Abstract
With the growing concern over increased carbon dioxide concentrations in our planet’s atmosphere, much research is being devoted to improving the methods of carbon capture and storage. Amine-based carbon capture techniques are advantageous due to amine’s ability to react directly and reversibly with carbon dioxide to form solid products. To better understand the composition of the solid products obtained by reactions of carbon dioxide with amines under different conditions, such as reactions with gaseous and solid CO2 (dry ice), spontaneous areal absorbance of CO2, and others, several such reactions were conducted. Single-crystal X-ray diffraction analysis of the seven previously and newly observed carbamate derivatives was carried out. The synthetic and structural results of solid products have been compared with published data on the same materials obtained by different methods. Peculiarities of hydrogen bonding were described for these materials based on the topological approach.
1. Introduction
Carbon dioxide (CO2) is a known greenhouse gas present in the atmosphere. According to the National Oceanic and Atmospheric Administration’s (NOAA’s) Annual Greenhouse Gas Index, CO2 dominates the planet’s total radiative forcing, meaning that it is currently the greatest contributor to warming [1]. Paleoclimatic CO2 concentrations within the atmosphere have been indirectly measured through the use of ice core gas extraction [2].
Alterations in atmospheric composition have been linked to rises in global temperature, seasonal weather variations, changes in the intensity or frequency of natural storm events, and the loss of polar ice caps [3]. The observed rise in global temperature is an important effect of increasing CO2 concentrations. As the largest source of U.S. greenhouse gas emissions, CO2 from fossil fuel combustion has accounted for approximately 77% of the total global warming potential emissions since 1990 [4]. As a direct response to the massive amount of CO2 released by combustive processes, recent attempts to engineer potential solutions to anthropogenic CO2 release have been made.
Carbon capture and storage (CCS) is a greenhouse gas abatement method designed to remove CO2 from the atmosphere or divert CO2 from emission sources. CCS, through physical or chemical sorption methods, has three main technological configurations: pre-combustion, post-combustion, or oxyfuel combustion. Other CO2 separation and purification techniques, such as those of ionic liquids, metal–organic frameworks, etc., are also in development [5,6,7]. However, those technologies have not yet been as widely applied as chemical absorption.
Post-combustion capture (PCC) is the leading form of CCS technology. In PCC, chemical solvents are used to extract CO2 from flue gas following the combustion process. Primary and secondary amines have been recognized as chemical solvents for PCC. Due to the generation of an ionic species, amine–CO2 reactions typically result in a zwitterionic carbamate, ammonium carbamate salt, their mixture, or even cyclization products (Figure 1) [8]. Additionally, many amine–CO2 reactions occur reversibly, meaning that the absorbed gas can be easily extracted for eventual storage. The most effective and favorable thermodynamic and kinetic properties for an amine compound used for PCC have been reviewed [9,10,11,12,13]. The best amine is one that demonstrates a high degree of CO2 capture, minimum energy input or amine-related emissions, low volatility, and a resistance to oxidative or thermal degradation [14,15]. Given these parameters, commonly researched amines for PCC applications include monoethanolamine, methyldiethanolamine, diethanolamine, and piperazine [16]. The latest literature focuses on multi-amine blends to improve CO2 absorption performance [17]. Many carbamate products reported in the literature were obtained intentionally upon aerial carbonation from the environment as a means of investigating the corresponding amines’ potential for CCS [7,18,19,20]. Others were reported as unintentional by-products upon the contact of a reaction solution mixture with the atmospheric air, thus demonstrating the easiness of such a reaction [14,15].
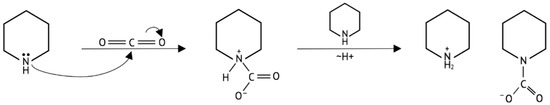
Figure 1.
Example mechanism of an amine–carbon dioxide reaction. The nitrogen lone pair bonds to the carbon of the CO2. A zwitterion is generated. Another amine molecule reacts with the species to provide chemical stability, and, following a proton rearrangement, two-component compounds are formed.
