A Systematic Study into the Effects of Long-Term Multicomponent Training on the Cognitive Abilities of Older Adults with Neurodegenerative Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Procedures/Strategies
2.2. Study Selection Criteria
(((((“physical exercise”[All Fields]) OR (“exercise 1”[All Fields] OR “exercise”[All Fields])) OR (multicomponent)) OR (“cognitive abilities”[All Fields])) OR (“cognition”[All Fields])) AND (“neurodegenerative diseases”[All Fields])
2.3. Main Concepts
2.4. Data Extraction
2.5. Method of Analysis of Results
2.6. Quality of Information
3. Results
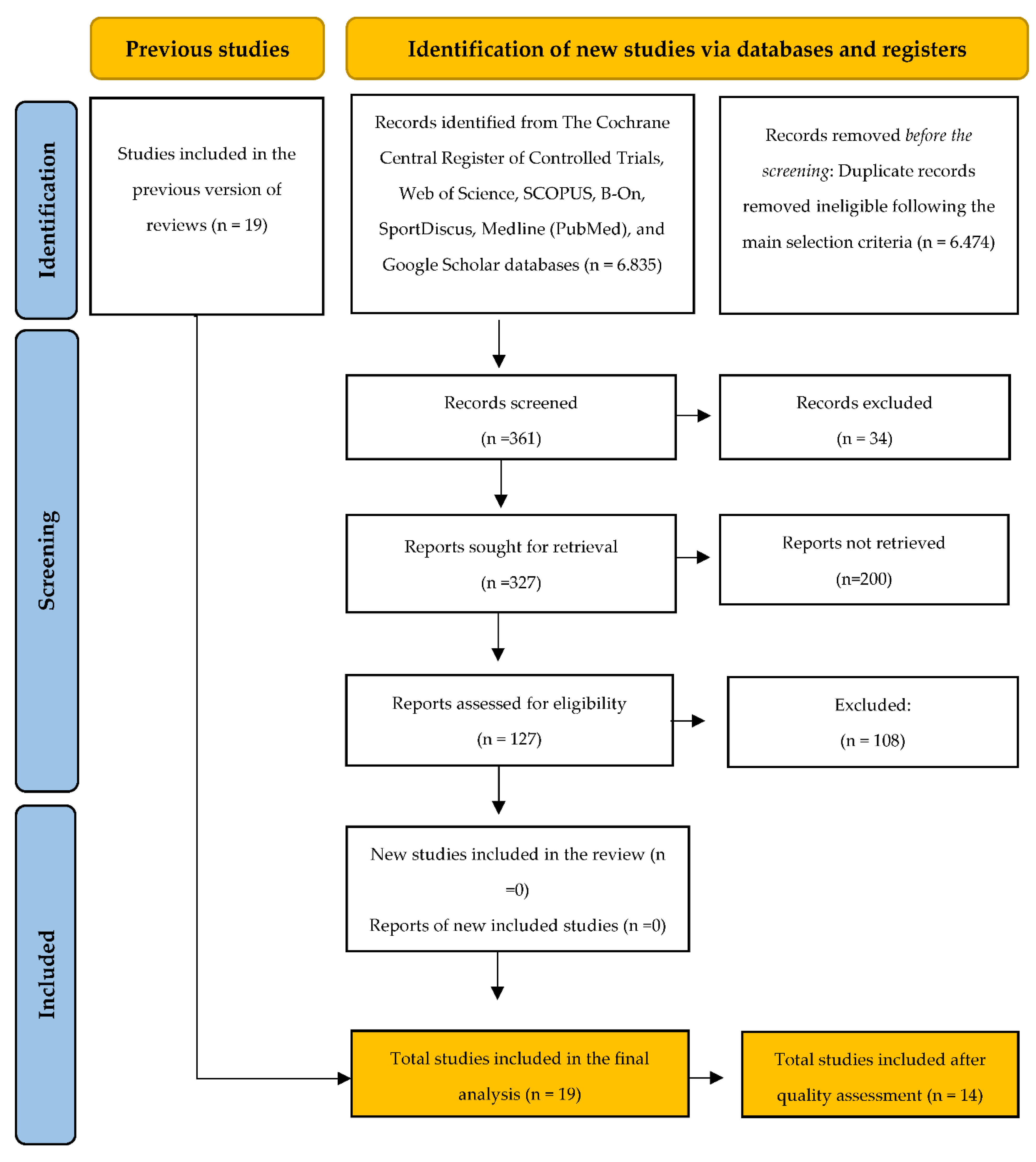
3.1. Study Selection
3.2. General Characteristics of Studies
3.3. Cognitive Performance Assessments
3.4. Multicomponent Interventions Effects
3.5. Short Description of Excluded Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolea, M.I.; Morris, J.C.; Galvin, J.E. Longitudinal Associations between Physical and Cognitive Performance among Community-Dwelling Older Adults. PLoS ONE 2015, 10, e0122878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murman, D.L. The Impact of Age on Cognition. Semin. Heart 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Fechine, B.; Vasconcelos, O.; Botelho, M.; Trompieri, N.; Carvalho, J. Memória, exercício físico e envelhecimento: Um estudo sobre a relação existente entre a memória visuomotora e idosos praticantes e não praticantes de atividade física. InterScience Place 2013, 1, 170–192. [Google Scholar] [CrossRef]
- Maffei, L.; Picano, E.; Andreassi, M.G.; Angelucci, A.; Baldacci, F.; Baroncelli, L.; Begenisic, T.; Bellinvia, P.F.; Berardi, N.; Biagi, L.; et al. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: The Train the Brain study. Sci. Rep. 2017, 7, 39471. [Google Scholar]
- Schure, M.B.; Borson, S.; Nguyen, H.Q.; Trittschuh, E.H.; Thielke, S.M.; Pike, K.C.; Adams, S.G.; Fan, V.S. Associations of cognition with physical functioning and health-related quality of life among COPD patients. Respir. Med. 2016, 114, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Klimova, B.; Valis, M.; Kuca, K. Cognitive decline in normal aging and its prevention: A review on non-pharmacological lifestyle strategies. Clin. Interv. Aging 2017, ume 12, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Borson, S. Cognition, Aging, and Disabilities: Conceptual Issues. Phys. Med. Rehabil. Clin. North Am. 2010, 21, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Raichlen, D.A.; Alexander, G.E. Adaptive Capacity: An Evolutionary Neuroscience Model Linking Exercise, Cognition, and Brain Health. Trends Neurosci. 2017, 40, 408–421. [Google Scholar] [CrossRef]
- Cieza, A.; Sabariego, C.; Bickenbach, J.; Chatterji, S. Rethinking Disability. BMC Med. 2018, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Uehara, E.; Fichman, H.; Landeira-Fernandez, J. Funções executivas: Um retrato integrativo dos principais modelos e teorias desse conceito / Executive functions? An integrative portrait of the main models and theories of this concept. Rev. Neuropsicol. Latinoam. 2013, 5, 25–37. [Google Scholar]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blader, J. Review of Executive Functions: What They Are, How They Work, and Why They Evolved, by Russell A. Barkley. J. Child Adolesc. Psychopharmacol. 2014, 24, 362–363. [Google Scholar] [CrossRef]
