Self-Reporting H2S Donors: Integrating H2S Release with Real-Time Fluorescence Detection
Abstract
1. Introduction
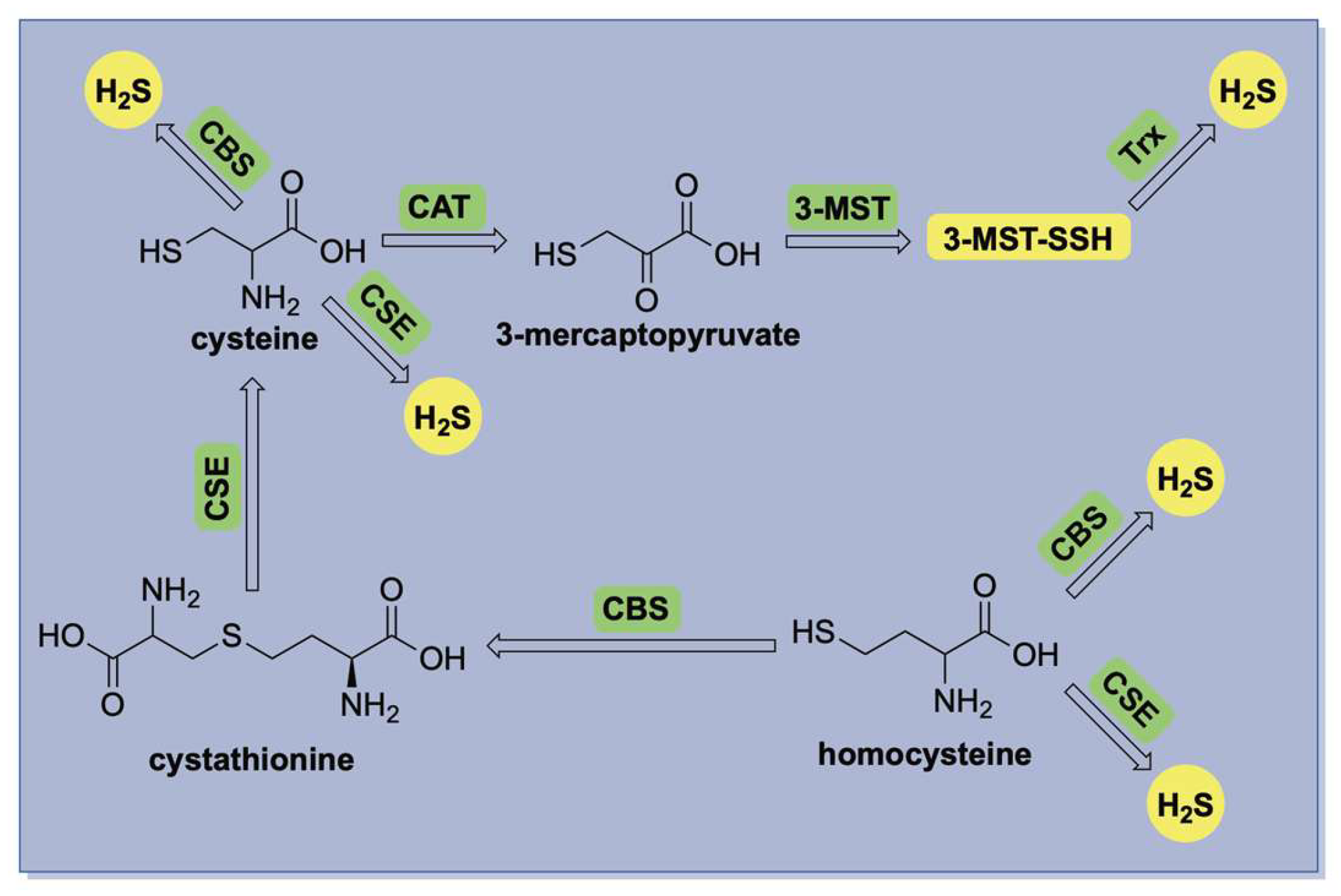
1.1. H2S Physical Characteristics and Endogenous Production
1.2. H2S Biological Activity
1.3. H2S-Specific Tools for Exploring Its Chemical Biology
2. Self-Reporting H2S Donors
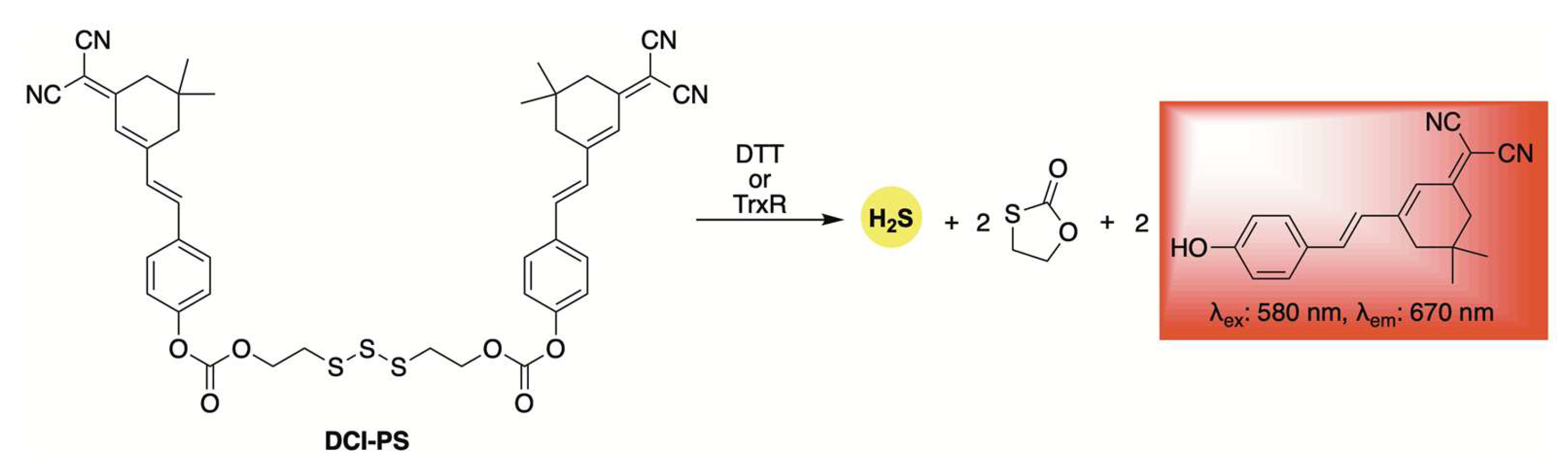
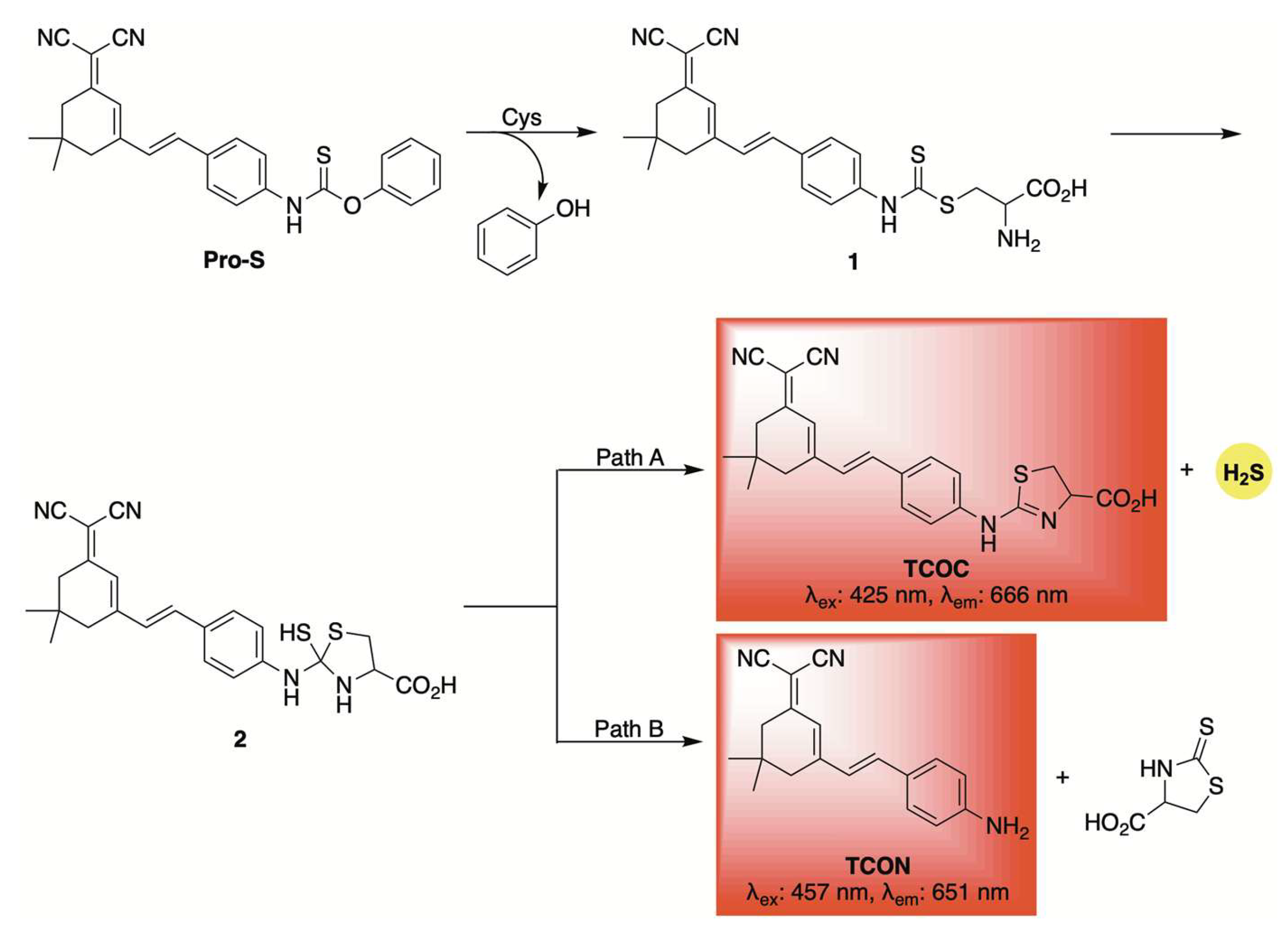
2.1. Thiol-Activation
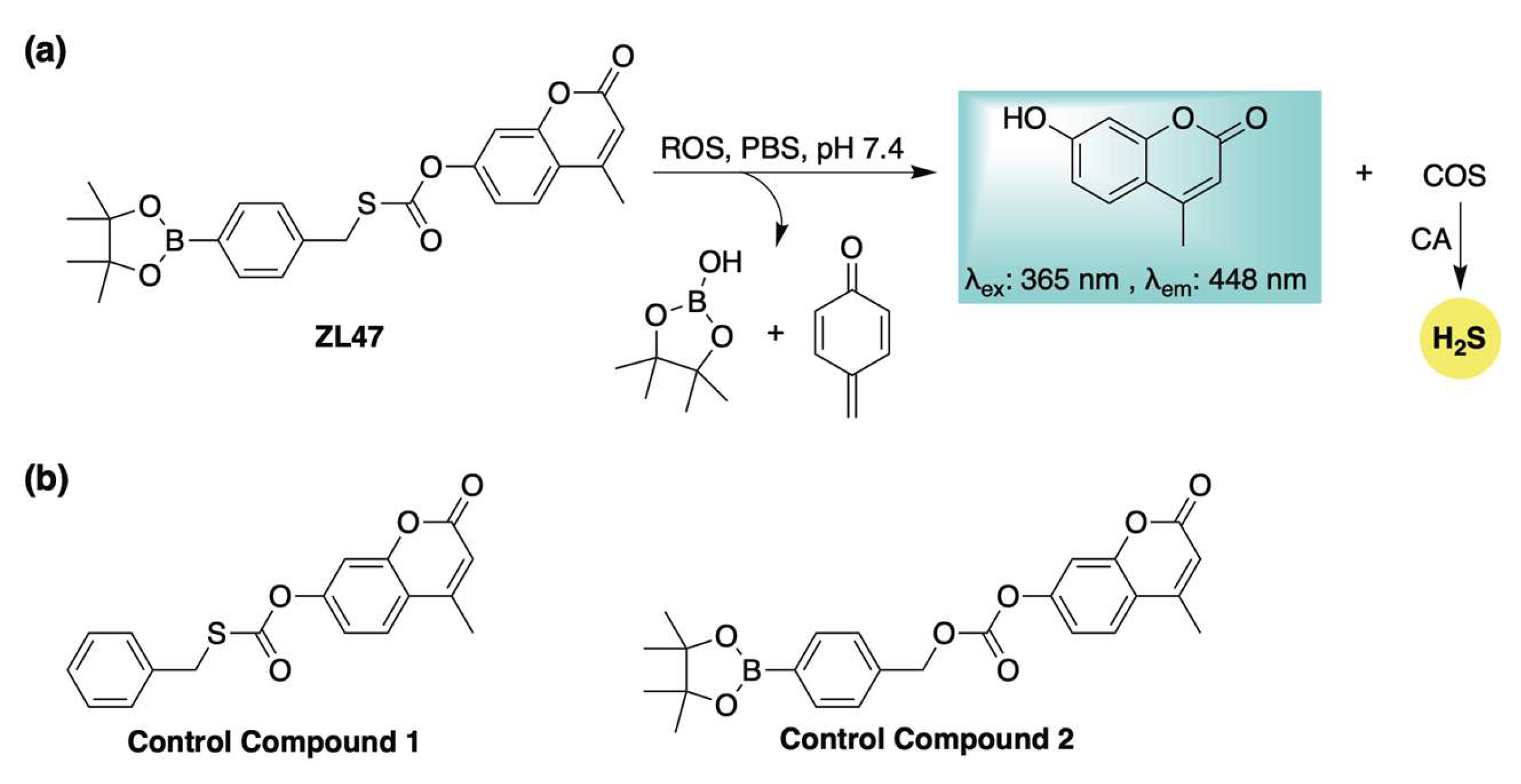
2.2. ROS-Activation
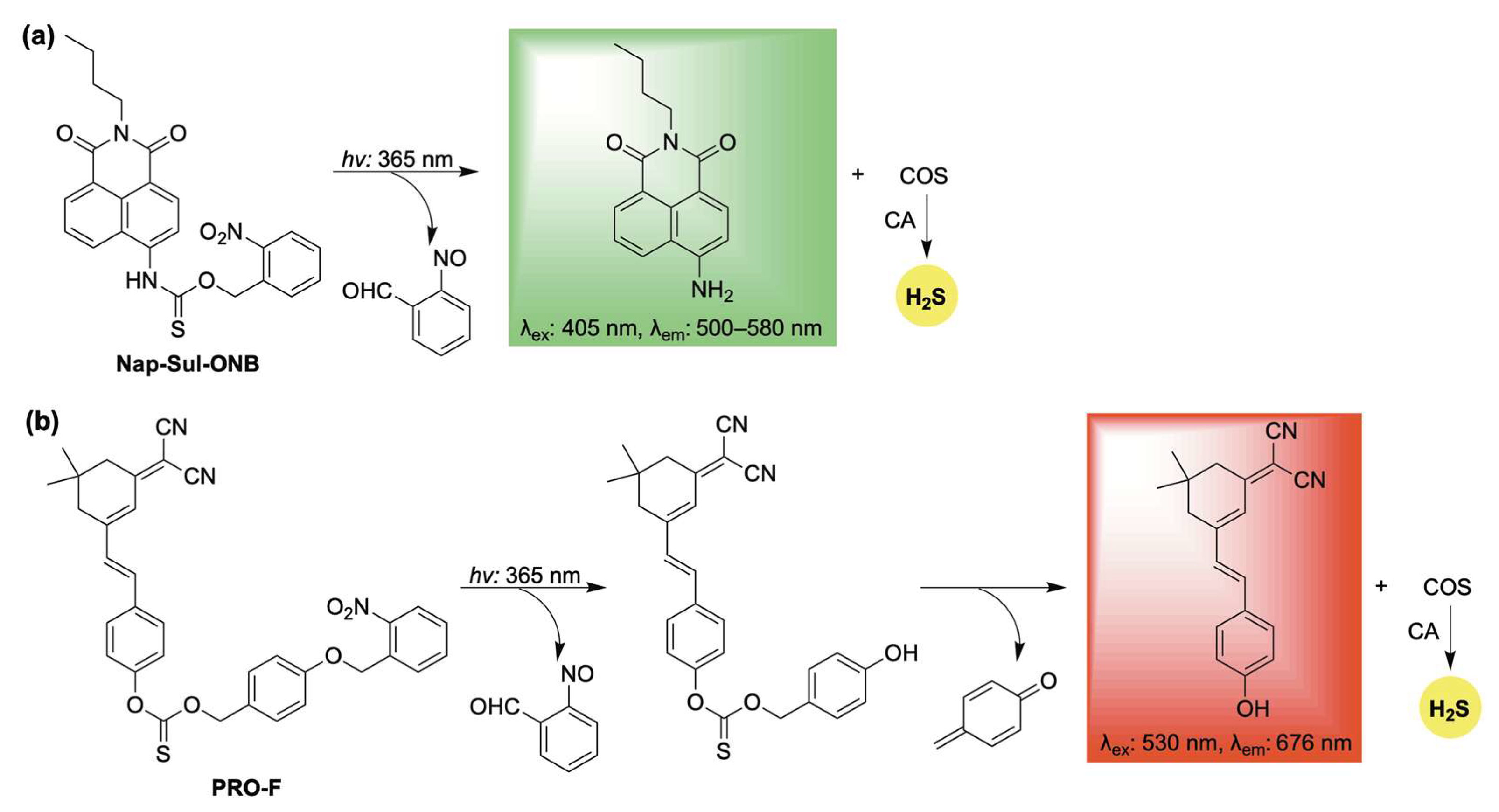
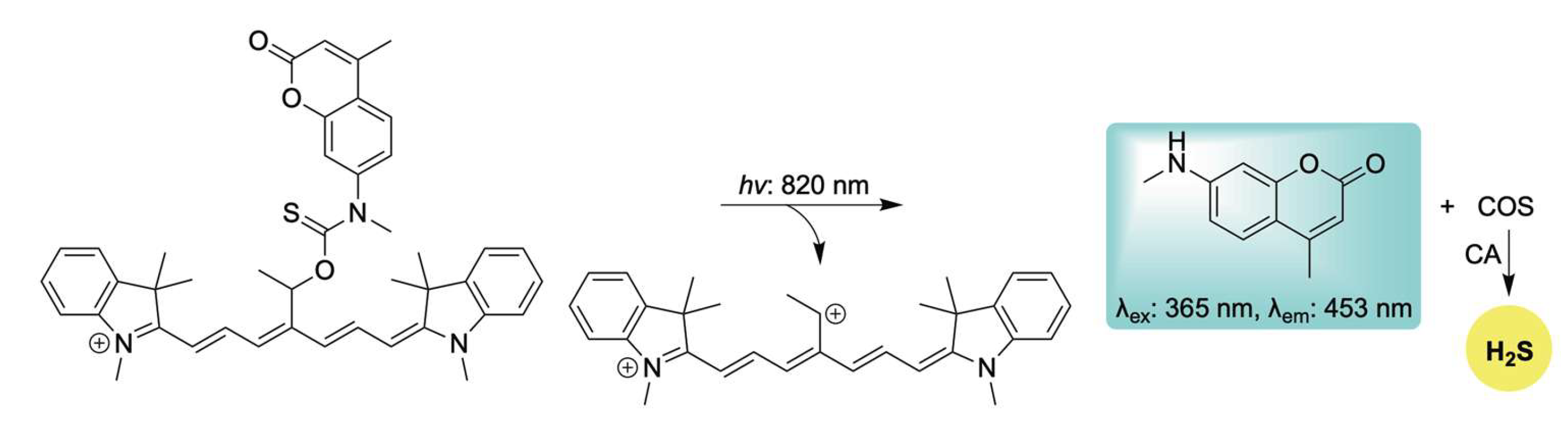
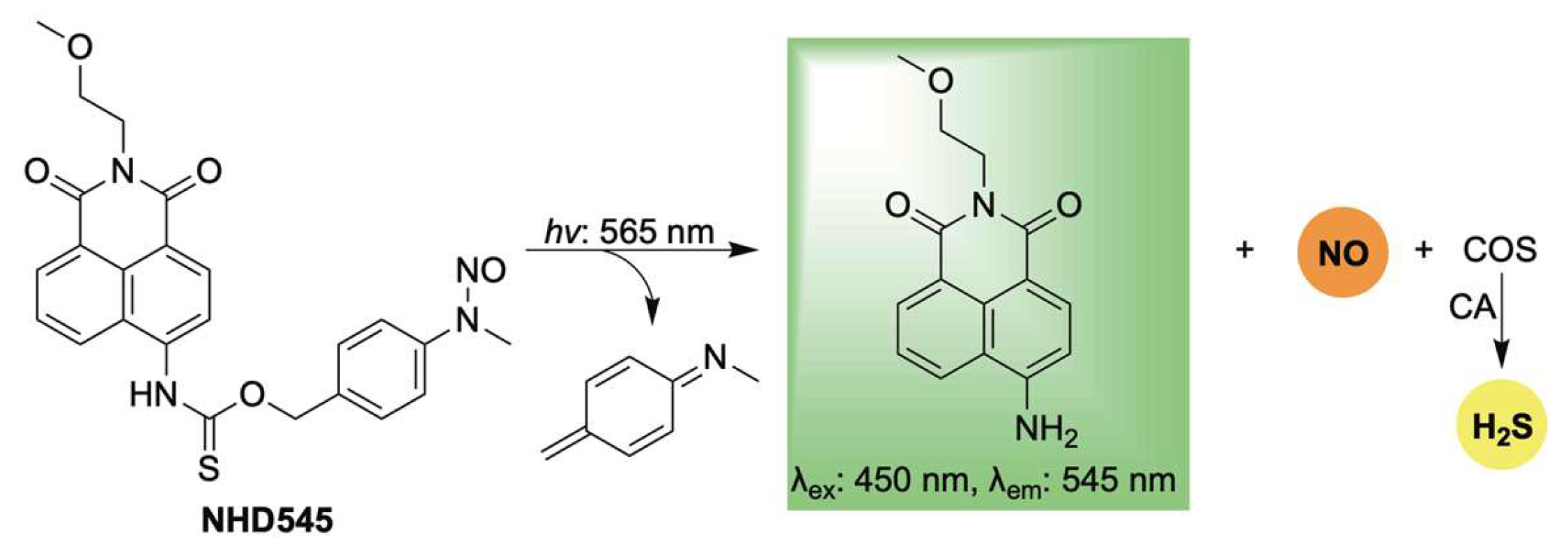
2.3. Light-Activation
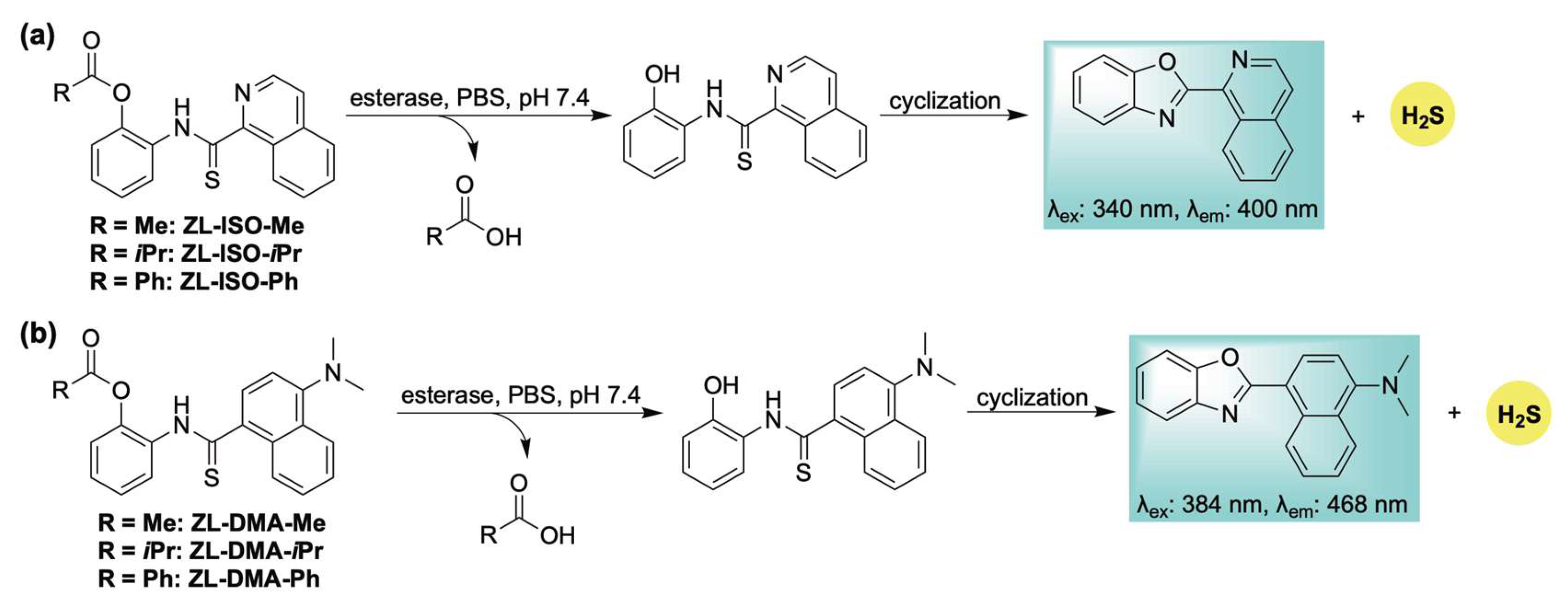
2.4. Enzyme-Activation
2.5. Bioorthogonal-Activation
3. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Szabo, C. A Timeline of Hydrogen Sulfide (H2S) Research: From Environmental Toxin to Biological Mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.W.; Davenport, S.J. Hydrogen Sulphide Literature. Public Health Rep. 1924, 39, 1–13. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A Critical Review of the Literature on Hydrogen Sulfide Toxicity. CRC Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of Hydrogen Sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef]
- Knight, L.D.; Presnell, S.E. Death by Sewer Gas: Case Report of a Double Fatality and Review of the Literature. Am. J. Forensic Med. Pathol. 2005, 26, 181–185. [Google Scholar] [CrossRef]
- Smith, R. A Short History of Hydrogen Sulfide. Am. Sci. 2010, 98, 6. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Loring, H.S.; Craft, H.A. The Oxidation of the Sulfur of Homocystine, Methionine, and S-Methylcysteine in the Animal Body. J. Biol. Chem. 1934, 105, 481–488. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Brown, G.B.; Chandler, J.P. The Synthesis of ll-S-(β-Amino-β-Carboxyethyl) Homocysteine and the Replacement by It of Cys-Tine in the Diet. J. Biol. Chem. 1942, 143, 59–64. [Google Scholar] [CrossRef]
- Binkley, F.; Du Vigneaud, V. The Formation of Cysteine from Homocysteine and Serine by Liver Tissue of Rats. J. Biol. Chem. 1942, 144, 507–511. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Wang, R. Two’s Company, Three’s a Crowd: Can H2S Be the Third Endogenous Gaseous Transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Wang, R. The Gasotransmitter Role of Hydrogen Sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Wang, R. Shared Signaling Pathways among Gasotransmitters. Proc. Natl. Acad. Sci. USA 2012, 109, 8801–8802. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Foresti, R.; Ferdinandy, P. Pharmacology of the ‘Gasotransmitters’ NO, CO and H2S: Translational Opportunities. Br. J. Pharmacol. 2015, 172, 1395–1396. [Google Scholar] [CrossRef]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and Working with Hydrogen Sulfide. Free Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Denicola, A.; Alvarez, B.; Möller, M.N. Solubility and Permeation of Hydrogen Sulfide in Lipid Membranes. PLoS ONE 2012, 7, e34562. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R. Persulfidation (S-Sulfhydration) and H2S. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Moore, P.K., Whiteman, M., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; Volume 230, pp. 29–59. ISBN 978-3-319-18143-1. [Google Scholar]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling Through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Vignane, T.; Filipovic, M.R. Emerging Chemical Biology of Protein Persulfidation. Antioxid. Redox Signal. 2023, 39, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.W.; Kraus, J.P. Cystathionine β-Synthase: Structure, Function, Regulation, and Location of Homocystinuria-Causing Mutations. J. Biol. Chem. 2004, 279, 29871–29874. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of Cystathionine γ-Lyase/Hydrogen Sulfide Pathway in Cardiovascular Disease: A Novel Therapeutic Strategy? Antioxid. Redox Signal. 2012, 17, 106–118. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Bhatia, M. Hydrogen Sulfide as a Vasodilator. IUBMB Life 2005, 57, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Popescu, C.; Vlădulescu-Trandafir, A.-I.; Onose, G. Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review. Antioxidants 2024, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- White, B.J.O.; Smith, P.A.; Dunn, W.R. Hydrogen Sulphide–Mediated Vasodilatation Involves the Release of Neurotransmitters from Sensory Nerves in Pressurized Mesenteric Small Arteries Isolated from Rats. Br. J. Pharmacol. 2013, 168, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W. The Vasorelaxant Effect of H2S as a Novel Endogenous Gaseous KATP Channel Opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Köhn, C.; Dubrovska, G.; Huang, Y.; Gollasch, M. Hydrogen Sulfide: Potent Regulator of Vascular Tone and Stimulator of Angiogenesis. Int. J. Biomed. Sci. 2012, 8, 81–86. [Google Scholar]
- Zhu, X.-Y.; Gu, H.; Ni, X. Hydrogen Sulfide in the Endocrine and Reproductive Systems. Expert Rev. Clin. Pharmacol. 2011, 4, 75–82. [Google Scholar] [CrossRef]
- Guo, W.; Kan, J.; Cheng, Z.; Chen, J.; Shen, Y.; Xu, J.; Wu, D.; Zhu, Y. Hydrogen Sulfide as an Endogenous Modulator in Mitochondria and Mitochondria Dysfunction. Oxidative Med. Cell. Longev. 2012, 2012, 878052. [Google Scholar] [CrossRef][Green Version]
- Huang, D.; Jing, G.; Zhu, S. Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants 2023, 12, 1644. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of Hydrogen Sulfide on Mitochondrial Function and Cellular Bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Li, M.; Mao, J.; Zhu, Y. New Therapeutic Approaches Using Hydrogen Sulfide Donors in Inflammation and Immune Response. Antioxid. Redox Signal. 2021, 35, 341–356. [Google Scholar] [CrossRef]
