter connecting to the O-chain. The outer core contains common hexose sugars, while the inner core consists of unusual sugars such as 3-deoxy-D-manno-octulosonic acid (Kdo) and L-glycero-D-manno heptose (Hep). Kdo is essential for the viability of GNB and connects the core to the lipid A region with a few exceptions utilizing 2-keto-D-glycero-D-talo-octonic acid (Ko) instead of Kdo. Lipid A is the innermost section of LPS, which typically consists of a phosphorylated β-1,6-linked glucosamine disaccharide with up to four attached acyl chains; these chains may be substituted with fatty acids allowing for up to seven acyl substituents (Figure 1). The use of synthetic lipid A in 1985 proved it is the endotoxic component of LPS by exerting the same biological effects as natural E. coli lipid A [23,24].
2.1.2. Relationship between Lipid A Structure and Activity of LPS
The bioactivity of LPS is directly related to the structure of lipid A via the three-dimensional conformation, which it assumes (Figure 2). The structure of lipid A can vary in terms of the type of disaccharide backbone, number and length of acyl groups, asymmetry of acyl group distribution, and the presence of phosphate groups. Highly active varieties of LPS, such as that found in E. coli, possess a lipid A structure that assumes a conical three-dimensional shape, while the lipid A of less active forms assume a cylindrical shape or an intermediate conical shape [24]. The lipid A structure found in the LPS of E. coli is a biphosphorylated β-1,6-linked glucosamine disaccharide substituted with 3-hydroxyl myristoyl (C14:0) groups at the 2, 2′, 3, and 3′ positions, of which the 2′ and 3′ fatty acyl chains are esterified with myristate and laurate (C12:0). This structure is highly conical and optimally recognized by the mammalian immune system; any deviation from this structure results in reduced bioactivity [23,24]. There is strong evidence indicating the intermediate and conical-shaped lipid A stimulate different toll-like receptors (TLRs) and thus result in different proinflammatory responses. While E. coli LPS stimulates cells through TLR4, LPS from Porphyromonas gingivalis, which is an intermediate shape between conical and cylindrical, stimulates TLR2 receptors. This differential receptor stimulation results in marked differences to gene transcription with E. coli LPS inducing the transcription of tumor necrosis factor α (TNF), interleukin (IL)-1, interferon (IFN)-γ, and the IL-12 subunit p40, while P. gingivalis, which exclusively binds to TLR2, induces only the transcription of TNF and IL-1 and to a lesser extent than E. coli [24]. Molecules, which have a completely cylindrical lipid A conformation, such as compound 406, have antagonistic properties and inhibit regular lipid A signalling [23,24].
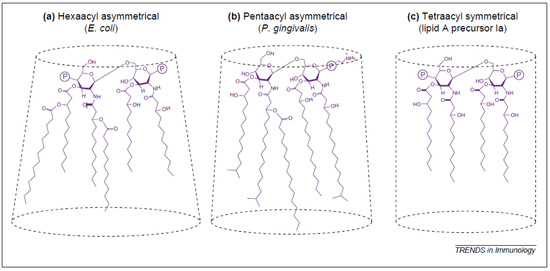
Figure 2.
Three-dimensional conformations of various lipid A structures: (a) conical conformation; (b) intermediary form; (c) cylindrical shape [24].
2.1.3. Structure of Lipoteichoic Acid
Lipoteichoic acid is an amphiphilic molecule expressed in most GPB and is considered the GP counterpart to LPS. It is suggested that LTA is involved in the proper growth and physiology of GPB, playing a role in homeostasis and virulence [25]. The general structure of LTA is a single unbranched polyglycerophosphate chain that is phosphodiester-linked to the non-reducing hexapyranosyl residue of the diacylglycerol anchor (Figure 3) [20]. The polyglycerophosphate chain is substituted with D-alanine and sugar residues [26]. Variation of LTA between different GPB species comes from the length of the acyl chains of the anchor and the length and carbohydrate composition of the glycerophosphate chain [20].
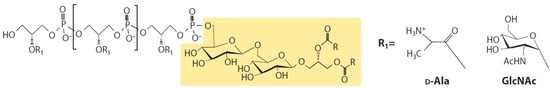
Figure 3.
Chemical structure of LTA from B. subtilis. The glycolipid anchor is shaded; R indicates a fatty acid, and R1 denotes backbone substitutions [25]; permission by original publisher obtained for use of this figure.
3. Sources of Endotoxins
3.1. Gastrointestinal Tract
The gastrointestinal tract (GIT) is the main source of endotoxin as the bovine rumen is a delicate ecosystem of microbiota, including both GN and GP species. Changes within the rumen environment alter the microbiota, disturb the balanced ecosystem, and negatively affect the overall health of the host [7,19]. A significant change to the rumen environment occurs during increased NFC feeding, a common practice to support high milk production, which depresses ruminal pH [2,7,19]. Recently, there have been many studies conducted using varying techniques to assess the alterations to the rumen microbiome in connection with decreased ruminal pH.
Fernando et al. [10] utilized terminal restriction fragment length polymorphism (T-RFLP), 16S rRNA gene libraries and quantitative real-time polymerase chain reaction (qRT-PCR) and observed that during adaptation to high-concentrate diets (high in NFC), there was a distinct increase in bacteria of the phylum Bacterioidetes and Proteobacteria, which are GNB, while individuals fed hay diets (low in NFC) had higher proportions of Fibrobacteres (GNB) and Firmicutes (GPB). Overall, there was a favoring towards growth of starch-digesting bacteria and GNB during high-NFC diets [10]. Another study evaluated changes to the microbiome, using T-RFLP of 16S rRNA genes and qRT-PCR, in connection with SARA induced via grain or alfalfa feeding. In general, they found that during SARA, regardless of induction method, there was a decrease in the proportion of GNB, mainly phylum Bacteroidetes and an increase in phylum Firmicutes. It was also determined that during severe SARA, the dominant microbiota were E. coli (GNB) and S. bovis (GPB), while mild SARA was dominated by Megasphaera elsdenii (GNB) and alfalfa-induced SARA was dominated by P. albensis (GNB) [25]. Similar results were attained through the use of 16SrRNA pyrosequencing of ruminal fluid following adaptation to SARA. While the proportions of GNB and GPB were found to be nearly equal in the control group, there was a distinct difference seen with the SARA-inducing diet. It was concluded that the SARA-inducing diet caused an increase in bacteria of phyla Firmicutes and Actinobacteria and a decrease in Proteobacteria and Bacteroidetes, in favor of amylotic bacterial species [19]. Overall, it seems to be the case that as NFC is slowly increased in the diet, there is a resulting increase in GNB, while the onset of SARA causes a major decrease in the GNB population [9]. The decrease in GNB can be linked to an increase in free ruminal LPS, likely due to the death and lysis of these bacteria [19]. It should be noted that there is a lack of information regarding the ruminal concentration of LTA and it is possible that this is also increased in the rumen following the shift in microbiota caused by acidosis.
Can LPS translocate through the rumen and colon barriers? In a study conducted by our lab, we proved that LPS is able to increase the permeability of rumen and colon tissue to 3H-mannitol by 6- and 5-fold, respectively, at ARA pH levels of 4.5 and 5.5 [5]. On the contrary, LPS was not able to affect permeability of rumen and colon to 3H-mannitol when pH of the media was at a normal level of 5.5 and 7.4 for rumen and colon, respectively. On the other hand, translocation of LPS was not pH-dependent. LPS was able to translocate freely if present at the mucosal side of rumen and colon [5]. Khafipour et al. [27] also tested whether SARA challenge would increase permeability to LPS. Indeed, they reported increased systemic circulatory LPS concentrations after cows were fed a diet that induced SARA. The next important question is, if LPS does translocate through intestinal barriers, what are some of the effects on the host intestinal permeability? Williams et al. [28] addressed this question and demonstrated the ability of systemic LPS to increase permeability of the small intestines of mice, which would allow the passage of LPS across it. It is physiologically normal for the intestine to shed epithelial cells at a consistent rate and the process is tightly regulated to maintain the integrity of the gut barrier. Intestinal epithelial cells (IECs) are produced in the crypts and move upwards towards the tip of the villi, where they are subsequently shed. Williams et al. [28] found that mice treated systemically with LPS had accelerated rates of IEC apoptosis and shedding exceeding the rate of production, disrupting the tight junctions of the gut epithelium and causing gaps through which LPS may translocate. This response was maximally observed at 1.5 h after administration of LPS. Overall, this study suggests that translocation of small amounts of LPS may result in conditions that provide the opportunity for more LPS to subsequently translocate.
3.2. Mammary Gland
The bovine mammary gland is another common source of endotoxin during bacterial infection, known as mastitis. Bovine mastitis is often caused by pathogenic coliform bacteria such as E. coli or species of Staphylococcus—the former being environmental while the latter is often acquired by contagious transfer. Mastitis induced by E. coli, although more severe, and acute compared to that induced by S. aureus, which causes a more chronic infection due to the ability of these bacteria to colonize within the mammary gland. Experimentally induced E. coli mastitis has been shown to result in a systemic acute phase response, initiated in the liver and indicative of LPS translocation [4,29]. Intramammary inoculation with E. coli also causes a significant increase in transcription of the following acute phase proteins (APPs), serum amyloid A (SAA), haptoglobin (Hp) and C-reactive protein (CRP) within the liver hepatocytes. In addition to the increase in APPs, there was also an early increase in transcription of genes involved in regulating cell death and gene transcription, as well as a late increase in those involved with the response to oxidative stress [30].
Until early 2000s, there was no evidence that LPS was able to translocate through the mammary gland to the systemic circulation. Several studies have proved that intrammary administration of LPS has been associated with disruption of the milk–blood barrier and translocation of LPS into systemic circulation [4,29,30,31]. Leitner et al. [32] evaluated the local and systemic effects of intramammary S. aureus infection. While factors such as SCC only increased in the infected quarters, specific immunoglobulins (IgG1, IgG2, IgM, and IgA) were found to increase in the milk of all quarters, both infected and non-infected, indicating a systemic immune response [32]. There is evidence confirming that LPS is able to translocate from the mammary gland to the circulation from Hakogi et al. [29] and Dosogne et al. [4], both finding increased LPS in the plasma during mammary gland infection. Hakogi et al. [29] reported that the plasma concentration of LPS during gangrenous mastitis was 85.2 ± 68.2 pg/mL, significantly higher than the control cow concentration of 4.7 ± 2.6 pg/mL [29]. Dosogne et al. [4] found that during intramammary LPS treatment used to experimentally induce mastitis, plasma concentrations of LPS were found to be within a range of 55–134 pg/mL while healthy cows maintained a level below 10 pg/mL [4]. This evidence supports the ability for LPS to translocate into the circulation from the mammary gland.
3.3. Uterus
Similar to the mammary gland, there has been some controversy regarding the absorption of endotoxin from the uterus. A study conducted on cows with puerperal endometritis evaluated the concentration of endotoxin in the uterine fluid as well as the plasma [6]. There was a positive correlation between the severity of endometritis, based on the characteristics of the lochia (postpartum vaginal discharge), and the concentration of LPS in the plasma. Endotoxin within the plasma was detected in one out of six mild endometritis cows and in all eight heavy endometritis cows at a range of 2–914 pg/mL. The results indicate translocation of endotoxin from the uterus to the blood stream during severe endometritis [6]. Prior to this, another study found that administration of healthy cows with an intrauterine infusion of 5 µg/kg body weight of E. coli resulted in the qualitative detection of endotoxin in the plasma using the limulus amebocyte lysate test (LAL). All control cows (n = 4) infused with saline were consistently negative for plasma endotoxin on day 5 postpartum, while all treated cows (n = 7) were positive at this time, suggesting that endotoxin was absorbed from the uteri of treated cows. It should be noted that at day 20 post-partum, this absorption was no longer seen in treated cows, indicating that early post-partum cows are more susceptible to endotoxin translocation than late post-partum cows [33].
4. Translocation of Endotoxin
The mechanisms by which endotoxin translocates into systemic circulation are not fully understood, although there are two main proposed pathways—paracellular and transcellular. Paracellular involves transport between IEC, while transcellular is the transport of endotoxin through the epithelial cells using receptor-mediated endocytosis [34].
The GI tract is exposed daily to billions of microorganisms and nutrients deriving from the diet fed to dairy cows. Maintenance of GI tract barrier functions is very important to prevent translocation of pathogenic bacteria and their by-products including endotoxins, and at the same time, allow absorption of nutrients. The protection barriers are built in layers in order to prevent both physical and chemical translocation of pathogenic bacteria and endotoxins. The first GI tract layer includes luminal antibacterial enzymes such as intestinal alkaline phosphatase (IAP), which functions to dephosphorylate and deactivate LPS [35]. The second layer involves the mucus layer that serves to physically prevent penetration of pathogenic bacteria and their by-products including endotoxins. The third physical barrier are tight junctions that bring epithelial cells tightly together and prevent translocation of bacteria and endotoxins in between the intestinal epithelial cells. The last barrier layer includes specialized cells such as Paneth cells and B cells that produce antibacterial peptides and secretory immunoglobulin A (sIgA), respectively [35]. The breakdown or dysfunction of GI tract barrier functions has local and systemic effects on the host related to direct interaction of bacteria and their toxic compounds with the epithelial cells, and translocation of endotoxins into the systemic circulation. One of those consequences is translocation of endotoxin into the systemic circulation associated with multiple health effects as we will discuss in detail below.
