Recent Advances in Materials for Uranium Extraction from Salt Lake Brine: A Review
Abstract
1. Introduction
2. Adsorption-Based Materials for Uranium Recovery
2.1. Inorganic and Carbon-Based Materials
2.2. Organic Polymers and Functional Group-Modified Materials
2.3. Biomass-Derived and Environmentally Friendly Adsorbents
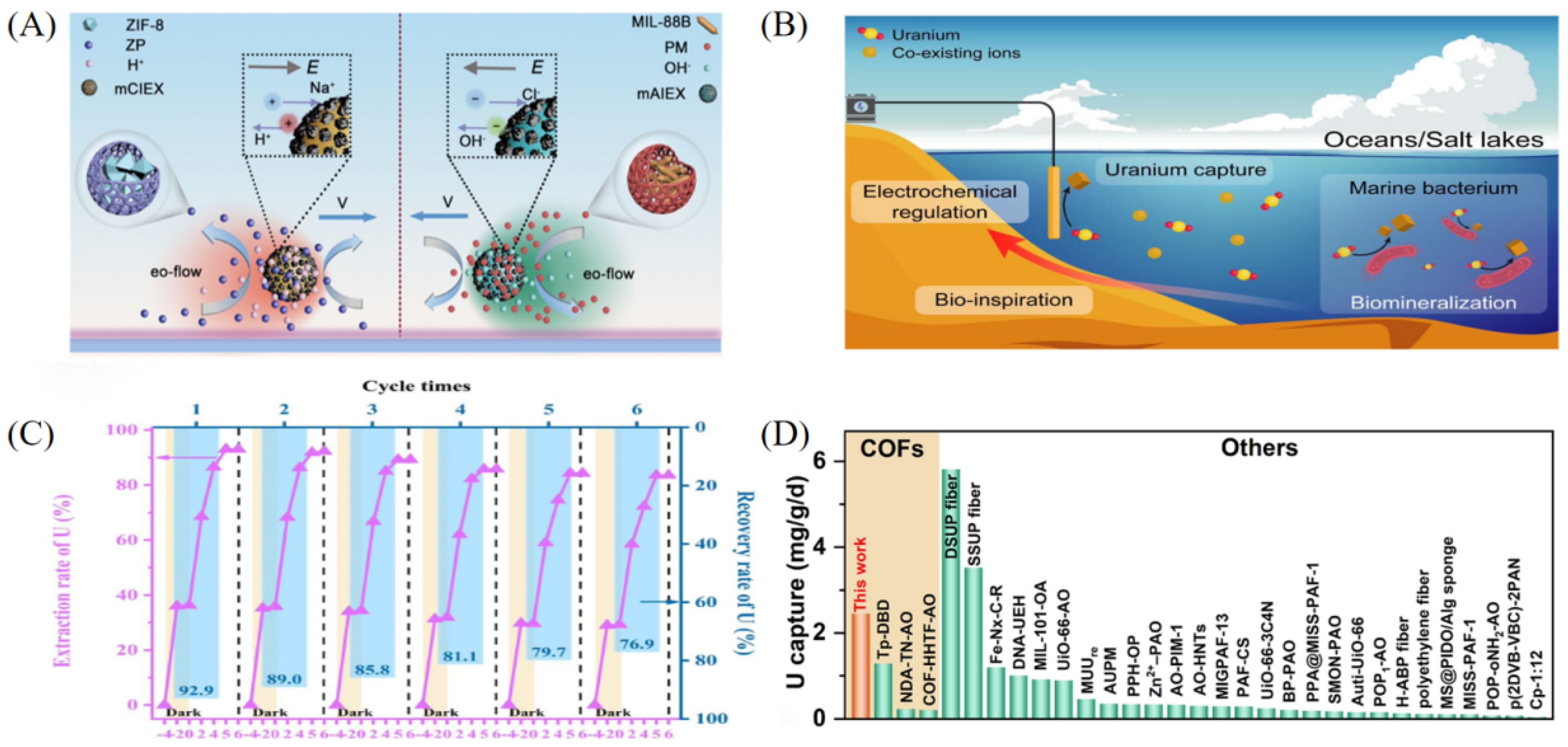
3. Applications of Other Materials in Uranium Extraction from Salt Lake Brine
4. Conclusions
Funding
Conflicts of Interest
References
- Alšauskas, O. World Energy Outlook 2024; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Wei, N.; Zheng, H.; Zhao, J.; Luo, P.; Luo, Q. The application progress and future recommendations of nuclear energy in the marine resource development field. Prog. Nucl. Energy 2025, 188, 105862. [Google Scholar] [CrossRef]
- Liu, B.; Peng, B.; Lu, F.; Hu, J.; Zheng, L.; Bo, M.; Shang, X.; Liu, W.; Zhang, Y.; Zhou, X.; et al. Critical review of nuclear power plant carbon emissions. Front. Energy Res. 2023, 11, 1147016. [Google Scholar] [CrossRef]
- Nuclear Energy Agency. Uranium 2022: Resources, Production and Demand; OECD Publishing: Paris, France, 2023. [Google Scholar]
- Li, L.; Hu, Z.; Guo, W.; Xia, H.; Wang, Y.; Wang, S.; Chen, G.; Qiu, M.; Hu, B. Recent advances in various adsorbents for the extraction of uranium from saline lakes: A review. J. Mol. Liq. 2024, 395, 123862. [Google Scholar] [CrossRef]
- Bai, J.; Chu, J.; Yin, X.; Wang, J.; Tian, W.; Huang, Q.; Jia, Z.; Wu, X.; Guo, H.; Qin, Z. Synthesis of amidoximated polyacrylonitrile nanoparticle/graphene composite hydrogel for selective uranium sorption from saline lake brine. Chem. Eng. J. 2020, 391, 123553. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, P.; Li, X.; Ning, Y.; Tan, L.; Edwards, R.L.; Yao, X.; Cheng, H. Distribution characteristics and influencing factors of uranium isotopes in saline lake waters in the northeast of Qaidam Basin. Minerals 2020, 10, 74. [Google Scholar] [CrossRef]
