Pea-Derived Antioxidant Peptides: Applications, Bioactivities, and Mechanisms in Oxidative Stress Management
Abstract
1. Introduction
2. Antioxidant Activity of Pea Peptides
2.1. In Vitro Antioxidant Activity and Reliability of Pea Peptides
2.1.1. In Vitro Antioxidant Activity of Pea Peptides
2.1.2. Reliability of In Vitro Methods for Evaluating the Antioxidant Activity of Peptides
2.2. Cellular Antioxidant Activity of Pea Peptides
2.3. In Vivo Antioxidant Activity of Pea Peptides
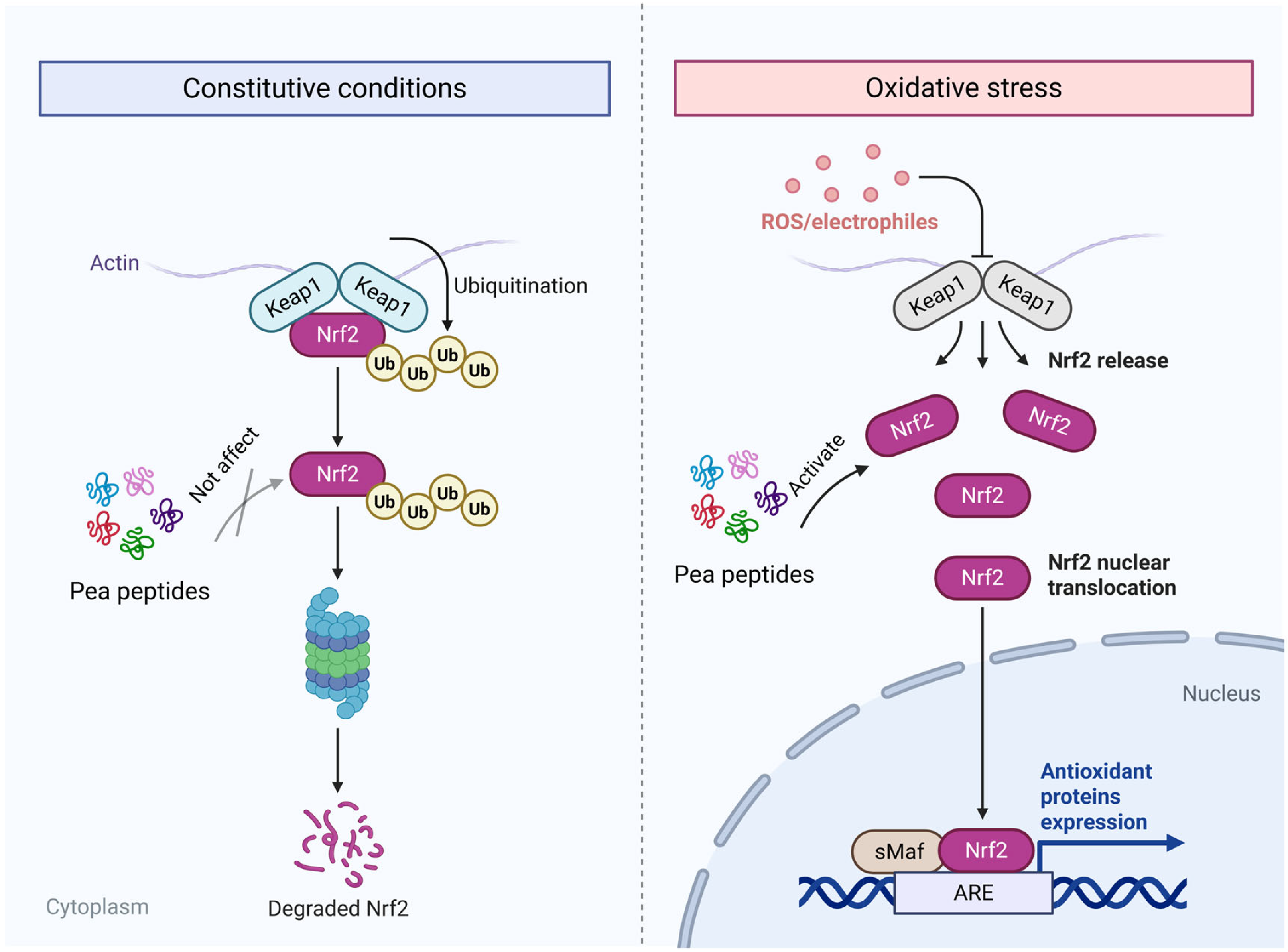
3. Antioxidant Mechanism of Pea Peptides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Omaye, S.T. Air pollutants, oxidative stress and human health. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.L.; Wang, B.; Wang, H.H.; Meng, L.B.; Zhao, Q.; Li, X.Y.; Xin, Y.; Jiang, X. Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms. Oxidative Med. Cell. Longev. 2019, 2019, 3010342. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.F.; Goto, S.; Koltai, E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol. Asp. Med. 2011, 32, 305–315. [Google Scholar] [CrossRef]
- Li, J.; Ran, X.C.; Zhou, M.D.; Wang, K.C.; Wang, H.; Wang, Y.Y. Oxidative stress and antioxidant mechanisms of obligate anaerobes involved in biological waste treatment processes: A review. Sci. Total Environ. 2022, 838, 156454. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Belyakov, V.A.; Fedorova, G.F.; Vasilev, R.F. A kinetic chemiluminescence study of the antioxidants evolved from polymeric materials into the gaseous-phase. J. Photochem. Photobiol. A Chem. 1993, 72, 73–81. [Google Scholar] [CrossRef]
- Fedorova, G.F.; Menshov, V.A.; Trofimov, A.V.; Tsaplev, Y.B.; Vasil’ev, R.F.; Yablonskaya, O.I. Chemiluminescence of Cigarette Smoke: Salient Features of the Phenomenon. Photochem. Photobiol. 2017, 93, 579–589. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costachescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S. Oxidative Stress and ROS Link Diabetes and Cancer. J. Mol. Pathol. 2024, 5, 96–119. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef]
- Chen, C.W.; Xia, P.P.; Gan, Y.M.; Zheng, X.H.; Yang, P.P.; Shi, A.; Liu, X.; Zhang, J.; Yu, P.; Zhang, D.J. Food-derived bioactive peptides as emerging therapeutic agents: Unlocking novel strategies for colorectal cancer treatment. Pharmacol. Res. 2025, 217, 107819. [Google Scholar] [CrossRef]
- Liu, J.B.; Han, L.; Cheng, D.K.; Li, S.R.; Chen, X.M.; Yu, Y.D.; Zhang, D.J.; Zhang, T. Incorporating ε-polylysine hydrochloride, tea polyphenols, nisin, and ascorbic acid into edible coating solutions: Effect on oxidation and structure of marinated egg proteins. Food Control 2024, 161, 110395. [Google Scholar] [CrossRef]
- Liu, J.B.; Cheng, D.K.; Zhang, D.J.; Han, L.; Gan, Y.M.; Zhang, T.; Yu, Y.D. Incorporating ε-Polylysine Hydrochloride, Tea Polyphenols, Nisin, and Ascorbic Acid into Edible Coating Solutions: Effect on Quality and Shelf Life of Marinated Eggs. Food Bioprocess Technol. 2022, 15, 2683–2696. [Google Scholar] [CrossRef]
- Domingos, A.K.; Saad, E.B.; Vechiatto, W.W.D.; Wilhelm, H.M.; Ramos, L.P. The influence of BHA, BHT and TBHQ on the oxidation stability of soybean oil ethyl esters (biodiesel). J. Braz. Chem. Soc. 2007, 18, 416–423. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Sewalt, V.J.H.; Robbins, K.L.; Houser, T.A. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005, 69, 289–296. [Google Scholar] [CrossRef]
- Botterweck, A.A.M.; Verhagen, H.; Goldbohm, R.A.; Kleinjans, J.; van den Brandt, P.A. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands cohort study. Food Chem. Toxicol. 2000, 38, 599–605. [Google Scholar] [CrossRef]
- Yang, X.X.; Sun, Z.D.; Wang, W.Y.; Zhou, Q.F.; Shi, G.Q.; Wei, F.S.; Jiang, G.B. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total Environ. 2018, 643, 559–568. [Google Scholar] [CrossRef]