A unified mechanism of the CO2–amine reaction, applicable to absorption in aqueous or nonaqueous solutions, was developed with details based on theoretical calculations and experimental evidence [21,22]. However, while many alkylammonium alkylcarbamates of cyclic and substituted amines and diamines have been isolated and characterized by IR and NMR spectroscopy, the list of crystallographic studies of organic carbamates is not very long (CSD version 5.43, November 2022 updates). Interesting examples include monoclinic and orthorhombic polymorphs of N-(2-ammonioethyl)carbamate and its monohydrate [8,18,19,20]; and a tetracarbamate derivative of cyclam, obtained by the bubbling of carbon dioxide into an aqueous solution of tetraazamacrocycle. The reaction was reversible, and in an aqueous solution, a decarboxylation process occurred with release of CO2 [23]. Despite the wide interest in the products of piperazine (Pz), the simplest cyclic diamine interacting with CO2 [14], no crystalline products corresponding to any stages of the kinetic equilibria of CO2 in the aqueous solutions of Pz have been reported until our research, which identified the zwitterionic piperazinium-4-carboxamide trihydrate (PzHCO2)·3H2O and two salts, piperazinium piperazine-4-carboxamide (PzH)(PzCO2) and bis-(piperazinium) piperazine-1,4-bis(carboxamide) trihydrate (PzH)2(Pz(CO2)2)·3H2O, as crystalline solids [24]. Recently, this list was complemented by the crystalline piperazine-1,4-diium-piperazinedicarboxylate, (PzH2)(Pz(CO2)2) [25].
In continuation of our previous research [24], experiments were conducted using a variety of amine reagents to determine their ability to react with CO2 under different conditions. Crystalline products obtained can give additional information for the description of a combination of reaction products in crystal and types of intermolecular interactions. Reagents used in this study are described in the Experimental Section. A schematic presentation of the molecular structures of products obtained from these reactions is given in Figure 2. These products have been characterized by single-crystal X-ray diffraction analysis. The goals of the diffraction study of crystalline products were to show the exact composition of the solid phase, ratio of obtained products and solvents, if any, conformation of the resulting molecules, and pattern of H bonds in crystal. We also hope to provide a structural basis for further kinetic and thermodynamic studies on the evaluation of amine candidates for CSS applications.
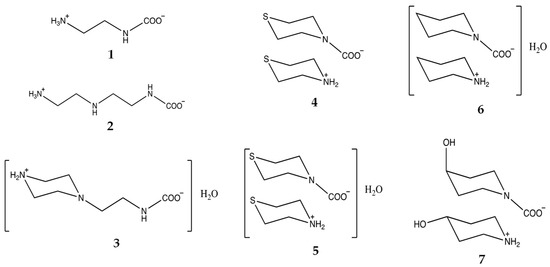
Figure 2.
Products obtained in this study.
2. Experimental
Preparation. All reagents used in this study were obtained from commercial sources and used as received. Thiomorpholine, 4-hydroxypiperidine, piperidine, diethylenetriamine, ethane-1,2-diamine, and 1-(2-aminoethyl)piperazine were purchased from Enamine Store, all with 95% purity. Piperidine was purchased from VWR®.
To obtain the crystalline products outlined in Figure 2, most solutions were prepared by dissolution of each amine in a solvent using a hotplate and VWR® Standard Vortex Mixer. Others were reacted in their pure form. Three primary methods of CO2 addition were employed: atmospheric, gaseous, and solid. Gaseous addition involved applying a stream of CO2 gas into the solution, whereas solid CO2 addition was the direct addition of dry ice to the solution. Ethylenediamine in a 1:1 water:methanol solvent reacted with gaseous CO2 to form compound 1. A small amount of pure diethylenetriamine was allowed to spontaneously react with atmospheric CO2 to form compound 2. Compound 3 was formed by reaction of an aqueous 1-(2-aminoethyl)piperazine solution with gaseous CO2; this product could also be formed through atmospheric methods. Pure liquid thiomorpholine reacted with atmospheric CO2 produce compound 4. Reaction of thiomorpholine with the other CO2 application methods yielded powder products that were not characterized in this study. Compound 5 was formed through reaction of an aqueous solution of morpholine and thiomorpholine with dry ice. Compound 6 was formed by the bubbling of CO2 gas in an aqueous piperidine solution. Dry ice was added to 4-hydroxypiperidine in a 1:1 water: ethanol mixture to produce compound 7. Single crystals suitable for the X-ray diffraction study were grown after approximately three weeks of slow evaporation at room temperature under a fume hood.