- Whiteman, M.; Winyard, P.G. Hydrogen Sulfide and Inflammation: The Good, the Bad, the Ugly and the Promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Blackler, R.W.; Chan, M.V.; Da Silva, G.J.; Elsheikh, W.; Flannigan, K.L.; Gamaniek, I.; Manko, A.; Wang, L.; Motta, J.-P.; et al. Anti-Inflammatory and Cytoprotective Actions of Hydrogen Sulfide: Translation to Therapeutics. Antioxid. Redox Signal. 2015, 22, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lukesh, J.C. H2S Donors with Cytoprotective Effects in Models of MI/R Injury and Chemotherapy-Induced Cardiotoxicity. Antioxidants 2023, 12, 650. [Google Scholar] [CrossRef]
- Carballal, S.; Trujillo, M.; Cuevasanta, E.; Bartesaghi, S.; Möller, M.N.; Folkes, L.K.; García-Bereguiaín, M.A.; Gutiérrez-Merino, C.; Wardman, P.; Denicola, A.; et al. Reactivity of Hydrogen Sulfide with Peroxynitrite and Other Oxidants of Biological Interest. Free Radic. Biol. Med. 2011, 50, 196–205. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, Y.; Meng, M.; Luo, M.; Zhao, H.; Sun, H.; Gao, S. GYY4137 Protects against Myocardial Ischemia/Reperfusion Injury via Activation of the PHLPP-1/Akt/Nrf2 Signaling Pathway in Diabetic Mice. J. Surg. Res. 2018, 225, 29–39. [Google Scholar] [CrossRef]
- Hu, Q.; Yammani, R.D.; Brown-Harding, H.; Soto-Pantoja, D.R.; Poole, L.B.; Lukesh, J.C. Mitigation of Doxorubicin-Induced Cardiotoxicity with an H2O2-Activated, H2S-Donating Hybrid Prodrug. Redox Biol. 2022, 53, 102338. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Qian, L.-L.; Wang, R.-X. Hydrogen Sulfide-Induced Vasodilation: The Involvement of Vascular Potassium Channels. Front. Pharmacol. 2022, 13, 911704. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Miljkovic, J.L.; Nauser, T.; Royzen, M.; Klos, K.; Shubina, T.; Koppenol, W.H.; Lippard, S.J.; Ivanović-Burmazović, I. Chemical Characterization of the Smallest S-Nitrosothiol, HSNO; Cellular Cross-Talk of H2S and S-Nitrosothiols. J. Am. Chem. Soc. 2012, 134, 12016–12027. [Google Scholar] [CrossRef]
- Chatzianastasiou, A.; Bibli, S.-I.; Andreadou, I.; Efentakis, P.; Kaludercic, N.; Wood, M.E.; Whiteman, M.; Di Lisa, F.; Daiber, A.; Manolopoulos, V.G.; et al. Cardioprotection by H2S Donors: Nitric Oxide-Dependent and -Independent Mechanisms. J. Pharmacol. Exp. Ther. 2016, 358, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Sojitra, B.; Bulani, Y.; Putcha, U.K.; Kanwal, A.; Gupta, P.; Kuncha, M.; Banerjee, S.K. Nitric Oxide Synthase Inhibition Abrogates Hydrogen Sulfide-Induced Cardioprotection in Mice. Mol. Cell. Biochem. 2012, 360, 61–69. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A Review of Hydrogen Sulfide (H2S) Donors: Chemistry and Potential Therapeutic Applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hamsath, A.; Neill, D.L.; Wang, Y.; Yang, C.; Xian, M. Strategies for the Design of Donors and Precursors of Reactive Sulfur Species. Chem. A Eur. J. 2019, 25, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Activatable Small-Molecule Hydrogen Sulfide Donors. Antioxid. Redox Signal. 2020, 32, 96–109. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137): New Insights into the Biology of Hydrogen Sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-Based Small-Molecule Fluorescent Probes for Chemoselective Bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical Probes for Molecular Imaging and Detection of Hydrogen Sulfide and Reactive Sulfur Species in Biological Systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- Chen, G.; Yu, J.; Wu, L.; Ji, X.; Xu, J.; Wang, C.; Ma, S.; Miao, Q.; Wang, L.; Wang, C.; et al. Fluorescent Small Molecule Donors. Chem. Soc. Rev. 2024, 53, 6345–6398. [Google Scholar] [CrossRef]
- Misra, R.; Bhuyan, H.J.; Dutta, A.; Bhabak, K.P. Recent Developments on Activatable Turn-On Fluorogenic Donors of Hydrogen Sulfide (H2S). ChemMedChem 2024, 19, e202400251. [Google Scholar] [CrossRef] [PubMed]
- Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M.D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138, 7256–7259. [Google Scholar] [CrossRef]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef] [PubMed]
- Henthorn, H.A.; Pluth, M.D. Mechanistic Insights into the H2S-Mediated Reduction of Aryl Azides Commonly Used in H2S Detection. J. Am. Chem. Soc. 2015, 137, 15330–15336. [Google Scholar] [CrossRef]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Development and Application of Carbonyl Sulfide-Based Donors for H2S Delivery. Acc. Chem. Res. 2019, 52, 2723–2731. [Google Scholar] [CrossRef]
- Zhao, Y.; Cerda, M.M.; Pluth, M.D. Fluorogenic Hydrogen Sulfide (H2S) Donors Based on Sulfenyl Thiocarbonates Enable H2S Tracking and Quantification. Chem. Sci. 2019, 10, 1873–1878. [Google Scholar] [CrossRef]
- Monks, T.J.; Hanzlik, R.P.; Cohen, G.M.; Ross, D.; Graham, D.G. Quinone Chemistry and Toxicity. Toxicol. Appl. Pharmacol. 1992, 112, 2–16. [Google Scholar] [CrossRef]
- Monks, T.; Jones, D. The Metabolism and Toxicity of Quinones, Quinonimines, Quinone Methides, and Quinone-Thioethers. Curr. Drug Metab. 2002, 3, 425–438. [Google Scholar] [CrossRef]
- Pluth, M.D. Moving Past Quinone-Methides: Recent Advances Toward Minimizing Electrophilic Byproducts from COS/H2S Donors. Curr. Top. Med. Chem. 2021, 21, 2882–2889. [Google Scholar] [CrossRef]
- Mahato, S.K.; Bhattacherjee, D.; Bhabak, K.P. The Biothiol-Triggered Organotrisulfide-Based Self-Immolative Fluorogenic Donors of Hydrogen Sulfide Enable Lysosomal Trafficking. Chem. Commun. 2020, 56, 7769–7772. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.K.; Bhattacherjee, D.; Barman, P.; Bhabak, K.P. Thioredoxin Reductase-Triggered Fluorogenic Donor of Hydrogen Sulfide: A Model Study with a Symmetrical Organopolysulfide Probe with Turn-On Near-Infrared Fluorescent Emission. J. Mater. Chem. B 2022, 10, 2183–2193. [Google Scholar] [CrossRef]