- Han, J.; Jiang, H.; Xu, J.; Hussain, S.A.; Yuan, X.; Qin, X. Hydraulic connection affects uranium distribution in the Gas Hure salt lake, Qaidam Basin, China. Environ. Sci. Pollut. Res. 2018, 25, 4881–4895. [Google Scholar] [CrossRef]
- Liu, N.; Liang, H.; Tian, W.; Li, C.; Gao, Q.; Wang, N.; Guo, R.; Mo, Z. An antibacterial and antifouling amidoxime-functionalized graphene oxide aerogel for selective uranium adsorption in Salt Lake water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129367. [Google Scholar] [CrossRef]
- Amralinova, B.; Agaliyeva, B.; Lozynskyi, V.; Frolova, O.; Rysbekov, K.; Mataibaeva, I.; Mizernaya, M. Rare-metal mineralization in salt lakes and the linkage with composition of granites: Evidence from Burabay rock mass (Eastern Kazakhstan). Water 2023, 15, 1386. [Google Scholar] [CrossRef]
- Altay, M.B.; Kalıpçıoğlu, C.; Kurt, Z. Comparative life cycle assessment of uranium recovery from brine. Resour. Conserv. Recycl. 2022, 181, 106237. [Google Scholar] [CrossRef]
- Timofeev, A.; Migdisov, A.A.; Williams-Jones, A.E.; Roback, R.; Nelson, A.T.; Xu, H. Uranium transport in acidic brines under reducing conditions. Nat. Commun. 2018, 9, 1469. [Google Scholar] [CrossRef] [PubMed]
- Gorman-Lewis, D.; Burns, P.C.; Fein, J.B. Review of uranyl mineral solubility measurements. J. Chem. Thermodyn. 2008, 40, 335–352. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhang, Y.; Wang, T.; Gao, P.; Lu, X. Uranyl speciation in carbonate-rich hydrothermal solutions: A molecular dynamics study. Inorg. Chem. 2024, 64, 50–57. [Google Scholar] [CrossRef]
- Lei, M.; Guo, J.; Chen, Q.; Liu, C.; Li, B.; Li, Y. Constructing a novel Ti3C2Tx nanocomposite via intercalation of guanidine phosphate to realize selective and effective uranyl extraction. Sep. Purif. Technol. 2025, 366, 132771. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Liu, S.; Zhou, Y.; Liu, D.; Fu, C.; Ye, L.; Fu, Y. UO22+ capture using amidoxime grafting low-cost activated carbon (AO-AC) from solution: Adsorption kinetic, isotherms and interaction mechanism. Inorg. Chim. Acta 2023, 544, 121226. