Several Crystalline Products of Carbon Dioxide Capture by Use of Amine Reagents
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, J.S.; Montzka, S.A. The Noaa Annual Greenhouse Gas Index (AGGI); NOAA Earth System Research Laboratory: Boulder, CO, USA, 2020. Available online: https://www.esrl.noaa.gov/gmd/aggi/aggi.html (accessed on 30 October 2025).
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. Clamate.gov; 2020. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 30 October 2025).
- National Aeronautics and Space Administration. Global Climate Change: Effects, 2018; National Aeronautics and Space Administration: Washington, DC, USA, 2018.
- Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks. In Public Review of Draft U.S. Inventory of Greenhouse Gas Emissions and Sinks: 1990–2021; Environmental Protection Agency: Washington, DC, USA, 2023. [Google Scholar]
- Murphy, L.J.; Robertson, K.N.; Kemp, R.A.; Tuononen, H.; Clyburne, J.A.C. Structurally simple complexes of CO2. Chem. Commun. 2015, 51, 3942–3956. [Google Scholar] [CrossRef]
- Bresciani, G.; Biancalana, L.; Pampaloni, G.; Marchetti, F. Recent Advances in the Chemistry of Metal Carbamates. Molecules 2020, 25, 3603. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, G.; Yan, H.; Zhao, Y. The latest development on amine functionalized solid adsorbents for post-combustion CO2 capture: Analysis review. Chin. J. Chem. Eng. 2021, 35, 17–43. [Google Scholar] [CrossRef]