- Zhao, X.; Ning, L.; Zhou, X.; Song, Z.; Zhang, J.; Guan, F.; Yang, X.-F. An Activatable Near-Infrared Fluorescence Hydrogen Sulfide (H2S) Donor for Imaging H2S Release and Inhibiting Inflammation in Cells. Anal. Chem. 2021, 93, 4894–4901. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Bai, X.; Li, P.; Su, D.; Zhang, W.; Zhang, W.; Tang, B. Highly Specific Cys Fluorescence Probe for Living Mouse Brain Imaging via Evading Reaction with Other Biothiols. Anal. Chem. 2019, 91, 8591–8594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Chen, Y.; Cai, X.; Sheng, W.; Zhu, H.; Jia, P.; Li, Z.; Huang, S.; Zhu, B. Visualization of the Cysteine Level During Golgi Stress Using a Novel Golgi-Targeting Highly Specific Fluorescent Probe. Chem. Commun. 2020, 56, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Bellagambi, F.G.; Ghimenti, S.; Breschi, M.C.; Calderone, V. Pharmacological Characterization of the Vascular Effects of Aryl Isothiocyanates: Is Hydrogen Sulfide the Real Player? Vasc. Pharmacol. 2014, 60, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S Donors Triggered by Cysteine: Reaction Mechanism and Structure and Activity Relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef]
- Hong, J.; Feng, G. Isothiocyanate Can Be Used as a Highly Specific Recognition Site for Fluorescent Cysteine Probes. Sens. Actuators B Chem. 2021, 326, 129016. [Google Scholar] [CrossRef]
- Ge, C.; Wang, H.; Ni, T.; Yang, Z.; Chang, K. Red-Emitting Fluorescent Turn-on Probe with Specific Isothiocyanate Recognition Site for Cysteine Imaging in Living Systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119826. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, C.; Rong, X.; Zhang, Y.; Su, M.; Wang, X.; Liu, M.; Zhang, X.; Sheng, W.; Zhu, B. A New Isothiocyanate-Based Golgi-Targeting Fluorescent Probe for Cys and Its Bioimaging Applications During the Golgi Stress Response. Bioorganic Chem. 2022, 122, 105741. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Fu, Y.; Meng, M.; Jin, H.; Zhao, W. An Isothiocyanate-Functionalized Self-Immolative Near-Infrared Fluorescence Probe for Cysteine Sensing and Bioimaging in Living Systems. Sens. Actuators B Chem. 2022, 366, 132013. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, M.; Ning, L.; Yuan, F.; Li, J.; Guo, Y.; Mu, Y.; Zhang, J. Biothiol-Triggered H2S Release from a Near-Infrared Fluorescent H2S Donor Promotes Cutaneous Wound Healing. Acta Mater. Medica 2022, 1, 476–485. [Google Scholar] [CrossRef]
- Mahato, S.K.; Barman, P.; Badirujjaman, M.; Bhabak, K.P. Cysteine-Responsive Prodrug of the Anti-Cancer Drug Amonafide: Fluorogenic Adjuvant Drug Delivery with Hydrogen Sulfide (H2S). Chem. Commun. 2023, 59, 4802–4805. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive Oxygen Species and Cell Signaling. Review. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond Oxidative Stress: An Immunologist’s Guide to Reactive Oxygen Species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Fang, Y.; Shi, W.; Li, X.; Chen, W.; Xian, M.; Ma, H. Reactive Oxygen Species-Triggered Off-On Fluorescence Donor for Imaging Hydrogen Sulfide Delivery in Living Cells. Chem. Sci. 2019, 10, 7690–7694. [Google Scholar] [CrossRef]
