Abstract
Due to high thermodynamic stability, the direct generation of formic acid by CO2 hydrogenation is not easy to achieve experimentally. However, when Nakahara and coworkers studied the equilibrium of formic acid reversibly decomposing into CO2 and H2, they found that using imidazolium formate ionic liquid as an additive could shift the reaction equilibrium to the formic acid side. Subsequently, imidazolium acetate ionic liquid and imidazolium bicarbonate ionic liquid have also been experimentally proven to be able to be used for CO2 hydrogenation to directly produce formic acid. In order to investigate the mechanism of action of ionic liquids in the process of CO2 catalyzed hydrogenation to formic acid, we performed DFT calculations. The results showed that, after the hydrogenation of CO2 to formic acid, the ionic liquids and formic acid molecules form adducts through hydrogen bonding, and then stabilize the product formic acid. The further use of methyl to replace H at the position of the cation R3 of the ionic liquids can improve the ability of the ionic liquids to stabilize formic acid, which also supports the experimental work of Nakahara and coworkers. In addition, among the three ionic liquids, the imidazolium acetate ionic liquid had the best stabilizing effect on formic acid, and the second best is the imidazolium formate ionic liquid, while the imidazolium bicarbonate ionic liquid has a relatively weak stabilizing ability.
1. Introduction
As a major greenhouse gas, excessive CO2 emissions significantly exacerbate global climate change [1]. However, CO2 is also a rich carbon raw material that can be catalytically hydrogenated to produce high-value chemicals, including formic acid (FA), methanol, etc. [2,3,4]. This process not only reduces the concentration of greenhouse gases in the atmosphere, but the FA produced also has excellent hydrogen storage density and outstanding safety performance [5,6], and is considered one of the most promising hydrogen storage materials [7,8]. Given these advantages, in recent years CO2 catalytic hydrogenation to produce FA has attracted extensive attention in the academic community [9,10,11,12].
In fact, since Inoue et al. first reported the catalytic hydrogenation of CO2 to formate by transition metal complexes [13], the catalytic applications of transition metal complexes in the field of CO2 hydrogenation conversion have been widely studied [14,15,16]. Unfortunately, due to the thermodynamic unfavorable of CO2 hydrogenation to FA (ΔG° = +32.9 kJ/mol) [17], the catalytic performance still has certain limitations. To this end, the researchers shift the reaction equilibrium by adding alkali to form formate in the reaction [18,19]. In 2009, Nozaki et al. used an Ir–PNP (PNP = 2,6-(di-iso-propylphosphinomethyl)-pyridine) complex catalyst to achieve efficient hydrogenation of CO2 to formate with KOH as an alkaline additive, and a turnover number (TON) of 3,500,000 and a turnover frequency (TOF) of 120,000 h−1 was achieved at 200 °C and 5.0 MPa [20]. In 2014, Pidko et al. successfully synthesized a Ru–PNP complex catalyst by replacing the metal center with Ru [21]. The catalyst exhibited excellent CO2 hydrogenation catalytic activity at 120 °C and 4.0 MPa under the reaction condition of DBU as an alkaline additive, and obtained a TOF of 1,100,000 h−1. Other alkaline additives, such as triethylamine (TEA) [22,23], NaOH [24,25], etc., have also been shown to be effective in promoting the hydrogenation of CO2 to formate. Although the addition of alkali can significantly promote the hydrogenation efficiency of CO2, the main product is formate rather than FA. Consequently, an acidification step is required to obtain the target product FA, which consumes substantial energy during separation and purification [26]. Moreover, this process generates an equivalent amount of organic or inorganic salts waste as waste products.
The following studies have explored the direct production of FA by hydrogenation of CO2 without alkaline additives [27,28]. In 2014, Laurenczy et al. reported a [RuCl2(PTA)4] (PTA = 1,3,5-triaza-7-phosphaadamantane) complex catalyst that used the solvation between FA and DMSO/Water system to achieve CO2 hydrogenation to directly generate FA at 60 °C and 100 bar, and obtained a TON of 749 [29]. Subsequently, Leitner et al. synthesized a highly active [Ru(Acriphos)(PPh3)(Cl)(PhCO2)][Acriphos = 4,5-bis(diphenylphosphino)acridine] catalyst in 2016 [30]. The direct hydrogenation of CO2 into FA was realized in DMSO/Water medium, and the TON of 16,310 and the TOF of 1019 h−1 were obtained at 60 °C and 120 bar. They further demonstrated through DFT calculations that the medium was thermodynamically stable to FA products. Although the above study successfully realized the direct conversion of CO2 hydrogenation to FA without alkaline additives, the TON and TOF values obtained were still significantly lower than those of the alkaline system. Therefore, it is of great significance to develop an additive that can not only target FA as the target product, but also achieve a high TON value for the optimization of CO2 hydrogenation reaction system.
Ionic liquids (ILs) have been widely used in the field of CO2 hydrogenation conversion [31,32,33,34,35,36] due to their excellent properties such as extremely low vapor pressure and good thermal stability [37,38]. In 2010, Nakahara studied the reversible decomposition equilibrium of FA using 1,3-dipropyl-2-methylimidazolium formate ionic liquid, and found that ILs solvents could stabilize FA molecules so that the equilibrium was more inclined to the FA side [26]. In 2021, Sans et al. used 1-butyl-2,3-dimethylimidazolium acetate as an acid buffer and synthesized Ru–CNC as a catalyst to achieve CO2 hydrogenation to directly generate FA [39]. At 120 °C and 60 bar, a TON of 833,800 and a TOF of 20,600 h−1 were obtained, which were the highest catalytic activities reported so far in the alkali-free homogeneous catalytic system. In 2022, Hu et al. reported a water–ionic liquids catalytic system for the direct generation of FA by CO2 hydrogenation [40]. Using the synergistic effect of Ir–PNP catalyst and imidazolium bicarbonate ionic liquid, the system achieved a TON of 364,249 and 86.8% FA yield at 120 °C and 7 MPa, and showed excellent catalytic efficiency in aqueous solution.