- Lu, W.; Chen, G.; Li, W. RETRACTED ARTICLE: Melanin-based biomimic photothermal nanoparticles for therapeutic application in diabetic nephropathy. J. Drug Target. 2021, 29, i–viii. [Google Scholar] [CrossRef] [PubMed]
- Hobson, J. The Montreal Cognitive Assessment (MoCA). Occup. Med. 2015, 65, 764–765. [Google Scholar] [CrossRef] [Green Version]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 10, a033118. [Google Scholar] [CrossRef] [Green Version]
- Von Bernhardi, R.; Eugenin-von Bernhardi, L.; Eugenin, J. Microglial cell dysregulation in Brain Aging and Neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, I.; Fayyaz, M.; Wajid, A.; Sohail, W.; Ali, A. Does Obesity Increase the Risk of Dementia: A Literature Review. Cureus 2018, 10, e2660. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Armstrong, M. Treatment of Parkinson’s Disease with Cognitive Impairment: Current Approaches and Future Directions. Behav. Sci. 2021, 11, 54. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer?s disease diagnosis and treatment [version 1; peer review: 2 approved]. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [Green Version]
- Orsini, M.; Ferreira, A.C.A.D.F.; De Assis, A.C.D.; Magalhães, T.; Teixeira, S.; Bastos, V.H.; Marinho, V.; Oliveira, T.; Fiorelli, R.; Oliveira, A.B.; et al. Cognitive Impairment in Neuromuscular Diseases: A Systematic Review. Neurol. Int. 2018, 10, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, D.C.B.; Fernandes, D.R.; Hernandez, S.S.; Soares, F.D.G.; de Figueiredo, K.; Coelho, F.G.D.M. Physical exercise is effective for neuropsychiatric symptoms in Alzheimer’s disease: A systematic review. Arq. De Neuro-Psiquiatr. 2021, 79, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; De-La-O, A.; Jurado-Fasoli, L.; Espuch-Oliver, A.; Robles-Gonzalez, L.; Navarro-Lomas, G.; de Haro, T.; Femia, P.; Castillo, M.J.; Gutierrez, A. Exercise training as S-Klotho protein stimulator in sedentary healthy adults: Rationale, design, and methodology. Contemp. Clin. Trials Commun. 2018, 11, 10–19. [Google Scholar] [CrossRef]
- Kirk-Sanchez, N.J.; McGough, E.L. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2014, 9, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Lin, F.V.; Salisbury, D.L.; Shah, K.N.; Chow, L.; Vock, D.; Nelson, N.W.; Porsteinsson, A.; Jack, C. Efficacy and mechanisms of combined aerobic exercise and cognitive training in mild cognitive impairment: Study protocol of the ACT trial. Trials 2018, 19, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Colcombe, S. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study—Revisited. Perspect. Psychol. Sci. 2018, 13, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Heyn, P.; Abreu, B.C.; Ottenbacher, K.J. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch. Phys. Med. Rehabil. 2004, 85, 1694–1704. [Google Scholar] [CrossRef]