- Lippert, A.R.; Van De Bittner, G.C.; Chang, C.J. Boronate Oxidation as a Bioorthogonal Reaction Approach for Studying the Chemistry of Hydrogen Peroxide in Living Systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. A Fluorogenic H2S Donor Activated by Reactive Oxygen Species for Real-Time Monitoring in Cells and In Vivo. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120243. [Google Scholar] [CrossRef] [PubMed]
- Sufian, A.; Badirujjaman, M.; Barman, P.; Bhabak, K.P. Dual-Stimuli-Activatable Hybrid Prodrug for the Self-Immolative Delivery of an Anticancer Agent and Hydrogen Sulfide with Turn-On Fluorescence. Chem. A Eur. J. 2023, 29, e202302197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hu, P.; Wang, Y.; Tang, Q.; Zheng, Q.; Wang, Z.; He, Y. A Reactive Oxygen Species (ROS) Activated Hydrogen Sulfide (H2S) Donor with Self-Reporting Fluorescence. ACS Sens. 2020, 5, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, M.; Zhao, F.; Song, X.; Ye, Y. ONOO−-Triggered Fluorescence H2S Donor for Mitigating Drug-Induced Liver Injury. Sens. Actuators B Chem. 2023, 378, 133131. [Google Scholar] [CrossRef]
- Li, J.; Lim, C.S.; Kim, G.; Kim, H.M.; Yoon, J. Highly Selective and Sensitive Two-Photon Fluorescence Probe for Endogenous Peroxynitrite Detection and Its Applications in Living Cells and Tissues. Anal. Chem. 2017, 89, 8496–8500. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, Z.; Wang, R.; Yu, F. Indication of Dynamic Peroxynitrite Fluctuations in the Rat Epilepsy Model with a Near-Infrared Two-Photon Fluorescent Probe. Anal. Chem. 2021, 93, 2490–2499. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, J.; Ji, C.; Shaibani, M.S.S.; Li, Z.; Lim, K.; Zhang, C.; Li, L.; Liu, Z. Ultrafast Detection of Peroxynitrite in Parkinson’s Disease Models Using a Near-Infrared Fluorescent Probe. Anal. Chem. 2020, 92, 4038–4045. [Google Scholar] [CrossRef]
- Zhu, C.; Suarez, S.I.; Lukesh, J.C. Illuminating and Alleviating Cellular Oxidative Stress with an ROS-Activated, H2S-Donating Theranostic. Tetrahedron Lett. 2021, 69, 152944. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, T.; Li, T.; Zhong, P.; Li, L.; Peng, M.; Li, Z.; Zhang, L.; Wang, H.; Hu, P.; et al. A Superoxide Anion Responsive and Self-Reporting Fluorescent H2S Donor for the Treatment of Diabetic Wound. Free Radic. Biol. Med. 2025, 231, 109–119. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, C.; Hankins, R.A.; Murmello, A.R.; Marrs, G.S.; Lukesh, J.C. An ROS-Responsive Donor That Self-Reports Its H2S Delivery by Forming a Benzoxazole-Based Fluorophore. J. Am. Chem. Soc. 2023, 145, 25486–25494. [Google Scholar] [CrossRef]
- Tian, H.; Qian, J.; Bai, H.; Sun, Q.; Zhang, L.; Zhang, W. Micelle-Induced Multiple Performance Improvement of Fluorescent Probes for H2S Detection. Anal. Chim. Acta 2013, 768, 136–142. [Google Scholar] [CrossRef]
- Choi, S.-A.; Park, C.S.; Kwon, O.S.; Giong, H.-K.; Lee, J.-S.; Ha, T.H.; Lee, C.-S. Structural Effects of Naphthalimide-Based Fluorescent Sensor for Hydrogen Sulfide and Imaging in Live Zebrafish. Sci. Rep. 2016, 6, 26203. [Google Scholar] [CrossRef]
