The Role of Ionic Liquids in Direct Synthesis of Formic Acid from CO2 Hydrogenation on Ru Complexes: A Theoretical Study
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
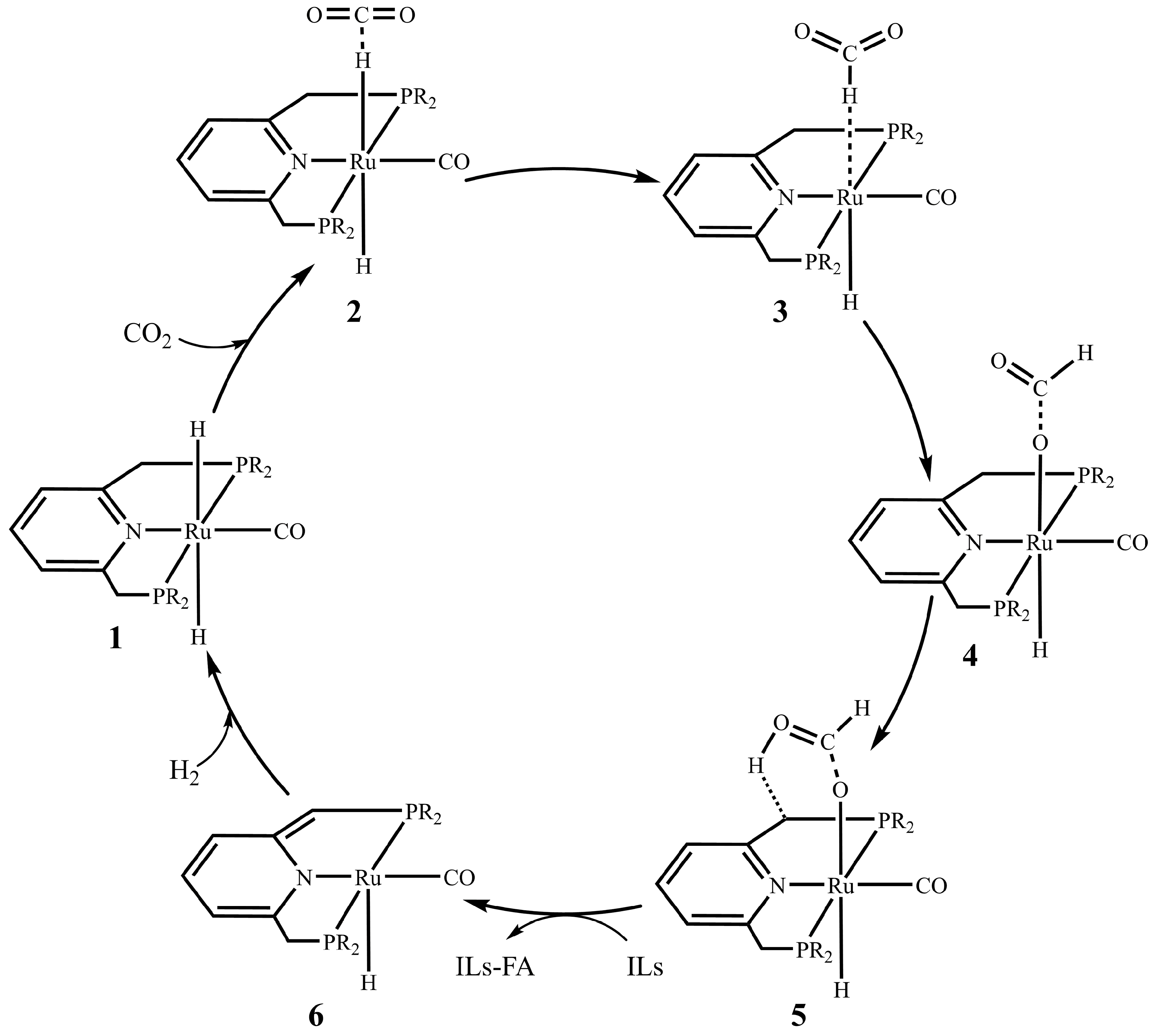
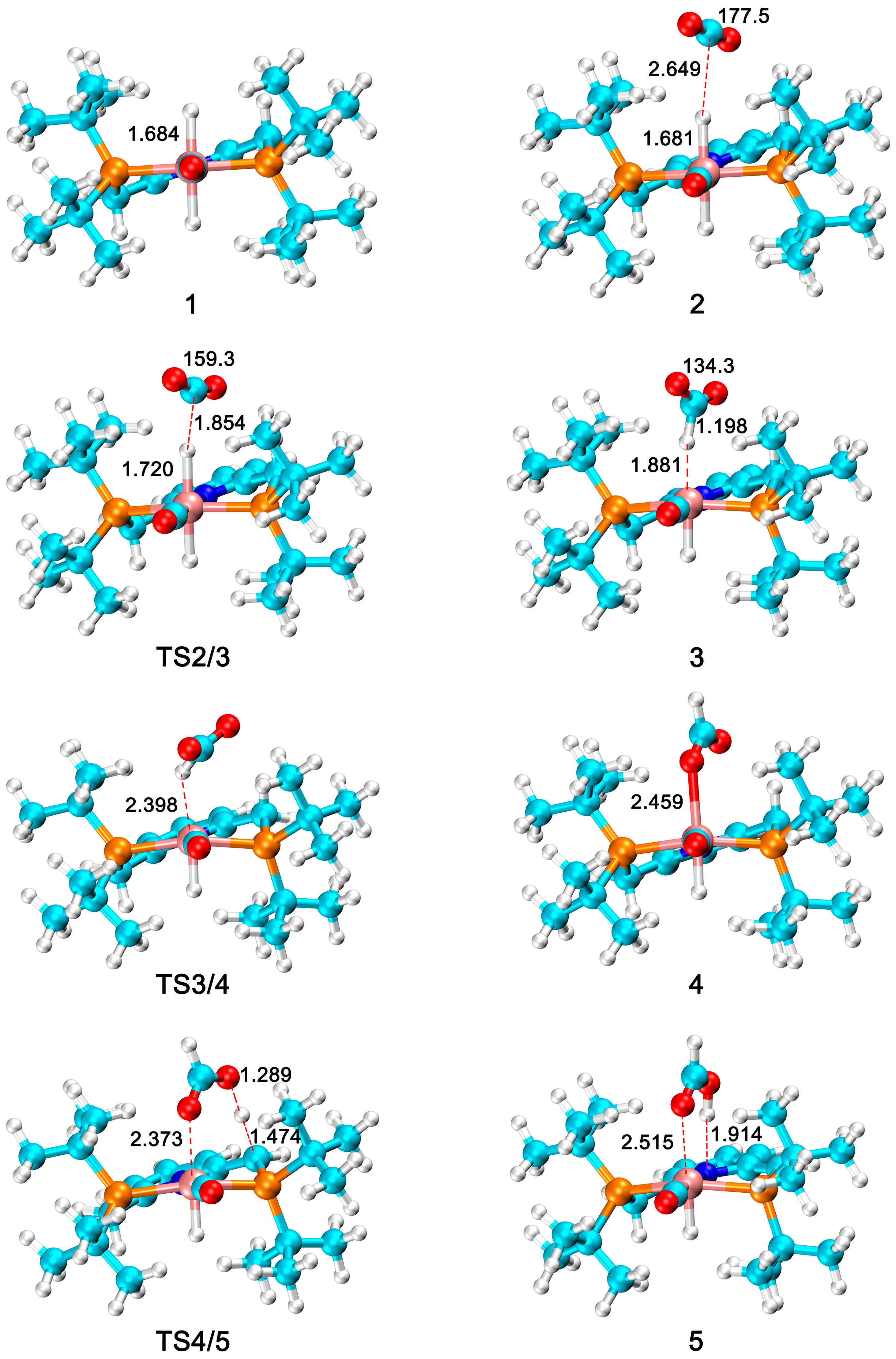
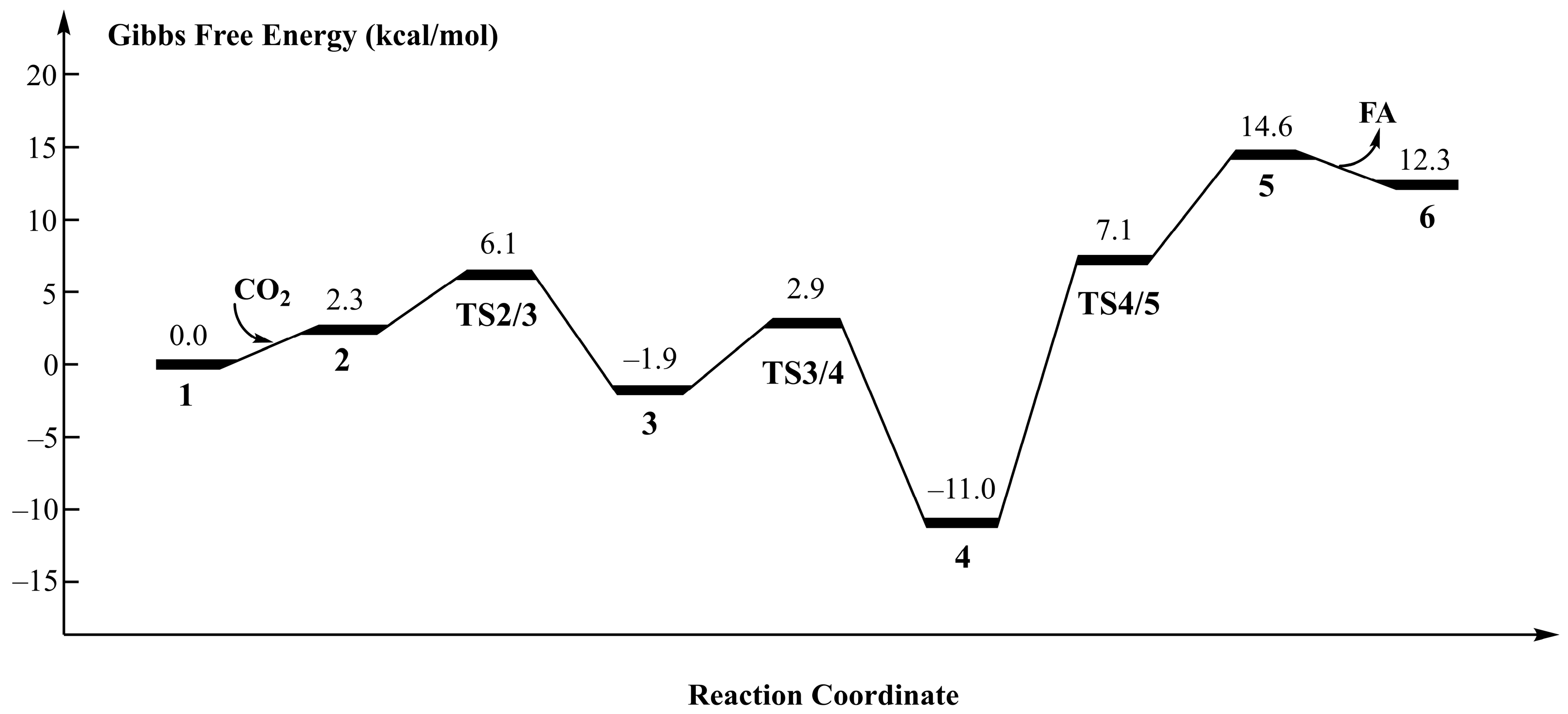
3.1. Current Mechanistic Assumptions
3.2. The Role of Ionic Liquids in CO2 Hydrogenation to Formic Acid
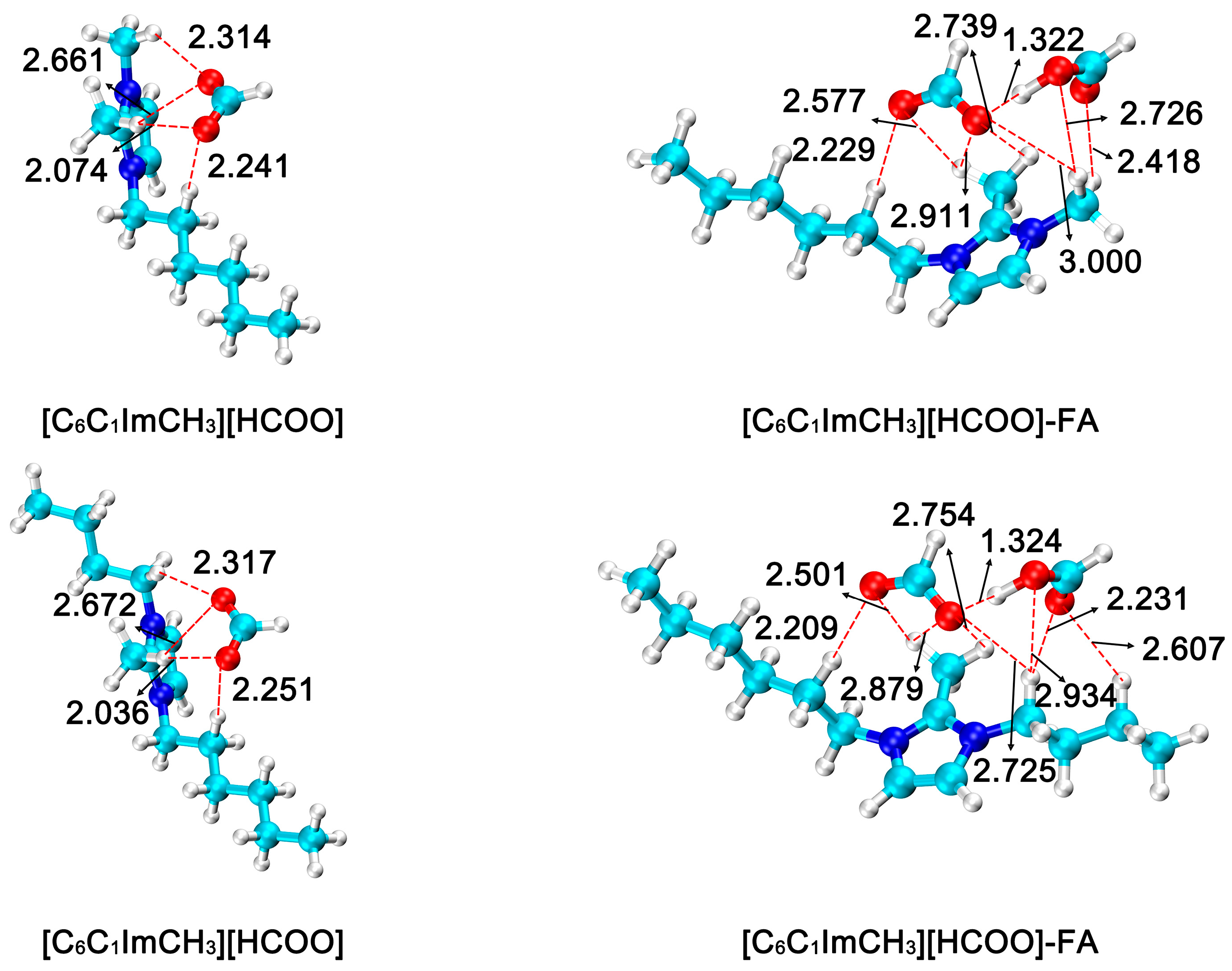
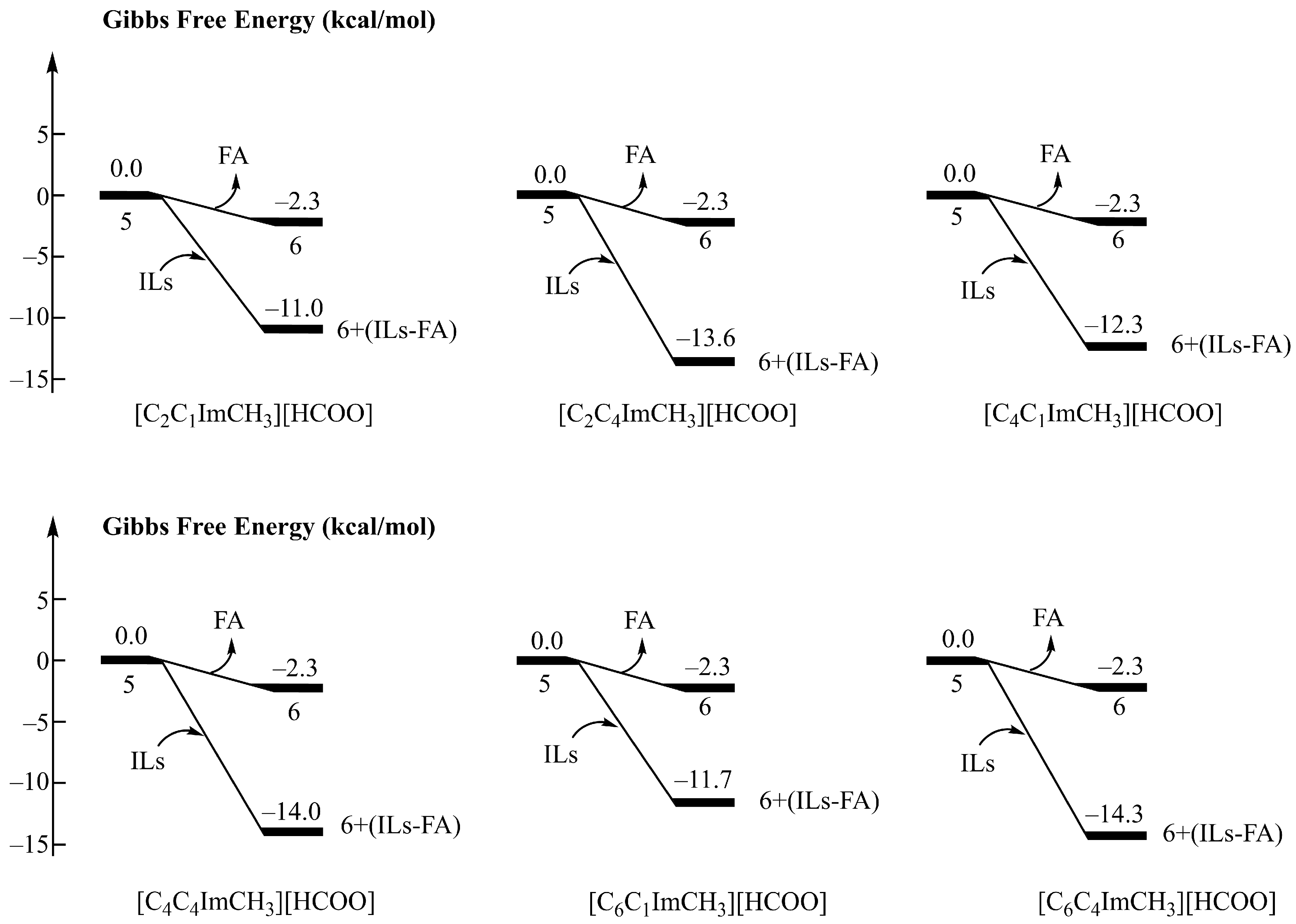
3.3. Effect of Cations in Ionic Liquids
3.4. Effect of Anions in Ionic Liquids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Wang, S.P.; Ma, X.B.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Wang, W.-H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 Hydrogenation to Formate and Methanol as an Alternative to Photo- and Electrochemical CO2 Reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Rana, J.; Sahoo, S.T.; Daw, P. Homogeneous first-row transition metal catalyst for sustainable hydrogen production and organic transformation from methanol, formic acid, and bio-alcohols. Tetrahedron 2021, 99, 132473. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dutta, I.; Lum, Y.W.; Lai, Z.P.; Huang, K.-W. Enabling storage and utilization of low-carbon electricity: Power to formic acid. Energy Environ. Sci. 2021, 14, 1194–1246. [Google Scholar] [CrossRef]
- Enthaler, S.; von Langermann, J.; Schmidt, T. Carbon dioxide and formic acid—The couple for environmental-friendly hydrogen storage? Energy Environ. Sci. 2010, 3, 1207–1217. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.-W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef]