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Z.; Geng, Y.; Li, H.; Wang, N.; Song, Y.; Wang, X.; Chen, J.; Wang, J.; Ma, S.; et al. Uranium extraction from seawater: Material design, emerging technologies and marine engineering. Chem. Soc. Rev. 2023, 52, 97–162. [Google Scholar] [CrossRef]
- Naik, M.-U.-D. Adsorbents for the uranium capture from seawater for a clean energy source and environmental safety: A review. ACS Omega 2024, 9, 12380–12402. [Google Scholar] [CrossRef] [PubMed]
- Sodaye, H.; Nisan, S.; Poletiko, C.; Prabhakar, S.; Tewari, P. Extraction of uranium from the concentrated brine rejected by integrated nuclear desalination plants. Desalination 2009, 235, 9–32. [Google Scholar] [CrossRef]
- Zhang, S.; Kano, N.; Mishima, K.; Okawa, H. Adsorption and desorption mechanisms of rare earth elements (REEs) by layered double hydroxide (LDH) modified with chelating agents. Appl. Sci. 2019, 9, 4805. [Google Scholar] [CrossRef]
- Feng, S.; Du, X.; Bat-Amgalan, M.; Zhang, H.; Miyamoto, N.; Kano, N. Adsorption of REEs from aqueous solution by EDTA-chitosan modified with zeolite imidazole framework (ZIF-8). Int. J. Mol. Sci. 2021, 22, 3447. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Mittal, H.; Alfantazi, A.; Alhassan, S.M. Recent developments in the adsorption of uranium ions from wastewater/seawater using carbon-based adsorbents. J. Environ. Chem. Eng. 2024, 12, 111705. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.; Chen, C. Adsorption of radionuclides on carbon-based nanomaterials. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 29, pp. 141–215. [Google Scholar]
- Mohamud, H. The Use of Carbon-Based Nanomaterials for the Removal of Radionuclides from Aqueous Solution. Ph.D. Thesis, University of Surrey, Guildford, UK, 2020. [Google Scholar]