- Hwang, J.; Han, D.; Oh, J.J.; Cheong, M.; Koo, H.J.; Lee, J.S.; Kim, H.S. Efficient Non-Catalytic Carboxylation of Diamines to Cyclic Ureas Using 2-Pyrrolidone as a Solvent and a Promoter. Adv. Synth. Catal. 2019, 361, 297–306. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations–A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Chaffee, A.; Knowles, G.; Liang, Z.; Zhang, J.; Xiao, P.; Webly, P. CO2 capture by adsorption: Materials and process development. Int. J. Greenh. Gas Control 2007, 1, 11–18. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 Capture with Chemical Absorption: A State-of-the-art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Ünveren, E.; Monkul, B.O.; Sarioglan, S.; Karademir, N. Solid amine sorbents for CO2 capture by chemical adsorption: A review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Conway, W.; Wang, X.; Fernandes, D.; Burns, R.; Lawrance, G.; Puxty, G.; Maeder, M. Toward Rational Design of Amine Solutions for PCC Applications: The Kinetics of the Reaction of CO2(aq) with Cyclic and Secondary Amines in Aqueous Solution. Environ. Sci. Technol. 2012, 46, 7422–7429. [Google Scholar] [CrossRef] [PubMed]
- Azzi, M.; Angove, D.; Dave, N.; Day, S.; Do, T.; Feron, P.; Zahra, M.A. Emissions to the Atmosphere from Amine-Based Post Combustion CO2 Capture Plant–Regulatory Aspects. Oil Gas Sci. Technol.-Rev. D’ifp Energ. Nouv. 2014, 69, 793–803. [Google Scholar] [CrossRef]
- Du, J.; Yang, W.; Xu, L.; Bei, L.; Lei, S.; Li, W.; Liu, H.; Weng, B.; Sun, L. Review on post-combustion CO2 capture by amine blended solvents and aqueous ammonia. Chem. Eng. J. 2024, 488, 150954. [Google Scholar] [CrossRef]