4.1. Possible Mechanisms of Paracellular Transport
The intestinal epithelium is a single layer of cells, called enterocytes, which act as a barrier from the external environment and are joined by junction complexes (Figure 4). Disruptions to the tight junction protein complexes between IECs decreases intestinal barrier function, thus increasing paracellular permeability, commonly referred to as “leaky gut”. The permeability of the intestinal epithelium can be altered by many factors such as ruminal acidosis, increased LPS in the luminal side, metabolic and environmental stressors, and inflammation [5,34,36].
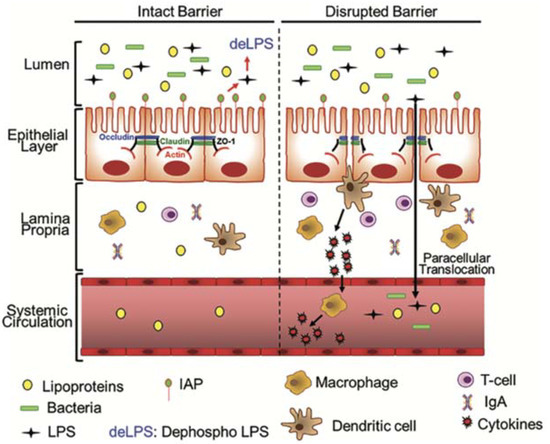
Figure 4.
Paracellular pathway of LPS translocation. Under the healthy status, the intestinal epithelial barrier is closed to bacteria and LPS. Intestinal alkaline phosphatase (IAP), an enzyme produced by epithelial cells, dephosphorylates and detoxifies LPS in the luminal space. When intestinal barrier functions are disrupted during ruminal acidosis, the presence of pathogenic bacteria or large amounts of LPS and the expression of tight junction proteins are suppressed then LPS can translocate. Immune cells (macrophages, dendritic cells, T cells, and B cells—not shown in the diagram) located in the lamina propria below the epithelium are engaged to remove LPS and produce specific immunoglobulin A (IgA) to neutralize LPS. Lipopolysaccharide and pathogenic bacteria also enter into systemic circulation, triggering a systemic inflammation with proinflammatory cytokines released by immune cells [35].
Endotoxin-induced alterations to gut permeability are thought to occur via increased expression of inducible nitric oxide synthase (iNOS), an enzyme which produces nitric oxide (NO) [37,38]. This phenomenon occurs in the epithelium of not only the gut and lungs but liver sinusoidal endothelial cells as well. The role of iNOS, NO, and peroxynitrite (ONOO−) in alteration of epithelial permeability have been observed through the use of iNOS−/− knockout mice, inhibitors of iNOS as well as scavengers of NO and ONOO−, which in all cases, prevented the increased intestinal permeability caused by endotoxemia [38]. Potoka et al. [39] showed that ONOO− can induce enterocyte apoptosis. Both NO and ONOO− have been shown to inhibit mitochondrial respiration resulting in decreased cellular ATP levels, and eventually, cell death [40]. In addition to its direct effects in inhibiting cellular respiration, ONOO− may also induce cell death by activating the cell’s apoptotic machinery [40].
Nitric oxide is an important mediator of inflammation and has protective effects when in low concentrations. Under normal conditions, only a small amount of NO is secreted as constitutive NO synthase (cNOS) and endothelial NO synthase (eNOS) produce much less NO than iNOS [37]. When in excessive concentrations, NO can impair localization and expression of important tight junction proteins such as the zonula occludens (ZO)-1 and ZO-3, and occludin [38]. Nitrogen oxide is also suggested to disrupt tight junctions by decreasing expression and function of Na+/K+ ATPase and causing Na+ dysregulation, leading to cell swelling and dysregulation of the tight junctions and protein expression [41].
4.2. Possible Mechanisms of Transcellular Transport
The second pathway by which endotoxin may enter the systemic circulation is transcellular, or through the epithelial cells. It has been shown that LPS is able to move across polarized intestinal cells while tight junctions remain intact and further evidence suggests this occurs via receptor-mediated endocytosis facilitated by TLR4 [42]. Multiple proteins are required for LPS signaling, including TLR4, myeloid differential protein 2 (MD2), and cluster of differentiation (CD14), which will be discussed further in detail. Under normal conditions there is minimal expression of these proteins by intestinal cells, preventing LPS signaling and causing hyporesponsiveness of healthy IECs to bacterial exposure [40]. Conversely, during states of inflammation, the expression of these signaling proteins is increased, causing increased responsiveness of IEC to LPS and possibly facilitating its endocytosis via TLR4 [43,44,45].
Only a small proportion of internalized LPS is transcytosed as the majority is either transported to endosomes and late lysosomes or recycled back to the apical side [46]. Ghoshal et al. [47] showed that long-chain fatty acids expediate absorption of LPS and its incorporation in epithelial cells (Caco-2 cells). The absorbed LPS was then incorporated in the newly formed chylomicrons in the Golgi apparatus and secreted from the basolateral side together with chylomicrons. Although the association of LPS with lipoproteins is typically a protective mechanism, it can also result in systemic inflammation when there is a significant amount of LPS in the circulation [47,48,49].
5. Periparturient Diseases and Their Connection to Endotoxin
5.1. Acute and Subacute Ruminal Acidosis
The main cause of ruminal acidosis is an increased intake of highly fermentable carbohydrates causing an imbalance of lactic-acid-producing and -utilizing bacteria, leading to the accumulation of volatile fatty acids (VFA) (subacute acidosis) or lactic acid (acute acidosis) [50]. A proportion of VFAs can be buffered through selective feeding, salivary buffering, and ruminal absorption but when concentrations become excessive ruminal pH drops [51,52]. Subacute ruminal acidosis is characterized by periods of ruminal pH below 5.8 to 5.0 for extended periods of time. This pH depression is attributed to an accumulation of VFAs but not lactic acid. The pH drop associated with acute ruminal acidosis (ARA), on the other hand, is caused by an increase in the concentration of lactic acid. The difference between acute and subacute ruminal acidosis is centred on the shifts of the rumen microbiome. When there are high levels of NFCs in the rumen, S. bovis rapidly grows and converts glucose to lactate, which is metabolized by other bacteria such as M. elsdenii into other VFAs. As pH continues to decrease below 5.0, however, the lactate-utilizing bacteria are inhibited and lactate accumulates in the rumen causing ARA [53,54,55]. During severe SARA, the dominant microbiota are E. coli and S. bovis, and as SARA persists, there is a decrease in GNB, leading to an increase in free LPS from E. coli, due to their lysis, which is conical and highly toxic [19,27,53].
The exact mechanisms of how LPS translocates through rumen or GI tract of cattle are not known. In a study conducted by our lab, we demonstrated that endotoxin is able to permeate rumen and colon walls, under Ussing chamber conditions, when present in the luminal side, independently of luminal pH of rumen or colon [5]. Presence of LPS on the luminal side of rumen and colon of cattle also increased permeability to 3H-mannitol by 6- and 5-fold, under acidic pH 4.5 and 5.5, respectively, suggesting LPS might disrupt tight junctions and cause leaky GI tract. In another study, we also showed that feeding diets containing 30% to 45% of the total mix ration (TMR) as barley grain increased ruminal concentration of LPS by 7.6- to 13.5-fold vs. cows that did not consume barley grain [36]. Also, feeding the same diet was associated with a systemic inflammatory status as indicated by increased acute-phase proteins (serum amyloid A, lipopolysaccharide binding protein and C-reactive protein).
5.2. Fatty Liver
Fatty liver is characterized by the storage of triglycerides (TGs) within hepatocytes. Conventionally, this disease is viewed as the result of the NEB that cows develop around parturition, causing increased mobilization of non-esterified fatty acids (NEFA) from adipose tissue. Increased NEFA in the circulation leads to increased hepatic uptake and oxidation of NEFA for energy; however, when the uptake is too high, the liver is only able to partially oxidize them, generating ketones, or converting them to TGs for storage [1,54,55].
Recent evidence suggests that, besides NEB, there might be other factors involved in the development of fatty liver. Interestingly, while all cows go through NEB around parturition, only some of them develop fatty liver and attempts to reduce the NEB through increased NFC diets during the far-off dry period can increase hepatic TG storage [7,55]. Connections have also been made between fatty liver and different peripartum diseases, some of which are not directly connected with NEB, such as mastitis and metritis, which are infectious diseases. However, the latter can affect feed intake (reduction) and indirectly contribute to NEB. It has been hypothesized that the endocytosis of TG-rich lipoproteins (i.e., chylomicrons) utilized for rapid removal of endotoxin from the circulation may contribute to the development of fatty liver [7].
Increasing evidence indicates a contributory role of inflammation triggered by endotoxin in the pathophysiology of fatty liver. Cows experiencing fatty liver have increased concentrations of TNF and SAA, as well as Hp, NEFA, and TG in the plasma with decreased calcitonin gene-related peptide (CGRP) and cholesterol. Serum amyloid A and Hp are acute-phase proteins, which bind toxins to aid their removal by the liver via lipoproteins, and bind hemoglobin to prevent the bacterial uptake of iron, respectively. Likely triggers of increased SAA and Hp are IL-1 and IL-6, which are produced in the liver by Kupffer cells when stimulated by endotoxin. Calcitonin gene-related peptide is also involved in the acute phase response and increases plasma concentration of glucose and lactate [7,56].
A study conducted by Bradford et al. [57] found that injection of TNF alone in late-lactation dairy cows promoted the storage of roughly double the amount of TGs in the liver compared to controls. Additionally, there was evidence of decreased glucose-6-phosphatase and phosphoenolpyruvate carboxykinase 1 transcription, two genes required for gluconeogenesis, which is consistent with the observed reduction in glucose production [57]. These observations further implicate endotoxins as TNF production is stimulated by both LPS and LTA [11,58,59]. Lastly, the role of inflammation and endotoxins in fatty liver disease development has been further identified through the study of its prevention utilizing probiotics. Two studies demonstrating positive results for the use of probiotics in preventing fatty liver utilized L. rhamnosus GG in mice and C. butyricum in rats. These probiotics helped to maintain the integrity of the gut barrier and thus reduce endotoxemia, inflammation and the development of fatty liver, which helps confirm the pivotal role of gut microbiota and endotoxemia in the development of this disease [60,61].
5.3. Mastitis
Mastitis is defined as inflammation of the udder, which is typically caused by bacterial infection [62]. The incidence of mastitis remains among the highest of all diseases affecting cattle and results in significant economic losses to the dairy industry [30,63,64]. In spite of many prevention strategies and therapies, bovine mastitis continues to be of high economic significance with annual losses of approximately $2 Billion in the USA alone. These strategies include antibiotic treatments, various vaccines specific to different pathogenic bacteria, dry or lactating cow therapies and non-specific prevention therapies. Regardless of the numerous strategies to treat and prevent mastitis, this disease continues to be a serious concern in the dairy industry, indicating a further need to prevent this disease [65].
Bovine mammary epithelial cells (bMEC) express TLR-2 and TLR-4 and during mastitis, the expression of these receptors is increased [66,67]. When stimulated by bacteria, the bMEC are able to produce chemokines, such as IL-8, to attract the migration of neutrophils. Additionally, upon stimulation with LPS or S. aureus, the bMEC can rapidly produce pro-inflammatory cytokines, namely TNF, which serve as mediators for inflammation. The coordinated production of cytokines and chemokines recruit neutrophils to the udder during infection by increasing the presence of adhesion molecules on endothelial cells that bind neutrophils and allows them to migrate into the mammary gland. Subclinical mastitis is diagnosed by measurement of somatic cell count (SCC) in the milk, which determines the level of neutrophil recruitment. An SCC above 200,000 cells/mL is indicative of inflammation of the udder and serves as a baseline for subclinical mastitis diagnosis [63,64,68].
Lipopolysaccharide has been found to induce major metabolic changes in bMEC when they are incubated with LPS [69]. The results of this study showed a total of 63 metabolites that differentiated the control cells from the treated ones. The most affected metabolites included glycerophosphocholine, glycerol-3-phosphate, L-carnitine, L-aspartate, glutathione, prostaglandin G2, α-linolenic acid and linoleic acid. They were mainly involved in eight pathways, including D-glutamine and D-glutamic acid metabolism; linoleic acid metabolism; α-linolenic metabolism; and phospholipid metabolism. The authors indicated that the differential metabolites were involved in eight pathways involved in lipid and energy metabolism. Additionally, findings suggested that the bMEC are able to regulate pro-inflammatory, anti-inflammatory, and antioxidation responses to maintain cell homeostasis to confront LPS challenge.