Many of the crystallized materials were extremely sensitive to air, and, as a result, structural characterization was performed at low temperatures with constant nitrogen gas flow. When possible, melting point determinations were conducted using an SRS OptiMelt apparatus.
X-ray Structure Determination. Data were collected using a Bruker APEX-II CCD diffractometer (λ(MoKα) radiation) equipped with a cryostat system (graphite monochromator, ω and φ scan mode). Data were corrected for absorption using the SADABS software [26]. The crystal structures were determined by direct methods and refined by a full-matrix least squares technique on F2 with anisotropic displacement parameters for non-hydrogen atoms. Hydrogen atoms in compounds 1–6 were defined using the difference Fourier methods; in compound 7, all hydrogen atoms were placed in calculated positions and refined within the riding model with fixed isotropic displacement parameters (Uiso(H) = 1.5Ueq(O) for the OH-groups and 1.2Ueq(C,N) for the other groups). Calculations were carried out using the SHELXTL program [27], and structural solution and refinement were conducted using the OLEX2 software [28]. Details of structural solution and refinement are provided in Table S1. Hydrogen bonding geometry in 1–7 is summarized in Table S2.
3. Results and Discussion
A unified mechanism of the CO2–amine reaction, applicable to absorption in aqueous or nonaqueous solutions, was developed with details based on theoretical calculations and experimental evidence (Figure 1) [21,22]. The nitrogen lone pair bonds to the carbon of CO2. A zwitterion is generated, another amine molecule reacts with the species to provide chemical stability, and, following a proton rearrangement, two-component compounds are formed. Many of the resulting products of this study, as shown in Figure 2, follow this mechanism. The ORTEP drawings, with numbering schemes for 1–7, are shown in Figure 3. The crystalline products 1–3, whose starting reagents contained amino-groups separated by an aliphatic chain, represent zwitterionic carbamates as anhydrous forms 1 and 2 and hydrated form 3. In the case of cyclic amines, the final compounds represent anhydrous 4 and 7 and hydrated 5 and 6 carbamate salts. Although some of the crystal structures have been already reported [7,15,16,17,26,27,28,29,30,31,32,33,34], they were included because of the different synthetic protocols (Table 1) and for discussion of some details in the hydrogen bonding systems and packing patterns.
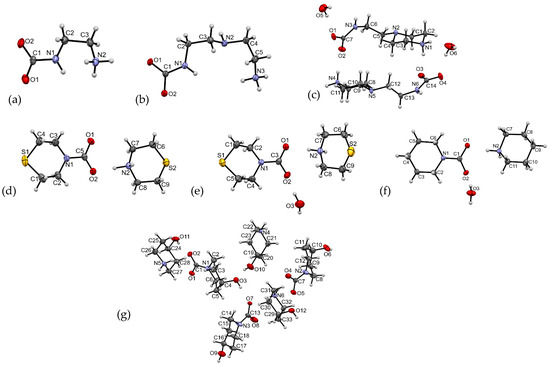
Figure 3.
The ORTEP drawings for the asymmetric units with numbering schemes for 1–7: (a) 1; (b) 2; (c) 3; (d) 4; (e) 5; (f) 6; and (g) 7.

Table 1.
Methods of syntheses of 1–7 and their literature reference analogs.
