Organogold(III) Complexes with Chelating Thiourea-Type Ligands
Abstract
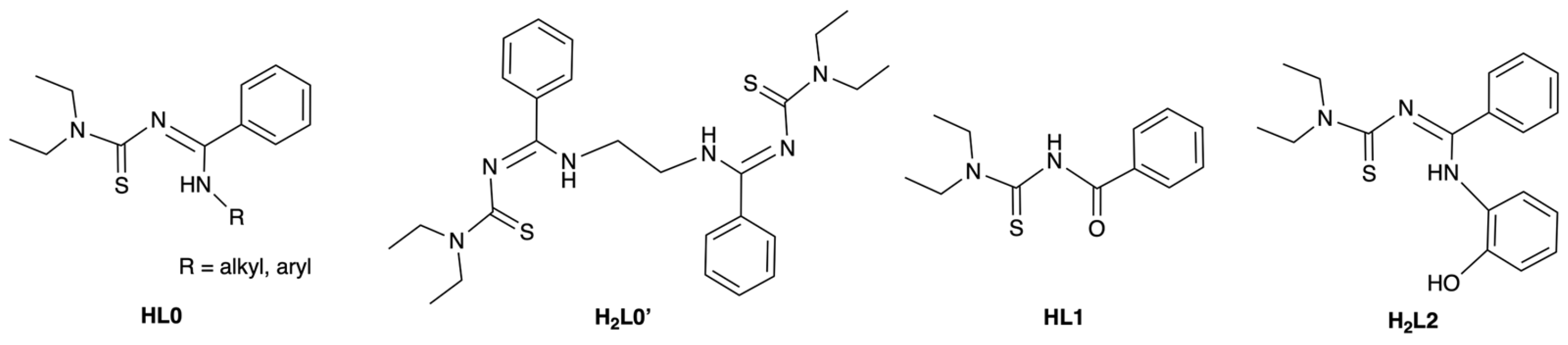
1. Introduction
2. Materials and Methods
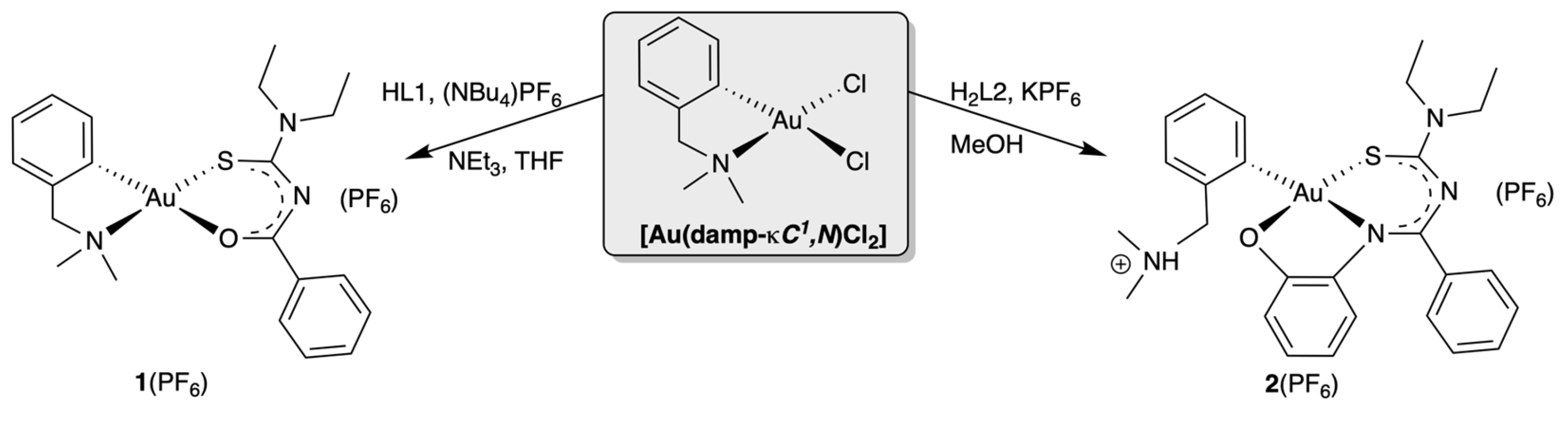
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera, R.P.; Gimeno, M.C. Main Avenues in Gold Coordination Chemistry. Chem. Rev. 2021, 121, 8311–8363. [Google Scholar] [CrossRef]
- Roccigiani, L.; Bochmann, M. Recent Advances in Gold(III) Chemistry: Structure, Bonding, Reactivity, and Role in Homogeneous Catalysis. Chem. Rev. 2021, 121, 8364–8451. [Google Scholar] [CrossRef] [PubMed]
- Lynch, W.E.; Padgett, C.W.; Quillian, B.; Haddock, J. A square-planar hydrated cationic tetrakis(methimazole)gold(III) complex. Acta Cryst. 2015, C71, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, S.; Koskinen, L.; Kultamaa, M.; Haukka, M.; Hirva, P. Persistence of oxidation state III of gold in thione coordination. Solid State Sci. 2017, 67, 37–45. [Google Scholar] [CrossRef]
- Abram, U.; Mack, J.; Ortner, K.; Müller, M. Reactions of dichloro[2-(dimethylaminomethyl)phenyl-C1,N]gold(III),[Au(damp-C1,N)Cl2], with heterocyclic thiols. Evidence for Au-N bond cleavage and protonation of the dimethylamino group. J. Chem. Soc. Dalton Trans. 1998, 1998, 1011–1019. [Google Scholar] [CrossRef]
- Smith, T.S.; Henderson, W.; Nicholson, B.K. Cycloaurated gold(III) complexes with monoanionic thiourea ligands. Inorg. Chim. Acta 2013, 408, 27–32. [Google Scholar] [CrossRef]
- Jia, W.-G.; Dai, Y.-C.; Zhang, H.-N.; Lu, X.; Sheng, E.-H. Synthesis and characterization of gold complexes. with pyridine-based SNS ligands and as homogeneous catalysts for reduction of 4-nitrophenol. RSC Adv. 2015, 5, 29491–29496. [Google Scholar] [CrossRef]
- Wie, J.-J.; Xiao, J.J.; Yu, J.-W.; Yi, X.-Y.; Liu, S.; Liu, G.-Y. Synthesis and structural characterization of silver(I) and gold(I) complexes of N,N′-diisobutyloxycarbonyl-N‘‘,N‘‘‘-(1,3-propylene)bisthiourea. Polyhedron 2017, 137, 176–181. [Google Scholar]