- Haeger, A.; Costa, A.S.; Romanzetti, S.; Kilders, A.; Trautwein, C.; Haberl, L.; Beulertz, M.; Hildebrand, F.; Schulz, J.B.; Reetz, K. Effect of a multicomponent exercise intervention on brain metabolism: A randomized controlled trial on Alzheimer’s pathology (Dementia-MOVE). Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6. [Google Scholar] [CrossRef]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging. Brain Plast. 2018, 4, 17–52. [Google Scholar] [CrossRef] [Green Version]
- Esnigdha, S.; Rivera, C.E.; Milgram, N.W.; Cotman, C.W. Exercise enhances memory consolidation in the aging brain. Front. Aging Neurosci. 2014, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Pinilla, F.; Hillman, C. The Influence of Exercise on Cognitive Abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederiksen, K.S.; Gjerum, L.; Waldemar, G.; Hasselbalch, S.G. Effects of Physical Exercise on Alzheimer’s Disease Biomarkers: A Systematic Review of Intervention Studies. J. Alzheimer’s Dis. 2018, 61, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Mudò, G.; Mäkelä, J.; Di Liberto, V.; Tselykh, T.V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Mälkiä, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell. Mol. Life Sci. 2012, 69, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Huang, M.; Névéol, A.; Lu, Z. Recommending MeSH terms for annotating biomedical articles. J. Am. Med. Inform. Assoc. 2011, 18, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Mulasso, A.; Roppolo, M.; Rainoldi, A.; Rabaglietti, E. Effects of a Multicomponent Exercise Program on Prevalence and Severity of the Frailty Syndrome in a Sample of Italian Community-Dwelling Older Adults. Healthcare 2022, 10, 911. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.J.; O’Brien, J.T. Depression and cognition in older adults. Curr. Opin. Psychiatry 2008, 21, 8–13. [Google Scholar] [CrossRef]
- Darling-Hammond, L.; Flook, L.; Cook-Harvey, C.; Barron, B.; Osher, D. Implications for educational practice of the science of learning and development. Appl. Dev. Sci. 2019, 24, 97–140. [Google Scholar] [CrossRef] [Green Version]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [Green Version]
- Awuah, R.B.; Aikins, A.D.-G.; Dodoo, F.N.-A.; Meeks, K.A.; Beune, E.J.; Klipstein-Grobusch, K.; Addo, J.; Smeeth, L.; Bahendeka, S.K.; Agyemang, C. Psychosocial stressors among Ghanaians in rural and urban Ghana and Ghanaian migrants in Europe. J. Health Psychol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Pagani, M. Exercise Prescription to Foster Health and Well-Being: A Behavioral Approach to Transform Barriers into Opportunities. Int. J. Environ. Res. Public Health 2021, 18, 968. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.K.; Atlantis, E.; Singh, M.A.F. Multi-modal exercise programs for older adults. Age Ageing 2007, 36, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, R.; Tew, G.; Aning, J.J.; Gilbert, S.E.; Lewis, L.; Saxton, J.M. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: Systematic review with meta-analysis. Br. J. Sport. Med. 2018, 54, 341–348. [Google Scholar]
- Panic, N.; Leoncini, E.; de Belvis, G.; Ricciardi, W.; Boccia, S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 2013, 8, e83138. [Google Scholar] [CrossRef] [Green Version]