At present, the reaction mechanism of CO2 hydrogenation to FA has been thoroughly studied [41,42,43]. The general assumption is that first the CO2 is co-ordinated with the Ru–H bond on the Ru catalyst, then the hydride is transferred directly to the CO2 to form the formate group, and then the formate is flipped to form a more stable M–OCOH configuration bound to the metal center with the O terminus (this configuration can be detected experimentally [12,44]), and the formate robs a proton on the ligand arm to form FA, as shown in Scheme 1. Due to the highly stable structure of M–OCOH, if FA is not separated from the reaction system in time, the final product tends to be a thermodynamically more stable formate rather than FA. Therefore, this paper considers the formation of a stable ionic liquid–formic acid adduct by using imidazolium formate ionic liquid to stabilize FA after FA formation. Furthermore, there are three substitution sites on the imidazole ring, named R1, R2, and R3, respectively. The use of a Ru center was informed by its recognized high activity in CO2 hydrogenation, its widespread application in diverse catalytic systems [45], and, crucially, by our group’s previous findings that confirmed its superior activity for this class of reactions [46].
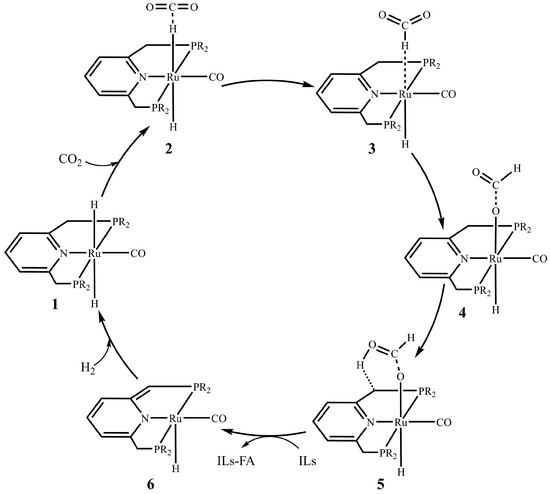
Scheme 1.
Proposed mechanism for CO2 hydrogenation to formic acid (R = tBu).
In addition, based on the fact that ILs can be functionalized by manipulating the pairing of cations and anions [47], we first investigated the effect of substituents on cations on the FA stabilization process. Two other ionic liquids that have been experimentally proven to stabilize FA, imidazolium acetate ionic liquid and imidazolium bicarbonate ionic liquid, were then considered. To elucidate the interactions between the ionic liquid and the formic acid molecule and to determine the respective roles of the cation and anion on the stabilization order, we compared the binding free energies of the ionic liquids with formic acid, as presented in Scheme 2.
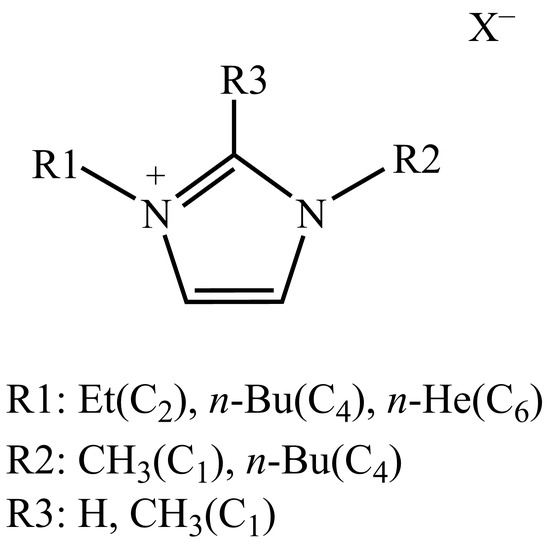
Scheme 2.
Imidazolium formate ionic liquid, imidazolium acetate ionic liquid, and imidazolium bicarbonate ionic liquid considered in this work (X− = HCOO−, OAc−, HCO3−).
2. Computational Methods
In this study, the geometries of all species were optimized using the B3LYP(D3–BJ) hybrid functional [48,49] in the Gaussian 09 program [50]. For Ru, the Stuttgart–Dresden pseudopotential basis set (SDD) was employed, supplemented by two sets of f functions and a set of g functions [51]. For the H, C, N, O, and P main group elements, the Dunning cc–pVDZ base set was applied [52]. This level of theory has been validated in previous studies [46,53,54]. Zero–point energy (ZPE) corrections were also performed at the same level of theory. Frequency calculations were performed to determine the minimum or transitional state at each stationary point and to obtain the thermochemical properties of all species. The Gibbs free energy was calculated under standard conditions (298.15 K, 1 atm). All transition states were verified by intrinsic reaction coordinates (IRC) calculations. The Polarized Continuum (PCM) model was used to consider the solvent effects of the aqueous solution environment [55,56]. All of the structural diagrams in this article were drawn using VMD 1.9.2 software [57].
The binding free energy is calculated using the following formula:
ΔG = G(Ru–PNP–CO2) − G(Ru–PNP) − G(CO2)
In the formula, G(Ru–PNP) represents the Gibbs free energy of the Ru–PNP complex, G(CO2) is the Gibbs free energy of the CO2 molecule, and G(Ru–PNP–CO2) is the Gibbs free energy of the adduct formed after CO2 coordinates to the Ru–PNP complex.