All carbamate anions have the typical flattened geometry of the carbamate residues usual for the sp2-hybridized nitrogen atom, with equalized C-O distances and reduced C-N distance. In our study compound 1 was obtained as a result of gaseous CO2 reaction with ethylene–diamine solution in H2O:MeOH as a monoclinic polymorph. Previously two crystalline polymorphs, monoclinic and orthorhombic, have been reported [18,19,20]. The orthorhombic form was found as the result of a gaseous reaction under pressure in the presence of 2-pyrrolidone [8] and in a mixture with the monoclinic form from methanol solution (see Table 1). Conformations of molecule 1 in both polymorphs are significantly different: the torsion angle around the C2–C3 bond in the monoclinic form is equal to 66.6 and in the orthorhombic form to 175.8 degrees. The overlapping diagrams for compounds composed of multiple independent formula units are shown in Figure S1. Additionally, the orthorhombic polymorph of 1 present in the extended conformation of the zwitterion crystallizes as the 3D H-bonded network, while the monoclinic polymorph adjusts the twisted conformation and crystallizes as the H-bonded layer.
Compound 2 was obtained by an atmospheric CO2 reaction with diethylenetriamine in our study and, in previous work, by exposure to air in the presence of phosphonium-based ionic liquids [29], but our work suggests that this media is not required to generate this crystalline product.
Compounds 3, 4, and 5 are novel crystallographic materials not reported prior to this work. Compound 3 comprises two independent zwitterions taking practically the same conformations.
Compounds 4 and 5 in crystal represent anhydrous and hydrated carbamate salts, respectively. The crystal structure of compound 6 was described in four publications [30,31,32,33].
Unlike the several reported synthetic protocols for compound 6, where the product was obtained via atmospheric CO2 interaction [30,31,32,33], we were able to replicate the structure only through the introduction of CO2 gas to an aqueous piperidine solution.
Similarly, compound 7 could only be obtained through the reaction of dry ice with 4-hydroxypiperidine in water: ethanol mixture as opposed to spontaneous aerial carbonation as reported by Döring et al. [34]. Compound 7 comprises three independent formula units, with the hydroxyl group situated in an axial position on one of the cations and two of the anions. It should be mentioned that in some literature sources, reaction conditions are presented without details. Different methods of synthesis or exposure to atmospheric air can give identical results in terms of final products, as demonstrated in Table 1. This observation allows us to suggest that for the amines mentioned in our study, various technological approaches can be considered for their applications.
Analysis of geometry parameters of molecules in crystals 1–7 (see tables of bond lengths and bond angles in CIF’s) revealed no hints of hindrances in these molecules, which is considered an indicator of their higher CO2 loading capacity and lower thermal stability, both of which are favorable properties for CO2 capture [35].
Hydrogen bonding patterns in crystals 1–7 are shown in Figure 4. The NH⋯O and OH⋯O (for hydrates) hydrogen bonds are the main driving forces for supramolecular assemblies in these compounds. The primary H-bonded association pattern in all reported compounds is an H-bonded ring in a chair conformation with R44(12) graph notation that includes two -NH2 fragments (from the terminal -NH3 or cyclic NH2-groups) and two -CO2 residues from two carbamate moieties. This H-bonded pattern is centrosymmetric in all compounds but 7, where one cation and one anion form the centrosymmetric tetramer, while the other two cations and two other anions form the pseudocentrosymmetric tetramer. The further extension of supramolecular motifs depends on the presence/absence of additional H donors and H acceptors in the systems, and in all but 4, this R44(12) synthon alternates with other H-bonded patterns, resulting in the H-bonded layers in 1 and 2, H-bonded chains (via bridging water molecules) in 5 and 6, and 3D H-bonded networks in 3 and 7. The crystal structure of 4, with its H donor–H acceptor equality, is built of the discrete H-bonded tetramers. In all but 4, an excess of H donors resulted in the involvement of oxygen atoms of carbamate residues in H bonding systems as multiple H acceptors (Table S2).
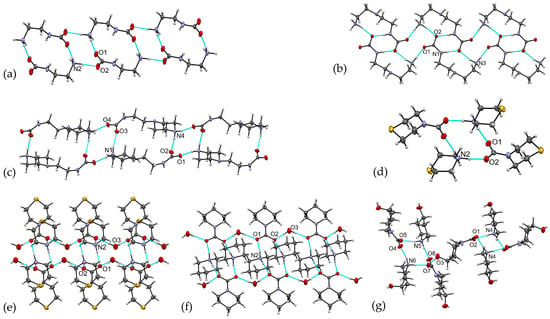
Figure 4.