- Dassarma, B.; Mahapatra, S.K.; Nandi, D.K.; Gangopadhyay, S.; Samanta, S. Protective role of butylated hydroxyanisole (BHA) and hydroxytoluene (BHT) against oxidative stress-induced inflammatory response in carbon tetrachloride-induced acute hepatorenal toxicity. Arch. Physiol. Biochem. 2025, 1–8. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Jiang, T.Y.; Xu, J.X.; Xi, W.J.; Shang, E.; Xiao, P.; Duan, J.A. The relationship between polysaccharide structure and its antioxidant activity needs to be systematically elucidated. Int. J. Biol. Macromol. 2024, 270, 132391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Li, Y.; Quan, Z.Z.; Xiao, P.; Duan, J.A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.J.; Yuan, Y.; Zeng, Q.D.; Xiong, J.; Gan, Y.M.; Jiang, K.; Xie, N. Plant protein-derived anti-breast cancer peptides: Sources, therapeutic approaches, mechanisms, and nanoparticle design. Front. Pharmacol. 2025, 15, 1468977. [Google Scholar] [CrossRef]
- Mao, Z.J.; Jiang, H.; Sun, J.A.; Zhao, Y.H.; Gao, X.; Mao, X.Z. Research progress in the preparation and structure-activity relationship of bioactive peptides derived from aquatic foods. Trends Food Sci. Technol. 2024, 147, 104443. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Wu, D.T.; Li, W.X.; Wan, J.J.; Hu, Y.C.; Gan, R.Y.; Zou, L. A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods 2023, 12, 2527. [Google Scholar] [CrossRef]
- Galves, C.; Gali, K.K.; Warkentin, T.; House, J.; Nickerson, M.T. High and low-protein pea genotypes: A comparative study of the composition, quality, and functionality of flour. Eur. Food Res. Technol. 2025, 251, 1279–1288. [Google Scholar] [CrossRef]
- Kumari, T.; Deka, S.C. Potential health benefits of garden pea seeds and pods: A review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Patil, C.; Awasthi, A.; Paul, M. Antioxidative and antimicrobial properties of pulse proteins and their applications in gluten-free foods and sports nutrition. Int. J. Food Sci. Technol. 2022, 57, 5571–5584. [Google Scholar] [CrossRef]
- Zhang, D.J.; Jiang, K.; Luo, H.; Zhao, X.R.; Yu, P.; Gan, Y.M. Replacing animal proteins with plant proteins: Is this a way to improve quality and functional properties of hybrid cheeses and cheese analogs? Compr. Rev. Food Sci. Food Saf. 2024, 23, e13262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.J.; Ling, X.L.; Jiang, K.; Zhao, X.R.; Gan, Y.M. Development of low-fat Mozzarella cheeses enriched with soy or pea protein hydrolysates: Composition, texture and functional properties during ageing. Int. J. Dairy Technol. 2024, 77, 165–182. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Tuccillo, F.; Lampi, A.M.; Knaapila, A.; Pulkkinen, M.; Kariluoto, S.; Coda, R.; Edelmann, M.; Jouppila, K.; Sandell, M.; et al. Flavor challenges in extruded plant-based meat alternatives: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2898–2929. [Google Scholar] [CrossRef]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re)formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Krause, S.; Asamoah, E.A.; Huc-Mathis, D.; Moulin, G.; Jakobi, R.; Rega, B.; Bonazzi, C. Applicability of pea ingredients in baked products: Links between formulation, reactivity potential and physicochemical properties. Food Chem. 2022, 386, 132653. [Google Scholar] [CrossRef]
- Husband, H.; Ferreira, S.; Bu, F.; Feyzi, S.; Ismail, B.P. Pea protein globulins: Does their relative ratio matter? Food Hydrocoll. 2024, 148, 109429. [Google Scholar] [CrossRef]
- Taylor, S.L.; Marsh, J.T.; Koppelman, S.J.; Kabourek, J.L.; Johnson, P.E.; Baumert, J.L. A perspective on pea allergy and pea allergens. Trends Food Sci. Technol. 2021, 116, 186–198. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Li, Y. Pea protein composition, functionality, modification, and food applications: A review. Adv. Food Nutr. Res. 2022, 101, 71–127. [Google Scholar]