3. Results and Discussion
In this study, imidazolium formate ionic liquid, imidazolium acetate ionic liquid, and imidazolium bicarbonate ionic liquid were used as additives, and their mechanism of action in the preparation of FA by CO2 catalyzed hydrogenation was explored through theoretical calculations. All calculations in this paper are performed in aqueous solution.
3.1. Current Mechanistic Assumptions
Based on DFT calculations, we have studied the reaction mechanism of Ru–PNP complexes catalyzing CO2 hydrogenation to FA. Figure 1 shows the optimized geometries for all intermediates and transition states in this catalytic cycle. The Gibbs free energy profile for the above process is shown in Figure 2.
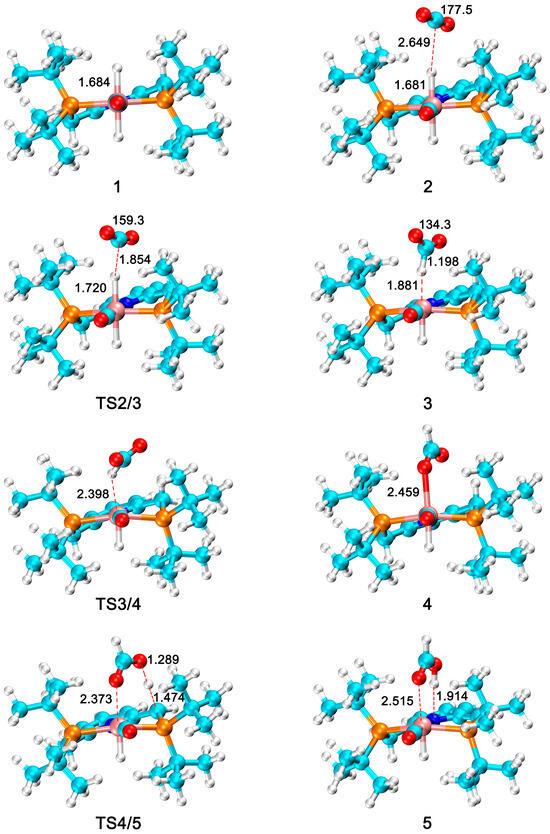
Figure 1.
The optimized geometries of all species in CO2 hydrogenation to formic acid catalyzed by Ru–PNP complexes. The bond distances are in angstrom (Å), and the bond angles are in degrees (◦). (Ru: pink; H: white; C: cyan; N: blue; O: red; P: orange).
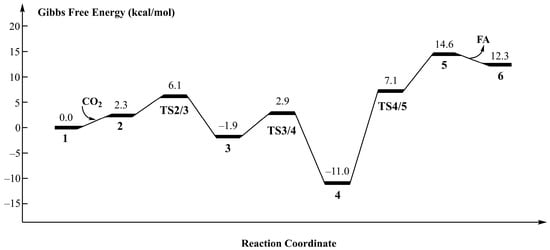
Figure 2.
Free energy profile for CO2 hydrogenation to formic acid catalyzed by Ru–PNP complexes.
The first step in the hydrogenation of CO2 to FA is the coordination of CO2, and CO2 interacts with the hydride of the M–H bond on complex 1 to form complex 2. At this time, the C atom of CO2 is 2.649 Å from the hydride. The O–C–O bond angle is 177.5°. The Gibbs free energy change for this process is 2.3 kcal/mol. Among them, the C–H bond distance is 1.209 Å, the distance between the hydride and the Ru metal center is 1.840 Å, and the O–C–O bond angle is 134.3°. The activation energy of this step is 4.2 kcal/mol with a Gibbs free energy barrier of 3.8 kcal/mol. The next step is to flip the formate group in complex 3 to produce a more stable formate complex 4. This step has an energy barrier of 3.0 kcal/mol and a relative energy reduction of 12.9 kcal/mol. From complexes 4 to 5 is the formation of FA, which is an energy absorption process of 25.6 kcal/mol and an activation energy of 18.1 kcal/mol. Finally, FA is removed from complex 5 and this step is an exergonic process of 2.3 kcal/mol.
Consistent with previous studies, complex 4 is the global minimum of the entire potential energy surface [53]. This indicates that the timely separation of FA in the reaction system is a critical step in obtaining the final product, FA. In the catalyst recovery stage, according to the theoretical calculations of the research group, water molecules as protons will accelerate this process [46,58]. At the same time, the influence of water bridge on the catalytic regeneration process was also considered in this study. The optimized geometries of all intermediates and transition states during Ru–PNP regeneration is shown in Figure S1 in the Supporting Information. Under the action of the water bridge, the activation energy barrier was reduced from 22.8 kcal/mol to 16.5 kcal/mol, as shown in Figure S2 in the Supporting Information.
3.2. The Role of Ionic Liquids in CO2 Hydrogenation to Formic Acid
Due to their unique physical and chemical properties, ionic liquids have shown significant advantages in catalyzing the hydrogenation of CO2 to FA. According to the substituents commonly used in the experiment, ethyl, n-butyl and n-hexyl groups were selected at the R1 position, and methyl and n-butyl groups were selected at the R2 position, and six different imidazolium formate ionic liquids [C2C1ImH][HCOO], [C2C4ImH][HCOO], [C4C1ImH][HCOO], [C4C4ImH][HCOO], [C6C1ImH][HCOO] and [C6C4ImH][HCOO], respectively. It is then applied to the reaction of CO2 hydrogenation to form FA, and the FA is stably formed through hydrogen bonding. Thereby facilitating the separation of formic acid from the reaction system and shifting the equilibrium for formic acid formation forward. The optimized geometries of all species in this process are shown in Figure 3. Among the six imidazolium formate acid ionic liquids, the distances between H at the R3 substitution position and the anionic formate group were 1.765 Å, 1.784 Å, 1.765 Å, 1.780 Å, 1.766 Å and 1.784 Å, respectively. In the ionic liquid–FA adduct formed, the distance increased to 1.933 Å, 1.962 Å, 1.939 Å, 1.963 Å, 1.939 Å, and 1.966 Å, respectively.