A study conducted by Moyes et al. [70] reported that IM LPS challenge in early postpartum cows was associated with expression of inflammatory genes in blood PMN leukocytes related with immune response, immune activation, leukotriene synthesis, and PMN adhesion or migration. The authors did not report whether LPS translocated or not in the systemic circulation. In addition, Paape et al. [71] demonstrated that 18 h after an IM challenge with LPS, a large percentage of immature PMN leukocytes were found in blood circulation. Immature PMN has been shown to have lower immune functions comparable with the mature PMN [71]. The recruitment of neutrophils is critical in the defence against bacterial infections as their release of reactive oxygen species (ROS) and various proteolytic enzymes are used to kill the invading bacteria. Unfortunately, it is through the recruitment of activated neutrophils that endotoxin causes damage to the mammary gland epithelium during mastitis when the mechanisms used to kill bacteria also harm the mammary gland tissue [71]. Studies suggest neutrophil-induced mammary gland damage is caused by proteases and toxic ROS, which severely damage secretory tissue, leading to the reduced milk production responsible for the bulk of economic loss associated with mastitis [72,73,74]. Additionally, Lavon et al. [75] found that both GNB and GPB mastitis are able to disrupt follicular function, linking mastitis to reproductive failure, which is the top cause of economic loss to dairy producers.
In dairy cattle, LPS has been used to mimic E. coli-induced mastitis, and it has been found to induce a systemic APR with increased levels of acute phase proteins in bloodserum [76]. Indeed, Hoeben et al. [77] reported that systemic effects of LPS or E. coli in the mammary gland might be reinforced by translocation of pro-inflammatory cytokines and induction of APP from the liver. Moreover, Minuti et al. [78] showed that IM challenge with LPS was associated with liver expression of genes related to APP including serum amyloid A, haptoglobin, and ceruloplasmin. Interestingly, IM challenge with LPS was able to increase accumulation of TG in the liver, supporting the hypothesis that translocation of LPS or cytokines from mammary gland can contribute to development of fatty liver. In support of this finding, Khovidhunkit et al. [79] showed that mammary administration LPS in rodent models increased markedly the uptake of NEFA from the liver.
On another note, Perkins et al. [80] treated mid-lactation cows with intramammary LPS and observed clinical responses. Interestingly, IM LPS lowered rumen motility. Lowered rumen motility is related to displaced abomasum (DA) in dairy cows. Indeed, in a review article, Doll et al. [81], summarized predisposing factors for DA and reports that mastitis is one of the predisposing factors of DA. The study by Perkins et al. [80] suggests that LPS derived from mammary gland during mastitis infection by GNB might explain association of mastitis with DA. Indeed, in a study conducted by our lab [82], we showed that parenteral administration of LPS increased the incidence of DA. Moreover, Vlaminck et al. [83] demonstrated that administration of E. coli LPS either intravenously or directly in the abomasum was associated with inhibition of abomasal motility in a dose-dependent manner. Additionally, Kaze et al. [84] showed that muscle tissue derived from the abomasal antrum of cows treated with endotoxin decreased contractility.
It has been known that peracute mastitis caused by GNB has been associated with a systemic inflammatory state triggered in part by LPS [85]. Moreover, proinflammatory cytokines that are involved in both local and systemic response to GNB mastitis are stimulated by LPS [86]. Upregulation of these cytokines has been related to interaction of LPS with APP including LBP and CD14. In a study by Bannerman et al. [68], they demonstrated that IM challenge of midlactating dairy cows with E. coli LPS was associated with increased lipopolysaccharide-binding (LBP) protein in the blood of those cows.
In addition, LPS has been shown to affect milk–blood barrier function. Thus, in a rodent model, Kobayashi et al. [31] showed that IM LPS challenge weakened milk–blood barrier functions 3 h after injection of LPS, as indicated by increased permeability to fluorescein isothiocyanate-conjugated (FITC)-albumin. Another interesting finding was that 3 h after LPS challenge, there was shedding of alveolar epithelial cells. The mechanism of LPS actions are related to its effects on claudin-7 and claudin-3 that are part of alveolar tight junctions. A more severe disruption of milk–blood barrier functions was observed 12 h after IM LPS injection, as indicated by ectopic localization of β-casein and larger amounts of FITC-albumin into the alveolar lumen.
A study conducted by Petzl et al. [87] pretreated cows with LPS 72 or 240 h prior to E. coli challenge. The results of the experiment revealed that pretreatment with LPS prevented development of fever and there were no alterations in the circulating leukocyte numbers. Additionally, there were no clinical signs of mastitis detectable in the groups pretreated with LPS versus the control group that showed clear signs of mastitis during the 24 h after challenge with E. coli. Moreover, bacterial load after E. coli challenge decreased significantly in cows pretreated with LPS versus control cows, where bacterial load remained high at 24 h after challenge. Lipopolysaccharide pretreatment also lowered expression of inflammation-related genes including TNF, CXCL-8, serum amyloid A3, IL-6, and β-defensin. Additionally, anti-inflammatory genes were up-regulated in the control group but not in the LPS-pretreatment groups.
The data presented clearly show that LPS plays an important role in the etiopathology of Gram-negative mastitis. Results presented show that LPS affects not only alveolar epithelial cells but is able to spill into systemic circulation and influence the activity of other organs, including the liver. LPS deriving from the mammary gland might contribute to the etiopathology of DA. Also, LPS deriving from mammary gland influences immune cells that translocate to the udder to help clean the infection caused by GNB.
5.4. Retained Placenta
Retained placenta (RP) is defined as failure to expel the placenta within 24 h after parturition. Promptly following calving, the immune system is responsible for degradation of the placentomes, which detaches the placental membranes from maternal tissue. The key event leading to RP is failure to breakdown the cotyledon–caruncle attachment after parturition due to immune dysfunction [88]. In cows with RP, there is evidence of impaired neutrophil activity and recruitment that can be seen prior to parturition. Kimura et al. [89] found that isolated neutrophils typically respond strongly to cotyledon supernatant. In cows that would later develop RP, however, neutrophils had decreased myeloperoxidase, indicating a reduced killing ability, which was evident throughout the sampling period from 2 weeks prepartum to 2 weeks postpartum. In addition, RP cows had a lower plasma concentration of IL-8, a chemoattractant that recruits neutrophils to cotyledons and increases collagenase secretion to help separate maternal and placental tissues [89]. LeBlanc [88] also describes evidence of decreased neutrophil chemotaxis and oxidative burst activity along with lower plasma IL-8 at 2 weeks prepartum in cows that later developed RP.
Cows affected by RP also have higher concentrations of LPS present in their lochia. Delayed uterine involution caused by RP allows the lochia to function as a medium for bacterial growth and favors the development of uterine infections [90]. The role of LPS in the etiology of RP was established in a study by Zebeli et al. [82] in which cows receiving parenteral administration of increasing doses of LPS over three consecutive weeks around parturition had a significantly higher incidence of RP compared to controls. It was proposed that this may be due to the exposure of neutrophils to LPS prior to calving causing them to develop LPS tolerance, a phenomenon where tolerized cells have reduced expression of TLR4 and thus have a weaker response to LPS [87]. Immune dysfunction may also be connected to LPS through reduced expression of L-selectin, which mediates the passage of neutrophils from the blood to the inflamed organ given the evidence that intramammary infusion of LPS reduces polymorphonuclear leukocyte expression of L-selectin in the mammary gland [91].
A recent study of gene expression in the utero-placental tissues found further evidence of immune dysfunction in cows with RP. When compared to cows that normally expelled placental tissues those with RP had lower expression of IL-1, IL-6, IL-8 and TNF. Additionally, there was reduced expression of intercellular adhesion molecule-1 (ICAM-1), an adhesion molecule responsible for the adhesion of leukocytes to vessels for movement from the circulation into the tissue [92]. This evidence also implicates the development of endotoxin tolerance in the pathogenesis of RP as the reduced cytokines are those typically stimulated by LPS and LTA [11,58].
5.5. Metritis and Endometritis
Bacterial infection of the uterus is common after parturition as 90% of cows experience uterine contamination with bacteria at this time [93]. Metritis, present in up to 40% of dairy cows, occurs within 21 days postpartum and is characterized by enlargement of the uterus and abnormal vaginal discharge. If infection persists longer than 21 days postpartum, as determined by the presence of purulent or mucopurulent vaginal discharge, it is classified as endometritis [93,94]. According to Sheldon et al. [94] cows that have no clinical sign of systemic disease but have a larger than normal uterus as well as vaginal purulent discharges, coming from the uterus, within the first 21 days after parturition may be diagnosed as having clinical metritis. On the other hand, if cows within the 21 days after calving have signs of systemic illness including high rectal temperature >39.5 °C, dullness, lowered milk production, or other associated signs of toxaemia, and a larger than normal uterus may be classified as puerperal metritis. The bacteria most commonly associated with uterine disease are E. coli and A. pyogenes, whose growth is supported by the postpartum uterine environment due to disruptions of the endometrium and retention of lochia [93]. Dohmen et al. [90] reported high concentrations of LPS in the lochia of dairy cows at 1 or 2 days after calving and its concentration was related to the presence of E. coli, Clostridium spp., and A. pyogenes. However, the same authors did not find increased LPS in the systemic circulation of cows with dystocia and RP. An earlier study by Peter et al. [33] showed increased levels of LPS in the plasma of healthy cows 4 h after infusion of E. coli endotoxin in the uterus at 5 days after calving.
Uterine infections are associated with a dramatic decrease in reproductive performance. Gilbert et al. [95] evaluated 141 Holstein cows over 5 herds for endometritis and reproductive performance. Utilizing cytological methods, 53% were diagnosed with subclinical endometritis and compared to healthy cows, there was a significant reduction in reproductive performance in cows with endometritis. Cows with infections had increased days open (118 vs. 206), delayed first service, decreased first service pregnancy rate and an increase in the number of inseminations [92]. More recently, Williams et al. [96] found that LPS reduces the production of estradiol (E2) by granulosa cells, while TNF decreased theca cell androstenedione production in addition to granulosa E2 in vitro, which are pivotal to ovulation. When applied in vivo animals treated with intrauterine LPS or TNF were less likely to ovulate, indicating impairment of ovarian function [96]. Uterine infections with E. coli have also been found to cause extension of the luteal phase through inducing a switch in progesterone production from PGF2α, which terminates the luteal phase, to PGE, which does not [97].
Another recent and interesting study was that of Purba et al. [98] that studied whether LPS infused intravenously (iv) or intrauterine (iu) would reach mammary gland. Data from the iv experiment demonstrated that LPS was immunolocalized in the microvessels of the lamina propria of the mammary gland 3 h after injection, as well as in the connective tissue and epithelial cells of alveoli 24 h after the iv administration. This suggests that intravenously injected LPS can be transported to the mammary glands. Results from the iu administration experiment, demonstrated that LPS had translocated in the connective tissues and interepithelial spaces of alveoli of the mammary gland 24 h after iu administration. This is a direct proof that LPS is able to translocate from the uterus to the mammary gland through systemic circulation.
A recent study found promise in the development of a subcutaneous vaccine against metritis in Holstein dairy cows utilizing bacterial proteins and inactivated whole cells from E. coli, F. necrophorum, and T. pyogenes. Results showed that the various subcutaneous vaccines lowered the incidence of metritis, increased likelihood of conception, and had increased serum IgG against the bacteria and proteins they were vaccinated against [99].
5.6. Laminitis
Laminitis is the inflammation of the dermal layers inside the foot regardless of infection and is one of the three major diseases, along with mastitis and uterine infections, which lead to the high culling rate of cows [7,100]. Laminitis occurs in acute, subclinical, and chronic forms, has a multifactorial etiology, and is believed to have many interdependent causal factors such as hormone changes, endotoxic insult, and environmental aspects [100].
There is general agreement that laminitis is a systemic disease with local manifestations in the hoof area [101]. Overall, laminitis is a disease that develops over three stages that overlap with each other. Phase 1, which starts with the release of vasoactive compounds that affect blood flow to the corium with damage to the dermal–epidermal junction. Phase 2 is associated with sinking and displacement of the third phalanx (i.e., P3) that damages corium and the cushion beneath. During this phase there is hemorrhage, thrombosis and different levels of necrosis. Phase 3 is characterized by development of sole ulcers and white line disease that takes 2–3 months to develop. The first two phases are subclinical and the third one is characterized by clinical lameness [102].
There have been various hypotheses with regard to etiopathology of laminitis. However, in this review, we will focus on the information related to the potential role of endotoxemia in the etiopathology of laminitis. The first experimental evidence for a potential role of systemic endotoxemia on the etiology of laminitis came from the study of Boosman et al. [103]. In their study, Holstein cows were injected intradermally (id) and intravenously (iv) with different doses of E. coli LPS (0111:B4), for three consecutive days. The researchers aimed to test whether endotoxemia, triggered by id or iv administration of LPS, would trigger acute laminitis. The doses of LPS used were similar to those that are known to cause an acute-phase response, ranging from the lowest 46 μg id and up to the highest dose of 6350 μg iv [103]. Data from this study showed that there were no clinical signs of laminitis, during pre-experimental claw trimming, in all 13 cows in the study. Histopathologic examination of the claws in cows administered LPS revealed lesions indicative of acute laminitis such as vacuolation of the stratum basale cells, infiltration of lymphocytes or polymorphonuclear lymphocytes, congestion, and hemorrhages. However, the authors concluded that although they were able to trigger laminitis they were not able to evoke acute lameness suggesting that other additional etiological factors besides endotoxin were involved.