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive Peptides and Hydrolysates from Pulses and Their Potential Use as Functional Ingredients. J. Food Sci. 2014, 79, R273–R283. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kadyan, S.; Ukhanov, V.; Cheng, J.J.; Nagpal, R.; Cui, L.Q. Recent advances in the health benefits of pea protein (Pisum sativum): Bioactive peptides and the interaction with the gut microbiome. Curr. Opin. Food Sci. 2022, 48, 100944. [Google Scholar] [CrossRef]
- Juárez-Chairez, M.F.; Cid-Gallegos, M.S.; Meza-Márquez, O.G.; Jiménez-Martínez, C. Biological functions of peptides from legumes in gastrointestinal health. A review legume peptides with gastrointestinal protection. J. Food Biochem. 2022, 46, e14308. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Decroos, K.; Van Wijmelbeke, L.; Verstraete, W. The impact of fermentation and in vitro digestion on the formation of angiotensin-I-converting enzyme inhibitory activity from pea and whey protein. J. Dairy Sci. 2003, 86, 429–438. [Google Scholar] [CrossRef]
- Aguilar, J.G.D.; de Castro, R.J.S.; Sato, H.H. Production of Antioxidant Peptides from Pea Protein Using Protease from Bacillus licheniformis LBA 46. Int. J. Pept. Res. Ther. 2020, 26, 435–443. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tang, Y.Q.; Cheng, H.; Zhang, J.J.; Zhang, S. Investigating structure, biological activity, peptide composition and emulsifying properties of pea protein hydrolysates obtained by cell envelope proteinase from Lactobacillus delbrueckii subsp. bulgaricus. Int. J. Biol. Macromol. 2023, 245, 125375. [Google Scholar] [CrossRef]
- Irankunda, R.; Echavarría, J.A.C.; El Hajj, S.; Paris, C.; Cakir-Kiefer, C.; Girardet, J.M.; Arnoux, P.; Muhr, L.; Canabady-Rochelle, L. Pisum Sativum protein hydrolysates: Production, Sensitive screening of nickel chelating peptides and evaluation of antioxidant properties. Food Biosci. 2025, 66, 106001. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Wong, E.A.; Webb, K.E. Board-invited review: Peptide absorption and utilization: Implications for animal nutrition and health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef]
- Li, N.N.; Yang, M.; Guo, Y.H.; Tong, L.T.; Wang, Y.Q.; Zhang, S.; Wang, L.L.; Fan, B.; Wang, F.Z.; Liu, L.Y. Physicochemical properties of different pea proteins in relation to their gelation ability to form lactic acid bacteria induced yogurt gel. LWT Food Sci. Technol. 2022, 161, 113381. [Google Scholar] [CrossRef]
- Ma, W.Y.; Zhang, C.M.; Kong, X.Z.; Li, X.F.; Chen, Y.M.; Hua, Y.F. Effect of pea milk preparation on the quality of non-dairy yoghurts. Food Biosci. 2021, 44, 101416. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.L. Purification, Identification and Evaluation of Antioxidant Peptides from Pea Protein Hydrolysates. Molecules 2023, 28, 2952. [Google Scholar] [CrossRef]
- Ding, J.; Liang, R.; Yang, Y.Y.; Sun, N.; Lin, S.Y. Optimization of pea protein hydrolysate preparation and purification of antioxidant peptides based on an in silico analytical approach. LWT 2020, 123, 109126. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of antioxidant peptides obtained from enzymatic pea protein hydrolysates and their ultrafiltration peptide fractions. J. Food Biochem. 2022, 46, e14289. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, M.A.; Tironi, V.A. Yellow pea flour and protein isolate as sources of antioxidant peptides after simulated gastrointestinal digestion. Legume Sci. 2020, 2, e59. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Pitre, M.; Karboune, S.; L’Hocine, L. Bioaccessibility and Antioxidant Activity of Faba Bean Peptides in Comparison to those of Pea and Soy after In Vitro Gastrointestinal Digestion and Transepithelial Transport across Caco-2 and HT29-MTX-E12 Cells. J. Agric. Food Chem. 2024, 72, 17953–17963. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Liu, H.R.; Wen, L.; Xu, Z.; Ding, L.; Cheng, Y.H.; Chen, M.L. Enhancing storage stability of pea peptides through encapsulation in maltodextrin and gum tragacanth via monitoring scavenge ability to free radicals. Int. J. Biol. Macromol. 2024, 276, 133736. [Google Scholar] [CrossRef]