- Bensch, W.; Schuster, M. Komplexierung von Gold mit N,N-Dialkyl-N′-benzoythioharnstoffen: Die Kristallstruktur von N,N-Diethyl-N′-benzoylformamidin tetrachloroaurat (III). Z. Anorg. Allg. Chem. 1992, 611, 95–98. [Google Scholar] [CrossRef]
- Bensch, W.; Schuster, M. Complex Formation of Gold with N,N-Dialkyl-N′-benzoylthioureas: The Crystal Structure of N,N-Diethyl-N′-benzoylthioureatogold(I) Chloride. Z. Anorg. Allg. Chem. 1992, 611, 99–102. [Google Scholar] [CrossRef]
- Khan, U.A.; Badshah, A.; Tahir, M.N.; Khan, E. Gold(I), silver(I) and copper(I) complexes of 2,4,6-trimethylphenyl-3-benzoylthiourea; synthesis and biological applications. Polyhedron 2020, 181, 114485. [Google Scholar] [CrossRef]
- Richter, R.; Schröder, U.; Kampf, M.; Hartung, J.; Beyer, L. Gold(I) Complexes of N-Thiocarbamoyl Benzamidines: Synthesis and Structures. Z. Anorg. Allg. Chem. 1997, 623, 1021–1026. [Google Scholar] [CrossRef]
- Schröder, U.; Richter, R.; Hartung, J.; Abram, U.; Beyer, L. Substituted 1,2,4-Thiadiazoliumdichloroaurates(I) and -tetrachloroaurates(III) as Products of the Reactions of N-Thiocarbamoyl-benzamidines with Tetrachlorogold(III) Compounds. Z. Naturforsch. 1997, 52b, 620–628. [Google Scholar] [CrossRef]
- Maia, P.I.S.; Nguyen, H.H.; Ponader, D.; Hagenbach, A.; Bergemann, S.; Gust, R.; Deflon, V.M.; Abram, U. Neutral Gold Complexes with Tridentate SNS Thiosemicarbazide Ligands. Inorg. Chem. 2012, 51, 1604–1613. [Google Scholar] [CrossRef]
- Ferreira Sucena, S.; Hagenbach, A.; Pham, C.T.; Abram, U. Binuclear Gold(I) Complexes with A Potentially Tetradentate S,N,N,S Ligand. Inorganics 2025, 13, 292. [Google Scholar] [CrossRef]
- Salvador-Gil, D.; Herrera, R.P.; Gimeno, M.C. Catalysis-free synthesis of thiazolidine–thiourea ligands for metal coordination (Au and Ag) and preliminary cytotoxic studies. Dalton Trans. 2023, 52, 7797–7808. [Google Scholar] [CrossRef]
- Risi, M.C.; Atiga, A.; Christopher, T.D.; Henderson, W.; Saunders, G.C. Diacylthioureas—An overlooked class of ligands; the coordination chemistry of diacylated thiourea with platinum(II), palladium(II) and gold(III). Dalton Trans. 2025, 54, 4977–4989. [Google Scholar] [CrossRef]