Evidence for a potential role of endotoxin in the etiology of laminitis came from another study conducted by Singh et al. [104]. The aim of that study was to test whether intra-arterial injection of LPS would induce lesions in the treated foot or in all of the feet. Researchers used one Holstein cross bull calf and two Charolais cross bulls in the study. The authors infused a 2 mg dose of E. coli endotoxin (0111:B4) in 1 L of Hartmann’s solution, within an hour, and monitored the animals for 10 h. The first bull was given another 3 L of Hartmann’s solution 24 h after the first infusion. Hoof biopsies were collected 2–3 days prior to endotoxin infusion and 15 days after infusion. Clinical observations indicated that 5 days after infusion of LPS, the hoofs looked red. At necropsy, there were hemorrhages in all the claws of the Holstein bull and these were severe in the wall and sole of hind claws. In Charolais bulls, the hemorrhages appeared as dark brown streaks throughout the sole. The authors concluded that experimental endotoxemia can induce hoof lesions in cattle, which may be a local effect of a systemic reaction.
Thoefner et al. [105] explored the possibility of developing laminitis by alimentary oligofructose overload. They used heifers in their study and provided three different oral doses of oligofructose at 13, 17, and 21 g/kg per group. Four of the six heifers fed oligofructose displayed clinical signs of laminitis starting 39 to 45 h after feeding. This finding was confirmed by another study by Danscher et al. [101], who showed that feeding oligofructose triggered laminitis and induced signs of lameness. In this study, claw pain was present, as shown by increased sensitivity in the hoof area. Data also showed joint effusion and concurrent systemic signs. The authors concluded that their data support the common perception that laminitis is a systemic disease with local manifestations and that oligofructose is a good model to study the etiology of laminitis in dairy cows. Although the mechanism of how oligofructose induces laminitis in cattle is not known, equid research suggests the potential involvement of endotoxin, released in the GI tract and its translocation into systemic circulation, as a potential factor. Bailey et al. [106] reported an increase in plasma concentration of LPS from the limit of detection (0.6 pg/mL) to a peak of 2.4 ± 1.0 pg/mL at 8 h after administration of LPS. Additionally, they reported that plasma TNF, a cytokine released by macrophages during endotoxemia, was detected in horses during oligofructose-induced laminitis, from 12 to 24 h after administration of LPS. These authors suggested that endotoxin may play an indirect role in the onset of laminitis through the activation of platelets, which can then activate neutrophils [101]. Platelets are 1000-times more sensitive to LPS than leukocytes are [107]. Platelets are capable of activating leukocytes by platelet-derived chemokines [108]. Additionally, an in vitro study by Reisinger et al. [109] found that culturing equine hoof explants with LPS caused a dose-dependent reduction in lamellar tissue integrity, further implicating endotoxins.
The association of ruminal acidosis with laminitis has been well established, although the pathophysiology remains poorly understood [7]. The mechanisms of development, as described by Nocek [100], are linked to acidosis through depression of systemic pH activating a vasoactive mechanism that increases blood pressure, which is exacerbated by factors such as histamine and endotoxin. Multiple studies have shown that feeding high-grain diets to dairy cows is associated with 16- to 20-fold increase in the amount of endotoxin in the rumen fluid [36]. We also demonstrated that LPS is able to translocate through rumen and colon walls independently of pH [5]. Endotoxin triggers a sustained increase in the blood pressure by inducing serum exudation, swelling and pain as the microvasculature of the foot begins to degrade and this vessel damage subsequently limits the nutrients to the epidermal cells, causing degradation of the corium and breakdown of the dermal–epidermis junction [97,110].
Lastly, cows treated orally with a vaccine against LPS alone or in combination with LTA have also been found to have lowered incidence of laminitis. The results from the combination treatment implicates LTA in the etiology of laminitis as it was found to further lower laminitis when compared to treating with LPS alone [7].
5.7. Displaced Abomasum
Displaced abomasum (DA) in dairy cows is the abnormal positioning of the abomasum from its normal position in the abdomen to the right (RDA) or left (LDA) side, with LDA being more frequently diagnosed than RDA. The incidence of DA ranges from 1.7% to 5.8% [111,112]. The ratio of left to right DA in Holstein dairy cows ranges between 3:1 and 7:1 [113,114,115]. Displaced abomasum is related to enlargement of the organ with fluid and/or gas. Once migrated, the abomasum usually becomes trapped in the abnormal position by the rumen and the torsion of the stomach can reduce or fully inhibit the passage of digesta [116,117]. Displaced abomasum is a multifactorial disease and has been reported to affect an average of 3%–5% of dairy cows in a herd [7,118,119,120]. The main cause of LDA is reduced motility leading to accumulation of gas in the organ [113] and the three main risk factors include: (1) hypocalcemia, (2) hypokalemia, and (3) endotoxemia [117].
Other factors that affect the incidence of LDA include decreased ruminal filling, increased NFC diets, and increased incidence of other diseases such as fatty liver, ketosis, RP, metritis and mastitis [119,121,122]. A lack of ruminal fill increases the opportunity for the abnormal positioning of the abomasum and can be caused by high NFC feeding in herds not fed a total mixed ration (TMR). In addition, high NFC diets also reduce abomasal motility and emptying via accumulation of VFAs [116,119]. Lastly, an increased risk of LDA occurs with multiple common periparturient diseases including ketosis, fatty liver, RP, mastitis, metritis, and milk fever. It has been proposed that some of these diseases lead to atony of the abomasum through hypocalcaemia (milk fever), or an increase in histamine (mastitis, metritis), while others may have similar causes such as prepartum feed intake depression followed by a slow increase in postpartum intake, which leads to decreased ruminal fill (ketosis, fatty liver); other connections are still unknown [116,119,122].
There is increasing evidence that endotoxin is involved in the etiology of LDA beginning with the high incidence of concurrent diseases such as acidosis, fatty liver, metritis, RP, and mastitis, which are all potentially caused by endotoxin or lead to its translocation [87,118,119,123]. The study conducted by Zebeli et al. [82], discussed previously, with regard to RP, also found an increased incidence of DA in cows treated intravenously with LPS [82]. Both in vitro and in vivo experiments have described the ability of endotoxin to reduce motility of the abomasum as well [83,84]. Reduced motility may be caused indirectly through reducing plasma calcium as there is a high association of hypocalcemia and DA [82,117,119,123]. When plasma concentrations of calcium are high, it may be involved in endotoxin clearance by binding endotoxin to form aggregates that are subsequently removed from the circulation via macrophages, which reduces calcium concentration in the circulation [7]. Research conducted by Zadnik et al. [123] found evidence of leukocytosis with neutrophilia (increased neutrophils in the blood) in cows with DA, which is also associated with the immune response against endotoxin [123]. Increased Hp in the plasma is evident during DA as well, further implicating the role of endotoxin and the inflammatory reaction against it in the development of DA [82,120].
Gram-positive bacteria may also be involved in the etiology of DA. During investigation of the reduced response to vasoconstrictors in connection with sepsis, it was established that LTA from S. aureus decreased smooth muscle contractility in the vasculature. It was concluded that the mechanism by which LTA inhibits contractility is through the activation of iNOS, which is present in the smooth muscle, a mechanism which may be applicable to motility of the GIT as well [124].
5.8. Milk Fever
Milk fever, also called parturient paresis, can be described as clinical hypocalcemia and is the most common disease present in dairy cows at parturition. On average, 5%–10% of cattle are affected by this disease postpartum, with 15% that are unresponsive to treatment with calcium borogluconate, resulting in downer cow syndrome (DCS), which will be discussed later [125]. Milk fever has been identified as a risk factor for other periparturient diseases such as mastitis, RP, ketosis, and DA. The current view for prevention of hypocalcemia is through the dietary cation-anion difference (DCAD) as well as the provision of dietary magnesium (Mg) [126]. However, there is evidence indicating that inflammation and possibly endotoxin are involved in the etiology of milk fever [7,83].
Endotoxemia has been linked to hypocalcemia and hypomagnesaemia, as well as other electrolyte disturbances. It has previously been shown that dairy cows infused with LPS experience reduced total calcium in the serum in a dose-dependent manner [127]. In addition, extensive evidence shows that hypocalcemia is common during sepsis in animals [128,129,130,131] as well as humans [132] and the administration of calcium during sepsis increases organ failure and lethality. Dietary magnesium is important in the prevention of milk fever and this may be further evidence of the endotoxin hypothesis. Magnesium deficiency has been shown to increase expression of CD14, an endotoxin receptor, in the small intestine and myocardium of rats [133].
There is evidence of inflammation in cows with milk fever as well, as they have increased plasma concentration of SAA, suggesting inflammation, and decreased plasma CGRP, an APP that decreases plasma calcium [7,134]. Gray et al. [125] suggests immunity is involved in the etiology of milk fever and serves as an explanation for the lower incidence of milk fever in heifers. Some of the evidence presented in support of their hypothesis includes the ability of IL-1β to trigger reduction in plasma calcium and the heritability of susceptibility as seen by breed predisposition [125]. Interestingly, recent research by our team identified a significant increase in factors of innate immunity, including IL-6, TNF, Hp, and LBP, up to 8 weeks prior to the development of clinical symptoms and diagnosis of milk fever [135]. This evidence strongly implicates a role of endotoxins in the etiopathogenesis as all of these factors are involved in the immune response against LPS [58,136,137].
5.9. Downer Cow Syndrome
The term “downer cow” is used in reference to a cow that is unable to get up from sternal recumbency due to hypocalcemia, calving paralysis, or a complication of other diseases such as milk fever, mastitis, and metritis. In addition to hypocalcemia, other suggested risk factors of DCS are stillbirth, dystocia and RP [138]. A review by Ametaj et al. [7] and Eckel et al. [139] was able to establish the possible role of endotoxin in the development of DCS through the measurement of plasma variables prepartum in a cow that developed DCS shortly after parturition. The sick cow had increased LBP and anti-LPS IgM, indicating an immune response against endotoxin [7]. A decrease in plasma cholesterol was also observed and may be indicative of bile production to protect against endotoxin, as previously discussed [56]. Additionally, all prepartum measurements of calcium concentration were lower, suggesting calcium dysregulation and the potential contribution of endotoxin to the hypocalcemia seen in cases of DCS. Lastly, during this study, there was an observed increase in plasma NEFA and BHBA postpartum [7].
Similar results were found in another study conducted on the connection of DCS with fatty liver. Of the 36 downer cows studied, there was a combined 88% suffering from moderate to severe fatty liver, suggesting a strong link between the two diseases. An observed increase in NEFA and BHBA with reductions in calcium and cholesterol were seen in downer cows concurrently suffering moderate to severe fatty liver [140]. Byrne et al. [141] found downer cows had a 3.3-fold higher incidence of E. coli O157:H7 in the feces and/or colon tissue when compared to healthy cows. This indicates the possibility of E. coli’s translocation from the colon into the blood may be involved in the development of DCS [7,141]. A case study also found a recumbent cow to have demyelination of the spinal cord, which has been induced by TNF in mice and rats, additionally implicating inflammation and endotoxin in downer cow syndrome [125]. It should be noted that there is no conclusive evidence yet that endotoxemia is involved in the etiology of downer cow syndrome.
6. Mucosal Immunity and Vaccination against Bacterial Antigens
6.1. Why Mucosal Vaccination Against LPS and LTA?
Mucosal surfaces are the entrance site of most infectious agents and as such the mucosal immune system is the first line of defense against pathogenic infection. Various vaccines, including parenteral and mucosal ones, have been developed to prevent bacterial or viral infections from mucosal sites. However, it should be noted that although parenteral vaccines have been very efficient in protecting against many bacterial or viral pathogens, they have been considered less efficient at inducing mucosal immunity [142]. Therefore, there is new interest in developing mucosal vaccinations in livestock animals. We are bringing to the research community the most recent development in the area of mucosal vaccination.
One of the benefits of mucosal vaccination is the induction of secretory immunoglobulin A (sIgA) production at specific mucosal sites. Immunoglobulin A is uniquely suited to function at mucosal sites as it is resistant to degradation by proteases, which are abundant in the external environment of the mucosal surface [143,144,145]. Additionally, lymphocytes that are primed at the mucosa express homing receptors that allow them to preferentially migrate back not only to the mucosal tissue where they were primed but also to distal mucosal tissues as well (further discussed in Section 6.4). These homing mechanisms help ensure that effector cells return to the initial site of infection to enhance protection at this tissue and can induce additional protection across other mucosal tissues. In addition to stimulating local responses in the mucosa, the activation of systemic immunity by mucosal vaccines is evident by the production of systemic immunoglobulin G (IgG) and the stimulation of a cell-mediated response with CD4+ T cells and CD8+ cytotoxic T cells, the latter contributing to protection against intracellular pathogens. Aside from these physiological advantages to mucosal vaccination, there are practical advantages as well, including their non-invasive nature, ease of administration, reduced adverse effects, and potentially lower production costs [143,145].