- Li, Y.; Chen, J.; Cai, P.; Wen, Z. An electrochemically neutralized energy-assisted low-cost acid-alkaline electrolyzer for energy-saving electrolysis hydrogen generation. J. Mater. Chem. A 2018, 6, 4948–4954. [Google Scholar] [CrossRef]
- Nezhad, M.M.; Semnani, A.; Tavakkoli, N.; Shirani, M. Selective and highly efficient removal of uranium from radioactive effluents by activated carbon functionalized with 2-aminobenzoic acid as a new sorbent. J. Environ. Manag. 2021, 299, 113587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.; Zhang, H.; Liu, Q.; Yu, J.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Anti-bacterial and super-hydrophilic bamboo charcoal with amidoxime modified for efficient and selective uranium extraction from seawater. J. Colloid Interface Sci. 2021, 598, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kim, K.H. Graphene-based materials for the adsorptive removal of uranium in aqueous solutions. Environ. Int. 2022, 158, 106944. [Google Scholar] [CrossRef]
- Jia, Z.; Cui, Y.; Chu, J.; Huang, Q.; Wang, J.; Tian, W.; Wu, X.; Qin, Z.; Bai, J. Amidoximated graphene/konjac glucomannan composite gel for uranium extraction from saline lake brine. Environ. Technol. Innov. 2022, 25, 102214. [Google Scholar] [CrossRef]
- Yin, X.; Bai, J.; Tian, W.; Li, S.; Wang, J.; Wu, X.; Wang, Y.; Fan, F.; Huang, Q.; Qin, Z. Uranium sorption from saline lake brine by amidoximated silica. J. Radioanal. Nucl. Chem. 2017, 313, 113–121. [Google Scholar] [CrossRef]
- Tu, J.; Peng, X.; Wang, S.; Tian, C.; Deng, H.; Dang, Z.; Lu, G.; Shi, Z.; Lin, Z. Effective capture of aqueous uranium from saline lake with magnesium-based binary and ternary layered double hydroxides. Sci. Total Environ. 2019, 677, 556–563. [Google Scholar] [CrossRef]
- Pan, H.B.; Kuo, L.J.; Wai, C.M.; Miyamoto, N.; Joshi, R.; Wood, J.R.; Strivens, J.E.; Janke, C.J.; Oyola, Y.; Das, S.; et al. Elution of uranium and transition metals from amidoxime-based polymer adsorbents for sequestering uranium from seawater. Ind. Eng. Chem. Res. 2016, 55, 4313–4320. [Google Scholar] [CrossRef]