- Ai, X.; Mu, J.; Xing, B. Recent Advances of Light-Mediated Theranostics. Theranostics 2016, 6, 2439–2457. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, H.; Gu, X.; Tang, B.Z. Photoactivatable Biomedical Materials Based on Luminogens with Aggregation-Induced Emission (AIE) Characteristics. Adv. Healthc. Mater. 2021, 10, 2101177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Moralès, O.; Mordon, S.; Delhem, N.; Boleslawski, E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers 2021, 13, 5176. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, Y.; Das, J.; Chaudhuri, A.; Karmakar, A.; Maiti, T.K.; Singh, N.D.P. Light Triggered Uncaging of Hydrogen Sulfide (H2S) with Real-Time Monitoring. Chem. Commun. 2018, 54, 3106–3109. [Google Scholar] [CrossRef]
- Dormán, G.; Prestwich, G.D. Using Photolabile Ligands in Drug Discovery and Development. Trends Biotechnol. 2000, 18, 64–77. [Google Scholar] [CrossRef]
- Edler, M.; Mayrbrugger, S.; Fian, A.; Trimmel, G.; Radl, S.; Kern, W.; Griesser, T. Wavelength Selective Refractive Index Modulation in a ROMP Derived Polymer Bearing Phenyl- and Ortho-Nitrobenzyl Ester Groups. J. Mater. Chem. C 2013, 1, 3931. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Bolton, S.G.; Pluth, M.D. Light-Activated COS/H2S Donation from Photocaged Thiocarbamates. Org. Lett. 2017, 19, 2278–2281. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Schwörer, M.A.; Wirz, J. Photochemical Reaction Mechanisms of 2-Nitrobenzyl Compounds: Methyl Ethers and Caged ATP. J. Am. Chem. Soc. 2004, 126, 4581–4595. [Google Scholar] [CrossRef]
- Aujard, I.; Benbrahim, C.; Gouget, M.; Ruel, O.; Baudin, J.-B.; Neveu, P.; Jullien, L. O-Nitrobenzyl Photolabile Protecting Groups with Red-Shifted Absorption: Syntheses and Uncaging Cross-Sections for One- and Two-Photon Excitation. Chem. Eur. J. 2006, 12, 6865–6879. [Google Scholar] [CrossRef]
- Bochet, C.G. Photolabile Protecting Groups and Linkers. J. Chem. Soc. Perkin Trans. 1 2002, 125–142. [Google Scholar] [CrossRef]
- Hua, W.; Zhao, J.; Gou, S. A Naphthalimide Derivative Can Release COS and Form H2S in a Light-Controlled Manner and Protect Cells against ROS with Real-Time Monitoring Ability. Analyst 2020, 145, 3878–3884. [Google Scholar] [CrossRef]
- Yuan, F.; He, X.; Lu, Y.; Ning, L.; Zhao, X.; Zhang, S.; Guan, F.; Guo, Y.; Zhang, J. Photoactivated Hydrogen Sulfide Donor with a Near-Infrared Fluorescence Report System for Accelerated Chronic Wound Healing. Anal. Chem. 2023, 95, 6931–6939. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Guo, A.; Wang, L.; Ning, L.; Guo, Y.; Zhang, J. Tunable Light-Activated Platform for Controlled Hydrogen Sulfide Release with Tracking. Angew. Chem. Int. Ed. 2025, 64, e202501685. [Google Scholar] [CrossRef] [PubMed]
- Hanc, K.; Janeková, H.; Štacko, P. Concurrent Subcellular Delivery of Hydrogen Sulfide and a Payload with Near-Infrared Light. JACS Au 2024, 4, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Lin, Z.; Cheng, Y.; Tang, Y.; Chen, Q.; Jiang, L.; Li, L.; Zhang, Z. A Photo-Triggered Dual-Gas Donor of Nitric Oxide and Hydrogen Sulfide with Fluorescence for Real-Time Monitoring of Its Release. Analyst 2025, 150, 378–385. [Google Scholar] [CrossRef]