- Qin, X.Y.; Zhang, J.T.; Li, G.M.; Zhou, M.; Gu, R.Z.; Lu, J.; Liu, W.Y. Structure and composition of a potential antioxidant obtained from the chelation of pea oligopeptide and sodium selenite. J. Funct. Foods 2020, 64, 103619. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.X.; Liu, F.R.; Ding, Y.Y.; Wang, R.; Luo, X.H.; Li, Y.N.; Chen, Z.X. Study of the functional properties and anti-oxidant activity of pea protein irradiated by electron beam. Innov. Food Sci. Emerg. Technol. 2017, 41, 124–129. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Liu, N.; Feng, C.X. Research on the effect of papain co-extrusion on pea protein and enzymolysis antioxidant peptides. J. Food Process. Preserv. 2017, 41, e13301. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, S.; Yang, F.; Zhou, K. Purification and characterization of antioxidative peptides prepared from pea protein with strong inhibitory activity on lipid oxidation. React. Oxyg. Species 2017, 3, 208–217. [Google Scholar] [CrossRef]
- Felix, M.; Perez-Puyana, V.; Romero, A.; Guerrero, A. Development of thermally processed bioactive pea protein gels: Evaluation of mechanical and antioxidant properties. Food Bioprod. Process. 2017, 101, 74–83. [Google Scholar] [CrossRef]
- Wah, L.L.H.; Flores, S.R.; Mosibo, O.K.; Aluko, R.E.; Fatoki, T.H.; Udenigwe, C.C. Peptide-Polyphenol Interactions: The Antagonistic Effect of Pea Pentapeptide (VNRFR) on the Antioxidant Properties of Quercetin and Rutin in Caenorhabditis elegans. Acs Food Sci. Technol. 2024, 4, 2080–2089. [Google Scholar] [CrossRef]
- Gallego, M.; Arnal, M.; Barat, J.M.; Talens, P. Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes. Foods 2021, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Kang, L.; Luo, D.X.; Fan, Y.T. Enhanced solubility, stability, bioaccessibility, and antioxidant activity of curcumin with hydrolyzed pea protein-based nano-micelles: pH-driven method vs ethanol-induced method. Int. J. Biol. Macromol. 2025, 291, 139106. [Google Scholar] [CrossRef]
- Girgih, A.T.; Chao, D.F.; Lin, L.; He, R.; Jung, S.; Aluko, R.E. Enzymatic protein hydrolysates from high pressure-pretreated isolated pea proteins have better antioxidant properties than similar hydrolysates produced from heat pretreatment. Food Chem. 2015, 188, 510–516. [Google Scholar] [CrossRef]
- Babini, E.; Tagliazucchi, D.; Martini, S.; Più, L.D.; Gianotti, A. LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins. Food Chem. 2017, 228, 186–196. [Google Scholar] [CrossRef]
- Cipollone, M.A.; Abraham, A.G.; Fontana, A.; Tironi, V.A. Autochthonous Fermentation as a Means to Improve the Bioaccessibility and Antioxidant Activity of Proteins and Phenolic Compounds of Yellow Pea Flour. Foods 2024, 13, 659. [Google Scholar] [CrossRef]
- Sun, W.Q.; Xiong, Y.L.L. Stabilization of cooked cured beef color by radical-scavenging pea protein and its hydrolysate. LWT Food Sci. Technol. 2015, 61, 352–358. [Google Scholar] [CrossRef]
- Wang, J.Q.; Hu, S.Z.; Nie, S.P.; Yu, Q.; Xie, M.Y. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants-A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Yu, W.C.; Zhao, L.X. Chemiluminescence detection of reactive oxygen species generation and potential environmental applications. Trac-Trends Anal. Chem. 2021, 136, 116197. [Google Scholar] [CrossRef]
- Bastos, E.L.; Romoff, P.; Eckert, C.R.; Baader, W.J. Evaluation of antiradical capacity by H2O2-hemin-induced luminol chemiluminescence. J. Agric. Food Chem. 2003, 51, 7481–7488. [Google Scholar] [CrossRef]
- Stanisavljevic, N.S.; Vukotic, G.N.; Pastor, F.T.; Suznjevic, D.; Jovanovic, Z.S.; Strahinic, I.D.; Fira, D.A.; Radovic, S.S. Antioxidant activity of pea protein hydrolysates produced by batch fermentation with lactic acid bacteria. Arch. Biol. Sci. 2015, 67, 1033–1042. [Google Scholar] [CrossRef]
- Anderson, D.; Phillips, B.J. Comparative in vitro and in vivo effects of antioxidants. Food Chem. Toxicol. 1999, 37, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Fahim, J.R.; Attia, E.Z.; Kamel, M.S. The phenolic profile of pea (Pisum sativum): A phytochemical and pharmacological overview. Phytochem. Rev. 2019, 18, 173–198. [Google Scholar] [CrossRef]