- Fuchita, Y.; Ieda, H.; Yasutake, M. First intramolecular aromatic substitution by gold(III) of a ligand other than pyridine derivatives. Synthesis and crystal structure of the novel five-membered cycloaurated complex of 1-ethyl-2-phenylimidazole. J. Chem. Soc. 2000, 2000, 271–274. [Google Scholar] [CrossRef]
- Garcia Santos, I.; Hagenbach, A.; Abram, U. Stable gold(III) complexes with thiosemicarbazone derivatives. Dalton Trans. 2004, 2004, 677–682. [Google Scholar] [CrossRef]
- Ortner, K.; Abram, U. Gold(III) complexes with diphenylthiocarbazonate. Synthesis and structures of [Au(Hdamp-C1) {PhNHNC(S)NNPh}Cl]Cl×H2O and [Au(Hdamp-C1){PhNNC(S)NNPh}(Smetetraz)] (Hdamp-C1=2-(dimethylammonium-methyl)phenyl; Smetetraz−=2-methylmercaptotetrazolate). Polyhedron 1999, 18, 749–754. [Google Scholar] [CrossRef]
- Abram, U.; Ortner, K.; Gust, R.; Sommer, K. Gold complexes with thiosemicarbazones: Reactions of bi- and tridentate thiosemicarbazones with dichloro[2-(dimethylaminomethyl)phenyl-C1,N]gold(III), [Au(damp-C1,N)Cl2]. J. Chem. Soc. Dalton Trans. 2000, 2000, 735–744. [Google Scholar] [CrossRef]
- Ortner, K.; Abram, U. Reactions of dichloro[2-(dimethylaminomethyl)phenyl-C1,N]gold(III), [Au(damp-C1,N)Cl2], with aromatic thiosemicarbazones. Structures and spectroscopical data of the first gold(III) thiosemicarbazone complexes. Inorg. Chem. Commun. 1998, 1, 251–253. [Google Scholar] [CrossRef]
- Maia, P.I.S.; Carneiro, A.; Lopes, C.D.; Oliveira, C.G.; Silva, J.S.; de Albuquerque, C.; Hagenbach, A.; Gust, R.; Deflon, V.M.; Abram, U. Organometallic gold(III) complexes with hybrid SNS-donating thiosemicarbazone ligands: Cytotoxicity and anti-Trypanosoma cruzi activity. Dalton Trans. 2017, 46, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Salsi, F.; Bulhões Portapilla, G.; Schutjajew, K.; Roca Jungfer, M.; Goulart, A.; Hagenbach, A.; de Albuquerque, S.; Abram, U. Organometallic Gold(III) Complexes with Tridentate Halogen-Substituted Thiosemicarbazones: Effects of Halogenation on Cytotoxicity and Anti-Parasitic Activity. Eur. J. Inorg. Chem. 2019, 2019, 4455–4462. [Google Scholar] [CrossRef]