- Bhardwai, R.; Kumar, A.; Choudhury, J. An all-aqueous and phosphine-free integrated amine-assisted CO2 capture and catalytic conversion to formic acid. Chem. Commun. 2022, 58, 11531–11534. [Google Scholar] [CrossRef]
- Guntermann, N.; Franciò, G.; Leitner, W. Hydrogenation of CO2 to formic acid in biphasic systems using aqueous solutions of amino acids as the product phase. Green Chem. 2022, 24, 8069–8075. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, W.; Zhao, Y.F.; Tang, M.H.; Chang, X.Q.; Xu, Y.T.; Li, R.X.; Liu, Z.M. Cellulose-Phytic Acid Composite Complexed Ru Catalyst for CO2 Hydrogenation to Free Formic Acid. ChemCatChem 2023, 15, e202201168. [Google Scholar] [CrossRef]
- Chang, J.R.; Mao, J.-X.; Ding, M.; Zhang, J.; Chen, X.N. Evaluating the Catalytic Activities of PNCNP Pincer Group 10 Metal Hydride Complexes: Pd-Catalyzed Reduction of CO2 to the Formic Acid Level with NH3·BH3 and NaBH4 under Ambient Conditions. Inorg. Chem. 2023, 62, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Izumida, H.; Sasaki, Y.; Hashimoto, H. Catalytic fixation of carbon dioxide to formic acid by transition–metal complexes under mild conditions. Chem. Lett. 1976, 5, 863–864. [Google Scholar] [CrossRef]
- Jessop, P.G.; Ikarlya, T.; Noyori, R. Homogeneous catalytic hydrogenation of supercritical carbon dioxide. Nature 1994, 368, 231–233. [Google Scholar] [CrossRef]
- Tai, C.-C.; Pitts, J.; Linehan, J.C.; Main, A.D.; Munshi, P.; Jessop, P.G. In Situ Formation of Ruthenium Catalysts for the Homogeneous Hydrogenation of Carbon Dioxide. Inorg. Chem. 2002, 41, 1606–1614. [Google Scholar] [CrossRef]