- de Souto Barreto, P.; Cesari, M.; Denormandie, P.; Armaingaud, D.; Vellas, B.; Rolland, Y. Exercise or Social Intervention for Nursing Home Residents with Dementia: A Pilot Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2017, 65, E123–E129. [Google Scholar] [CrossRef]
- de Oliveira Silva, F.; Ferreira, V.; Plácido, J.; Sant’anna, P.; Araújo, J.; Marinho, V.; Laks, J.; Deslandes, A.C. Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: A randomized controlled trial. Maturitas 2019, 126, 28–33. [Google Scholar] [CrossRef]
- Öhman, H.; Savikko, N.; Strandberg, T.E.; Kautiainen, H.; Raivio, M.M.; Laakkonen, M.L.; Tilvis, R.; Pitkälä, K.H. Effects of Exercise on Cognition: The Finnish Alzheimer Disease Exercise Trial: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2016, 64, 731–738. [Google Scholar] [CrossRef]
- Gobbi, L.T.B.; Pelicioni, P.H.S.; Lahr, J.; Lirani-Silva, E.; Teixeira-Arroyo, C.; Dos Santos, P.C.R. Effect of different types of exercises on psychological and cognitive features in people with Parkinson’s disease: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2021, 64, 101407. [Google Scholar] [CrossRef]
- Volpe, D.; Signorini, M.; Marchetto, A.; Lynch, T.; Morris, M.E. A comparison of Irish set dancing and exercises for people with Parkinson’s disease: A phase II feasibility study. BMC Geriatr. 2013, 13, 54–56. [Google Scholar] [CrossRef] [Green Version]
- Mavrommati, F.; Collett, J.; Franssen, M.; Meaney, A.; Sexton, C.; Dennis-West, A.; Betts, J.F.; Izadi, H.; Bogdanovic, M.; Tims, M.; et al. Exercise response in Parkinson’s disease: Insights from a cross-sectional comparison with sedentary controls and a per-protocol analysis of a randomised controlled trial. BMJ Open 2017, 7, e017194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Ito, K.; Shimokata, H.; Washimi, Y.; Endo, H.; Kato, T. A Randomized Controlled Trial of Multicomponent Exercise in Older Adults with Mild Cognitive Impairment. PLoS ONE 2013, 8, e61483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarazona-Santabalbina, F.J.; Gómez-Cabrera, M.C.; Pérez-Ros, P.; Martínez-Arnau, F.M.; Cabo, H.; Tsaparas, K.; Salvador-Pascual, A.; Rodriguez-Mañas, L.; Viña, J. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2016, 17, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, M.; Zeng, H.; Pan, L. Multi-component exercise training improves the physical and cognitive function of the elderly with mild cognitive impairment: A six-month randomized controlled trial. Ann. Palliat. Med. 2021, 10, 8919–8929. [Google Scholar] [CrossRef]
- Bae, S.; Harada, K.; Lee, S.; Harada, K.; Makino, K.; Chiba, I.; Park, H.; Shimada, H. The Effect of a Multicomponent Dual-Task Exercise on Cortical Thickness in Older Adults with Cognitive Decline: A Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1312. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Murukesu, R.R.; Singh, D.K.A.; Shahar, S.; Subramaniam, P. A Multi-Domain Intervention Protocol for the Potential Reversal of Cognitive Frailty: “WE-RISE” Randomized Controlled Trial. Front. Public Health 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Suzuki, T. Cognitive function affects trainability for physical performance in exercise intervention among older adults with mild cognitive impairment. Clin. Interv. Aging 2013, 8, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Makizako, H.; Shimada, H.; Yoshida, D.; Tsutsumimoto, K.; Uemura, K.; Suzuki, T. Does a multicomponent exercise program improve dual-task performance in amnestic mild cognitive impairment? A randomized controlled trial. Aging Clin. Exp. Res. 2012, 24, 640–646. [Google Scholar] [CrossRef]