- Mack, J.; Ortner, K.; Abram, U.; Parish, R.V. Gold(III) Complexes with 2- (N,N-dimethylaminomethy1)phenyl (damp−). Syntheses and Structures of [Au(damp-C,N)C12], [Au(damp-C,N)(OOCCH3)2] and [Au(damp-C,N)(mnt)] (mnt2− = 1.2-dicyanoethene-1.2-dithiolate). Z. Anorg. Allg. Chem. 1997, 623, 873–879. [Google Scholar] [CrossRef]
- Beyer, L.; Hoyer, E.; Hennig, H.; Kirmse, R.; Hartmann, H.; Liebscher, J. Synthese und Charakterisierung neuartiger Übergangsmetall-chelate von 1,1-Dialkyl-3-benzoyl-thioharnstoffen. J. Prakt. Chem. 1975, 317, 829–839. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Grewe, J.; Schroer, J.; Kuhn, B.; Abram, U. Rhenium and Technetium Complexes with Tridentate N-[(N′′,N′′-Dialkylamino)(thiocarbonyl)]-N′-substituted Benzamidine Ligands. Inorg. Chem. 2008, 47, 5136–5144. [Google Scholar]
- APEX 3 Software Suite, version 1.0; Bruker: Billerica, MA, USA, 2016.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Vicente, J.; Chicote, M.T.; Bermudez, M.D.; Sachez-Santano, M.J.; Jones, P.G. Synthesis of [2-{(dimethylamino)methyl}phenyl-C‘N]-(phenyl)gold(III) complexes. Crystal structure of two modifications of chloro[2-{(dimethylamino)methyl}phenyl-C’N](phenyl)gold(III). J. Organomet. Chem. 1988, 354, 381–390. [Google Scholar] [CrossRef]
- Henderson, W.; Nicholson, B.K.; Oliver, A.G. Synthesis and characterisation of four- and eight-membered ring auralactam complexes. J. Organomet. Chem. 2001, 620, 182–189. [Google Scholar] [CrossRef]
- Price, G.A.; Brisdon, A.K.; Randall, S.; Lewis, E.; Whittaker, D.W.; Pritchard, R.G.; Muryn, C.A.; Flower, K.R.; Quayle, P. Some insights into the gold-catalysed A3-coupling reaction. Organomet. Chem. 2017, 846, 251–262. [Google Scholar] [CrossRef]
- Dinger, M.B.; Henderson, W. Organogold(III) metallacyclic chemistry. Part 1. Synthesis of the first auraoxodimethylene methane(auracyclobutan-3-one) and aurathietane-3,3-dioxide complexes. Crystal structure of [{C6H3(CH2NMe2)-2-(OMe)-5}Au{CH(COPh)S(O)2CH(COPh)}].C6H6. J. Organomet. Chem. 1997, 547, 243–252. [Google Scholar] [CrossRef]