- Tai, C.-C.; Chang, T.; Roller, B.; Jessop, P.G. High-Pressure Combinatorial Screening of Homogeneous Catalysts: Hydrogenation of Carbon Dioxide. Inorg. Chem. 2003, 42, 7340–7341. [Google Scholar] [CrossRef]
- Bello, T.O.; Bresciani, A.E.; Nascimento, C.A.O.; Alves, R.M.B. Thermodynamic analysis of carbon dioxide hydrogenation to formic acid and methanol. Chem. Eng. Sci. 2021, 242, 116731. [Google Scholar] [CrossRef]
- Huff, C.A.; Sanford, M.S. Catalytic CO2 Hydrogenation to Formate by a Ruthenium Pincer Complex. ACS Catal. 2013, 3, 2412–2416. [Google Scholar] [CrossRef]
- Guan, C.; Pan, Y.P.; Ang, E.P.L.; Hu, J.S.; Yao, C.G.; Huang, M.-H.; Li, H.F.; Lai, Z.P.; Huang, K.-W. Conversion of CO2 from air into formate using amines and phosphorus-nitrogen PN3P-Ru(II) pincer complexes. Green Chem. 2018, 20, 4201–4205. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamashita, M.; Nozaki, K. Catalytic Hydrogenation of Carbon Dioxide Using Ir(III)–Pincer Complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. [Google Scholar] [CrossRef]
- Filonenko, G.A.; van Putten, R.; Schulpen, E.N.; Hensen, E.J.M.; Pidko, E.A. Highly Efficient Reversible Hydrogenation of Carbon Dioxide to Formates Using a Ruthenium PNP–Pincer Catalyst. ChemCatChem 2014, 6, 1526–1530. [Google Scholar] [CrossRef]
- Federsel, C.; Boddien, A.; Jackstell, R.; Jennerjahn, R.; Dyson, P.J.; Scopelliti, R.; Laurenczy, G.; Beller, M. A Well-Defined Iron Catalyst for the Reduction of Bicarbonates and Carbon Dioxide to Formates, Alkyl Formates, and Formamides. Angew. Chem. Int. Ed. 2010, 49, 9777–9780. [Google Scholar] [CrossRef]