It should be noted that although the current paradigm in vaccine development is that vaccines delivered by needles (or otherwise known as parenteral vaccination) are not efficient in inducing mucosal immune responses, recent developments indicate that this is not the case [146]. Several clinical and experimental studies have shown that induction of mucosal immune responses after systemic vaccination are not a rare occurrence. This opens new horizons for parenteral vaccination and for the way vaccines can be delivered. However, the focus of this review article is to discuss new developments on mucosal vaccination in dairy cows (for more details, read 146). However, as indicated above, we will focus our discussion on mucosal immunity and some recent developments in dairy cows. Our team also has gathered data that when both parenteral and mucosal vaccination against LPS and LTA were applied, the results were better than parenteral or mucosal vaccination alone (see discussion on Section 6.5).
6.2. Secretory Immunoglobulin A (sIgA)
The production of secretory immunoglobulin A (sIgA) is the hallmark of the mucosal immune system and is the dominant functional antibody in mucosal secretions. The lamina propria is the submucosal region of mucosal surfaces and is the major site of antibody production, containing the Peyer’s patches where the majority of activated B cells are located [147]. Unlike other antibodies, sIgA is uniquely suited for functioning in the harsh, protease-rich environment of the mucosal surfaces as it is resistant to degradation [147,148]. Secretory IgA is almost exclusively found in dimers, which are connected by a joining chain (J-chain) [147]. The basolateral surface of IECs expresses a polymetric immunoglobulin receptor (pIgR) that mediates the transport of IgA across the cell to the apical side. Immunoglobulin A is then secreted via proteolytic cleavage of pIgR, which generates the secretory component, a polypeptide that remains associated with IgA and is thought to prevent degradation of the antibody [142,147,149].
The main function of sIgA is to maintain the integrity of the mucosal barrier and is effective in preventing viral and bacterial infection [144]. A vital feature of IgA is its ability to promote “immune exclusion”, involves the entrapment of pathogens within the mucous, preventing direct contact of microorganisms with the mucosal surface [142,144]. During pIgR-mediated transport, sIgA may also intercept invading pathogens contained in epithelial cell vesicular compartments. Dimeric IgA is additionally present in the interstitial fluid underlying the epithelial barrier as it is synthesized locally by IgA-secreting plasma cells. This may be used to transport pathogens, which have breached the epithelial barrier back into the lumen using pIgR or by mediating the destruction of infected cells via antibody-dependent cell-mediated cytotoxicity [142].
Research conducted on V. cholerae and the role of IgA in the protection against it found that antibodies against LPS are important to the prevention of this bacterial infection. The antibody is known to bind to the bacterial surface and cause aggregation of the live bacteria, which prevents their colonization in the small intestine and enhances their clearance from the GIT by peristalsis [150]. Lipopolysaccharides are considered to be T cell-independent (TI) antigens due to their ability to activate B cells independently of T cells. T cell-dependent activation of B cells requires the binding of an antigen to the B cell receptor (BCR) and binding of the CD40 ligand on a T cell with the CD40 receptor on a B cell. T-independent antigens, on the other hand, can activate B cells without the help of T cells. In the case of LPS, the second signal received by the B cell comes from the activation of TLRs. Simultaneous engagement of TLR4 and the BCR by LPS stimulates the production of both polyclonal and antigen-specific antibodies [151].
Oral administration of LTA from L. rhamnosus GG has also been found to increase the number of IgA producing cells in the lamina propria in mice [146]. Thus, the production of sIgA is pivotal in the prevention of both GN and GP bacterial disease at the mucosal surface. Additionally, a preliminary study of repeated oral administration of LPS and LTA in dairy cows found that it caused an increase in total salivary sIgA and a decrease in acute-phase proteins, plasma anti-LPS immunoglobulins, and the cytokine TNF. These results indicated an increase in mucosal protection against endotoxins and reduced markers of systemic endotoxin exposure in the plasma [13].
6.3. Induction of the Mucosal Immune Responses
The induction of a mucosal immune responses requires the presence of organized lymphoid tissues, such as Peyer’s patches and mesenteric lymph nodes [142,148]. Organized inductive sites are concentrated in areas where pathogens are most likely to enter and areas of high microbial density. The presence of an organized mucosal lymphoid follicle induces the differentiation of a follicle-associated epithelium (FAE), which contains microfold cells (M cells) [142]. These M cells are rich in endocytotic vesicles to uptake lumen contents and transport them to their intraepithelial pockets or directly to underlying dendritic cells (DCs), which reside in the subepithelial dome (SED) [142,147]. The intraepithelial pocket of M cells contains lymphocytes and macrophages, which interact with the transcytosed antigens and microorganisms. There is evidence indicating that DCs can also directly sample from the luminal environment [149].
Dendritic cells are professional antigen-presenting cells (APCs) that express large amounts of major histocompatibility complexes I and II (MHC I and MHC II) that allow for the presentation of soluble antigens to both naïve and primed T cells. Particulate antigens are likely phagocytosed by macrophages, present in the intraepithelial pocket, which then present the antigens to T cells or release the antigen to DCs for presentation [147]. Dendritic cells can exit the mucosa through the lymphatic system and present antigens to T cells within the lymphoid tissues of the draining lymph nodes, thus allowing for interaction with the systemic immune system and not just local mucosa (Figure 5) [142]. The presentation of antigens to naïve T cells results in the activation of both CD4+ and CD8+ T cells. T helper cells (CD4+) differentiate into five main profiles including Th1, Th2, Th17, Treg and Tfh subsets, which are characterized by their cytokine production. At mucosal sites, activation of T helper cells by DCs promotes the differentiation of Th2 cells, which produce cytokines that cause B cell isotype switching to IgA production [147,152].
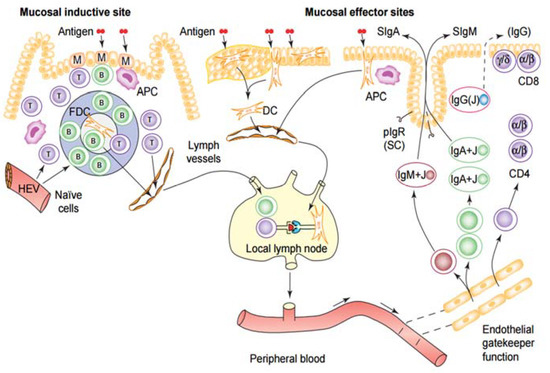
Figure 5.
Diagram summarizing the mucosal immune response. Lumen contents are sampled from inductive sites by M-cells within the mucosa-associated lymphoid tissue (MALT) and transported to underlying antigen-presenting cells. Dendritic cells are able to directly sample lumen contents and capture antigens as well. Naïve B and T cells arrive at the MALT and lymph nodes via high endothelial venules. After being primed to become memory/effector B and T cells, they migrate from MALT and lymph nodes to the peripheral blood for subsequent extravasation at the mucosal effector site. This process is mediated by local vascular adhesion molecules and chemokines, collectively known as homing receptors. Within the mucosal effector site of the gut (the lamina propria) are B lymphocytes, plasma cells, and T helper cells. Immunoglobulin A and M (IgA and IgM) produced by plasma cells is secreted into the lumen through interaction with pIgR-mediated transport [153].
6.4. Connectivity and Compartmentalization of the Mucosal Immune System
Lymphocytes, which have been activated at the mucosa preferentially migrate to other mucosal effector sites throughout the body. This resulted in the idea of a common mucosal immune system [147]. Lymphocytes activated at mucosal sites upregulate the expression of tissue-specific adhesion molecules as well as chemokine receptors, which act as ‘homing receptors’ to guide the cells back to the mucosa. For example, B cells activated in the mucosa express CCR10, a chemokine receptor for CCL28, which is secreted by epithelial cells of the intestines, salivary glands, tonsils, respiratory tract and lactating mammary glands. This allows for CCR10+IgA+ B cells to migrate to all of these tissues. Thus, immunization at one site can result in the secretion of specific IgA antibodies in other mucosal or glandular tissue [147].
In addition to the common system, there are chemokines, integrins, and cytokines that are differentially expressed among mucosal tissues, resulting in compartmentalization of certain mucosal tissues [148]. This keeps an immune response focused at the site of initial induction through receptor-mediated recognition systems [142]. Lymphocytes activated in the gut mucosa, for example, may express L-selectin and α4β7 integrin, which are two major adhesion molecules that bind to mucosal addressin cell adhesion molecule 1 (MAdCAM-1) expressed in venules of the small and large intestines [142,147]. The homing signals from the gut-associated lymphoid tissue (GALT) differ from those of the bronchus-associated lymphoid tissue (BALT) and nasal-associated lymphoid tissue (NALT) (Figure 6) [154].
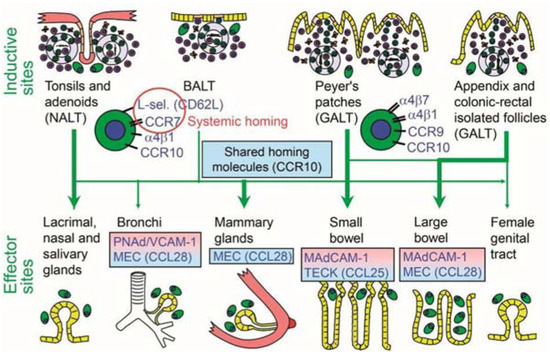
Figure 6.
Homing properties of mucosal memory/effector B cells. Putative scheme for the compartmentalized migration of B cells from inductive sites to effector sites with more or less preferred pathways depicted (graded arrows). The principal homing receptor profiles of the respective B-cell populations, and adhesion/chemokine cues directing extravasation at different effector sites, are indicated (pink and blue panels) [154].
Further evidence of this regionalization was seen in immunization studies with the cholera toxin B subunit. Oral immunization was found to induce an antibody response in the small intestine, ascending colon, mammary and salivary glands with an inefficient induction of IgA in the distal large intestine, tonsils and the female genital tract. Nasal immunization, on the other hand, resulted in strong responses in the upper respiratory tract, female genital tract and the salivary and nasal secretions with no response in the gut. Lastly, rectal and vaginal immunizations are found to have mostly local effects with little spread to further mucosal tissues [148].
6.5. Optimal Route of Immunization
Due to the connectivity and compartmentalization of the mucosal immune system, there are constraints to the route of immunization, depending on the target site for increasing immunity [149]. There is variation in the magnitude of both local and systemic immune responses at different vaccination sites [154,155]. The four major mucosal immunization routes are oral, nasal, vaginal, and rectal and while all produce local responses, the most effective vaccination routes are those that also produce strong systemic immunity and mucosal immunity at distal sites [12].
Administration of a mucosal vaccine through the female reproductive tract is difficult as the immunological features of the area are altered by hormonal fluctuations during the menstrual cycle [12]. The vaginal route has been found to induce a localized effect, causing little or no response in the respiratory and gastrointestinal tract. Rectal immunization, similar to vaginal, produces a strong localized response in the rectum with little response in the small intestine and the proximal colon [148]. The lack of a distal response from these routes eliminated them as optimal choices to generate a wide-spread effect throughout the mucosa [12,142,148].
Oral and nasal vaccinations have been found to effectively induce both mucosal and systemic immunity through cellular and humoral responses [12]. Research performed by Hopkins et al. [156] immunized mice with S. typhimurium, carrying the hybrid form of the hepatitis B virus core antigen (HBc), by four different routes—oral, nasal, rectal, and vaginal. The immunization induced specific antibodies against both LPS and HBc, although they were independent of each other. Evaluation of the differences in immune response to all four routes determined that oral and nasal routes were the most effective at inducing immunity in the saliva and intestines [156].
6.6. Endotoxin and Immunological Memory
The foundation of vaccination is to induce immunological memory and subsequently allow for a more rapid and efficient response against pathogenic infections [157]. The development of B cell memory (or plasma cells - PC) is the hallmark of adaptive immunity and was previously thought to require a T cell dependent (TD) response (Figure 7). This belief led to the thought that bacterial toxins, which are TI antigens, could not be used as vaccines as they could not elicit long-term protection [158,159]. An increasing body of evidence, however, shows that B cell memory can also be stimulated by TI antigens, although not in the classical TD fashion. In 2004, B-1b memory cells were isolated from T-deficient donors infected with B. hermsii, which is sufficient to establish the development of long-term protection in the absence of a TD response [158]. Obukhanych and Nussenzweig [160] found that polysaccharide antigens stimulate production of PC that are distinct from those induced by TD responses. Upon a secondary challenge with the polysaccharide, it was found that the activation of the B memory cells is regulated by antigen-specific IgG.