Fragments of crystal structures in 1–7. (a) Catenation motif via alternating H-bonded rings R44(12)R22(14) in 1; (b) catenation motif via alternating H-bonded rings R44(12)R22(8)R21(10) in 2; (c) catenation motif via alternating H-bonded rings R44(12)R22(20) in 23; (d) H-bonded tetramer in 4; (e) H-bonded chain in 5; (f) H-bonded chain in 6; (g) association of centrosymmetric and noncentrosymmetric tetramers in 7.
Compounds with similar molecular structures yielded similar crystal packings. Zwitterionic compounds 1 and 2 crystallize in the same monoclinic P21/c space group and are associated in the H-bonded layers with all meaningful H bonds realized within the layers and a lack of strong interactions between the layers. In both structures, the layers stack along the a axes (Figure 5). Compounds 4, 5, and 6 form very similar arrangements of tetramers. Compounds 3 and 7 contain much more complex arrangements, with their packing showing three-dimensional hydrogen bonded networks.
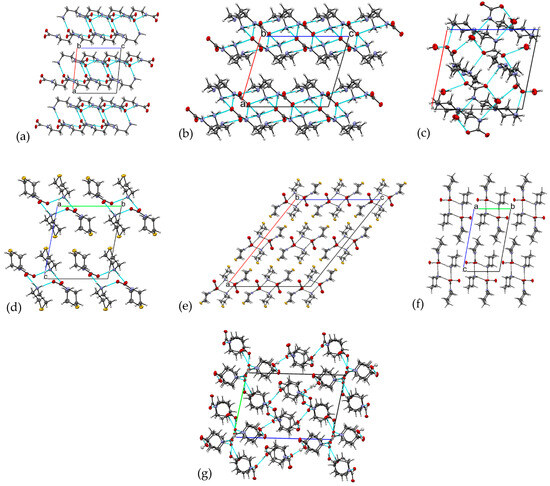
Figure 5.
Packing motifs in 1–7. (a) Packing of the H-bonded layers in 1; (b) packing of H-bonded layers in 2; (c) 3D H-bonded network in 3; (d) packing of H-bonded tetramers in 4; (e) packing of H-bonded chains in 5; (f) packing of H-bonded chains in 6; (g) 3D H-bonded network in 7.
4. Conclusions
The amines investigated herein were able to react with and capture CO2. The single crystal X-ray structural study provides evidence for a variety of solid amine–CO2 products. Different amine solutions, as well as two-component mixtures of amines, should be studied further to determine which can form crystalline materials and which exhibit the most advantageous physical and chemical properties for CCS applications. It was suggested [35] that higher CO2 loading capacity and lower thermal stability, both of which are favorable properties for CO2 capture, are connected with hindrances in these molecules. Analysis of geometry parameters of molecules in crystals 1–7 (see tables of bond lengths and bond angles in CIF’s) revealed no significant deviation of these parameters from standard values, so no hints of hindrances in these were detected.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry7060176/s1, Figure S1: The ORTEP drawings and overlapping diagrams for compounds comprised of multiple independent formula units. (a) 3; (b) overlapping diagram for two zwitterions in 3; (c) 7; (d) overlapping diagram of cations in 7; (e) overlapping diagram of anions in 7. Hydrogens are omitted for clarity; Table S1: Detailed crystal data and structure refinement parameters for 1–7; Table S2: Hydrogen bonds in 1–7. CCDC 2262778-2262784 contain the supplementary crystallographic data for 1–7. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/ (accessed on 13 May 2023), or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Author Contributions
All authors were involved in conceptualization, methodology, and data analysis for the manuscript; V.S. synthesized all the materials in this paper; the single crystal X-ray characterization was carried out by V.S. and M.S.F.: original draft preparation was done by V.S.; crystallographic pictures from CIF files were derived using Mercury software by M.S.F. All authors contributed during the writing process of final version of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSF via DMR-2122108 (PREM) and DMR-2320830 grants. In addition M.S.F. thanks the subprogram 011202 for support.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the authors.