- Shao, X.Z.; Yang, X.F.; Xu, J.M.; Liu, S.; Miao, S.; Liu, X.Y.; Su, X.; Duan, H.M.; Huang, Y.Q.; Zhang, T. Iridium Single-Atom Catalyst Performing a Quasi-homogeneous Hydrogenation Transformation of CO2 to Formate. Chem 2019, 5, 693–705. [Google Scholar] [CrossRef]
- Zhu, F.X.; Zhu-Ge, L.; Yang, G.F.; Zhou, S.L. Iron–Catalyzed Hydrogenation of Bicarbonates and Carbon Dioxide to Formates. ChemSusChem 2015, 8, 609–612. [Google Scholar] [CrossRef]
- Kothandaraman, J.; Czaun, M.; Goeppert, A.; Haiges, R.; Jones, J.-P.; May, R.B.; Prakash, G.K.S.; Olah, G.A. Amine-Free Reversible Hydrogen Storage in Formate Salts Catalyzed by Ruthenium Pincer Complex without pH Control or Solvent Change. ChemSusChem 2015, 8, 1442–1451. [Google Scholar] [CrossRef]
- Yasaka, Y.; Wakai, C.; Matubayasi, N.; Nakahara, M. Controlling the Equilibrium of Formic Acid with Hydrogen and Carbon Dioxide Using Ionic Liquid. J. Phys. Chem. A 2010, 114, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Wesselbaum, S.; Hintermair, U.; Leitner, W. Continuous-Flow Hydrogenation of Carbon Dioxide to Pure Formic Acid using an Integrated scCO2 Process with Immobilized Catalyst and Base. Angew. Chem. Int. Ed. 2012, 51, 8585–8588. [Google Scholar] [CrossRef] [PubMed]
- Jens, C.M.; Scott, M.; Liebergesell, B.; Westhues, C.G.; Schäfer, P.; Franciò, G.; Leonhard, K.; Leitner, W.; Bardow, A. Rh-Catalyzed Hydrogenation of CO2 to Formic Acid in DMSO-based Reaction Media: Solved and Unsolved Challenges for Process Development. Adv. Synth. Catal. 2019, 361, 307–316. [Google Scholar] [CrossRef]