- Dinger, M.; Henderson, W.; Organogold(III) metallacyclic chemistry. Part 41. Synthesis, characterisation, and biological activity of gold(III)-thiosalicylate and -salicylate complexes. J. Organomet. Chem. 1998, 560, 233–243. [Google Scholar] [CrossRef]
- Price, G.A.; Flower, K.R.; Pritchard, R.G.; Brisdon, A.K.; Quayle, P. First structurally confirmed example of the formation of a gold(III) carbon bond via transmetallation with a boroxine. Dalton Trans. 2011, 40, 11696–11697. [Google Scholar] [CrossRef]
- Contel, M.; Nobel, D.; Spek, A.L.; van Koten, G. Reactivity of [2,6-Bis((dimethylamino)methyl)phenyl]gold(I), an Unusual Intermolecularly Stabilized Bis(amino)aryl Gold(I) Dimer, toward Alkyl Halides. X-ray Crystal Structures of Its Iodomethane and Methylene Diiodide Adducts. Organometallics 2000, 19, 3288–3295. [Google Scholar] [CrossRef]
- Vicente, J.; Bermudez, M.D.; Escribano, J.; Carillo, M.P.; Jones, P.G. Synthesis of Intermediates in the C-H Activation of Acetone with 2-PhenylazophenylgoId(iii) Complexes and in the C-C Coupling of Aryl Groups from Diarylgold(iii) Complexes. Crystal and Molecular Structures of [Au{C6H3(N=NC6H4Me-4′)-2-Me-5}(acac-C)CI] (acac = acetylacetonate), cis-[Au(C6H4N-NPh-2)Cl2(PPh3)], and [Au(C6H4CH2NMe2-2)(C6F5)Cl. J. Chem. Soc. Dalton Trans. 1990, 1990, 3083–3089. [Google Scholar]
- Bonnardel, P.A.; Parish, R.V.; Pritchard, R.G. Synthesis, characterisation and substitution reactions of gold(III) C,N-chelates. J. Chem. Soc. Dalton Trans. 1996, 1996, 3185–3193. [Google Scholar] [CrossRef]
- Contel, M.; Stol, M.; Casado, M.A.; van Klink, G.P.M.; Ellis, D.D.; Spek, A.L.; van Koten, G. A Bis(ortho-amine)aryl-Gold(I) Compound as an Efficient, Nontoxic, Arylating Reagent. Organometallics 2002, 21, 4556–4559. [Google Scholar] [CrossRef]
- Hejda, M.; Dostál, L.; Jambor, R.; Ruzicka, A.; Jirásko, R.; Holeceka, J. Synthesis, Structure and Transmetalation Activity of Various C,N-Chelated Organogold(I) Compounds. Eur. J. Inorg. Chem. 2012, 2012, 2578–2587. [Google Scholar] [CrossRef]