- Patil, N.D.; Bains, A.; Sridhar, K.; Sharma, M.; Dhull, S.B.; Goksen, G.; Chawla, P.; Inbaraj, B.S. Recent advances in the analytical methods for quantitative determination of antioxidants in food matrices. Food Chem. 2025, 463, 141348. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.T.; Dong, Q.; Yu, C.X.; Chen, H.Y.; Zhao, Y.; Zhang, B.S.; Yu, P.L.; Chen, M.J. Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants 2024, 13, 479. [Google Scholar] [CrossRef]
- Liu, J.B.; Chen, Z.F.; He, J.; Zhang, Y.; Zhang, T.; Jiang, Y.Q. Anti-oxidative and anti-apoptosis effects of egg white peptide, Trp-Asn-Trp-Ala-Asp, against H2O2-induced oxidative stress in human embryonic kidney 293 cells. Food Funct. 2014, 5, 3179–3188. [Google Scholar] [CrossRef]
- Ryu, B.I.; Kim, K.T. Antioxidant activity and protective effect of methyl gallate against t-BHP induced oxidative stress through inhibiting ROS production. Food Sci. Biotechnol. 2022, 31, 1063–1072. [Google Scholar] [CrossRef]
- Wu, J.H.; Sun, B.G.; Luo, X.L.; Zhao, M.M.; Zheng, F.P.; Sun, J.Y.; Li, H.H.; Sun, X.T.; Huang, M.Q. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Jin, J.E.; Chang, H.L.; Huang, K.C.E.; Chiang, Y.F.; Ali, M.; Hsia, S.M. Antioxidative Activity of Soy, Wheat and Pea Protein Isolates Characterized by Multi-Enzyme Hydrolysis. Nanomaterials 2021, 11, 1509. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Cui, C.X.; Yan, F.; Li, N.; Sun, X.F.; Wang, F.Y.; Wang, C.F. In Vitro Protective Effect of Pea-Derived Peptides (PPs) via the Keap1/Nrf2 Signaling Pathway on Alpha-Gliadin-Sensitizing Peptide Induced Cacao-2 Cells. Mol. Nutr. Food Res. 2024, 68, e2400010. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wen, L.D.; Wang, F.Y.; Li, T.E.; Zheng, H.D.; Wang, T.L.; Qiao, M.W.; Huang, X.Q.; Song, L.J.; Bukyei, E.; et al. Alleviating effects of pea peptide on oxidative stress injury induced by lead in PC12 cells via Keap1/Nrf2/TXNIP signaling pathway. Front. Nutr. 2022, 9, 964938. [Google Scholar] [CrossRef]
- Wang, X.; Bhullar, K.S.; Fan, H.B.; Liao, W.; Qiao, Y.J.; Su, D.; Wu, J.P. Regulatory Effects of a Pea-Derived Peptide Leu-Arg-Trp (LRW) on Dysfunction of Rat Aortic Vascular Smooth Muscle Cells against Angiotensin II Stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef]
- Hsieh, L.S.; Hsu, Y.C.; Chiang, W.D. Neuroprotective peptides isolated from flavourzyme-pea protein hydrolysate protect human SH-SY5Y cells from Aß1-42 induced apoptosis. J. Funct. Foods 2023, 108, 105755. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.X.; Wei, Y.; Cai, M.Y.; Gu, R.Z.; Wang, Y.C.; Ma, Y.; Chen, L. Pea-derived peptides, VLP, LLP, VA, and LL, improve insulin resistance in HepG2 cells via activating IRS-1/PI3K/AKT and blocking ROS-mediated p38MAPK signaling. J. Food Biochem. 2020, 44, e13454. [Google Scholar] [CrossRef]
- Zhang, J.J.; Song, L.J.; Li, T.G.; Zhu, L.; Wang, T.L.; Zhao, P.J.; Ma, Y.; Zhao, J.S.; Huang, X.Q. Steam explosion modified pea peptides alleviates hepatosteatosis by regulating lipid metabolism pathways and promoting autophagy. Food Res. Int. 2025, 208, 116182. [Google Scholar] [CrossRef]
- Zhang, D.J.; Xiong, J.; Zhao, X.R.; Gan, Y.M. Anti-fatigue activities of γ-aminobutyric acid-enriched soymilk in an acute exercise-treated mouse model via regulating AMPK/PGC-1a pathway. Food Biosci. 2023, 55, 103060. [Google Scholar] [CrossRef]
- Zhang, D.J.; Yuan, Y.; Xiong, J.; Zeng, Q.D.; Gan, Y.M.; Jiang, K.; Xie, N. Anti-breast cancer effects of dairy protein active peptides, dairy products, and dairy protein-based nanoparticles. Front. Pharmacol. 2024, 15, 1486264. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Luo, J.J.; Qi, J.Y.; Zhao, X.Y.; An, P.; Luo, Y.T.; Wang, G.S. The Role and Mechanism of Polysaccharides in Anti-Aging. Nutrients 2022, 14, 5330. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Wang, Y.Y.; Deng, S.L.; Lian, Z.X.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Feng, A.Q.; Zhao, Z.W.; Liu, C.F.; Du, C.; Gao, P.Y.; Liu, X.G.; Li, D.Q. Study on characterization of Bupleurum chinense polysaccharides with antioxidant mechanisms focus on ROS relative signaling pathways and anti-aging evaluation in vivo model. Int. J. Biol. Macromol. 2024, 266, 131171. [Google Scholar] [CrossRef]