- Moret, S.; Dyson, P.J.; Laurenczy, G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat. Commun. 2014, 5, 4017. [Google Scholar] [CrossRef]
- Rohmann, K.; Kothe, J.; Haenel, M.W.; Englert, U.; Hölscher, M.; Leitner, W. Hydrogenation of CO2 to Formic Acid with a Highly Active Ruthenium Acriphos Complex in DMSO and DMSO/Water. Angew. Chem. Int. Ed. 2016, 55, 8966–8969. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Xie, Y.; Li, W.J.; Hu, S.Q.; Song, J.L.; Jiang, T.; Han, B.X. Hydrogenation of Carbon Dioxide is Promoted by a Task-Specific Ionic Liquid. Angew. Chem. Int. Ed. 2008, 47, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Weilhard, A.; Qadir, M.I.; Sans, V.; Dupont, J. Selective CO2 Hydrogenation to Formic Acid with Multifunctional Ionic Liquids. ACS Catal. 2018, 8, 1628–1634. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhao, Y.F.; Wang, H.; Yu, B.; Yu, X.X.; Zhang, H.Y.; Liu, Z.M. 110th Anniversary: Ionic Liquid Promoted CO2 Hydrogenation to Free Formic Acid over Pd/C. Ind. Eng. Chem. Res. 2019, 58, 6333–6339. [Google Scholar] [CrossRef]
- Webber, R.; Qadir, M.I.; Sola, E.; Martín, M.; Suárez, E.; Dupont, J. Fast CO2 hydrogenation to formic acid catalyzed by an Ir(PSiP) pincer hydride in a DMSO/water/ionic liquid solvent system. Catal. Commun. 2020, 146, 106125. [Google Scholar] [CrossRef]
- Piccirilli, L.; Rabell, B.; Padilla, R.; Riisager, A.; Das, S.; Nielsen, M. Versatile CO2 Hydrogenation–Dehydrogenation Catalysis with a Ru–PNP/Ionic Liquid System. J. Am. Chem. Soc. 2023, 145, 5655–5663. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Hu, J.L.; Jia, Y.X.; Yao, C.F.; Hu, X.B. Highly Efficient Catalyzing and Separation System for CO2 Hydrogenation to Formic Acid in Formate-Based Ionic Liquids. Ind. Eng. Chem. Res. 2025, 64, 9608–9616. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sust. Energ. Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Weilhard, A.; Argent, S.P.; Sans, V. Efficient carbon dioxide hydrogenation to formic acid with buffering ionic liquids. Nat. Commun. 2021, 12, 231. [Google Scholar] [CrossRef]
- Ma, W.T.; Hu, J.L.; Zhou, L.; Wu, Y.T.; Geng, J.; Hu, X.B. Efficient hydrogenation of CO2 to formic acid in water without consumption of a base. Green Chem. 2022, 24, 6727–6732. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Hensen, E.J.M.; Pidko, E.A. Mechanism of CO2 hydrogenation to formates by homogeneous Ru–PNP pincer catalyst: From a theoretical description to performance optimization. Catal. Sci. Technol. 2014, 4, 3474–3485. [Google Scholar] [CrossRef]
- Rawat, K.S.; Pathak, B. Aliphatic Mn–PNP complexes for the CO2 hydrogenation reaction: A base free mechanism. Catal. Sci. Technol. 2017, 7, 3234–3242. [Google Scholar] [CrossRef]
- Ramos, V.M.; de Oliveira–Filho, A.G.S.; de Lima Batista, A.P. Homogeneous Catalytic CO2 Hydrogenation by [Fe]–Hydrogenase Bioinspired Complexes: A Computational Study. J. Phys. Chem. A 2022, 126, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Filonenko, G.A.; Conley, M.P.; Copéret, C.; Luta, M.; Hensen, E.J.M.; Pidko, E.A. The impact of Metal–Ligand Cooperation in Hydrogenation of Carbon Dioxide Catalyzed by Ruthenium PNP Pincer. ACS Catal. 2013, 3, 2522–2526. [Google Scholar] [CrossRef]