Acknowledgments
The authors are grateful to New Mexico Highlands University for opportunity to use facilities at Chemistry Department.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Butler, J.S.; Montzka, S.A. The Noaa Annual Greenhouse Gas Index (AGGI); NOAA Earth System Research Laboratory: Boulder, CO, USA, 2020. Available online: https://www.esrl.noaa.gov/gmd/aggi/aggi.html (accessed on 30 October 2025).
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. Clamate.gov; 2020. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 30 October 2025).
- National Aeronautics and Space Administration. Global Climate Change: Effects, 2018; National Aeronautics and Space Administration: Washington, DC, USA, 2018.
- Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks. In Public Review of Draft U.S. Inventory of Greenhouse Gas Emissions and Sinks: 1990–2021; Environmental Protection Agency: Washington, DC, USA, 2023. [Google Scholar]
- Murphy, L.J.; Robertson, K.N.; Kemp, R.A.; Tuononen, H.; Clyburne, J.A.C. Structurally simple complexes of CO2. Chem. Commun. 2015, 51, 3942–3956. [Google Scholar] [CrossRef]
- Bresciani, G.; Biancalana, L.; Pampaloni, G.; Marchetti, F. Recent Advances in the Chemistry of Metal Carbamates. Molecules 2020, 25, 3603. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, G.; Yan, H.; Zhao, Y. The latest development on amine functionalized solid adsorbents for post-combustion CO2 capture: Analysis review. Chin. J. Chem. Eng. 2021, 35, 17–43. [Google Scholar] [CrossRef]
- Hwang, J.; Han, D.; Oh, J.J.; Cheong, M.; Koo, H.J.; Lee, J.S.; Kim, H.S. Efficient Non-Catalytic Carboxylation of Diamines to Cyclic Ureas Using 2-Pyrrolidone as a Solvent and a Promoter. Adv. Synth. Catal. 2019, 361, 297–306. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations–A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Chaffee, A.; Knowles, G.; Liang, Z.; Zhang, J.; Xiao, P.; Webly, P. CO2 capture by adsorption: Materials and process development. Int. J. Greenh. Gas Control 2007, 1, 11–18. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 Capture with Chemical Absorption: A State-of-the-art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Ünveren, E.; Monkul, B.O.; Sarioglan, S.; Karademir, N. Solid amine sorbents for CO2 capture by chemical adsorption: A review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Conway, W.; Wang, X.; Fernandes, D.; Burns, R.; Lawrance, G.; Puxty, G.; Maeder, M. Toward Rational Design of Amine Solutions for PCC Applications: The Kinetics of the Reaction of CO2(aq) with Cyclic and Secondary Amines in Aqueous Solution. Environ. Sci. Technol. 2012, 46, 7422–7429. [Google Scholar] [CrossRef] [PubMed]
- Azzi, M.; Angove, D.; Dave, N.; Day, S.; Do, T.; Feron, P.; Zahra, M.A. Emissions to the Atmosphere from Amine-Based Post Combustion CO2 Capture Plant–Regulatory Aspects. Oil Gas Sci. Technol.-Rev. D’ifp Energ. Nouv. 2014, 69, 793–803. [Google Scholar] [CrossRef][Green Version]
- Du, J.; Yang, W.; Xu, L.; Bei, L.; Lei, S.; Li, W.; Liu, H.; Weng, B.; Sun, L. Review on post-combustion CO2 capture by amine blended solvents and aqueous ammonia. Chem. Eng. J. 2024, 488, 150954. [Google Scholar] [CrossRef]
- Wai, S.K.; Nwaoha, C.; Saiwan, C.; Idem, R.; Supap, T. Absorption heat, solubility, absorption and desorption rates, cyclic capacity, heat duty, and absorption kinetic modeling of AMP-DETA blend for post-combustion CO2 capture. Sep. Purif. Tech. 2018, 194, 89–95. [Google Scholar] [CrossRef]