- Tang, H.; Saunders, G.C.; Henderson, W. Platinum(II), palladium(II), and gold(III) complexes of dianionic, secondary dithiooxamide ligands. J. Coord. Chem. 2019, 72, 2550–2561. [Google Scholar] [CrossRef]
- Vicente, J.; Chicote, M.T.; Martinez-Viviente, E.; Martinez-Martinez, A.J.; Sanchez-Moya, N. 2-(Aminomethyl)phenyl Complexes of Au(III), Mixed Au(III)/Ag(I), and Pd(II) with the 2,2-Diacetyl-1,1-Ethylenedithiolato Ligand: Dancing of Palladacycles around a Juggler Ligand. Inorg. Chem. 2010, 49, 8099–8111. [Google Scholar] [CrossRef]
- Parish, R.V.; Howe, B.P.; Wright, J.P.; Mack, J.; Pritchard, R.G. Chemical and Biological Studies of Dichloro(2-((dimethylamino)methyl)phenyl)gold(III). Inorg. Chem. 1996, 35, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Beyer, L.; Hoyer, E.; Liebscher, J.; Hartmann, H. Komplexbildung mit N-Acyl-thioharnstoffen. Z. Chem. 1981, 21, 81–91. [Google Scholar] [CrossRef]
- Koch, K.R. New chemistry with old ligands: N-alkyl- and N, N-dialkyl-N′-acyl(aroyl)thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord. Chem. Rev. 2001, 216−217, 473–488. [Google Scholar] [CrossRef]
- Nierstenhöfer, M.C.; Mohr, F. Palladium complexes with a chelating monoanionic sulfur ylide. J. Organomet. Chem. 2023, 990, 122659. [Google Scholar] [CrossRef]
- Hayes, T.R.; Powell, A.S.; Barnes, C.L.; Benny, P.D. Synthesis and stability of 2+1 complexes of N,N-diethylbenzoylthiourea with [MI(CO)3]+ (M = Re, 99mTc). J. Coord. Chem. 2015, 68, 3432–3448. [Google Scholar] [CrossRef]
- Sacht, C.; Datt, M.S.; Otto, S.; Roodt, A. Chiral and achiral platinum(II) complexes for potential use as chemotherapeutic agents: Crystal and molecular structures of cis-[Pt(L1)2] and [Pt(L1)Cl(MPSO)] [HL1 = N,N-diethyl-N’-benzoylthiourea]. J. Chem. Soc. 2000, 2000, 727–733. [Google Scholar] [CrossRef]
- Richter, R.; Beyer, L.; Kaiser, J. Crystal and Molecular Structure of Bis(l,l-diethyl-3-benzoyl-thioureato)copper(II). Z. Anorg. Allg. Chem. 1980, 461, 67–73. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Beyer, L. 1H-NMR-Untersuchung der behinderten Rotation um die C-N-Bindung in 1,1’-Diethyl-3-benzoyl-harnstoff-Derivaten. J. Prakt. Chem. 1975, 317, 938–942. [Google Scholar] [CrossRef]