- Doi, T.; Makizako, H.; Shimada, H.; Yoshida, D.; Tsutsumimoto, K.; Sawa, R.; Misu, S.; Suzuki, T. Effects of multicomponent exercise on spatial–temporal gait parameters among the elderly with amnestic mild cognitive impairment (aMCI): Preliminary results from a randomized controlled trial (RCT). Arch. Gerontol. Geriatr. 2013, 56, 104–108. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Lyu, X.; Lin, Q.-R.; Wang, Z.-W.; Tang, L.; Zhao, Y.; Lyu, Q.-Y. Effects of a multicomponent intervention to slow mild cognitive impairment progression: A randomized controlled trial. Int. J. Nurs. Stud. 2021, 125, 104110. [Google Scholar] [CrossRef] [PubMed]
- Siega, J.; Iucksch, D.D.; Israel, V.L. Multicomponent Aquatic Training (MAT) Program for People with Parkinson’s Disease: A Protocol for a Controlled Study. Int. J. Environ. Res. Public Health 2022, 19, 1727. [Google Scholar] [CrossRef] [PubMed]
- Siega, J.; Iucksch, D.D.; Da Silva, A.Z.; Zotz, T.G.G.; Israel, V.L. Parkinson’s disease and multicomponent aquatic exercise: Effects on motor aspects, functional mobility, muscle function and aquatic motor skills. J. Bodyw. Mov. Ther. 2021, 27, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Mahameed, I.; Weiss, I.; Rosengarten, D.; Balmor, G.R.; Heching, M.; Kramer, M.R. Effects of a 12-week combined aerobic and strength training program in ambulatory patients with amyotrophic lateral sclerosis: A randomized controlled trial. J. Neurol. 2021, 268, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Ferri, A.; Lanfranconi, F.; Corna, G.; Bonazzi, R.; Marchese, S.; Magnoni, A.; Tremolizzo, L. Tailored Exercise Training Counteracts Muscle Disuse and Attenuates Reductions in Physical Function in Individuals With Amyotrophic Lateral Sclerosis. Front. Physiol. 2019, 10, 1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Orozco, K.; Bonilla-Vargas, K.; Cruz, F.; Mancera, O.; Ruiz, M.; Alvarez, L.; Pardo, R.; Arboleda, H. Cognitive Assessment Test: Validation of a Short Cognitive Test for the Detection of Mild Cognitive Disorder. Int. J. Alzheimer’s Dis. 2018, 2018, 3280621. [Google Scholar] [CrossRef] [Green Version]
- Borges-Machado, F.; Silva, N.; Farinatti, P.; Poton, R.; Ribeiro, Ó.; Carvalho, J. Effectiveness of Multicomponent Exercise Interventions in Older Adults With Dementia: A Meta-Analysis. Gerontologist 2020, 61, e449–e462. [Google Scholar] [CrossRef]
- Venegas-Sanabria, L.C.; Cavero-Redondo, I.; Martínez-Vizcaino, V.; Cano-Gutierrez, C.A.; Álvarez-Bueno, C. Effect of multicomponent exercise in cognitive impairment: A systematic review and meta-analysis. BMC Geriatr. 2022, 22, 617. [Google Scholar] [CrossRef]