- Garbauskas, M.F.; Goehner, R.P.; Davis, A.M. The structure of two polymorphs of N-(2-ammonioethyl)carbamate, C3H8N2O2. Acta Crystallogr. 1983, C39, 1684–1686. [Google Scholar] [CrossRef]
- Antsyshkina, A.S.; Sadikov, G.G.; Solonina, I.A.; Rodinkova, M.N. Synthesis and crystal structures of tetraacetylethylenediamine and N-(2-ammoniumethyl)carbamate. Russ. J. Inorg. Chem. 2007, 52, 1561–1566. [Google Scholar] [CrossRef]
- Shao, B.; Wang, H.B. N-(2-Azaniumylethyl)carbamate monohydrate. Acta Crystallogr. 2011, 67, o3201. [Google Scholar] [CrossRef]
- Said, R.B.; Kolle, J.M.; Essalah, K.; Tangour, B.; Sayari, A. A Unified Approach to CO2–Amine Reaction Mechanisms. ACS Omega 2020, 5, 26125–26133. [Google Scholar] [CrossRef]
- Said, R.B.; Rahali, S.; Yan, C.; Seydou, M.; Tangour, B.; Sayari, A. CO2 Capture by Diamines in Dry and Humid Conditions: A Theoretical Approach. J. Phys. Chem. A 2023, 127, 7756–7763. [Google Scholar] [CrossRef]
- Alves, L.G.; Munhá, R.F.; Martins, A.M. Synthesis and structural characterization of N,N′,N′′,N′′′-tetrasubstituted cyclams. Chem. Heterocyc. Comp. 2021, 57, 871–874. [Google Scholar] [CrossRef]
- Fonari, M.S.; Antal, S.; Ordonez, C.; Timofeeva, T.V. Crystalline products of CO2 capture by piperazine aqueous solutions. CrystEngComm 2016, 18, 6282–6289. [Google Scholar] [CrossRef]
- Sim, J.; Jo, E.; Jhon, Y.H.; Jang, S.G.; Shim, J.-G.; Jang, K.-R.; Paek, K.; Kim, J. Isolation and crystal structure determination of piperazine dicarbamate obtained from a direct reaction. Bull. Korean Chem. Soc. 2016, 37, 1854–1857. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, version 2.03; Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Harper, N.D.; Nizio, K.D.; Hendsbee, A.D.; Masuda, J.D.; Robertson, K.N.; Murphy, L.J.; Johnson, M.B.; Pye, C.C.; Clyburne, J.A.C. Survey of Carbon Dioxide Capture in Phosphonium-Based Ionic Liquids and End-Capped Polyethylene Glycol Using DETA (DETA = Diethylenetriamine) as a Model Absorbent. Ind. Eng. Chem. Res. 2011, 50, 2822–2830. [Google Scholar] [CrossRef]
- Jiang, H.; Novak, I. Piperidine–CO2–H2O molecular complex. J. Mol. Struct. 2003, 645, 177–183. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Effendy; Skelton, B.W.; Somers, N.; White, A.H. Syntheses, structures and vibrational spectroscopy of some unusual silver(I) (pseudo-) halide/unidentate nitrogen base polymers. Inorg. Chim. Acta 2005, 358, 4307–4326. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Jin, R.; Ma, Y. New Crystal Structure of Molecular Complex 1-Piperidine Carboxylate-Piperidinium-H2O Studied by X-Ray Single Crystal Diffraction. Wuhan Univ. J. Nat. Sci. 2007, 12, 1099–1104. [Google Scholar] [CrossRef]
- Mondal, R.; Bhunia, M.K. Crystal Chemistry of 1:1 Molecular Complexes of Carbamate Salts Formed by Slow Aerial Carbonation of Amines. J. Chem. Crystallogr. 2008, 38, 787–792. [Google Scholar] [CrossRef]
- Döring, C.; Näther, C.; Jess, I.; Ibrom, K.; Jones, P.G. Two polymorphs of 4-hydroxypiperidine with different NH configurations. CrystEngComm 2015, 17, 5206–5215. [Google Scholar] [CrossRef]
- Kortunov, P.V.; Siskin, M.; Paccagnini, M.; Thomann, H. CO2 Reaction Mechanisms with Hindered Alkanolamines: Control and Promotion of Reaction Pathways. Energy Fuels 2016, 30, 1223–1236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).