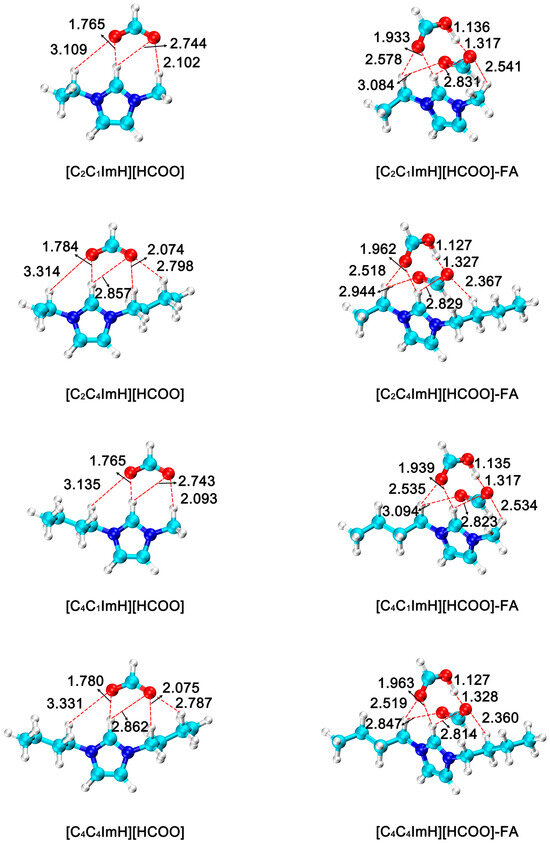
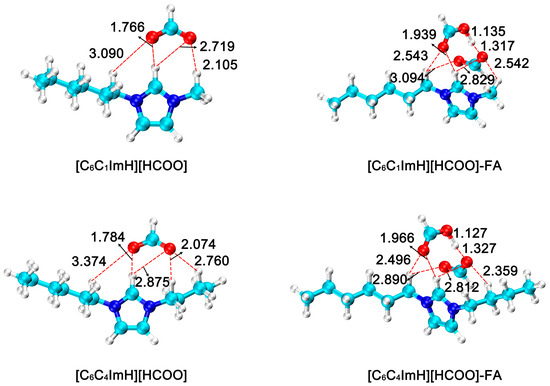
Figure 3.
Optimized geometries of imidazolium formate ionic liquid (R3 = H) and ionic liquid–formic acid adduct. The bond distances are in angstrom (Å). (H: white; C: cyan; N: blue; O: red).
Figure 4 shows the binding free energy of imidazolium formate ionic liquid to FA molecule, and the direct removal of FA from complex 5 is an exergonic process of 2.3 kcal/mol without the addition of ionic liquids. After the addition of ionic liquids, the binding free energies to FA molecules were −11.1 kcal/mol, −10.9 kcal/mol, −10.5 kcal/mol, −11.5 kcal/mol, −10.8 kcal/mol, and −11.3 kcal/mol, respectively. This indicates that ionic liquids can effectively stabilize FA by forming intermolecular hydrogen bonds with FA.
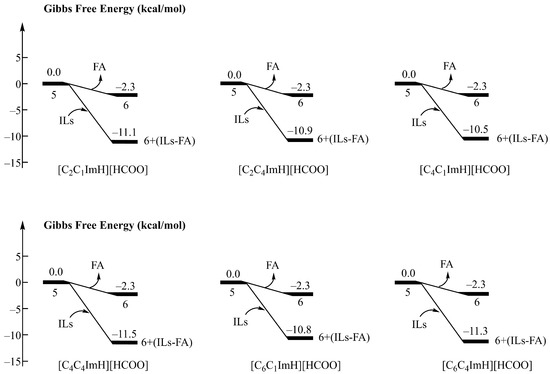
Figure 4.
Binding free energy of six imidazolium formate ionic liquids (R3 = H) to formic acid.
Indeed, the addition of ionic liquids can influence the system after the formic acid formation step. However, the introduction of the ionic liquid not only introduces potential interactions with the ruthenium-formate complex but also alters the reaction environment, for instance, by changing the dielectric constant. Quantifying the specific value of this change is challenging and was not measured in our experimental setup. Therefore, we have not accounted for the influence of the ionic liquid on the ruthenium–formate complex in our current discussion.
3.3. Effect of Cations in Ionic Liquids
Based on the stabilizing effect of imidazolium formate ionic liquid on FA, the effect of cationic R3 substituent structure on FA stabilization was investigated in this study. After replacing the substituent at the R3 position from H to methyl. Six different imidazolium formate ionic liquids [C2C1ImCH3][HCOO], [C2C4ImCH3][HCOO], [C4C1ImCH3][HCOO], [C4C4ImCH3][HCOO] and [C6C1ImCH3][HCOO] were obtained, and then the FA product was stabilized by forming intermolecular hydrogen bonds. The optimized geometries of all species in this process is shown in Figure 5. In the ionic liquid–formic acid adduct, the hydrogen bond lengths formed by anions and FA in the ionic liquids are 1.320 Å, 1.322 Å, 1.322 Å, 1.325 Å, 1.322 Å, and 1.324 Å, respectively.