- Farhani, F.; Shahrbanian, S.; Auais, M.; Hekmatikar, A.H.A.; Suzuki, K. Effects of Aerobic Training on Brain Plasticity in Patients with Mild Cognitive Impairment: A Systematic Review of Randomized Controlled Trials. Brain Sci. 2022, 12, 732. [Google Scholar] [CrossRef]
- Girotra, P.; Behl, T.; Sehgal, A.; Singh, S.; Bungau, S. Investigation of the Molecular Role of Brain-Derived Neurotrophic Factor in Alzheimer’s Disease. J. Mol. Neurosci. 2021, 72, 173–186. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimnejad, M.; Azizi, P.; Alipour, V.; Zarrindast, M.-R.; Vaseghi, S. Complicated Role of Exercise in Modulating Memory: A Discussion of the Mechanisms Involved. Neurochem. Res. 2022, 47, 1477–1490. [Google Scholar] [CrossRef]
- Venegas-Sanabria, L.C.; Martínez-Vizcaino, V.; Cavero-Redondo, I.; Chavarro-Carvajal, D.A.; Cano-Gutierrez, C.A.; Álvarez-Bueno, C. Effect of physical activity on cognitive domains in dementia and mild cognitive impairment: Overview of systematic reviews and meta-analyses. Aging Ment. Health 2020, 25, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T. Physical Activity and Cognitive Function in Mild Cognitive Impairment. ASN Neuro 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Biduski, G.M.; Bertoli, J.; Sousa, M.V.; Diefenthaeler, F.; Freitas, C.D.L.R. Effects of 6-weeks of detraining on functional capacity and rapid torque production in older women. J. Bodyw. Mov. Ther. 2021, 29, 167–173. [Google Scholar] [CrossRef]
- Ilardi, C.R.; Chieffi, S.; Iachini, T.; Iavarone, A. Neuropsychology of posteromedial parietal cortex and conversion factors from Mild Cognitive Impairment to Alzheimer’s disease: Systematic search and state-of-the-art review. Aging Clin. Exp. Res. 2021, 34, 289–307. [Google Scholar] [CrossRef]
| Key-Terms | Concepts |
|---|---|
| Cognition | Involves the entire process through which a person becomes aware of their situation, requirements, goals, and required activities, and then applies this knowledge to problem-solving tactics in everyday life [38] |
| Cognitive abilities | The concept of skill can be broadly defined as a method of action and generalized strategy for coping with situations and difficulties. These can be of various types, and the conceptual field to solve the problem is not uniform. Cognitive talents are the abilities that enable an individual to be competent and engage symbolically with his environment [39]. |
| Neurocognitive disorders | It is characterized as a group of conditions frequently leading to impaired brain functioning due to a medical disease other than a psychiatric illness. It is often used synonymously (but incorrectly) with dementia [40]. |
| Older adults | According to the World Health Organization, the older are defined as people who are 60 years or older in developing nations and 65 years or older in industrialized countries [41]. |
| Physical Exercise | Comprises all the conscious, systematic, and planned practice of physical activity, performed with a specific objective (e.g., to improve health) and well delineates the time of practice, duration, intensity, and type. It can be performed with or without a prescription [42]. |