- Wai, S.K.; Nwaoha, C.; Saiwan, C.; Idem, R.; Supap, T. Absorption heat, solubility, absorption and desorption rates, cyclic capacity, heat duty, and absorption kinetic modeling of AMP-DETA blend for post-combustion CO2 capture. Sep. Purif. Tech. 2018, 194, 89–95. [Google Scholar] [CrossRef]
- Garbauskas, M.F.; Goehner, R.P.; Davis, A.M. The structure of two polymorphs of N-(2-ammonioethyl)carbamate, C3H8N2O2. Acta Crystallogr. 1983, C39, 1684–1686. [Google Scholar] [CrossRef]
- Antsyshkina, A.S.; Sadikov, G.G.; Solonina, I.A.; Rodinkova, M.N. Synthesis and crystal structures of tetraacetylethylenediamine and N-(2-ammoniumethyl)carbamate. Russ. J. Inorg. Chem. 2007, 52, 1561–1566. [Google Scholar] [CrossRef]
- Shao, B.; Wang, H.B. N-(2-Azaniumylethyl)carbamate monohydrate. Acta Crystallogr. 2011, 67, o3201. [Google Scholar] [CrossRef]
- Said, R.B.; Kolle, J.M.; Essalah, K.; Tangour, B.; Sayari, A. A Unified Approach to CO2–Amine Reaction Mechanisms. ACS Omega 2020, 5, 26125–26133. [Google Scholar] [CrossRef]
- Said, R.B.; Rahali, S.; Yan, C.; Seydou, M.; Tangour, B.; Sayari, A. CO2 Capture by Diamines in Dry and Humid Conditions: A Theoretical Approach. J. Phys. Chem. A 2023, 127, 7756–7763. [Google Scholar] [CrossRef]
- Alves, L.G.; Munhá, R.F.; Martins, A.M. Synthesis and structural characterization of N,N′,N′′,N′′′-tetrasubstituted cyclams. Chem. Heterocyc. Comp. 2021, 57, 871–874. [Google Scholar] [CrossRef]
- Fonari, M.S.; Antal, S.; Ordonez, C.; Timofeeva, T.V. Crystalline products of CO2 capture by piperazine aqueous solutions. CrystEngComm 2016, 18, 6282–6289. [Google Scholar] [CrossRef]