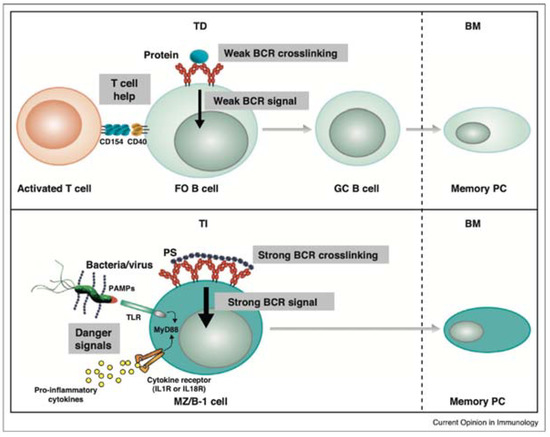
Figure 7.
T-dependent (TD) and T-independent (TI) production of plasma cells (PC) or memory B cells. One model proposes life-long TD memory PC cells associated with the strength of B-cell receptor (BCR) signal, provided there is T cell help. The antigens (Ag) for TD response are proteins which activate follicular (FO) B cells by aggregation of their BCR and accessory signals by CD4+ T-cell. These cells are differentiated into germinal center (GC) B cells prior to converting into PC. A model proposed by Defrance et al. [159] suggests that differentiation of B-1b or marginal zone (MZ) B cells into TI memory PC combines a strong BCR signal that is induced by multiple BCR crosslinking and accessory danger signals presented by toll-like receptor (TLR) agonists or inflammatory cytokines. The strong BCR crosslinking is related to repetitive structure of TI Ag. Dangers signals for the latter are provided directly by bacterial pathogen (pathogen-associated molecular patterns—PAMPS) or through proinflammatory cytokines by accessory cells. IL1R = Interleukin-1 receptor; IL-18R = Interleukin-18 receptor [160].
Further evidence of the role LPS plays in the development of immunological memory is present from research done with regard to human infection with V. cholerae and S. flexneri 2a. The presence of LPS-specific IgG memory B cells in the circulation was associated with a 68% decrease in risk of infection. Lipopolysaccharide-specific IgA antibodies are also associated with increased protection against V. cholerae infection. Due to the large increase in protection, which comes from LPS-specific memory B cells, it can be inferred that the use of LPS as a vaccine can help develop a strong and long-lasting immune response against subsequent infections [161]. Additionally, a study done on an oral vaccine for S. flexneri 2a showed promising results with detectable memory B cells to LPS present 28 days after exposure [162]. These studies show that vaccinations with bacteria or their components are able to elicit long-term immune protection.
Research conducted by our lab has demonstrated that oral administration of LPS and LTA in dairy cows has modulated both innate and adaptive immune responses [15]. In this study, 30 cows were split into a control group treated with oral saline only and a treated group that received five oral treatments of three increasing doses of LPS from E. coli 0111:B4 during week 4, 3, and 2 prior to calving and one flat dose of LTA from Bacillus subtilis administered orally during the same weeks prior to calving. The results of this study showed that cows treated with bacterial LPS and LTA increased concentrations of immunoglobulin-(Ig)A in the saliva. Treatment also lowered concentrations of anti-LPS IgA, IgG, and IgM Abs in the plasma as well as concentrations of tumor necrosis factor-alpha (TNF-α). The results of this study demonstrated that repeated mucosal administration of dairy cows to bacterial LPS and LTA is able to trigger both innate and humoral immune responses in periparturient dairy cows.
Another study by our lab (unpublished data) showed that cows treated with a combination of oral and parenteral LPS and LTA lowered the incidence of multiple periparturient diseases of dairy cows. Data showed that cows treated with a combination of LPS and LTA both orally and subcutaneously had 60% lower clinical cases of mastitis, 12% lower clinical cases of endometritis, 8% lower cases of udder edema, 33% lower number of dead and culled cows, and 27% lower number of cows removed from the herd by 27%. Additionally, the number of healthy cows in the group was 150% higher than the control group of cows. These data indicate that bacterial endotoxins (LPS and LTA) play a role in the periparturient health of dairy cows, increasing the susceptibility of them to disease.
7. Conclusions
Overall, increasing evidence suggest that bacterial endotoxins are involved in multiple periparturient diseases of dairy cows. Endotoxins include membrane components deriving from both Gram-negative and Gram-positive bacteria including LPS and LTA. The sources of endotoxins in dairy cows might be various, including rumen, when cattle are fed diets containing high amounts of grain, or infected uterus and udder in the days following calving. Given that endotoxins translocate into the systemic circulation of the host through mucosal membranes, knowledge about mucosal immunity and mucosal vaccination is of utmost importance to prevent health consequences of endotoxins.
Author Contributions
Conceptualization, B.N.A. and E.F.E.; Resources, B.N.A.; Writing-Original Draft Preparation, E.F.E.; Writing-Review & Editing, B.N.A.; Supervision, B.N.A.; Project Administration, B.N.A.; Funding Acquisition, B.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alberta Livestock and Meat Agency Ltd. grant number 2013F016R.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 1, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.R.; Bell, A.W.; Overton, T.R.; Loor, J.J. Nutritional management of the transition cow in the 21st century—A paradigm shift in thinking. Anim. Prod. Sci. 2013, 53, 1000–1023. [Google Scholar] [CrossRef]
- Mallard, B.A.; Dekkers, J.C.; Ireland, M.J.; Leslie, K.E.; Sharif, S.; Lacey Vankampen, C.; Wagter, L.; Wilkie, B.N. Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calf health. J. Dairy Sci. 1998, 81, 585–595. [Google Scholar] [CrossRef]
- Dosogne, H.; Meyer, E.; Sturk, A.; van Loon, J.; Massart-Leen, A.M.; Burvenich, C. Effect of enrofloxacin treatment on plasma endotoxin during bovine Escherichia coli mastitis. Inflamm. Res. 2002, 51, 201–205. [Google Scholar] [CrossRef]
- Emmanuel, D.G.V.; Madsen, K.L.; Churchill, T.A.; Dunn, S.M.; Ametaj, B.N. Acidosis and lipopolysaccharide from Escherichia coli B:055 cause hyperpermeability of rumen and colon tissues. J. Dairy Sci. 2007, 90, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Mateus, L.; Lopes da Costa, L.; Diniz, P.; Ziecik, A.J. Relationship between endotoxin and prostaglandin (PGE2 and PGFM) concentrations and ovarian function in dairy cows with puerperal endometritis. Anim. Reprod. Sci. 2003, 76, 143–154. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Zebeli, Q.; Iqbal, S. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. R. Bras. Zootec. 2010, 39, 433–444. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef]
- Garrett, E.F.; Nordlund, K.V.; Goodger, W.J.; Oetzel, G.R. A cross-sectional field study investigating the effect of periparturient dietary management on ruminal pH in early lactation dairy cows. J. Dairy Sci. 1997, 80, 169. [Google Scholar]
- Fernando, S.C.; Purvis, H.T., 2nd; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; Desilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef]
- Draing, C.; Sigel, S.; Deininger, S.; Traub, S.; Munke, R.; Mayer, C.; Hareng, L.; Hartung, T.; von Aulock, S.; Hermann, C. Cytokine induction by Gram-positive bacteria. Immunobiology 2008, 213, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Kumar, A.; Diaz-Mitoma, F.; Mestecky, J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010, 6, 1001147. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Zebeli, Q.; Mansmann, D.A.; Dunn, S.M.; Ametaj, B.N. Oral administration of LPS and lipoteichoic acid prepartum modulated reactants of innate and humoral immunity in periparturient dairy cows. Innate Immun. 2014, 20, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Sivaraman, S.; Dunn, S.M.; Zebeli, Q. Repeated oral administration of lipopolysaccharide from Escherichia coli 0111: B4 modulated humoral immune responses in periparturient dairy cows. Innate Immun. 2012, 18, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Zebeli, Q.; Mansmann, D.A.; Dunn, S.M.; Ametaj, B.N. Oronasal administration of lipopolysaccharide prepartum modulated plasma metabolite patterns in periparturient dairy cows. Open J. Anim. Sci. 2013, 3, 184–192. [Google Scholar] [CrossRef][Green Version]
- Zebeli, Q.; Mansmann, D.; Sivaraman, S.; Dunn, S.M.; Ametaj, B.N. Oral challenge with increasing doses of LPS modulated the patterns of plasma metabolites and minerals in periparturient dairy cows. Innate Immun. 2013, 19, 298–314. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Jing, L.; Zhang, R.; Liu, Y.; Zhu, W.; Mao, S. Intravenous lipopolysaccharide challenge alters ruminal bacterial microbiota and disrupts ruminal metabolism in dairy cattle. Br. J. Nutr. 2014, 112, 170–182. [Google Scholar] [CrossRef]
- Mao, S.Y.; Zhang, R.Y.; Wang, D.S.; Zhu, W.Y. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar] [CrossRef]
- Van Amersfoort, E.S.; Van Berkel, T.J.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef]
- Nankar, S.A.; Pande, A.H. Physicochemical properties of bacterial pro-inflammatory lipids influence their interaction with apolipoprotein-derived peptides. Biochim. Biophys. Acta 2013, 4, 853–862. [Google Scholar] [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Netea, M.G.; van Deuren, M.; Kullberg, B.J.; Cavaillon, J.M.; Van der Meer, J.W. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002, 23, 135–139. [Google Scholar] [CrossRef]
- Percy, M.G.; Grundling, A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Morath, S.; von Aulock, S.; Hartung, T. Structure/function relationships of lipoteichoic acids. J. Endotoxin Res. 2005, 11, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Duckworth, C.A.; Watson, A.J.; Frey, M.R.; Miguel, J.C.; Burkitt, M.D.; Sutton, R.; Hughes, K.R.; Hall, L.J.; Caamano, J.H.; et al. A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis. Model. Mech. 2013, 6, 1388–1399. [Google Scholar] [CrossRef]
- Hakogi, E.; Tamura, H.; Tanaka, S.; Kohata, A.; Shimada, Y.; Tabuchi, K. Endotoxin levels in milk and plasma of mastitis-affected cows measured with a chromogenic limulus test. Vet. Microbiol. 1989, 20, 267–274. [Google Scholar] [CrossRef]
- Jorgensen, H.B.; Buitenhuis, B.; Rontved, C.M.; Jiang, L.; Ingvartsen, K.L.; Sorensen, P. Transcriptional profiling of the bovine hepatic response to experimentally induced E. coli mastitis. Physiol. Genom. 2012, 44, 595–606. [Google Scholar] [CrossRef]
- Kobayashi, K.; Oyama, S.; Numata, A.; Rahman, M.M.; Kumura, H. Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions. PLoS ONE 2013, 8, e62187. [Google Scholar] [CrossRef]
- Leitner, G.; Yadlin, B.; Glickman, A.; Chaffer, M.; Saran, A. Systemic and local immune response of cows to intramammary infection with Staphylococcus aureus. Res. Vet. Sci. 2000, 69, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T.; Bosu, W.T.; Gilbert, R.O. Absorption of Escherichia coli endotoxin (lipopolysaccharide) from the uteri of postpartum dairy cows. Theriogenology 1990, 33, 1011–1014. [Google Scholar] [CrossRef]
- Mani, V.; Weber, T.E.; Baumgard, L.H.; Gabler, N.K. Growth and Development Symposium: Endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 2012, 90, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, D.G.; Dunn, S.M.; Ametaj, B.N. Feeding high proportions of barley grain stimulates an inflammatory response in dairy cows. J. Dairy Sci. 2008, 91, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.A.; Li, J.S.; Li, Y.S.; Zhu, N.T.; Liu, F.N.; Tan, L. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J. Gastroenterol. 2004, 10, 2373–2378. [Google Scholar] [CrossRef]
- Han, X.; Fink, M.P.; Yang, R.; Delude, R.L. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock 2004, 21, 261–270. [Google Scholar] [CrossRef]
- Potoka, D.A.; Upperman, J.S.; Nadler, E.P.; Wong, C.T.; Zhou, X.; Zhang, X.R.; Ford, H.R. NF-kappaB inhibition enhances peroxynitrite-induced enterocyte apoptosis. J. Surg. Res. 2002, 106, 7–14. [Google Scholar] [CrossRef]
- Potoka, D.A.; Nadler, E.P.; Upperman, J.S.; Ford, H.R. Role of nitric oxide and peroxynitrite in gut barrier failure. World J. Surg. 2002, 26, 806–811. [Google Scholar] [CrossRef]