| MulticomponentExercise | It is a type of physical exercise intervention designed to improve muscle strength, balance, and walking retraining, respecting a set of exercises conducted usually in the same session [43]. |
| Long-term (exercise) interventions | Considering the recent scientific literature, we assume that long-term interventions (with physical exercise) have 24 weeks (6 months) or more since the definition is still not wholly clear [44]. |
| P | Older adults with neurodegenerative disorders |
| I | Group class, supervised, and long-term MEP. |
| C | No contact, attention control, sham (placebo) physical exercise, or alternative active management |
| O | Any validated neuropsychological cognition test was used to assess at least one cognition abilities outcome measured at baseline and follow-up. |
| S | Randomized controlled trials |
| Authors | Population | Characteristics of MET Intervention | Comparison | Outcomes | Main Results |
|---|---|---|---|---|---|
| Alzheimer’s disease | |||||
| de Souto Barreto et al. (2017) [47] France | (n = 91) | Twice a week, for 24 weeks | Social intervention | MMSE | No improvements in cognitive performance |
| Öhman et al. (2016) [49] Finland | (n = 210) | Twice a week for 1 year | Home-based health literacy intervention | Clock Design Test, Verbal Fluency, Clinical Dementia rating test, and MMSE | Modest effects on executive function |
| Parkinson’s disease | |||||
| Gobbi et al. (2021) [32] Brazil | (n = 107) | Twice a week, for 32 weeks | Functional Mobility Mental/Leisure | MMSE, Hoehn and Yahr Stage scale, UPDRS, Baecke Questionnaire. | A short delay in the decline of global cognitive function |
| Volpe et al. (2013) [51] Italy | (n = 24) | Once a week for 24 weeks. | Irish set dancing | MMSE and PQD—39. | Improvements in neuromotor tasks related to balance and motor disability |
| Mavrommatiet al. (2017) [63] United Kingdom | (n = 138) | Twice a week for 24 weeks | Control group(non-exercise) | UPDRS | No improvements in cognitive performance |
| Mild Cognitive impairment | |||||
| Makizako et al. (2012) [60] Japan | (n = 50) | Twice a week for 24 weeks | Control group (non-exercise) | MMSE | No significant difference in the MMSE score. |
| Suzuki et al. (2013) Japan [53] | (n = 100) | Twice a week for 24 weeks | Control group (non-exercise) | MMSE and Alzheimer’s Disease Assessment Scale | No improvements in cognitive performance |
| Doi et al. (2013) Japan [61] | (n = 50) | Twice a week for 24 weeks | Control group (non-exercise) | MMSE | No significant difference in the MMSE score. |
| Tarazona-Santabalbina et al. (2016) Spain [54] | (n = 100) | Five days per week for 24 weeks | Control group (non-exercise) | MMSE | Increase in the global cognition |
| Li et al. (2021) China [55] | (n = 84) | 5 times a week for 24 weeks | The Control group received regular educational health instruction | MMSE, MoCA, CM-PPT; Mini-Physical Performance Test | No improvements in cognitive performance |
| Bae et al. (2020) Japan [56] | (n = 280) | Once a week 40 weeks | Control group (non-exercise) | Gerontology-Functional Assessment Tool (NCGG-FAT) | No improvements in cognitive performance |
| Yang et al. (2021) China [62] | (n = 112) | Twice a week for 24 weeks | Control group (non-exercise) | MoCA | No improvements in global cognitive performance |