- Sim, J.; Jo, E.; Jhon, Y.H.; Jang, S.G.; Shim, J.-G.; Jang, K.-R.; Paek, K.; Kim, J. Isolation and crystal structure determination of piperazine dicarbamate obtained from a direct reaction. Bull. Korean Chem. Soc. 2016, 37, 1854–1857. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, version 2.03; Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Harper, N.D.; Nizio, K.D.; Hendsbee, A.D.; Masuda, J.D.; Robertson, K.N.; Murphy, L.J.; Johnson, M.B.; Pye, C.C.; Clyburne, J.A.C. Survey of Carbon Dioxide Capture in Phosphonium-Based Ionic Liquids and End-Capped Polyethylene Glycol Using DETA (DETA = Diethylenetriamine) as a Model Absorbent. Ind. Eng. Chem. Res. 2011, 50, 2822–2830. [Google Scholar] [CrossRef]
- Jiang, H.; Novak, I. Piperidine–CO2–H2O molecular complex. J. Mol. Struct. 2003, 645, 177–183. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Effendy; Skelton, B.W.; Somers, N.; White, A.H. Syntheses, structures and vibrational spectroscopy of some unusual silver(I) (pseudo-) halide/unidentate nitrogen base polymers. Inorg. Chim. Acta 2005, 358, 4307–4326. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Jin, R.; Ma, Y. New Crystal Structure of Molecular Complex 1-Piperidine Carboxylate-Piperidinium-H2O Studied by X-Ray Single Crystal Diffraction. Wuhan Univ. J. Nat. Sci. 2007, 12, 1099–1104. [Google Scholar] [CrossRef]
- Mondal, R.; Bhunia, M.K. Crystal Chemistry of 1:1 Molecular Complexes of Carbamate Salts Formed by Slow Aerial Carbonation of Amines. J. Chem. Crystallogr. 2008, 38, 787–792. [Google Scholar] [CrossRef]
- Döring, C.; Näther, C.; Jess, I.; Ibrom, K.; Jones, P.G. Two polymorphs of 4-hydroxypiperidine with different NH configurations. CrystEngComm 2015, 17, 5206–5215. [Google Scholar] [CrossRef]