- Feng, T.; Huang, Y.Y.; Tang, Z.H.; Wei, D.D.; Mo, J.M. Anti-fatigue effects of pea (Pisum sativum L.) peptides prepared by compound protease. J. Food Sci. Technol. 2021, 58, 2265–2272. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef]
- Wei, S.S.; Pei, Y.C.; Wang, Y.R.; Guan, H.; Huang, Y.L.; Xing, T.; Johnson, R.W.; Wang, D.Y. Role of human Keap1 S53 and S293 residues in modulating the binding of Keap1 to Nrf2. Biochimie 2019, 158, 73–81. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhang, Y.; Zhang, M.Y.; Yu, C.F.; Yang, P.P.; Xu, M.X.; Ling, J.T.; Wu, Y.T.; Zhu, Z.C.; Chen, Y.X.; et al. Food-derived peptides as novel therapeutic strategies for NLRP3 inflammasome-related diseases: A systematic review. Crit. Rev. Food Sci. Nutr. 2025, 65, 1433–1464. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhang, J.; Yan, J.W.; Qi, X.R.; Wang, Y.H.; Zheng, Z.T.; Liang, J.Q.; Ling, J.T.; Chen, Y.X.; Tang, X.Y.; et al. Application of fermented Chinese herbal medicines in food and medicine field: From an antioxidant perspective. Trends Food Sci. Technol. 2024, 148, 104410. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Li, M.Y.; Chiu, J.F.; Kelsen, A.; Lu, S.C.; Fukagawa, N.K. Identification and Characterization of an Nrf2-Mediated ARE Upstream of the Rat Glutamate Cysteine Ligase Catalytic Subunit Gene (GCLC). J. Cell. Biochem. 2009, 107, 944–954. [Google Scholar] [CrossRef]
- Hu, B.; Chen, R.; Jiang, M.; Xiong, S.T.; Xie, A.; Liu, X.Q.; Fu, B. MTX-211 Inhibits GSH Synthesis through Keap1/NRF2/GCLM Axis and Exerts Antitumor Effects in Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 7608. [Google Scholar] [CrossRef]
| Sources | Processing | Sequences | Antioxidant Capacity | References |
|---|---|---|---|---|
| PPH | 7% pea protein, 3.8% (enzyme/substrate ratio) alcalase protease, 50 °C for 3 h | YLVN, EEHLCFR, TFY | DPPH: IC50: EEHLCFR = 0.027 mg/mL; TFY = 1.492 mg/mL; ABTS: IC50: YLVN = 0.002 mg/mL; EEHLCFR = 0.019 mg/mL; TFY = 0.006 mg/mL; OH·: IC50: EEHLCFR = 2.796 mg/mL; O2-: IC50: YLVN = 1.357 mg/mL; EEHLCFR = 1.247 mg/mL; ORAC: IC50: YLVN = 1.12 μmol TE/μmol Peptide; EEHLCFR = 0.921 TE/μmol Peptide; TFY = 0.484 µmol TE/μmol Peptide | [52] |
| PPH | Alkaline proteases; substrate concentration (2, 3, 4, and 5%), enzyme to substrate ratio (4, 6, 8, 10, and 12%), temperature (40, 45, 50, 55, and 60 °C), and pH (7.0, 8.0, 9.0, and 10.0) | YSSPIHIW, ADLYNPR, HYDSEAILF, AGVLPGIK, GHYPNPDIEYG, SQISPLPVLK, KFTPPHVI, KINPDAPLDKV, RDDNEDLRVL, HTDADYILV; ATDDQIMDGVR, QIENPVKEL, HIISPELQ, TVVVNFSV | DPPH increased as enzyme to substrate ratio increase; DPPH increased and then decreased as pH, temperature and substrate concentration increase; the antioxidant activity of peptides followed the order of YSSPIHIW > ADLYNPR > HYDSEAILF | [53] |
| PPH | Alcalase (50 °C, pH 8.0), pepsin (37 °C, pH 2.0), trypsin (37 °C, pH 8.0), chymotrypsin (37 °C, pH 8.0), flavourzyme (50 °C, pH 8.0), and pancreatin (37 °C, pH 7.5) | Pea protein peptides with MW < 1 kDa and 1–3 kDa | High HRSA and SRSA, high linoleic acid peroxidation inhibition activity | [54] |
| PPH | pH 6, 6.58 and 8.0, 60 °C for 120 min | Peptide mixture | High FRAP, ORAC and DPPH value | [46] |
| PPIH | Sample was mixed with 3.5 mL of electrolite solution, 0.5 mL of α-amylase, 25 μL 0.3 mol/L CaCl2, and 975 μL H2O and incubated at 37 °C for 2 min. After that, the resulting solution was mixed with 7.5 mL of simulated gastric fluid and 1.6 mL of pepsin solution at 37 °C for 2 h and then mixed with 11.0 mL of the simulated intestinal fluid and 5.0 mL of pancreatin solution at 37 °C for 2 h | Peptide mixture | ORAC: ranged from 0.07 to 0.31 mg/mL; IC50 of HRSA: 3–10 mg/mL | [55] |