| Murukesu et al. (2020) Malaysia [58] | (n = 42) | Twice a week for 24 weeks | Control group (non-exercise) | MMSE | No improvements in global cognitive performance |
| Uemura et al. 2013 (Japan) [59] | MCI (n = 44) | Twice a week for 24 weeks | Pre and post-physical exercise program | MMSE, Trail Making Test Part B, | No improvements in global cognitive performance. |
| Authors | Population | Characteristics of MET Intervention | Comparison | Outcomes | Outcomes/Main Results |
|---|---|---|---|---|---|
| Ferri et al. 2019 [67] (Italy) | Amyotrophic lateral sclerosis (n = 11) | 12 weeks 3 times a week | The Usual Care control group | UPDRS, ALSFRS-R | Tailored moderate-intensity exercise is not detrimental for patients with ALS and can counteract muscle disuse. |
| Kalron et al. 2021 [66] (Israel) | Amyotrophic lateral sclerosis (n = 32) | Twice a week for 12 weeks | Flexibility training group | ALSFRS-R | The ALSFRS-R is in favour of the aerobic-strength group. Exercise is far superior to flexibility and well-being in ambulatory ALS patients. |
| Siega et al. 2021 [65] (Brazil) | Parkinson disease (n = 22) | MET aquatic twice a week for 12 weeks | Control group (non-exercise) | UPDRS | Exercise program was capable increasing UPDRS scores |
| Siega et al. 2022 [64] (Brazil) | Adults with Parkinson | Multicomponent aquatic training 12 weeks, twice a week | Control group (non-exercise) | Parkinson Disease Questionnaire | Exercise program provides positive results in terms of controlling the progress of PD symptoms with the parameters assessed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldo-Silva, A.; Vieira-Pedrosa, A.; Simões, J.; Monteiro-Júnior, R.S.; Pimenta, N.; Sampaio, A.R.; Teques, P.; Amoroso, J.P.; Furtado, G.E. A Systematic Study into the Effects of Long-Term Multicomponent Training on the Cognitive Abilities of Older Adults with Neurodegenerative Disorders. Psych 2022, 4, 760-773. https://doi.org/10.3390/psych4040056
Caldo-Silva A, Vieira-Pedrosa A, Simões J, Monteiro-Júnior RS, Pimenta N, Sampaio AR, Teques P, Amoroso JP, Furtado GE. A Systematic Study into the Effects of Long-Term Multicomponent Training on the Cognitive Abilities of Older Adults with Neurodegenerative Disorders. Psych. 2022; 4(4):760-773. https://doi.org/10.3390/psych4040056
Chicago/Turabian StyleCaldo-Silva, Adriana, Ana Vieira-Pedrosa, Joel Simões, Renato Sobral Monteiro-Júnior, Nuno Pimenta, António Rodrigues Sampaio, Pedro Teques, José Pedro Amoroso, and Guilherme Eustáquio Furtado. 2022. "A Systematic Study into the Effects of Long-Term Multicomponent Training on the Cognitive Abilities of Older Adults with Neurodegenerative Disorders" Psych 4, no. 4: 760-773. https://doi.org/10.3390/psych4040056
APA StyleCaldo-Silva, A., Vieira-Pedrosa, A., Simões, J., Monteiro-Júnior, R. S., Pimenta, N., Sampaio, A. R., Teques, P., Amoroso, J. P., & Furtado, G. E. (2022). A Systematic Study into the Effects of Long-Term Multicomponent Training on the Cognitive Abilities of Older Adults with Neurodegenerative Disorders. Psych, 4(4), 760-773. https://doi.org/10.3390/psych4040056