- Sugi, K.; Musch, M.W.; Field, M.; Chang, E.B. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology 2001, 120, 1393–1403. [Google Scholar] [CrossRef]
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A.; et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006, 176, 3070–3079. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Arnold, E.T.; Thomas, L.S.; Gonsky, R.; Zhou, Y.; Hu, B.; Arditi, M. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J. Biol. Chem. 2002, 277, 20431–20437. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Brown, D.; McKee, M.; Lynch-Devaney, K.; Gerken, G.; Podolsky, D.K. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am. J. Pathol. 2002, 160, 165–173. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.L.; Meresse, S.; Gounon, P.; Davoust, J.; Mounier, J.; Sansonetti, P.J.; Gorvel, J.P. Trafficking of Shigella lipopolysaccharide in polarized intestinal epithelial cells. J. Cell Biol. 1999, 145, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef]
- Grunfeld, C.; Feingold, K.R. Endotoxin in the gut and chylomicrons: Translocation or transportation? J. Lipid Res. 2009, 50, 1–2. [Google Scholar] [CrossRef]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Meissner, H.H.; Henning, P.H.; Horn, C.H.; Leeuw, K.-J.; Hagg, F.M.; Fouché, G. Ruminal acidosis: A review with detailed reference to the controlling agent Megasphaera elsdenii NCIMB 41125. S. Afr. J. Anim. Sci. 2010, 40, 79–100. [Google Scholar] [CrossRef]
- Krause, K.M.; Oetzel, G.R. Understanding and preventing subacute ruminal acidosis in dairy herds: A review. Anim. Feed Sci. Technol. 2006, 126, 215–236. [Google Scholar] [CrossRef]
- Maulfair, D.D.; McIntyre, K.K.; Heinrichs, A.J. Subacute ruminal acidosis and total mixed ration preference in lactating dairy cows. J. Dairy Sci. 2013, 96, 6610–6620. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, D.G.V.; Shanthipoosan, S.; Ametaj, B.N. High grain diets perturb rumen and plasma metabolites and induce inflammatory responses in early lactation dairy cows. Ital. J. Anim. Sci. 2010, 6, 424–426. [Google Scholar] [CrossRef]
- Goff, J.P. Major advances in our understanding of nutritional influences on bovine health. J. Dairy Sci. 2006, 89, 1292–1301. [Google Scholar] [CrossRef]
- Grummer, R.R. Nutritional and management strategies for the prevention of fatty liver in dairy cattle. Vet. J. 2008, 176, 10–20. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Bradford, B.J.; Bobe, G.; Nafikov, R.A.; Lu, Y.; Young, J.W.; Beitz, D.C. Strong relationships between mediators of the acute phase response and fatty liver in dairy cows. Can. J. Anim. Sci. 2005, 85, 165–175. [Google Scholar] [CrossRef]
- Bradford, B.J.; Mamedova, L.K.; Minton, J.E.; Drouillard, J.S.; Johnson, B.J. Daily injection of tumor necrosis factor-increases hepatic triglycerides and alters transcript abundance of metabolic genes in lactating dairy cattle. J. Nutr. 2009, 139, 1451–1456. [Google Scholar] [CrossRef]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Wellnitz, O.; Arnold, E.T.; Bruckmaier, R.M. Lipopolysaccharide and lipoteichoic acid induce different immune responses in the bovine mammary gland. J. Dairy Sci. 2011, 94, 5405–5412. [Google Scholar] [CrossRef]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the probiotics for the gut-liver axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef]
- Ritze, Y.; Bárdos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef]
- Aitken, S.L.; Corl, C.M.; Sordillo, L.M. Immunopathology of mastitis: Insights into disease recognition and resolution. J. Mammary Gland Biol. Neoplasia 2011, 16, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, H.; Khan, A.A.; Banday, M.T.; Ashraf, I.; Handoo, N.; Shah, A.B.; Hamadani, A. Bovine mastitis—A disease of serious concern for dairy farmers. Int. J. Livest. Res. 2013, 3, 42–55. [Google Scholar] [CrossRef]
- Rainard, P.; Riollet, C. Innate immunity of the bovine mammary gland. Vet. Res. 2006, 37, 369–400. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.G.; Babra, C.; Tiwari, H.K.; Williams, V.; Wet, S.D.; Gibson, J.; Paxman, A.; Morgan, E.; Costantino, P.; Sunagar, R.; et al. Trends in therapeutic and prevention strategies for management of bovine mastitis: An overview. J. Vaccines Vaccin. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Goldammer, T.; Zerbe, H.; Molenaar, A.; Schuberth, H.J.; Brunner, R.M.; Kata, S.R.; Seyfert, H.M. Mastitis increases mammary mRNA abundance of beta-defensin 5, toll-like-receptor 2 (TLR2), and TLR4 but not TLR9 in cattle. Clin. Diagn. Lab. Immunol. 2004, 11, 174–185. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Lee, J.W.; Ibeagha, A.E.; Bannerman, D.D.; Paape, M.J.; Zhao, X. Bacterial lipopolysaccharide induces increased expression of toll-like receptor (TLR) 4 and downstream TLR signaling molecules in bovine mammary epithelial cells. Vet. Res. 2008, 39, 11. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Paape, M.J.; Lee, J.W.; Zhao, X.; Hope, J.C.; Rainard, P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin. Diagn. Lab. Immunol. 2004, 11, 463–472. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, L.; Jiang, J.; Xu, Q.; Luo, Z.; Luo, Q.; Yu, S.; Yao, X.; Ren, Z.; Hu, Y.; et al. Metabolomic Profiles of Bovine Mammary Epithelial Cells Stimulated by Lipopolysaccharide. Sci. Rep. 2019, 9, 19131. [Google Scholar] [CrossRef]
- Moyes, K.M.; Larsen, T.; Sørensen, P.; Ingvartsen, K.L. Changes in various metabolic parameters in blood and milk during experimental Escherichia coli mastitis for primiparous Holstein dairy cows during early lactation. J. Anim. Sci. Biotechnol. 2014, 5, 47. [Google Scholar] [CrossRef]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar]
- Zhao, X.; Lacasse, P. Mammary tissue damage during bovine mastitis: Causes and control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, K.; Zhao, X.; Bouetard, A.; Delbecchi, L.; Paquette, B.; Lacasse, P. Antioxidants to prevent bovine neutrophil-induced mammary epithelial cell damage. J. Dairy Sci. 2005, 88, 4295–4303. [Google Scholar] [CrossRef]
- Mehrzad, J.; Desrosiers, C.; Lauzon, K.; Robitaille, G.; Zhao, X.; Lacasse, P. Proteases involved in mammary tissue damage during endotoxin-induced mastitis in dairy cows. J. Dairy Sci. 2005, 88, 211–222. [Google Scholar] [CrossRef]
- Lavon, Y.; Leitner, G.; Moallem, U.; Klipper, E.; Voet, H.; Jacoby, S.; Glick, G.; Meidan, R.; Wolfenson, D. Immediate and carryover effects of Gram-negative and Gram-positive toxin-induced mastitis on follicular function in dairy cows. Theriogenology 2011, 76, 942–953. [Google Scholar] [CrossRef]
- Jacobsen, S.; Toelboell, T.; Andersen, P.H. Dose dependency and individual variability in selected clinical, haematological and blood biochemical responses after systemic lipopolysaccharide challenge in cattle. Vet. Res. 2005, 36, 167–178. [Google Scholar] [CrossRef]
- Hoeben, D.; Burvenich, C.; Trevisi, E.; Bertoni, G.; Hamann, J.; Bruckmaier, R.M.; Blum, J.W. Role of endotoxin and TNF-alpha in the pathogenesis of experimentally induced coliform mastitis in periparturient cows. J. Dairy Res. 2000, 67, 503–514. [Google Scholar] [CrossRef]
- Minuti, A.; Zhou, Z.; Graugnard, D.E.; Rodriguez-Zas, S.L.; Palladino, A.R.; Cardoso, F.C.; Trevisi, E.; Loor, J.J. Acute mammary and liver transcriptome responses after an intramammary Escherichia coli lipopolysaccharide challenge in postpartal dairy cows. Physiol. Rep. 2015, 4, e12388. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef]
- Perkins, K.H.; VandeHaar, M.J.; Burton, J.L.; Liesman, J.S.; Erskine, R.J.; Elsasser, T.H. Clinical responses to intramammary endotoxin infusion in dairy cows subjected to feed restriction. J. Dairy Sci. 2002, 85, 1724–1731. [Google Scholar] [CrossRef]
- Doll, K.; Sickinger, M.; Seeger, T. New aspects in the pathogenesis of abomasal displacement. Vet. J. 2009, 181, 90–96. [Google Scholar] [CrossRef]
- Zebeli, Q.; Sivaraman, S.; Dunn, S.M.; Ametaj, B.N. Intermittent parenteral administration of endotoxin triggers metabolic and immunological alterations typically associated with displaced abomasum and retained placenta in periparturient dairy cows. J. Dairy Sci. 2011, 94, 4968–4983. [Google Scholar] [CrossRef] [PubMed]
- Vlaminck, K.; van Meirhaeghe, H.; van den Hende, C.; Oyaert, W.; Muylle, E. Effect of endotoxins on abomasal emptying in cattle. Dtsch. Tierarztl. Wochenschr. 1985, 92, 392–395. [Google Scholar] [PubMed]
- Kaze, C.; Mevissen, M.; Hirsbrunner, G.; Steiner, A. Effect of endotoxins on contractility of smooth muscle preparations from the bovine abomasal antrum. Dtsch. Tierarztl. Wochenschr. 2004, 111, 28–35. [Google Scholar] [PubMed]
- Ziv, G. Treatment of peracute and acute mastitis. Vet. Clin. N. Am. Food Anim. Pract. 1992, 8, 1–15. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Kudo, K.; Mori, K.; Nagai, F.; Hatsugaya, A.; Tajima, M.; Tamura, K.; Hoshi, F.; Koiwa, M.; Kawamura, S. Acute phase response in naturally occurring coliform mastitis. J. Vet. Med. Sci. 2001, 63, 675–678. [Google Scholar] [CrossRef]
- Petzl, W.; Günther, J.; Pfister, T.; Sauter-Louis, C.; Goetze, L.; von Aulock, S.; Hafner-Marx, A.; Schuberth, H.J.; Seyfert, H.M.; Zerbe, H. Lipopolysaccharide pretreatment of the udder protects against experimental Escherichia coli mastitis. Innate Immun. 2012, 18, 467–477. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Postpartum uterine disease and dairy herd reproductive performance: A review. Vet. J. 2008, 176, 102–114. [Google Scholar] [CrossRef]
- Kimura, K.; Goff, J.P.; Kehrli, M.E., Jr.; Reinhardt, T.A. Decreased neutrophil function as a cause of retained placenta in dairy cattle. J. Dairy Sci. 2002, 85, 544–550. [Google Scholar] [CrossRef]
- Dohmen, M.J.; Joop, K.; Sturk, A.; Bols, P.E.; Lohuis, J.A. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology 2000, 54, 1019–1032. [Google Scholar] [CrossRef]
- Diez-Fraile, A.; Meyer, E.; Duchateau, L.; Burvenich, C. L-selectin and beta2-integrin expression on circulating bovine polymorphonuclear leukocytes during endotoxin mastitis. J. Dairy Sci. 2003, 86, 2334–2342. [Google Scholar] [CrossRef]
- Boro, P.; Kumaresan, A.; Singh, A.K.; Gupta, D.; Kumar, S.; Manimaran, A.; Mohanty, A.K.; Mohanty, T.K.; Pathak, R.; Attupuram, N.M.; et al. Expression of short chain fatty acid receptors and pro-inflammatory cytokines in utero-placental tissues is altered in cows developing retention of fetal membranes. Placenta 2014, 35, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Azawi, O.I. Postpartum uterine infection in cattle. Anim. Reprod. Sci. 2008, 105, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Sibley, K.; Miller, A.N.; Lane, E.A.; Fishwick, J.; Nash, D.M.; Herath, S.; England, G.C.; Dobson, H.; Sheldon, I.M. The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function. Am. J. Reprod. Immunol. 2008, 60, 462–473. [Google Scholar] [CrossRef]
- Herath, S.; Lilly, S.T.; Fischer, D.P.; Williams, E.J.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2 to prostaglandin E2 in bovine endometrium. Endocrinology 2009, 150, 1912–1920. [Google Scholar] [CrossRef]
- Purba, F.Y.; Ueda, J.; Nii, T.; Yoshimura, Y.; Isobe, N. Effects of intrauterine infusion of bacterial lipopolysaccharides on the mammary gland inflammatory response in goats. Vet. Immunol. Immunopathol. 2020, 219, 109972. [Google Scholar] [CrossRef]
- Machado, V.S.; Bicalho, M.L.; Meira Junior, E.B.; Rossi, R.; Ribeiro, B.L.; Lima, S.; Santos, T.; Kussler, A.; Foditsch, C.; Ganda, E.K.; et al. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes prevents puerperal metritis in Holstein dairy cows. PLoS ONE 2014, 9, e91734. [Google Scholar] [CrossRef]
- Nocek, J.E. Bovine acidosis: Implications on laminitis. J. Dairy Sci. 1997, 80, 1005–1028. [Google Scholar] [CrossRef]
- Danscher, A.M.; Enemark, J.M.; Telezhenko, E.; Capion, N.; Ekstrøm, C.T.; Thoefner, M.B. Oligofructose overload induces lameness in cattle. J. Dairy Sci. 2009, 92, 607–616. [Google Scholar] [CrossRef]