- Kortunov, P.V.; Siskin, M.; Paccagnini, M.; Thomann, H. CO2 Reaction Mechanisms with Hindered Alkanolamines: Control and Promotion of Reaction Pathways. Energy Fuels 2016, 30, 1223–1236. [Google Scholar] [CrossRef]

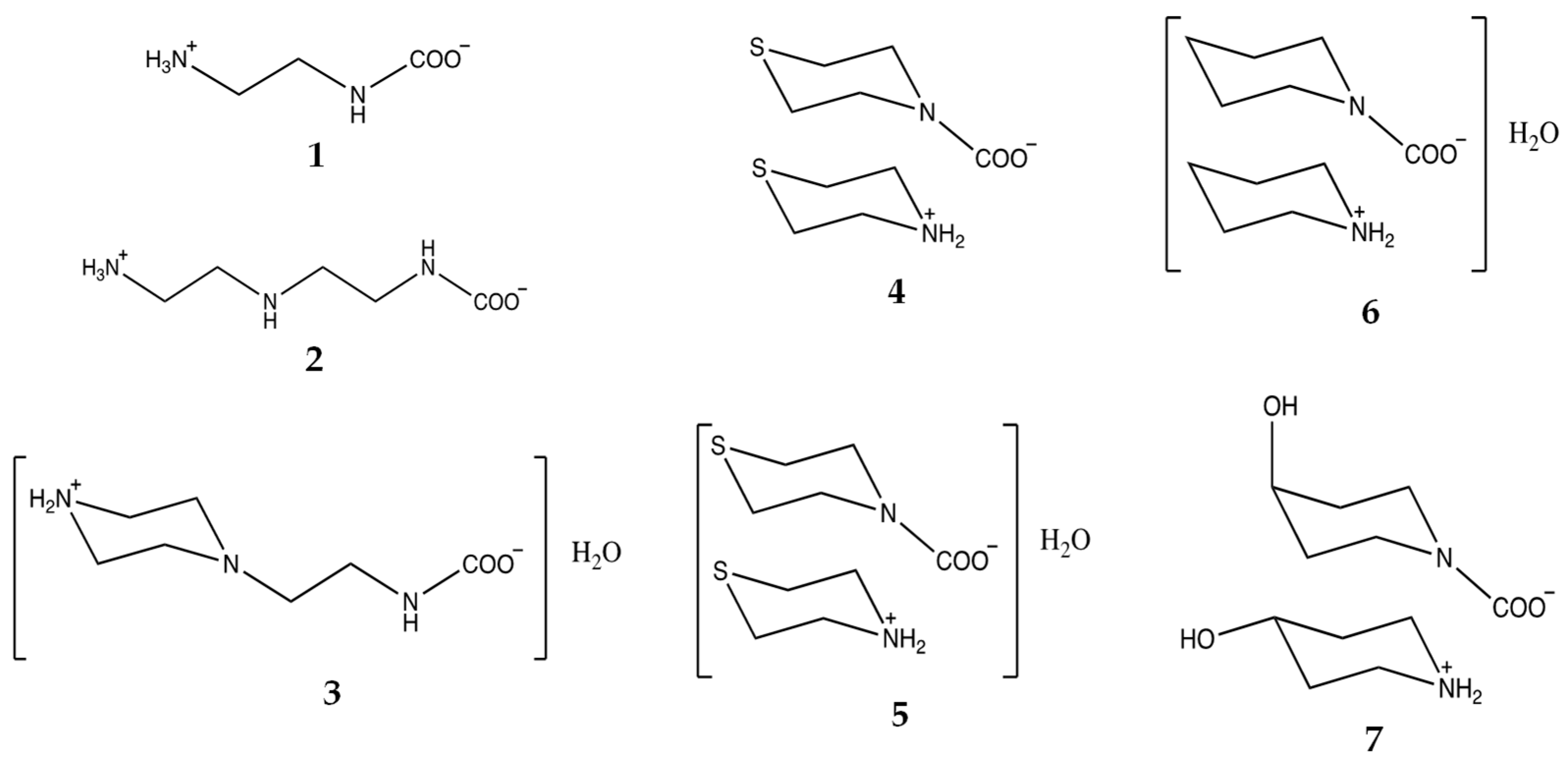
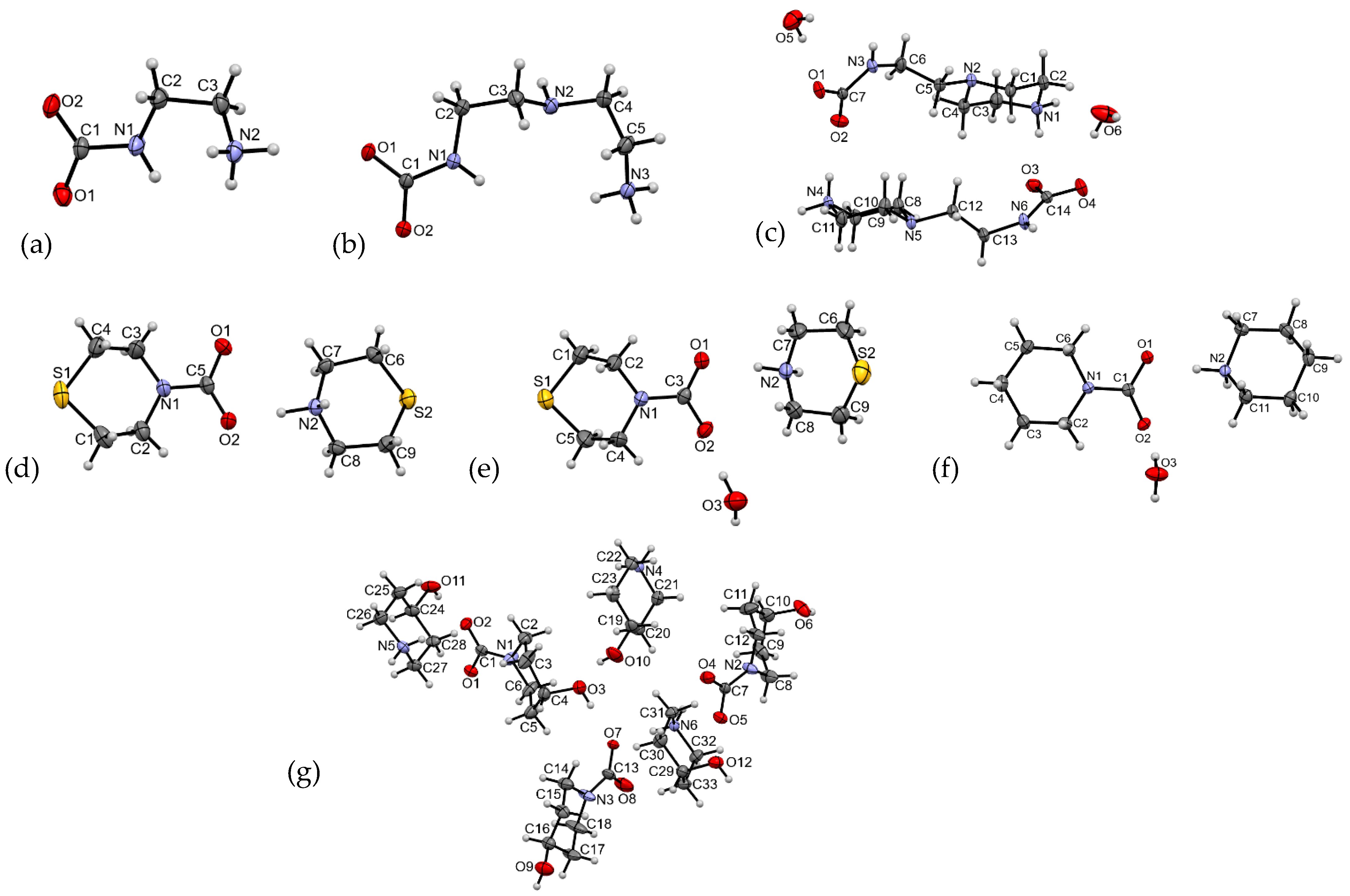

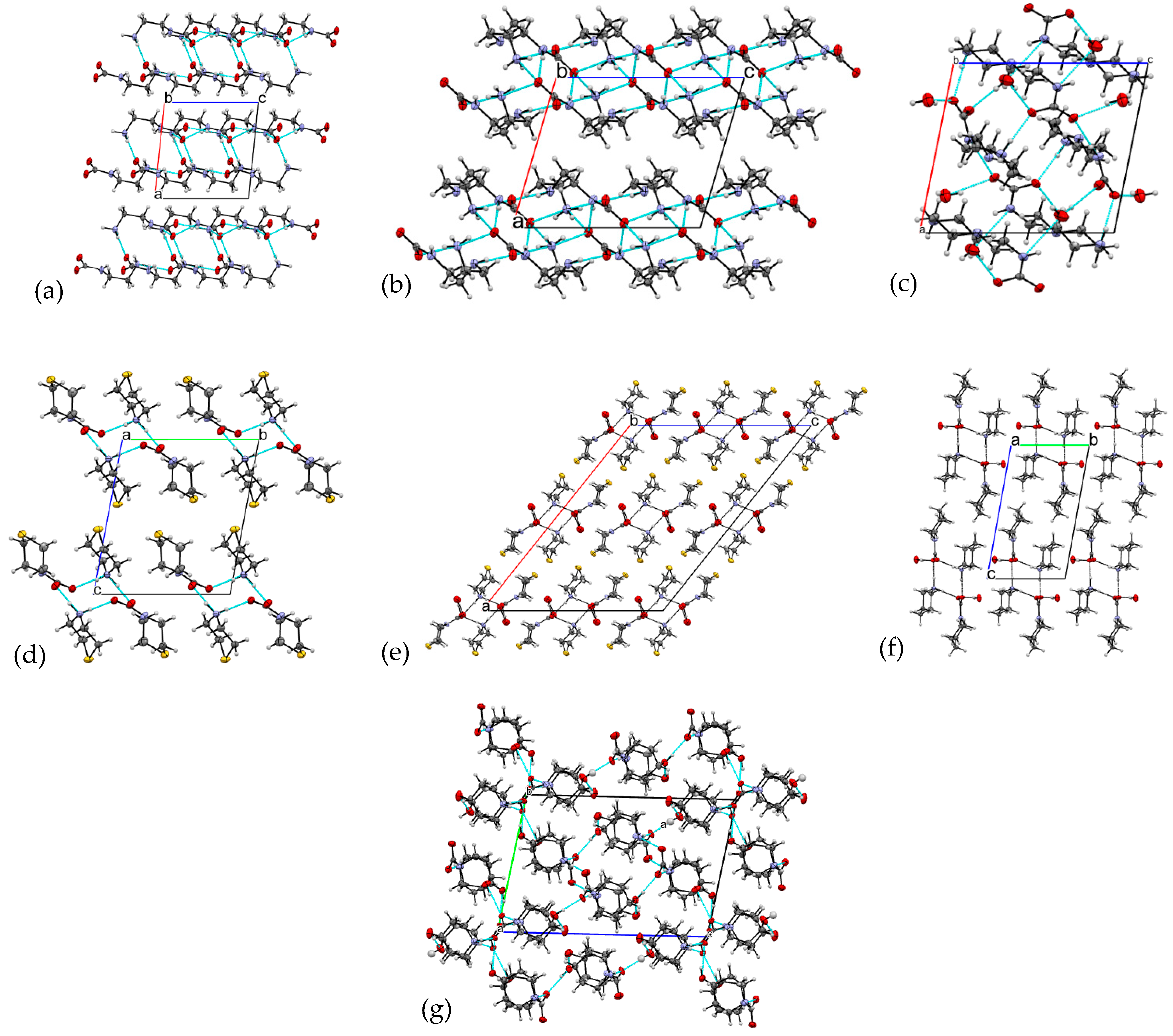
| Compound | CCDC ID | Sp.gr. | Method | Reference |
|---|---|---|---|---|
| 1 N-(2-ammonioethyl) carbamate | 2262778 | Monoclinic P21/c | Gaseous CO2 reaction with ethylenediamine in H2O:MeOH | Present work |
| CAXMOD CAXMOD01 | Orthorhombic Pna21 Monoclinic P21/a | Both form from 95% EtOH solution; orthorhombic formed from MeOH | [18] | |
| CAXMOD02 | Monoclinic P21/c | Isolated as a minor admixture upon attempt to synthesize ethylenediamine complex of lanthanum nitrate | [19] | |
| CAXMOD03 | Orthorhombic Pna21 | In the presence of 2-pyrrolidone, gaseous under pressure | [8] | |
| OCESEW (monohydrate) | Monoclinic P21/c | Exposure to air in xylenol | [20] | |
| 2 Diethylenetriammonia carbamate | 2262781 | Monoclinic P21/c | Atmospheric CO2 reaction with diethylenetriamine | Present work |
| KEFRIY | Monoclinic P21/c | Exposure to air in the presence of phosphonium-based ionic liquids | [29] | |
| 3 Aminoethylpiperazinium carbamate | 2262784 | Triclinic P-1 | Gaseous or atmospheric CO2 reaction with 1-(2-aminoethyl)piperazine in H2O | Present work |
| 4 Thiomorpholinium thiomorpholine carbamate | 2262779 | Triclinic P-1 | Atmospheric CO2 reaction with thiomorpholine | Present work |