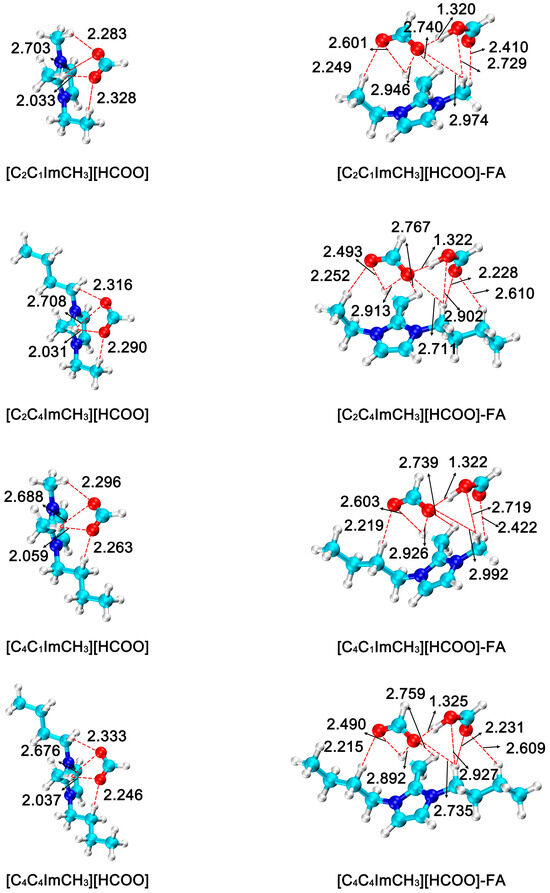
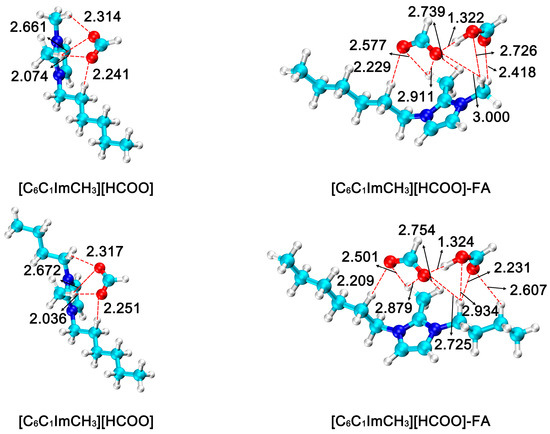
Figure 5.
Optimized geometries of imidazolium formate ionic liquid (R3 = methyl) and ionic liquid–formic acid adduct. The bond distances are in angstrom (Å). (H: white; C: cyan; N: blue; O: red).
As shown in Figure 6, the binding free energies of the ionic liquids to the FA molecule are −11.0 kcal/mol, −13.6 kcal/mol, −12.3 kcal/mol, −14.0 kcal/mol, −11.7 kcal/mol, and −14.3 kcal/mol, respectively. This suggests that the substitution of H at R3 with methyl improves the ability to stabilize FA. This is likely because replacing the hydrogen substituent at the R3 position with a methyl group leads to an increased number of hydrogen bonds formed between the ionic liquid and the formic acid molecule, and consequently enhances the capability to stabilize formic acid. This shows good agreement with the results of the experiment reported by Nakahara et al. in 2010 [26].
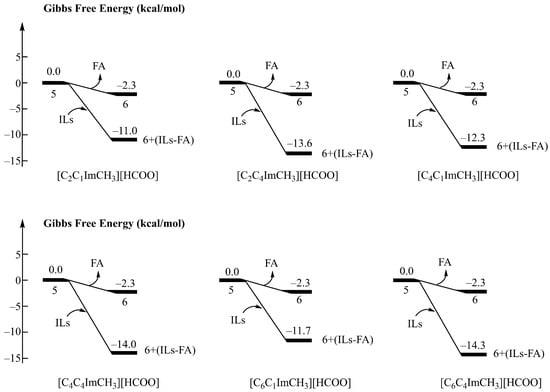
Figure 6.
Binding free energy of six imidazolium formate ionic liquids (R3 = methyl) to formic acid.
3.4. Effect of Anions in Ionic Liquids
For ionic liquids, the structure of the anion is equally important. Sans et al. obtained the highest catalytic activity observed to date for an alkali-free system using an imidazolium acetate ionic liquid [39]. Similarly, Hu et al. also facilitated the direct generation of FA from imidazolium bicarbonate ionic liquid [40]. Therefore, we investigated the effect of different anions on the molecular stabilization of FA. Three anions that have been experimentally proven to stabilize FA are used: formate anion, acetate anion, and bicarbonate anion The optimized geometries of all species in the process of FA stabilization by imidazolium formate ionic liquid is shown in Figure 3. The optimal geometries of all species in the process of FA stabilization by imidazolium acetate ionic liquid and imidazolium bicarbonate ionic liquid are shown in Figures S3 and S4 in the Supporting Information.
The binding free energies of different ionic liquids to FA are shown in Table 1. For example, when the group at the R1 position is ethyl and the group at the R2 position is methyl, the binding free energies of the three ionic liquids [C2C1ImH][HCOO], [C2C1ImH][OAc] and [C2C1ImH][HCO3] to FA molecules are −11.1 kcal/mol, −12.8 kcal/mol, −10.3 kcal/mol, respectively. The order of stability of FA in these three ionic liquids is shown as follows: imidazolium acetate ionic liquid > imidazolium formate ionic liquid > imidazolium bicarbonate ionic liquid. And when methyl is used to replace H at the R3 position, the order of the ability to stabilize the FA molecule still follows the above rule. This also confirms the experimental results reported by Dupont et al. in 2018 that imidazolium acetate ionic liquid are superior to imidazolium formate ionic liquid [32]. This may be attributed to the higher pKa of the acetate anion. We agree with this perspective. When the substituent at R3 is methyl, the optimal geometries of all species in the process of FA stabilization by imidazolium acetate ionic liquid and imidazolium bicarbonate ionic liquid are shown in Figures S5 and S6 in the Supporting Information.