| Pea flour | 1.2 g portion of legume flour was mixed with 1.8 g of water, and the suspension was mixed in a ratio 1:1 with digestive fluid (oral, gastric, duodenal, and jejunal-ileal digestion phases) to perform the digestion | NYDEGSEPR, NQLDSTPR, EDVPNHGT, GGSSTHPYP, NDLGNPDHGEH, LGNPDSGENH, NDLGNPDSGENH, IGANEPSEH, LGNPDSGENH, NDLGNPDHGEH, NYDEGSEPR, YDEGSEPR, NDLGNPDHGEH, SDDEDTAPPR, GDGMPGGGSNGSGPGPK, QEEDEDEEKQPR, KEDEDEDEEEEE, NDLGNPDSGENH | Pea peptides had lower antioxidant capacity than faba bean, but higher antioxidant capacity than soybean | [56] |
| Pea peptides | Purchased from Shuangta Biochemical Technology | 200–5000 Da | The HRSA, SRSA and ABTS activity of pea peptides were improved after encapsulated in maltodextrin and gum tragacanth | [57] |
| PPH | pH 6.2, 0–8 h in a 43 °C water bath | GRNEDEEKGAIVKVKGGL, GRRGGQQQEEESEEQNEGNSVLSG, KDFPFPSSAL, LGGNPETEFPETQEEQQGRHRQ, QRPVKELAFPG, RRGGQQQEEESEEQNEGNSVL, RRGGQQQEEESEEQNEGNSVLSGF, SLDLWDPFQ, SLDVWDPLK | IC50: 0.774 mg/mL (DPPH), 0.305 mg/mL (ABTS) | [47] |
| Pea oligopeptide | Alcalase 2.4 L, pH 9.0, 80 °C for 30 min | TGRGAP, PPKIYP, HQMPKP, TSSLP | Se-pea oligopeptide had higher DPPH and HRSA than pea oligopeptide and sodium selenite | [58] |
| PPH | 1% Alcalase® (1 h, pH 8.0) + Flavourzyme® (1 h, pH 7.0) or Protamex® (2 h, pH 7.0) + Flavourzyme® (2 h, pH 7.0), 55 °C | APQE, ELTP, LPQ, LSSIL, NVISQ, PNY, QLEEL, SEPFN, SLSLL, SPDIY, TPGEVL, TPVIA | IC50: 14.57–14.99 mg/mL (iron chelating activity); 4.24–5.62 mg/mL (reducing activity); 0.041–0.045 mg/mL (ABTS) | [48] |
| PPH | Enzyme to substrate ratio 1:100, pH 7.0, 50 °C, 1.5 h | >5 kDa; 3–5 kDa; 1–3 kDa; <1 kDa | 55% (DPPH); the 50 kGy pre-irradiated hydrolysates have the strongest ABTSscavenging effect | [59] |
| PPH | Enzyme concentration of 12.0%, temperature of 60.2 °C, pH 6.5, substrate concentration of 7.1% | Peptide mixture | 98.1% (DPPH) at the optimal hydrolysis conditions | [60] |
| PPH | 2% enzyme/substrate (neutral protease/validase/alkaline protease), 6 h, 55 °C, pH 7.0 | >10 kDa; 3–10 kDa; 1–3 kDa; <1 kDa | Peptides with MW < 1 kDa had the highest ORAC (55.2–81.6 μmol TE/g), DPPH (70.5%), ABTS (52.5 μmol TE/g) and lipid peroxidation values | [61] |
| PPH | 1% enzyme/substrate, pH 8.0, 25 min/120 min | Peptide mixture | The lowest antioxidant activity was found at pH 8.0; when the hydrolysis takes place to a great extent, the antioxidant activity decreases significantly against any reagent (DPPH, ABTS or FC) | [62] |
| Pea Pentapeptide | Chemical synthesis | VNRFR | VNRFR had lower DPPH and ABTS compared to quercetin and rutin; but when VNRFR was bound with quercetin or rutin, their antioxidant capacity decreased due to antagonistic effects between them | [63] |
| Pea seed hydrolysate | The seeds were cooked in water at a legume:water ratio of 1:6 (w/v), by three different household processing methods: (a) ordinary cooking, at 100 °C for 40 min; (b) pressure cooking, at 8.7 psi for 15 min; and (c) microwave cooking, at 800 W for 30 min | Peptide mixture | Peptide mixture had high DPPH, ABTS and FRAP value | [64] |
| PPH | 30 min, 50 °C, pH 7.0, enzyme/substrate (E/S) ratio of 1:50 | Peptide mixture | The ABTS and reducing powder of PPH increased after loading with curcumin and treated using pH-driven method and ethanol-induced method | [65] |
| PPH | 4% alcalase, 4 h, pH 4.0, 50 °C | Peptide mixture | The ORAC, DPPH, SRSA, HRSA, FRAP and MCA of PPH increased after 400 and/or 600 Mpa pretreatment; heat pretreatment of PPH resulted in reduced DPPH | [66] |
| PPH | pH 8 and 55 °C for Alcalase; pH 6.5 and 40 °C for Neutrase; pH 6 and 60 °C for Flavourzyme | TVTSLDLPVLRW, IGPSSSPDIYNPEAGRIK, ENLQNYRLL, GPIYSNEFGKFF, AEYVRLY, TVTSLDLPVLRW, NIGPSSSPDIYNPEAGRIK, AMFVPH, GPIYSNEFGKFF, NILEASYNTR | The ABTS, DPPH, SRSA and HRSA of TVTSLDLPVLRW were 579.5, 5078.2, 422.0 and 1533.8 mmol GSH/mol peptide, respectively; AEYVRLY were 492.3, 5316.4, 1394.4 and 1584.7 mmol GSH/mol peptide, respectively; and FVPY were 491.9, 6108.4, 1088.7 and 976.3 mmol GSH/mol peptide, respectively | [67] |