- Beyer, L.; Behrendt, S.; Kleinpeter, E.; Borsdorf, R.; Hoyer, E. d-N.M.R. Studies of Hindered Rotation on N-C(X) Bond Increment. VIII. Metal Complexes of 1,1-Diethy1-3-benzoylthio-and selenourea. Z. Anorg. Allg. Chem. 1977, 437, 282–288. [Google Scholar] [CrossRef]
- Behrendt, S.; Beyer, L.; Dietze, F.; Kleinpeter, E.; Hoyer, E.; Ludwig, E.; Uhlemann, E. Rotational Barriers in Metal Chelates. Hindered Rotation about the Terminal NC(X)-Bond in Ni-Coordinated Isomeric Unsaturated 1,3-(S,O)-Ligands. Inorg. Chim. Acta 1980, 43, 141–144. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Hazin, K. Synthesis and Characterization of Unusual Oxidorhenium(V) Cores. Eur. J. Inorg. Chem. 2011, 2011, 78–82. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Deflon, V.M.; Deflon, V.M. Mixed-Ligand Complexes of Technetium and Rhenium with Tridentate Benzamidines and Bidentate Benzoylthioureas. Eur. J. Inorg. Chem. 2009, 2009, 3179–3187. [Google Scholar] [CrossRef]
- Vijayan, P.; Viswanathamurthi, P.; Sugumar, P.; Ponnuswamy, M.N.; Malecki, J.G.; Velmurugan, K.; Nandhakumar, R.; Balakumaran, M.D.; Kalaichelvan, P.D. Solvent-assisted formation of ruthenium(II)/copper(I) complexes containing thiourea derivatives: Synthesis, crystal structure, density functional theory, enzyme mimetics and in vitro biological perspectives. Appl. Organometal Chem. 2017, 31, e3652. [Google Scholar] [CrossRef]
- Barolli, J.P.; Maia, P.I.S.; Colina-Vegas, L.; Moreira, J.; Plutin, A.M.; Mocelo, R.; Deflon, V.M.; Cominetti, M.R.; Camargo-Mathias, M.I.; Batista, A.A. Heteroleptic tris-chelate ruthenium(II) complexes of N,N-disubstituted-N′-acylthioureas: Synthesis, structural studies, cytotoxic activity and confocal microscopy studies. Polyhedron 2017, 126, 33–41. [Google Scholar] [CrossRef]
- Gomes, L.R.; Santos, L.M.N.B.F.; Coutinho, J.A.P.; Schröder, B.; Low, J.N. N′-Benzoyl-N,N-diethylthiourea: A monoclinic polymorph. Acta Cryst. 2010, E66, o870. [Google Scholar] [CrossRef]
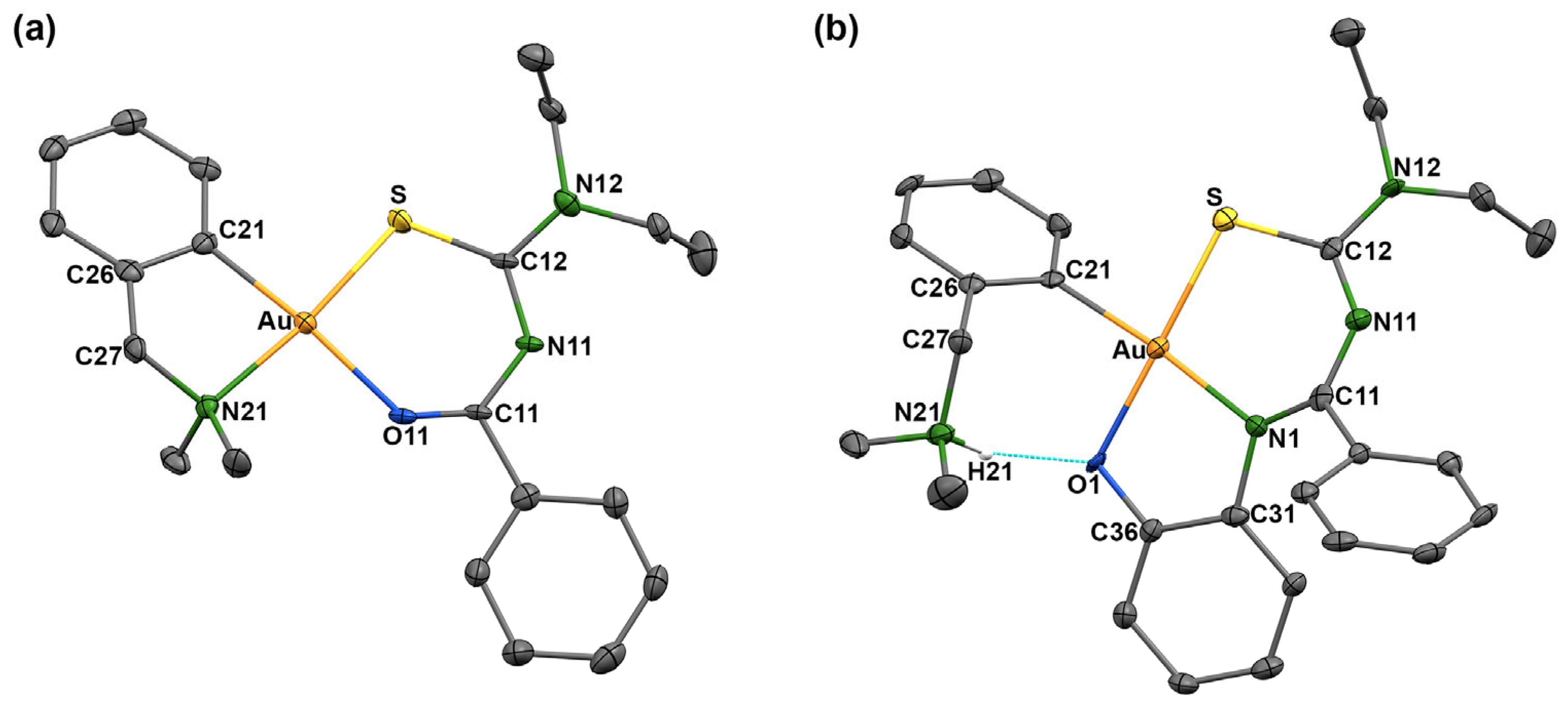
| [Au(damp-κC1,N)(L1-κS,O)]+ | |||||||