Table 1.
The binding free energy of ionic liquids to formic acid.
4. Conclusions
In this study, we used imidazolium formate ionic liquid, imidazolium acetate ionic liquid, and imidazolium bicarbonate ionic liquid as additives to study the mechanism of ionic liquids in the process of CO2 catalytic hydrogenation to FA through theoretical calculations. The results show that the three ionic liquids can effectively stabilize the FA molecule due to the intermolecular hydrogen bonding between the ionic liquids and the FA molecule.
Because of the designability of ionic liquids, we further investigated the effects of cations and anions on the stabilization of FA in ionic liquids. First, the binding free energy of [C2C1ImH][HCOO] to FA was −11.5 kcal/mol when the cation R3 substitution position was H, and −14.0 kcal/mol after the replacement of methyl group [C2C1ImCH3][HCOO] to FA. This indicates that the methyl group can improve the stability with formic acid after substituting the hydrogen atom at the R3 position. For the stabilizing effect of different anions on FA, the binding free energies of [C4C1ImH][HCOO], [C4C1ImH][OAc], [C4C1ImH][HCO3] and FA molecules were −10.5 kcal/mol, −12.2 kcal/mol, and −10.4 kcal/mol, respectively. The best ability of these three ionic liquids to stabilize FA is imidazolium acetate ionic liquid, followed by imidazolium carbamate ionic liquid, and imidazolium bicarbonate ionic liquid has poor FA stabilization ability. This study investigates the binding energies between formic acid and ionic liquids, which indicate the presence of hydrogen-bonding interactions. It further explores the influence of cation and anion structures on the stabilization step, thereby offering a theoretical rationale for the related experimental research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry7060182/s1. Figure S1: Optimized geometries of all species during the regeneration of Ru–PNP catalysts; Figure S2: Gibbs free energy profile during the regeneration of Ru–PNP complexes; Figure S3: Optimized geometries of imidazolium acetate ionic liquid and ionic liquid–formic acid adduct in aqueous solution (R3 = H); Figure S4: Optimized geometries of imidazolium acetate ionic liquid and ionic liquid–formic acid adduct in aqueous solution (R3 = methyl); Figure S5: Optimized geometries of imidazolium bicarbonate ionic liquid and ionic liquid–formic acid adduct in aqueous solution (R3 = H); Figure S6: Optimized geometries of imidazolium bicarbonate ionic liquid and ionic liquid–formic acid adduct in aqueous solution (R3 = methyl); Table S1: Cartesian coordinates for optimized geometries of all species in aqueous solution.
Author Contributions
Conceptualization, J.L.; methodology, J.L.; investigation, P.G.; writing—original draft preparation, P.G.; writing—review and editing, J.L. and P.G.; supervision, J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 21776123 and the Project for Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
The data presented in this study are available in the Supporting Information.
Acknowledgments
We are thankful to the High-Performance Computing Center of Nanjing Tech University for supporting the computational resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, W.; Wang, S.P.; Ma, X.B.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Wang, W.-H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 Hydrogenation to Formate and Methanol as an Alternative to Photo- and Electrochemical CO2 Reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Rana, J.; Sahoo, S.T.; Daw, P. Homogeneous first-row transition metal catalyst for sustainable hydrogen production and organic transformation from methanol, formic acid, and bio-alcohols. Tetrahedron 2021, 99, 132473. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dutta, I.; Lum, Y.W.; Lai, Z.P.; Huang, K.-W. Enabling storage and utilization of low-carbon electricity: Power to formic acid. Energy Environ. Sci. 2021, 14, 1194–1246. [Google Scholar] [CrossRef]
- Enthaler, S.; von Langermann, J.; Schmidt, T. Carbon dioxide and formic acid—The couple for environmental-friendly hydrogen storage? Energy Environ. Sci. 2010, 3, 1207–1217. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.-W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef]
- Bhardwai, R.; Kumar, A.; Choudhury, J. An all-aqueous and phosphine-free integrated amine-assisted CO2 capture and catalytic conversion to formic acid. Chem. Commun. 2022, 58, 11531–11534. [Google Scholar] [CrossRef]
- Guntermann, N.; Franciò, G.; Leitner, W. Hydrogenation of CO2 to formic acid in biphasic systems using aqueous solutions of amino acids as the product phase. Green Chem. 2022, 24, 8069–8075. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, W.; Zhao, Y.F.; Tang, M.H.; Chang, X.Q.; Xu, Y.T.; Li, R.X.; Liu, Z.M. Cellulose-Phytic Acid Composite Complexed Ru Catalyst for CO2 Hydrogenation to Free Formic Acid. ChemCatChem 2023, 15, e202201168. [Google Scholar] [CrossRef]