| Yellow pea flour hydrolysates | In the first one, flour was dispersed in distilled water in a ratio of 1/1.75 (similar to tube assay), and the mixture was incubated at 30 °C for 24 h (FF1). In the second, the flour/distilled water ratio was 1/6, and the fermentation was conducted at 37 °C for 24 h (FF2) | Peptide mixture | IC50: FF1: 0.071 mg SP/mL, FF2: 0.033 mg SP/mL (ORAC) | [68] |
| PPH | 0.5 h, flavourzyme, 50 °C, pH 6.0, 1% enzyme-protein ratio | Peptide mixture | PPH had higher ABTS and reducing power than PF and PPI; the production of TBARS and protein carbonyls were inhibited | [69] |
| Sources | Processing | Sequences | Model | Antioxidant Capacity | Mechanism | References |
|---|---|---|---|---|---|---|
| Pea peptides | Chemical synthesis | EFEGMTFLL, KGOTPLFPR, KYSSPIHIW, KKADLYNPR, EHYDSEAILF, KYGPTPVRDGFK | Pb treated PC12 cells | Pea peptides increased cell viability and inhibited ROS generation; pea peptides increased SOD, CAT, GR and GSH-Px accumulation and inhibited MDA levels | Activating Nrf2 pathway | [84] |
| Pea peptides | Chemical synthesis | KEDDEEEEQGEEE, GQTPLFPR, IPVNRPGQLQ, VTPGATDDQIMDGVRK, YGPTPVRDGFK, HYDSEAILF, ADLYNPR, IR, YSSPIHIW, KF, EF | Caco-2 cells | Cell viability, SOD, CAT, GST, GSH-Px ↑; ROS, MDA ↓ | Activating Nrf2 pathway | [83] |
| PPH | Bromelain (1000 CDU/mL), Neutrase (0.0024 AU-N/mL) and Flavourzyme (3.3 LAPU/mL); 45 °C for 24 h | Peptide mixture | H2O2-injured C2C12 cells | Cell viability, crystal violet intensity ↑ | / | [82] |
| PPH | 1% (w of enzyme/w of pea protein) Flavourzyme® at 50 °C for 6 h | NKFGKFF, GGPFKSPF and RPVLGGSSTFPYP | Retinoic acid-injured human neuroblastoma SH-SY5Y cells | Cell viability ↑; ROS ↓ | Nrf2/HO-1 pathway ↑ | [86] |
| Pea Pentapeptide | Chemical synthesis | VNRFR | C. elegans species exposed to fresh NGM medium plates containing 400 mM juglone | VNRFR improved survival rate of C. elegans species and inhibited its ROS production | / | [63] |
| Pea peptides | Chemical synthesis | VLP, LLP, VA, LL | HepG2 containing insulin | Cell viability increased at low peptide concentrations but decreased at high VA and LL concentrations; ROS ↓ | / | [87] |
| PPH | 7% pea protein, 3.8% (enzyme/substrate ratio) alcalase protease, 50 °C for 3 h | YLVN, EEHLCFR, TFY | 0.5, 1, 2, 4, 6, and 8 mmol/L of H2O2 injured LO2 cells; | Cell viability, GSH-Px, CAT, SOD ↑; ROS ↓ | Binding with keap1; binding energy: YLVN = −8.2 kcal/mol; EEHLCFR = −7.2 kcal/mol; TFY = −8.9 kcal/mol | [52] |
| Pea peptides | The pea protein was loaded into the steam explosion equipment and treated at 1.0 MPa for 40 s | Peptide mixture | HepG2 cells treated with FFA; C57BL/6 J mice fed with high-fat diet | Cell viability ↑ | Nrf2/HO-1 pathway ↑ | [88] |
| Pea peptides | Chemical synthesis | LRW | A7r5 cells treated with Ang II | Ang II-stimulated oxidative stress ↓ | NF-κB pathway ↓ | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Xie, N.; Zhang, D. Pea-Derived Antioxidant Peptides: Applications, Bioactivities, and Mechanisms in Oxidative Stress Management. Chemistry 2025, 7, 141. https://doi.org/10.3390/chemistry7050141
Gan Y, Xie N, Zhang D. Pea-Derived Antioxidant Peptides: Applications, Bioactivities, and Mechanisms in Oxidative Stress Management. Chemistry. 2025; 7(5):141. https://doi.org/10.3390/chemistry7050141
Chicago/Turabian StyleGan, Yiming, Ni Xie, and Deju Zhang. 2025. "Pea-Derived Antioxidant Peptides: Applications, Bioactivities, and Mechanisms in Oxidative Stress Management" Chemistry 7, no. 5: 141. https://doi.org/10.3390/chemistry7050141
APA StyleGan, Y., Xie, N., & Zhang, D. (2025). Pea-Derived Antioxidant Peptides: Applications, Bioactivities, and Mechanisms in Oxidative Stress Management. Chemistry, 7(5), 141. https://doi.org/10.3390/chemistry7050141