- Lei, F.; Zhou, Y.; Geng, L.; Li, B.; Chen, J.; Liu, Y.; Hu, Y.; Liu, T.; Shi, K.; Wu, W.; et al. Phospho-Enriched amidoxime adsorbents utilizing synergistic multifunctional groups for enhanced uranium removal from wastewater. Chem. Eng. J. 2024, 488, 151045. [Google Scholar] [CrossRef]
- Liu, N.; Li, C.; Bai, J.; Liang, H.; Gao, Q.; Wang, N.; Guo, R.; Qin, Z.; Mo, Z. A high-capacity amidoxime-functionalized magnetic composite for selective uranium capture in Salt Lake water. J. Environ. Chem. Eng. 2021, 9, 106688. [Google Scholar] [CrossRef]
- Ma, C.; Gao, J.; Wang, D.; Yuan, Y.; Wen, J.; Yan, B.; Zhao, S.; Zhao, X.; Sun, Y.; Wang, X.; et al. Sunlight polymerization of poly (amidoxime) hydrogel membrane for enhanced uranium extraction from seawater. Adv. Sci. 2019, 6, 1900085. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y.; Zhang, M.; Yuan, X.; Peng, X.; Sun, G.; Cui, Y. Amidoxime-Schiff base synergistic coordination and skeleton structure modification to construct porous polymer adsorbent materials for highly selective adsorption of uranium (VI). Desalination 2025, 607, 118802. [Google Scholar] [CrossRef]
- Zhang, W.; Che, X.; Pei, D.; Zhang, X.; Chen, Y.; Li, M.; Li, C. Biofibrous nanomaterials for extracting strategic metal ions from water. In Proceedings of the Exploration; Wiley Online Library: Hoboken, NJ, USA, 2022; Volume 2, p. 20220050. [Google Scholar]
- Zuo, B.; Wang, R.; Zuo, J.; Abdelfattah, W.; Helal, M.H.; Yan, G.; Liu, S.; Wang, T.; Bao, J.; Bai, L.; et al. Thermally-switchable adsorbent for selective uranium extraction from wastewater. Chem. Eng. J. 2024, 500, 156484. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yang, K.; Haleem, A.; Sun, Y.; Pan, J. Significantly enhanced uranium extraction by intelligent light-driven nanorobot catchers with precise controllable moving trajectory. J. Hazard. Mater. 2024, 469, 133908. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, L.; Feng, J.; Dong, H.; Zhang, C.; Ma, F.; Wang, Q. Efficient uranium adsorption by amidoximized porous polyacrylonitrile with hierarchical pore structure prepared by freeze-extraction. J. Mol. Liq. 2021, 328, 115304. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Wu, H.; Yu, W.; Guo, Z.; Liang, X. Amidoxime-based polymer microspheres with high selectivity for uranium from saline lake brine. ACS Appl. Polym. Mater. 2023, 5, 4380–4387. [Google Scholar] [CrossRef]
- Yang, L.; Qian, Y.; Zhang, Z.; Li, T.; Lin, X.; Fu, L.; Zhou, S.; Kong, X.Y.; Jiang, L.; Wen, L. A marine bacteria-inspired electrochemical regulation for continuous uranium extraction from seawater and salt lake brine. Chem. Sci. 2024, 15, 4538–4546. [Google Scholar] [CrossRef]
- Bai, J.; Yin, X.; Zhu, Y.; Fan, F.; Wu, X.; Tian, W.; Tan, C.; Zhang, X.; Wang, Y.; Cao, S.; et al. Selective uranium sorption from salt lake brines by amidoximated Saccharomyces cerevisiae. Chem. Eng. J. 2016, 283, 889–895. [Google Scholar] [CrossRef]
- Liang, H.; Tian, W.; Wang, N.; Zhang, H.; Wang, R.; Guo, R.; Mo, Z.; Liu, N. Amidoxime-grafted cotton fibers with anti-microbial sludge for efficient uranium recovery. Int. J. Biol. Macromol. 2024, 272, 132776. [Google Scholar] [CrossRef]
- Ma, M.; Luo, Q.; Han, R.; Wang, H.; Yang, J.; Liu, C. A Phosphorylated Dendrimer-Supported Biomass-Derived Magnetic Nanoparticle Adsorbent for Efficient Uranium Removal. Nanomaterials 2024, 14, 810. [Google Scholar] [CrossRef]
- Liang, H.; Wang, N.; Tian, W.; Zhang, H.; Li, C.; Guo, R.; Mo, Z.; Liu, N. Amidoxime functionalized magnetic MXene/chitosan composites for efficient uranium extraction. Process Saf. Environ. Prot. 2024, 188, 1235–1245. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.U.D.; Khan, R.; Haneklaus, N. Efficient and sustainable extraction of uranium from aquatic solution using biowaste-derived active carbon. Front. Chem. 2023, 11, 1327212. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Li, M.; Ge, H.; Li, Y.; Duan, T.; Zhu, W. Biomass-derived composite aerogels with novel structure for removal/recovery of uranium from simulated radioactive wastewater. Nanotechnology 2019, 30, 455602. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Feng, K.; Li, Q.; Qin, M.; Yang, J.; Zhang, X.; Chen, L.; Gong, J.; Qu, J.; Niu, R. Ion-exchange induced multiple effects to promote uranium uptake from nonmarine water by micromotors. J. Hazard. Mater. 2024, 480, 136464. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, J.; Liang, J.; Pan, D.; Li, P.; Fan, Q. Efficient recovery of uranium from saline lake brine through photocatalytic reduction. J. Mol. Liq. 2020, 308, 113007. [Google Scholar] [CrossRef]
- Wang, C.; Jiao, H.; Wu, Y.; Na, P. Amidoxime-functionalized TiO2 nanotube arrays plate photocatalyst for efficient extracting and recovering uranium from salt lakes: Bench-scale experiments and theoretical calculation. Sep. Purif. Technol. 2024, 339, 126556. [Google Scholar] [CrossRef]
- Zhu, W.; Li, X.; Wang, D.; Fu, F.; Liang, Y. Advanced photocatalytic uranium extraction strategies: Progress, challenges, and prospects. Nanomaterials 2023, 13, 2005. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Xie, Y.; Liu, X.; Chen, Z.; Yang, H.; Waterhouse, G.I.; Ma, S.; Wang, X. Modulating uranium extraction performance of multivariate covalent organic frameworks through donor–acceptor linkers and amidoxime nanotraps. JACS Au 2023, 3, 239–251. [Google Scholar] [CrossRef] [PubMed]
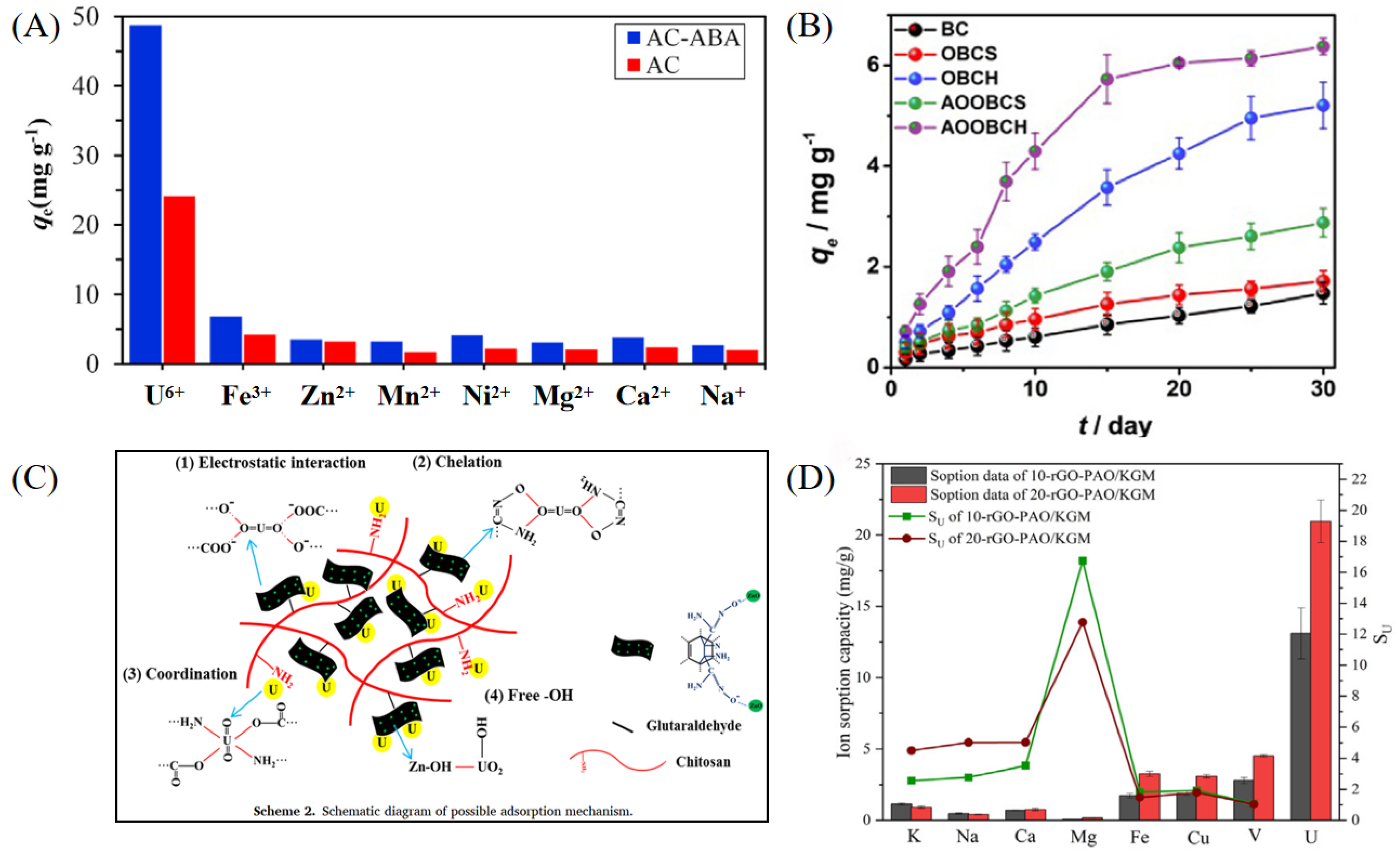
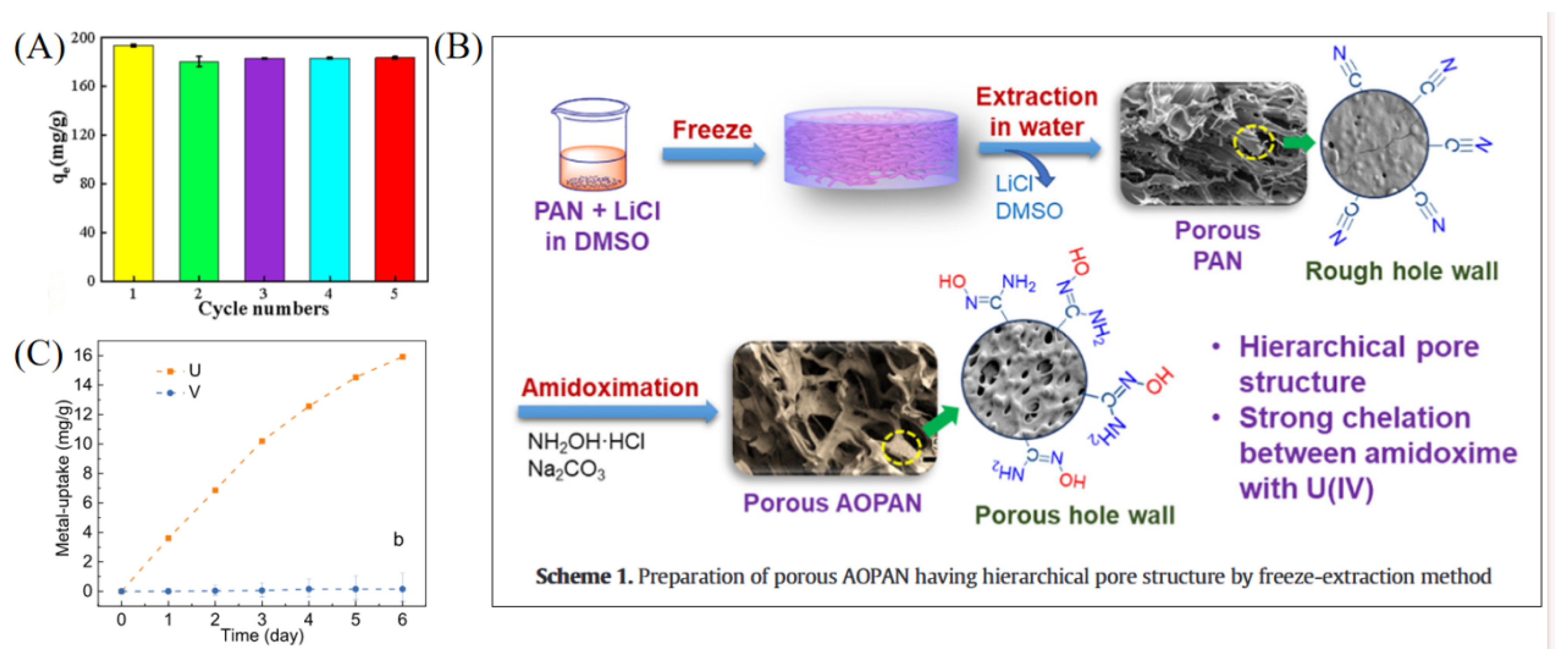
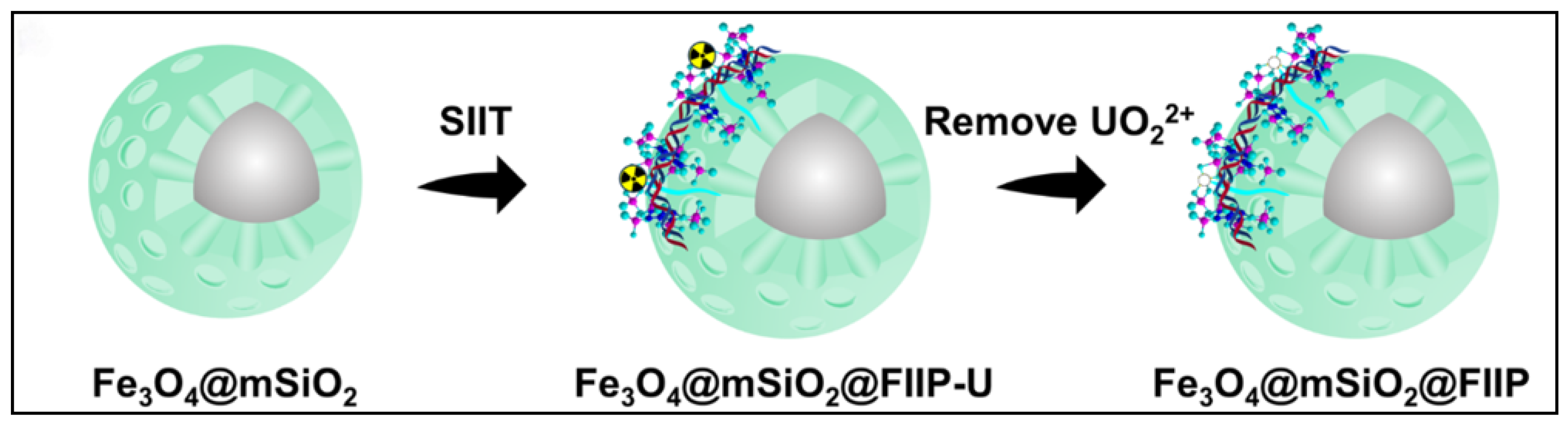
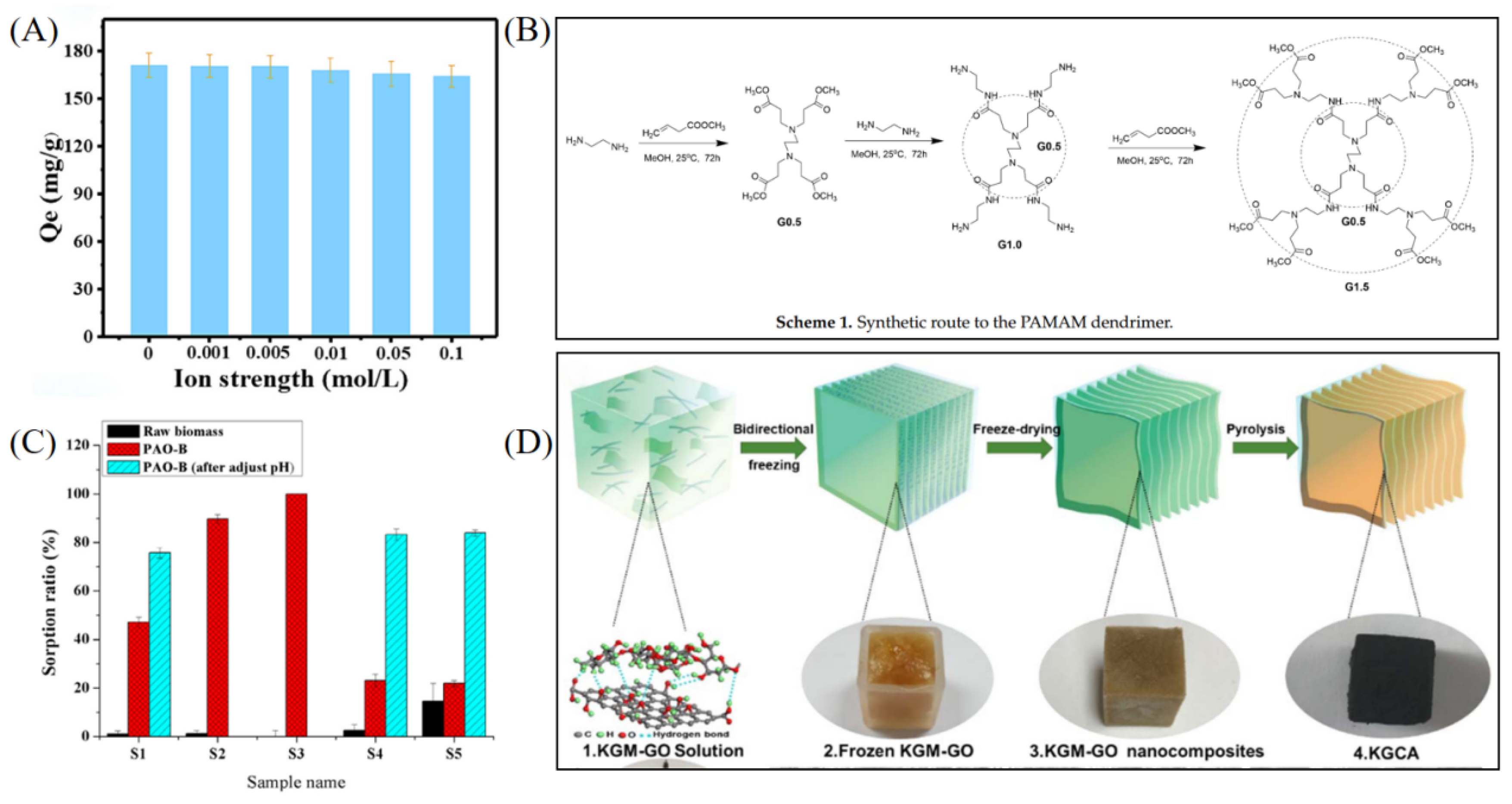
| Adsorbent Name | Type | Experimental Conditions | Kd (Static) | Kd (Dynamic) | SF (or SU/M) |
|---|---|---|---|---|---|
| AC-ABA [27] | Carbon-based material | pH 4.5; 40 °C; U(VI) 25–125 mg L−1; multiple coexisting ions | Not provided | – | Selectivity coefficient |
| MgAl-LDH/MgAlFe-LDH [32] | Inorganic layered double hydroxide | pH 6; 25 °C; simulated brine; U(VI) 0.2–30 mg L−1; high salinity environment | 377.31/434.78 L · g−1 | – | ∼110 vs. Ca2+; up to 2.93 × 104 vs. Na+, K+, Mg2+ |