- Chang, J.R.; Mao, J.-X.; Ding, M.; Zhang, J.; Chen, X.N. Evaluating the Catalytic Activities of PNCNP Pincer Group 10 Metal Hydride Complexes: Pd-Catalyzed Reduction of CO2 to the Formic Acid Level with NH3·BH3 and NaBH4 under Ambient Conditions. Inorg. Chem. 2023, 62, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Izumida, H.; Sasaki, Y.; Hashimoto, H. Catalytic fixation of carbon dioxide to formic acid by transition–metal complexes under mild conditions. Chem. Lett. 1976, 5, 863–864. [Google Scholar] [CrossRef]
- Jessop, P.G.; Ikarlya, T.; Noyori, R. Homogeneous catalytic hydrogenation of supercritical carbon dioxide. Nature 1994, 368, 231–233. [Google Scholar] [CrossRef]
- Tai, C.-C.; Pitts, J.; Linehan, J.C.; Main, A.D.; Munshi, P.; Jessop, P.G. In Situ Formation of Ruthenium Catalysts for the Homogeneous Hydrogenation of Carbon Dioxide. Inorg. Chem. 2002, 41, 1606–1614. [Google Scholar] [CrossRef]
- Tai, C.-C.; Chang, T.; Roller, B.; Jessop, P.G. High-Pressure Combinatorial Screening of Homogeneous Catalysts: Hydrogenation of Carbon Dioxide. Inorg. Chem. 2003, 42, 7340–7341. [Google Scholar] [CrossRef]
- Bello, T.O.; Bresciani, A.E.; Nascimento, C.A.O.; Alves, R.M.B. Thermodynamic analysis of carbon dioxide hydrogenation to formic acid and methanol. Chem. Eng. Sci. 2021, 242, 116731. [Google Scholar] [CrossRef]
- Huff, C.A.; Sanford, M.S. Catalytic CO2 Hydrogenation to Formate by a Ruthenium Pincer Complex. ACS Catal. 2013, 3, 2412–2416. [Google Scholar] [CrossRef]
- Guan, C.; Pan, Y.P.; Ang, E.P.L.; Hu, J.S.; Yao, C.G.; Huang, M.-H.; Li, H.F.; Lai, Z.P.; Huang, K.-W. Conversion of CO2 from air into formate using amines and phosphorus-nitrogen PN3P-Ru(II) pincer complexes. Green Chem. 2018, 20, 4201–4205. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamashita, M.; Nozaki, K. Catalytic Hydrogenation of Carbon Dioxide Using Ir(III)–Pincer Complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. [Google Scholar] [CrossRef]
- Filonenko, G.A.; van Putten, R.; Schulpen, E.N.; Hensen, E.J.M.; Pidko, E.A. Highly Efficient Reversible Hydrogenation of Carbon Dioxide to Formates Using a Ruthenium PNP–Pincer Catalyst. ChemCatChem 2014, 6, 1526–1530. [Google Scholar] [CrossRef]
- Federsel, C.; Boddien, A.; Jackstell, R.; Jennerjahn, R.; Dyson, P.J.; Scopelliti, R.; Laurenczy, G.; Beller, M. A Well-Defined Iron Catalyst for the Reduction of Bicarbonates and Carbon Dioxide to Formates, Alkyl Formates, and Formamides. Angew. Chem. Int. Ed. 2010, 49, 9777–9780. [Google Scholar] [CrossRef]
- Shao, X.Z.; Yang, X.F.; Xu, J.M.; Liu, S.; Miao, S.; Liu, X.Y.; Su, X.; Duan, H.M.; Huang, Y.Q.; Zhang, T. Iridium Single-Atom Catalyst Performing a Quasi-homogeneous Hydrogenation Transformation of CO2 to Formate. Chem 2019, 5, 693–705. [Google Scholar] [CrossRef]
- Zhu, F.X.; Zhu-Ge, L.; Yang, G.F.; Zhou, S.L. Iron–Catalyzed Hydrogenation of Bicarbonates and Carbon Dioxide to Formates. ChemSusChem 2015, 8, 609–612. [Google Scholar] [CrossRef]
- Kothandaraman, J.; Czaun, M.; Goeppert, A.; Haiges, R.; Jones, J.-P.; May, R.B.; Prakash, G.K.S.; Olah, G.A. Amine-Free Reversible Hydrogen Storage in Formate Salts Catalyzed by Ruthenium Pincer Complex without pH Control or Solvent Change. ChemSusChem 2015, 8, 1442–1451. [Google Scholar] [CrossRef]
- Yasaka, Y.; Wakai, C.; Matubayasi, N.; Nakahara, M. Controlling the Equilibrium of Formic Acid with Hydrogen and Carbon Dioxide Using Ionic Liquid. J. Phys. Chem. A 2010, 114, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Wesselbaum, S.; Hintermair, U.; Leitner, W. Continuous-Flow Hydrogenation of Carbon Dioxide to Pure Formic Acid using an Integrated scCO2 Process with Immobilized Catalyst and Base. Angew. Chem. Int. Ed. 2012, 51, 8585–8588. [Google Scholar] [CrossRef] [PubMed]
- Jens, C.M.; Scott, M.; Liebergesell, B.; Westhues, C.G.; Schäfer, P.; Franciò, G.; Leonhard, K.; Leitner, W.; Bardow, A. Rh-Catalyzed Hydrogenation of CO2 to Formic Acid in DMSO-based Reaction Media: Solved and Unsolved Challenges for Process Development. Adv. Synth. Catal. 2019, 361, 307–316. [Google Scholar] [CrossRef]
- Moret, S.; Dyson, P.J.; Laurenczy, G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat. Commun. 2014, 5, 4017. [Google Scholar] [CrossRef]