- Shearer, J.K.; van Amstel, S.R. Pathogenesis and treatment of sole ulcers and white line disease. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Boosman, R.; Mutsaers, C.W.; Klarenbeek, A. The role of endotoxin in the pathogenesis of acute bovine laminitis. Vet. Q. 1991, 13, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Murray, R.D.; Ward, W.R. Gross and histopathological study of endotoxin-induced hoof lesions in cattle. J. Comp. Pathol. 1994, 110, 103–115. [Google Scholar] [CrossRef]
- Thoefner, M.B.; Pollitt, C.C.; Van Eps, A.W.; Milinovich, G.J.; Trott, D.J.; Wattle, O.; Andersen, P.H. Acute bovine laminitis: A new induction model using alimentary oligofructose overload. J. Dairy Sci. 2004, 87, 2932–2940. [Google Scholar] [CrossRef]
- Bailey, S.R.; Adair, H.S.; Reinemeyer, C.R.; Morgan, S.J.; Brooks, A.C.; Longhofer, S.L.; Elliott, J. Plasma concentrations of endotoxin and platelet activation in the developmental stage of oligofructose-induced laminitis. Vet. Immunol. Immunopathol. 2009, 129, 167–173. [Google Scholar] [CrossRef]
- Brooks, A.C.; Menzies-Gow, N.J.; Wheeler-Jones, C.; Bailey, S.R.; Cunning-ham, F.M.; Elliott, J. Endotoxin-induced activation of equine platelets: Evidence for direct activation of p38 MAPK pathways and vasoactive mediator production. Inflamm. Res. 2007, 56, 154–161. [Google Scholar] [CrossRef]
- Brandt, E.; Petersen, F.; Ludwig, A.; Ehlert, J.E.; Bock, L.; Flad, H.D. The beta-thromboglobulins and platelet factor 4: Blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J. Leukoc. Biol. 2000, 67, 471–478. [Google Scholar] [CrossRef]
- Reisinger, N.; Schaumberger, S.; Nagl, V.; Hessenberger, S.; Schatzmayr, G. Concentration Dependent Influence of Lipopolysaccharides on Separation of Hoof Explants and Supernatant Lactic Acid Concentration in an Ex Vivo/In Vitro Laminitis Model. PLoS ONE 2015, 10, e0143754. [Google Scholar] [CrossRef]
- Eades, S.C. Overview of current laminitis research. Vet. Clin. N. Am. Equine Pract. 2010, 26, 51–63. [Google Scholar] [CrossRef]
- Kelton, D.F.; Lissemore, K.D.; Martin, R.E. Recommendations for recording and calculating the incidence of selected clinical diseases of dairy cattle. J. Dairy Sci. 1998, 81, 2502–2509. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Herman, J.A.; Johnson, G.W.; McArt, J.A.A. Herd-Level monitoring and prevention of displaced abomasum in dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.H. Diseases of the abomasum associated with current feeding practices. J. Am. Vet. Med. Assoc. 1969, 154, 1203–1205. [Google Scholar]
- Markusfeld, O. The association of displaced abomasum withvarious periparturient factors in dairy cows. A retrospective study. Prev. Vet. Med. 1986, 4, 173–183. [Google Scholar] [CrossRef]
- Constable, P.D.; Miller, G.Y.; Hoffsis, G.F.; Hull, B.L.; Rings, D.M. Risk factors for abomasal volvulus and left abomasal displace-ment in cattle. Am. J. Vet. Res. 1992, 53, 1184–1192. [Google Scholar] [PubMed]
- Coppock, C.E. Displaced abomasum in dairy cattle: Etiological factors. J. Dairy Sci. 1974, 57, 926–933. [Google Scholar] [CrossRef]
- Detilleux, J.C.; Grohn, Y.T.; Eicker, S.W.; Quaas, R.L. Effects of left displaced abomasum on test day milk yields of Holstein cows. J. Dairy Sci. 1997, 80, 121–126. [Google Scholar] [CrossRef]
- Cameron, R.E.; Dyk, P.B.; Herdt, T.H.; Kaneene, J.B.; Miller, R.; Bucholtz, H.F.; Liesman, J.S.; Vandehaar, M.J.; Emery, R.S. Dry cow diet, management, and energy balance as risk factors for displaced abomasum in high producing dairy herds. J. Dairy Sci. 1998, 81, 132–139. [Google Scholar] [CrossRef]
- Shaver, R.D. Nutritional risk factors in the etiology of left displaced abomasum in dairy cows: A review. J. Dairy Sci. 1997, 80, 2449–2453. [Google Scholar] [CrossRef]
- Stengarde, L.; Holtenius, K.; Traven, M.; Hultgren, J.; Niskanen, R.; Emanuelson, U. Blood profiles in dairy cows with displaced abomasum. J. Dairy Sci. 2010, 93, 4691–4699. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic predictors of displaced abomasum in dairy cattle. J. Dairy Sci. 2005, 88, 159–170. [Google Scholar] [CrossRef]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef] [PubMed]
- Zadnik, T. A comparative study of the hemato-biochemical parameters between clinically healthy cows and cows with displacement of the abomasum. Acta Vet. Beogr. 2003, 53, 297–310. [Google Scholar]
- Tsuneyoshi, I.; Kanmura, Y.; Yoshimura, N. Lipoteichoic acid from Staphylococcus aureus depresses contractile function of human arteries in vitro due to the induction of nitric oxide synthase. Anesth. Analg. 1996, 82, 948–953. [Google Scholar] [PubMed]
- Gray, C.P.; St George, T.D.; Jonsson, N.N. Milk fever in dairy cattle: A novel hypothesis for an immune mediated aetiology. Cattle Pract. 2007, 15, 277–282. [Google Scholar]
- DeGaris, P.J.; Lean, I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008, 176, 58–69. [Google Scholar] [CrossRef]
- Waldron, M.R.; Nonnecke, B.J.; Nishida, T.; Horst, R.L.; Overton, T.R. Effect of lipopolysaccharide infusion on serum macromineral and vitamin D concentrations in dairy cows. J. Dairy Sci. 2003, 86, 3440–3446. [Google Scholar] [CrossRef]
- Holowaychuk, M.K.; Hansen, B.D.; DeFrancesco, T.C.; Marks, S.L. Ionized hypocalcemia in critically ill dogs. J. Vet. Intern. Med. 2009, 23, 509–513. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. Calcium: A regulator of the inflammatory response in endotoxemia and sepsis. New Horiz. 1996, 4, 58–71. [Google Scholar]
- Malcolm, D.S.; Zaloga, G.P.; Holaday, J.W. Calcium administration increases the mortality of endotoxic shock in rats. Crit. Care Med. 1989, 17, 900–903. [Google Scholar] [CrossRef]
- Zaloga, G.P.; Malcolm, D.; Chernow, B.; Holaday, J.W. Endotoxin-induced hypocalcemia results in defective calcium mobilization in rats. Circ. Shock 1988, 24, 143–148. [Google Scholar]
- Collage, R.D.; Howell, G.M.; Zhang, X.; Stripay, J.L.; Lee, J.S.; Angus, D.C.; Rosengart, M.R. Calcium supplementation during sepsis exacerbates organ failure and mortality via calcium/calmodulin-dependent protein kinase kinase signaling. Crit. Care Med. 2013, 41, e352–e360. [Google Scholar] [CrossRef] [PubMed]
- Chmielinska, J.J.; Tejero-Taldo, M.I.; Mak, I.T.; Weglicki, W.B. Intestinal and cardiac inflammatory response shows enhanced endotoxin receptor (CD14) expression in magnesium deficiency. Mol. Cell. Biochem. 2005, 278, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zebeli, Q.; Beitz, D.C.; Bradford, B.J.; Dunn, S.M.; Ametaj, B.N. Peripartal alterations of calcitonin gene-related peptide and minerals in dairy cows affected by milk fever. Vet. Clin. Pathol. 2013, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Dervishi, E.; Ametaj, B.N. Milk fever in dairy cows is preceded by activation of innate immunity and alterations in carbohydrate metabolism prior to disease occurrence. Res. Vet. Sci. 2018, 117, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Hosseini, A.; Odhiambo, J.F.; Zebeli, Q.; Deng, Q.; Sharma, S.; Iqbal, S.; Dunn, S.M.; Lam, T.H.; Farooq, U. Application of Acute Phase Proteins for Monitoring Inflammatory States in Cattle; Veas, F., Ed.; InTech Europe: Rijeka, Croatia, 2011; pp. 299–354. ISBN 978-953-307-873-1. [Google Scholar]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets-an updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Correa, M.T.; Erb, H.N.; Scarlett, J.M. Risk factors for downer cow syndrome. J. Dairy Sci. 1993, 76, 3460–3463. [Google Scholar] [CrossRef]
- Eckel, E.F.; Ametaj, B.N. Invited review: Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 2016, 99, 5967–5990. [Google Scholar] [CrossRef]
- Kalaitzakis, E.; Panousis, N.; Roubies, N.; Giadinis, N.; Kaldrymidou, E.; Georgiadis, M.; Karatzias, K. Clinicopathological evaluation of downer dairy cows with fatty liver. Can. Vet. J. 2010, 51, 615–622. [Google Scholar]
- Byrne, C.M.; Erol, I.; Call, J.E.; Kaspar, C.W.; Buege, D.R.; Hiemke, C.J.; Fedorka-Cray, P.J.; Benson, A.K.; Wallace, F.M.; Luchansky, J.B. Characterization of Escherichia coli O157:H7 from downer and healthy dairy cattle in the upper Midwest region of the United States. Appl. Environ. Microbiol. 2003, 69, 4683–4688. [Google Scholar] [CrossRef]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- De Magistris, M.T. Mucosal delivery of vaccine antigens and its advantages in pediatrics. Adv. Drug Deliv. Rev. 2006, 58, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Dlugonska, H.; Grzybowski, M. Mucosal vaccination—An old but still vital strategy. Ann. Parasitol. 2012, 58, 1–8. [Google Scholar] [PubMed]
- Gerdts, V.; Mutwiri, G.K.; Tikoo, S.K.; Babiuk, L.A. Mucosal delivery of vaccines in domestic animals. Vet. Res. 2006, 37, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.D.; Freytag, L.C. Parenteral Vaccination Can Be an Effective Means of Inducing Protective Mucosal Responses. Clin. Vaccine Immunol. 2016, 23, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Simecka, J.W. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv. Drug Deliv. Rev. 1998, 34, 235–259. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Wershil, B.K.; Furuta, G.T. 4. Gastrointestinal mucosal immunity. J. Allergy Clin. Immunol. 2008, 121, S380–S383. [Google Scholar] [CrossRef]
- Apter, F.M.; Michetti, P.; Winner, L.S., 3rd; Mack, J.A.; Mekalanos, J.J.; Neutra, M.R. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect. Immun. 1993, 61, 5279–5285. [Google Scholar] [CrossRef]
- Hanihara-Tatsuzawa, F.; Miura, H.; Kobayashi, S.; Isagawa, T.; Okuma, A.; Manabe, I.; MaruYama, T. Control of Toll-like receptor–mediated T cell–independent type 1 antibody responses by the inducible nuclear protein IκB-ζ. J. Biol. Chem. 2014, 289, 30925–30936. [Google Scholar] [CrossRef]
- Weill, F.S.; Cela, E.M.; Paz, M.L.; Ferrari, A.; Leoni, J.; Gonzalez Maglio, D.H. Lipoteichoic acid from Lactobacillus rhamnosus GG as an oral photoprotective agent against UV-induced carcinogenesis. Br. J. Nutr. 2013, 109, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007, 25, 5467–5484. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Secretory immunity with special reference to the oral cavity. J. Oral Microbiol. 2013, 5, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L.; Faden, H.; Welliver, R.C. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 2001, 14, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.; Kraehenbuhl, J.P.; Schodel, F.; Potts, A.; Peterson, D.; de Grandi, P.; Nardelli-Haefliger, D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 1995, 63, 3279–3286. [Google Scholar] [CrossRef]
- Deauvieau, F.; Dussurgey, S.; Rossignol, D.; de Montfort, A.; Burdin, N.; Guy, B. Memory B and T cell responses induced by serotype 4 Streptococcus pneumoniae vaccines: Longitudinal analysis comparing responses elicited by free polysaccharide, conjugate and carrier. Vaccine 2009, 28, 576–582. [Google Scholar] [CrossRef]
- Alugupalli, K.R.; Leong, J.M.; Woodland, R.T.; Muramatsu, M.; Honjo, T.; Gerstein, R.M. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 2004, 21, 379–390. [Google Scholar] [CrossRef]
- Defrance, T.; Taillardet, M.; Genestier, L. T cell-independent B cell memory. Curr. Opin. Immunol. 2011, 23, 330–336. [Google Scholar] [CrossRef]
- Obukhanych, T.V.; Nussenzweig, M.C. T-independent type II immune responses generate memory B cells. J. Exp. Med. 2006, 203, 305–310. [Google Scholar] [CrossRef]
- Patel, S.M.; Rahman, M.A.; Mohasin, M.; Riyadh, M.A.; Leung, D.T.; Alam, M.M.; Chowdhury, F.; Khan, A.I.; Weil, A.A.; Aktar, A.; et al. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin. Vaccine Immunol. 2012, 19, 842–848. [Google Scholar] [CrossRef]
- Simon, J.K.; Wahid, R.; Maciel, M., Jr.; Picking, W.L.; Kotloff, K.L.; Levine, M.M.; Sztein, M.B. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine 2009, 27, 565–572. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).