| CS-Ppy-Fe3O4-AO [35] | Organic–magnetic composite | pH 6; 25 °C; U(VI) 25–600 mg L−1; tested in salt lake water | Not specified | – | SU/M |
| AOPAN [41] | Organic polymer material | pH 6; 25 °C; U(VI) 10–117 ppm; multiple ions coexist | Not specified | – | Significant U selectivity |
| Fe3O4-P-CMC/PAMAM [46] | Biomass–organic–magnetic composite | pH 5.5; 25 °C; U(VI) 10–200 mg L−1; multiple ions coexist | Not specified | – | SU/M |
| MCF-AO [47] | Organic–inorganic hybrid composite | pH 4; 25 °C; U(VI) 100 mg L−1; tested in salt lake water | Not specified | – | SU/M |
| S-COF membrane electrode [43] | Organic framework-carbon-based electrochemical material | Natural seawater/salt lake brine; pH ≈ 8.2; electrochemical operation for 21–32 days | – | 48.04 mg g−1 (21 d, seawater); 75.72 mg g−1 (32 d, brine) | High selectivity |
| TNTPAO [52] | Inorganic–organic photocatalytic material | Salt lake water; pH ≈ 8; U(VI) ∼200 ppb; photocatalysis + adsorption; 96 h | – | Extraction efficiency 94.5%, recovery 93.6% | High selectivity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Lei, M.; Huang, J.; Li, Y.; Li, Y.; Guo, J. Recent Advances in Materials for Uranium Extraction from Salt Lake Brine: A Review. Chemistry 2025, 7, 142. https://doi.org/10.3390/chemistry7050142
Wang P, Lei M, Huang J, Li Y, Li Y, Guo J. Recent Advances in Materials for Uranium Extraction from Salt Lake Brine: A Review. Chemistry. 2025; 7(5):142. https://doi.org/10.3390/chemistry7050142
Chicago/Turabian StyleWang, Panting, Miao Lei, Junhang Huang, Yuanhao Li, Ye Li, and Junpeng Guo. 2025. "Recent Advances in Materials for Uranium Extraction from Salt Lake Brine: A Review" Chemistry 7, no. 5: 142. https://doi.org/10.3390/chemistry7050142
APA StyleWang, P., Lei, M., Huang, J., Li, Y., Li, Y., & Guo, J. (2025). Recent Advances in Materials for Uranium Extraction from Salt Lake Brine: A Review. Chemistry, 7(5), 142. https://doi.org/10.3390/chemistry7050142