| 5 Thiomorpholinium thiomorpholine carbamate monohydrate | 2262783 | Monoclinic C2/c | Solid CO2 (dry ice) reaction with morpholine and thiomorpholine in H2O | Present work |
| 6 Piperidinium piperidine-N-carboxylate monohydrate | 2262780 | Triclinic P-1 | Gaseous CO2 reaction with piperidine in H2O | Present work |
| HABJIE | Triclinic P-1 | Exposure to air (MP undetectable) | [30] | |
| HABJIE01 | Triclinic P-1 | As a by-product of piperidine solution interaction with air | [31] | |
| HABJIE02 | Triclinic P-1 | Exposure to air (MP undetectable) | [32] | |
| HABJIE03 | Triclinic P-1 | Aerial carbonation | [33] | |
| 7 4-Hydroxypiperidinium 4-hydroxypiperidine-N-carboxylate | 2262782 | Triclinic P-1 | Solid CO2 (dry ice) reaction with 4-hydroxypiperidine in H2O:EtOH | Present work |
| NUNPUJ | Triclinic P-1 | Exposure to air | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sena, V.; Fonari, M.S.; Timofeeva, T.V. Several Crystalline Products of Carbon Dioxide Capture by Use of Amine Reagents. Chemistry 2025, 7, 176. https://doi.org/10.3390/chemistry7060176
Sena V, Fonari MS, Timofeeva TV. Several Crystalline Products of Carbon Dioxide Capture by Use of Amine Reagents. Chemistry. 2025; 7(6):176. https://doi.org/10.3390/chemistry7060176
Chicago/Turabian StyleSena, Victoria, Marina S. Fonari, and Tatiana V. Timofeeva. 2025. "Several Crystalline Products of Carbon Dioxide Capture by Use of Amine Reagents" Chemistry 7, no. 6: 176. https://doi.org/10.3390/chemistry7060176
APA StyleSena, V., Fonari, M. S., & Timofeeva, T. V. (2025). Several Crystalline Products of Carbon Dioxide Capture by Use of Amine Reagents. Chemistry, 7(6), 176. https://doi.org/10.3390/chemistry7060176