- Yang, Y.; Aloysius, H.; Inoyama, D.; Chen, Y.; Hu, L. Enzyme-Mediated Hydrolytic Activation of Prodrugs. Acta Pharm. Sin. B 2011, 1, 143–159. [Google Scholar] [CrossRef]
- Chauhan, P.; Bora, P.; Ravikumar, G.; Jos, S.; Chakrapani, H. Esterase Activated Carbonyl Sulfide/Hydrogen Sulfide (H2S) Donors. Org. Lett. 2017, 19, 62–65. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2016, 55, 4514–4518. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, C.; Weaver, D.E.; Lukesh, J.C. Esterase-Activated Hydrogen Sulfide Donors with Self-Reporting Fluorescence Properties and Highly Tunable Rates of Delivery. ACS Chem. Biol. 2024, 19, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, X.; Peng, W.; Liu, J.; Jia, Z.; Cheng, M.; Li, J.; Guo, W.; Zheng, Y. A Thiocoumarin Based Self-Reporting Sulfide Prodrug Strategy with a Favorable Safety Profile. Eur. J. Med. Chem. 2025, 289, 117426. [Google Scholar] [CrossRef] [PubMed]
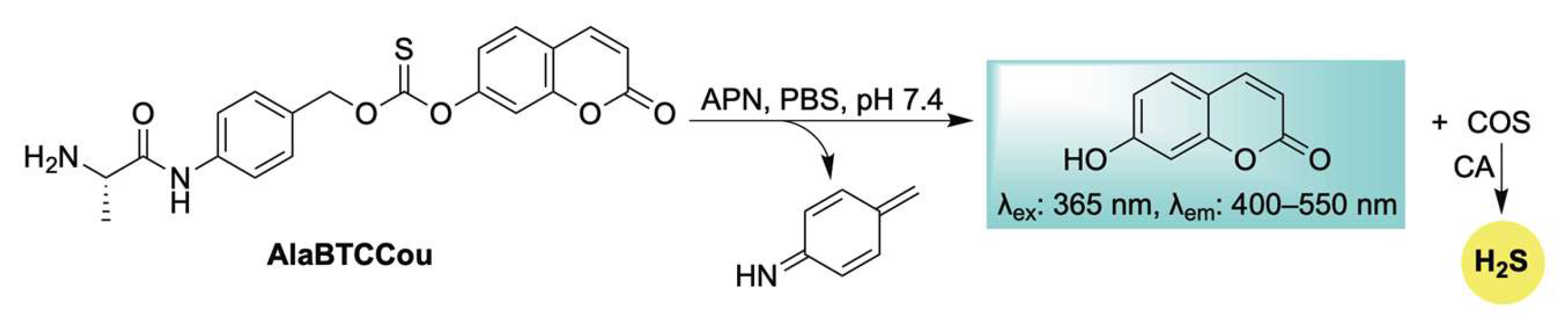
- Rong, F.; Bao, W.; Li, G.; Ge, Y.; Zhu, W.; Hao, B.; Zhao, Y.; Yuan, Y.; Wang, Y. Aminopeptidase N-Activated Self-Immolative Hydrogen Sulfide Donor for Inflammatory Response-Specific Wound Healing. Angew. Chem. Int. Ed. 2025, 64, e202423527. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Prescher, J.A.; Dube, D.H.; Bertozzi, C.R. Chemical Remodelling of Cell Surfaces in Living Animals. Nature 2004, 430, 873–877. [Google Scholar] [CrossRef]
- Nguyen, S.S.; Prescher, J.A. Developing Bioorthogonal Probes to Span a Spectrum of Reactivities. Nat. Rev. Chem. 2020, 4, 476–489. [Google Scholar] [CrossRef]
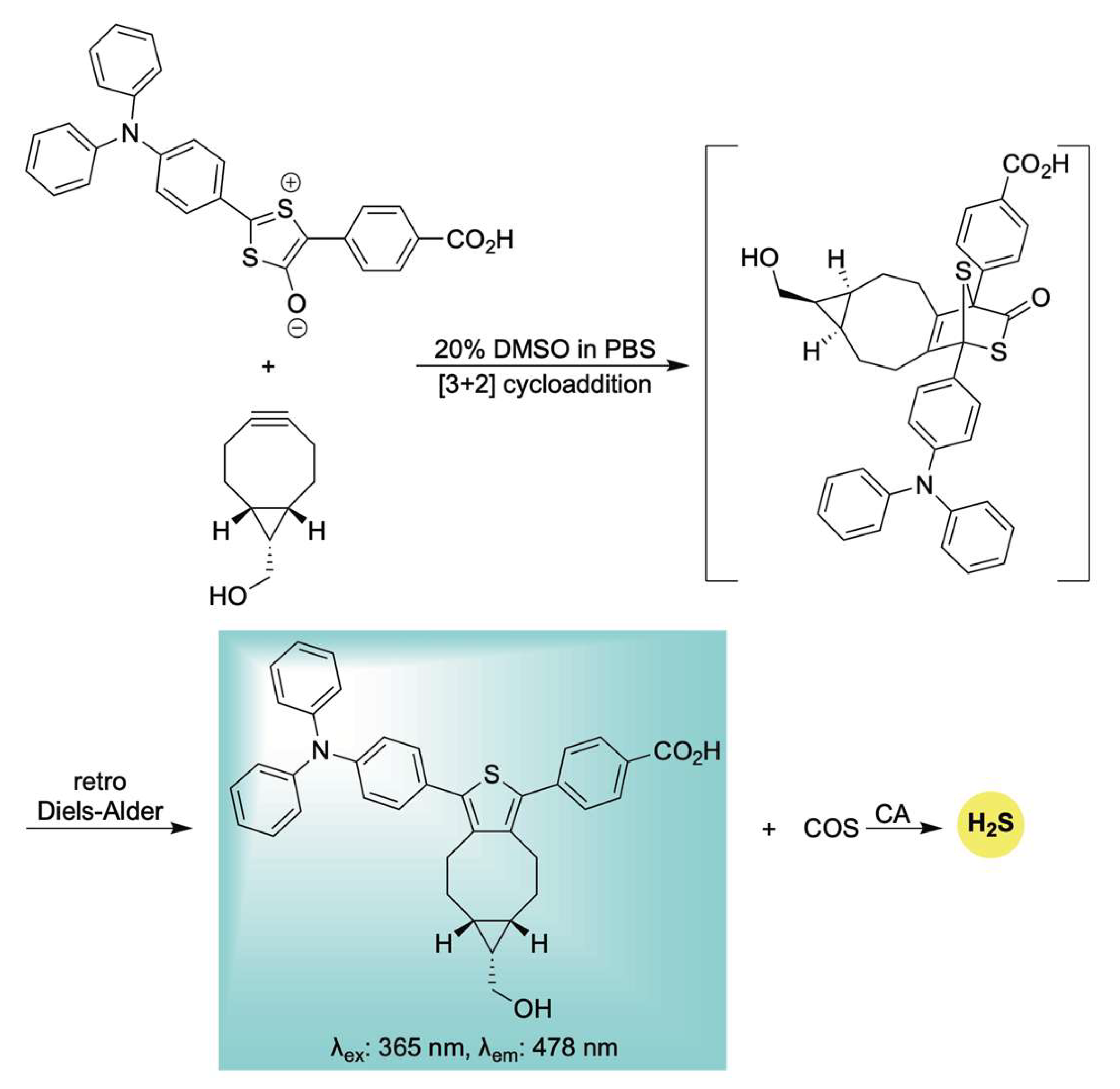
- Steiger, A.K.; Yang, Y.; Royzen, M.; Pluth, M.D. Bio-Orthogonal “Click-and-Release” Donation of Caged Carbonyl Sulfide (COS) and Hydrogen Sulfide (H2S). Chem. Commun. 2017, 53, 1378–1380. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, R.; Tang, C.; Zhang, C.; Xu, W.; Wu, L.; Wang, Y.; Ye, D.; Liang, Y. Design and Development of a Bioorthogonal, Visualizable and Mitochondria-Targeted Hydrogen Sulfide (H2S) Delivery System. Angew. Chem. Int. Ed. 2022, 61, e202112734. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Lukesh, J.C. Self-Reporting H2S Donors: Integrating H2S Release with Real-Time Fluorescence Detection. Chemistry 2025, 7, 116. https://doi.org/10.3390/chemistry7040116
Zhu C, Lukesh JC. Self-Reporting H2S Donors: Integrating H2S Release with Real-Time Fluorescence Detection. Chemistry. 2025; 7(4):116. https://doi.org/10.3390/chemistry7040116
Chicago/Turabian StyleZhu, Changlei, and John C. Lukesh. 2025. "Self-Reporting H2S Donors: Integrating H2S Release with Real-Time Fluorescence Detection" Chemistry 7, no. 4: 116. https://doi.org/10.3390/chemistry7040116
APA StyleZhu, C., & Lukesh, J. C. (2025). Self-Reporting H2S Donors: Integrating H2S Release with Real-Time Fluorescence Detection. Chemistry, 7(4), 116. https://doi.org/10.3390/chemistry7040116