- Rohmann, K.; Kothe, J.; Haenel, M.W.; Englert, U.; Hölscher, M.; Leitner, W. Hydrogenation of CO2 to Formic Acid with a Highly Active Ruthenium Acriphos Complex in DMSO and DMSO/Water. Angew. Chem. Int. Ed. 2016, 55, 8966–8969. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Xie, Y.; Li, W.J.; Hu, S.Q.; Song, J.L.; Jiang, T.; Han, B.X. Hydrogenation of Carbon Dioxide is Promoted by a Task-Specific Ionic Liquid. Angew. Chem. Int. Ed. 2008, 47, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Weilhard, A.; Qadir, M.I.; Sans, V.; Dupont, J. Selective CO2 Hydrogenation to Formic Acid with Multifunctional Ionic Liquids. ACS Catal. 2018, 8, 1628–1634. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhao, Y.F.; Wang, H.; Yu, B.; Yu, X.X.; Zhang, H.Y.; Liu, Z.M. 110th Anniversary: Ionic Liquid Promoted CO2 Hydrogenation to Free Formic Acid over Pd/C. Ind. Eng. Chem. Res. 2019, 58, 6333–6339. [Google Scholar] [CrossRef]
- Webber, R.; Qadir, M.I.; Sola, E.; Martín, M.; Suárez, E.; Dupont, J. Fast CO2 hydrogenation to formic acid catalyzed by an Ir(PSiP) pincer hydride in a DMSO/water/ionic liquid solvent system. Catal. Commun. 2020, 146, 106125. [Google Scholar] [CrossRef]
- Piccirilli, L.; Rabell, B.; Padilla, R.; Riisager, A.; Das, S.; Nielsen, M. Versatile CO2 Hydrogenation–Dehydrogenation Catalysis with a Ru–PNP/Ionic Liquid System. J. Am. Chem. Soc. 2023, 145, 5655–5663. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Hu, J.L.; Jia, Y.X.; Yao, C.F.; Hu, X.B. Highly Efficient Catalyzing and Separation System for CO2 Hydrogenation to Formic Acid in Formate-Based Ionic Liquids. Ind. Eng. Chem. Res. 2025, 64, 9608–9616. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sust. Energ. Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Weilhard, A.; Argent, S.P.; Sans, V. Efficient carbon dioxide hydrogenation to formic acid with buffering ionic liquids. Nat. Commun. 2021, 12, 231. [Google Scholar] [CrossRef]
- Ma, W.T.; Hu, J.L.; Zhou, L.; Wu, Y.T.; Geng, J.; Hu, X.B. Efficient hydrogenation of CO2 to formic acid in water without consumption of a base. Green Chem. 2022, 24, 6727–6732. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Hensen, E.J.M.; Pidko, E.A. Mechanism of CO2 hydrogenation to formates by homogeneous Ru–PNP pincer catalyst: From a theoretical description to performance optimization. Catal. Sci. Technol. 2014, 4, 3474–3485. [Google Scholar] [CrossRef]
- Rawat, K.S.; Pathak, B. Aliphatic Mn–PNP complexes for the CO2 hydrogenation reaction: A base free mechanism. Catal. Sci. Technol. 2017, 7, 3234–3242. [Google Scholar] [CrossRef]
- Ramos, V.M.; de Oliveira–Filho, A.G.S.; de Lima Batista, A.P. Homogeneous Catalytic CO2 Hydrogenation by [Fe]–Hydrogenase Bioinspired Complexes: A Computational Study. J. Phys. Chem. A 2022, 126, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Filonenko, G.A.; Conley, M.P.; Copéret, C.; Luta, M.; Hensen, E.J.M.; Pidko, E.A. The impact of Metal–Ligand Cooperation in Hydrogenation of Carbon Dioxide Catalyzed by Ruthenium PNP Pincer. ACS Catal. 2013, 3, 2522–2526. [Google Scholar] [CrossRef]
- Taran, O.P.; Miroshnikova, A.V.; Baryshnikov, S.V.; Kazachenko, A.S.; Skripnikov, A.M.; Sychev, V.V.; Malyar, Y.N.; Kuznetsov, B.N. Reductive Catalytic Fractionation of Spruce Wood over Ru/C Bifunctional Catalyst in the Medium of Ethanol and Molecular Hydrogen. Catalysts 2022, 12, 1384. [Google Scholar] [CrossRef]
- Meng, L.Q.; Yao, L.H.; Li, J. Theoretical Study of Reversible Hydrogenation of CO2 to Formate Catalyzed by Ru(II)–PN5P, Fe(II)–PN5P, and Mn(I)–PN5P Complexes: The Effect of the Transition Metal Center. Catalysts 2024, 14, 440. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Becke, A.D. Density–functional exchange–energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision. D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Martin, J.M.L.; Sundermann, A. Correlation consistent valence basis sets for use with the Stuttgart–Dresden–Bonn relativistic effective core potentials: The atoms Ga–Kr and In–Xe. J. Chem. Phys. 2001, 114, 3408–3420. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical hybrid density functional with perturbative second–order correlation. J. Chem. Phys. 2006, 124, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Meng, L.Q.; Gong, P.C.; Li, J.; Zhou, Y. Theoretical Study of the Reversible Hydrogenation of Carbon Dioxide to Formate on Mn–Pincer Complexes. ChemPhysChem 2025, 26, e202400906. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, J.; Yang, Z. Substituent’s Effects of PNP Ligands in Ru(II)-Catalyzed CO2 Hydrogenation to Formate: Theoretical Analysis Considering Steric Hindrance and Promotion of Hydrogen Bonding. Catalysts 2022, 12, 760. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Li, J.; Yoshizawa, K. Catalytic Hydrogenation of Carbon Dioxide with a Highly Active Hydride on Ir(III)–Pincer Complex: Mechanism for CO2 Insertion and Nature of Metal–Hydride Bond. Bull. Chem. Soc. Jpn. 2011, 84, 1039–1048. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).