- Taran, O.P.; Miroshnikova, A.V.; Baryshnikov, S.V.; Kazachenko, A.S.; Skripnikov, A.M.; Sychev, V.V.; Malyar, Y.N.; Kuznetsov, B.N. Reductive Catalytic Fractionation of Spruce Wood over Ru/C Bifunctional Catalyst in the Medium of Ethanol and Molecular Hydrogen. Catalysts 2022, 12, 1384. [Google Scholar] [CrossRef]
- Meng, L.Q.; Yao, L.H.; Li, J. Theoretical Study of Reversible Hydrogenation of CO2 to Formate Catalyzed by Ru(II)–PN5P, Fe(II)–PN5P, and Mn(I)–PN5P Complexes: The Effect of the Transition Metal Center. Catalysts 2024, 14, 440. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Becke, A.D. Density–functional exchange–energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision. D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Martin, J.M.L.; Sundermann, A. Correlation consistent valence basis sets for use with the Stuttgart–Dresden–Bonn relativistic effective core potentials: The atoms Ga–Kr and In–Xe. J. Chem. Phys. 2001, 114, 3408–3420. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical hybrid density functional with perturbative second–order correlation. J. Chem. Phys. 2006, 124, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Meng, L.Q.; Gong, P.C.; Li, J.; Zhou, Y. Theoretical Study of the Reversible Hydrogenation of Carbon Dioxide to Formate on Mn–Pincer Complexes. ChemPhysChem 2025, 26, e202400906. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, J.; Yang, Z. Substituent’s Effects of PNP Ligands in Ru(II)-Catalyzed CO2 Hydrogenation to Formate: Theoretical Analysis Considering Steric Hindrance and Promotion of Hydrogen Bonding. Catalysts 2022, 12, 760. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Li, J.; Yoshizawa, K. Catalytic Hydrogenation of Carbon Dioxide with a Highly Active Hydride on Ir(III)–Pincer Complex: Mechanism for CO2 Insertion and Nature of Metal–Hydride Bond. Bull. Chem. Soc. Jpn. 2011, 84, 1039–1048. [Google Scholar] [CrossRef]





| Binding Free Energy (kcal/mol) | Imidazolium Formate | Imidazolium Acetate | Imidazolium Bicarbonate |
|---|---|---|---|
| R1 = Et, R2 = CH3 | −11.1 | −12.8 | −10.3 |
| R1 = Et, R2 = n-Bu | −10.9 | −11.5 | −10.4 |
| R1 = n-Bu, R2 = CH3 | −10.5 | −12.2 | −10.4 |
| R1 = n-Bu, R2 = n-Bu | −11.5 | −12.3 | −10.4 |
| R1 = n-He, R2 = CH3 | −10.8 | −12.3 | −9.7 |
| R1 = n-He, R2 = n-Bu | −11.3 | −13.2 | −10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Li, J. The Role of Ionic Liquids in Direct Synthesis of Formic Acid from CO2 Hydrogenation on Ru Complexes: A Theoretical Study. Chemistry 2025, 7, 182. https://doi.org/10.3390/chemistry7060182
Gong P, Li J. The Role of Ionic Liquids in Direct Synthesis of Formic Acid from CO2 Hydrogenation on Ru Complexes: A Theoretical Study. Chemistry. 2025; 7(6):182. https://doi.org/10.3390/chemistry7060182
Chicago/Turabian StyleGong, Pengcheng, and Jun Li. 2025. "The Role of Ionic Liquids in Direct Synthesis of Formic Acid from CO2 Hydrogenation on Ru Complexes: A Theoretical Study" Chemistry 7, no. 6: 182. https://doi.org/10.3390/chemistry7060182
APA StyleGong, P., & Li, J. (2025). The Role of Ionic Liquids in Direct Synthesis of Formic Acid from CO2 Hydrogenation on Ru Complexes: A Theoretical Study. Chemistry, 7(6), 182. https://doi.org/10.3390/chemistry7060182