|---|---|---|---|---|---|---|---|
| Au–S | 2.266(1) | Au–O11 | 2.110(3) | Au–C21 | 2.005(4) | Au–N21 | 2.115(3) |
| O11–C11 | 1.183(5) | C11–N11 | 1.360(5) | N11–C12 | 1.349(5) | C12–N12 | 1.317(5) |
| C12–S | 1.759(4) | S–Au–O11 | 94.00(8) | S–Au–C21 | 91.2(1) | S–Au–N21 | 173.5(1) |
| O11–Au–C21 | 174.7(2) | O11–Au–N21 | 92.0(1) | C21–Au–N21 | 82.9(2) | Au–O11–C11 | 129.0(3) |
| O11–C11–N11 | 131.2(4) | C11–N11–C12 | 126.8(4) | N11–C12–N12 | 116.2(4) | N11–C12–S | 128.6(3) |
| C12–S–Au | 106.3(1) | Au–C21–C26 | 113.0(3) | Au–N21–C27 | 105.8(3) | ||
| [Au(Hdamp-κC1)(L2-κS,N,O)]+ | |||||||
| Au–S | 2.269(2) | Au–N1 | 2.050(5) | Au–O1 | 2.031(4) | Au–C21 | 2.041(6) |
| O1–C36 | 1.364(7) | C31–N1 | 1.437(7) | N1–C11 | 1.331(7) | C11–N11 | 1.338(8) |
| N11–C12 | 1.343(8) | C12–S | 1.745(6) | S–Au–N1 | 98.1(1) | S–Au–O1 | 179.3(1) |
| S–Au–C21 | 86.9(2) | N1–Au–O1 | 82.6(2) | N1–Au–C21 | 174.7(2) | O1–Au–C21 | 92.4(2) |
| Au–O1–C36 | 111.2(3) | O1–C36–C31 | 119.9(5) | C36–C31–N1 | 114.9(5) | C31–N1–Au | 110.5(4) |
| C31–N1–N11 | 125.2(5) | Au–N1–C11 | 110.5(4) | N1–C11–N11 | 125.3(5) | C11–N11–C12 | 130.0(5) |
| N11–C12–N12 | 115.4(5) | N11–C12–S | 127.4(5) | C12–S–Au | 103.3(5) | Au–C21–C26 | 121.6(4) |
| N21–H21 | 1.00 | O1∙∙∙H21 | 1.77 | O1∙∙∙H21–N21 | 150.2 | N21…O1 | 2.688(6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sucena, S.F.; Hagenbach, A.; Abram, U. Organogold(III) Complexes with Chelating Thiourea-Type Ligands. Chemistry 2025, 7, 174. https://doi.org/10.3390/chemistry7060174
Sucena SF, Hagenbach A, Abram U. Organogold(III) Complexes with Chelating Thiourea-Type Ligands. Chemistry. 2025; 7(6):174. https://doi.org/10.3390/chemistry7060174
Chicago/Turabian StyleSucena, Suelen Ferreira, Adelheid Hagenbach, and Ulrich Abram. 2025. "Organogold(III) Complexes with Chelating Thiourea-Type Ligands" Chemistry 7, no. 6: 174. https://doi.org/10.3390/chemistry7060174
APA StyleSucena, S. F., Hagenbach, A., & Abram, U. (2025). Organogold(III) Complexes with Chelating Thiourea-Type Ligands. Chemistry, 7(6), 174. https://doi.org/10.3390/chemistry7060174








