1. Introduction
Over the past few decades, with rapid economic development and improved living standards, fruit consumption has significantly increased, and consumer demands for fruit quality have also risen [
1]. Soluble solids content (SSC) in fruits refers to the total content of substances dissolved in fruit juice, including sugars (such as glucose, fructose, and sucrose), organic acids, and minerals. Since sugars dominate this composition, SSC is commonly referred to as soluble sugar content. The level of soluble sugar content is one of the core indicators for evaluating fruit sweetness, maturity, and overall quality [
2]. Despite technological advancements, the lack of standardized protocols for cross-variety model generalization remains a critical gap in industrial applications.
By measuring SSC, it is possible to assess fruit sweetness, maturity, and storability, providing scientific guidance for harvesting, storage, and processing. For instance, Qiu et al. predicted the maturity of pineapples by estimating their SSC, thereby determining the optimal harvest time [
3]. Similarly, Guo et al. used near-infrared transmittance spectroscopy to measure SSC and other parameters in apples under different temperatures, achieving non-destructive monitoring of storage quality [
4].
Moreover, in the fruit market, SSC serves as a critical indicator of fruit quality, directly impacting consumer taste experiences and purchasing decisions. It is also a key basis for producers to optimize cultivation management and enhance market competitiveness. Additionally, research on and the optimization of SSC holds significant importance for agricultural research and variety improvement, driving the optimization of cultivation techniques and the development of new fruit varieties [
5].
In this context, how to detect the SSC content in fruits has become a research focus. Traditional fruit sugar content detection techniques, such as juice chemical analysis [
6,
7], Abbe refractometry [
8,
9,
10], and handheld refractometry [
11,
12], have laboratory-level precision (typical error of ±0.2 °
Brix). However, their technical limitations severely restrict industrial applications, as destructive testing causes complete sample loss. Traditional methods lead to a fruit loss rate of up to 100%, causing a postharvest economic loss of 0.5–1.2%. It takes 5–10 min to detect a single fruit, involving multiple steps like sample preparation, instrument calibration, and data reading. The detection throughput is less than 20 samples per h, which cannot match the processing capacity of modern sorting equipment (≥300 samples per h). Offline detection cannot achieve real-time quality monitoring on postharvest sorting conveyor belts. These shortcomings have driven researchers to develop non-destructive sensing technologies based on new optical principles to meet the fruit industry’s demand for on-site, rapid (detection time < 10 s), high-throughput (>300 samples per h), and high-precision (±0.5 °
Brix) detection.
Non-destructive detection technology (NDT) has attracted significant attention in recent years due to its ability to obtain the internal and external quality parameters of fruits without causing damage. By leveraging physical interactions such as electromagnetic waves and mechanical waves, NDT provides a non-invasive approach to measuring key indicators like sugar content. Compared to traditional methods, NDT has many advantages (as shown in
Table 1).
These technological advancements provide core support for building IoT-based intelligent sorting systems, promoting the transformation of postharvest processing from experience-based decision-making to data-driven paradigms. Near-infrared spectroscopy (NIRS), with its speed (single detection < 3 s), high precision (
R2 > 0.92), and capability for multi-indicator simultaneous detection, has already been commercialized for sugar content detection in major fruits such as apples and citrus [
13]. To systematically evaluate these modalities,
Table 1 summarizes the performance metrics across critical parameters.
Currently, non-destructive detection tech for fruit SSC has become multi-modal, covering NIRS, HSI, CV, EN, LIF, DLS, etc. These technologies, combining specific physical interactions with chemometric modeling and machine learning, enable rapid (detection time < 5 s), non-destructive (sample integrity 100%) detection with an accuracy of ±0.3–0.8 °Brix (R2 > 0.85).
Despite great potential, practical NDT application faces challenges like spectral background noise, multi-component interference, and poor model generalization, which affect detection accuracy and reliability. High equipment costs, the need for skilled operators, and high surface requirements for some methods also hinder wide application. Future research will focus on multi-modal tech fusion (e.g., combining visible imaging with HSI), portable device development, and AI algorithm optimization to break traditional destructive detection bottlenecks and boost fruit quality detection towards intelligence and portability.
Notably, deep-learning-empowered third-generation NDT is emerging. For instance, Xiao et al. [
14] validated that fusing hyperspectral and fluorescence hyperspectral data via optimized deep learning (WOA-CNN-LSTM) achieves significant advances in non-destructive SSC prediction for kiwifruit. This work pioneers a dual-path evolution for next-generation fruit quality monitoring: multi-modal optical sensing integration establishes a comprehensive biochemical mapping foundation, while embedded AI-hardware co-design bridges laboratory precision with field scalability. Ultimately, such intelligent systems will drive autonomous orchard management and dynamic freshness control across global supply chains.
Consequently, this review aims to conduct an in-depth exploration of non-destructive sugar content detection in fruits. We have read and summarized a large amount of the relevant literature and research according to the standard [
15] (as shown in
Figure 1) and systematically examined the underlying principles, current applications, advantages, limitations, and future development directions of these technologies. This research is crucial for advancing the intelligent, efficient, and sustainable development of the fruit industry and provides valuable guidance for researchers and practitioners.
2. Materials and Methods
Non-destructive detection technologies for fruit quality are based on physical principles like spectroscopy, acoustics, electromagnetism, and image processing. By analyzing spectral absorption and reflection, acoustic wave propagation, or image features, these technologies enable the rapid, non-destructive assessment of both internal and external fruit quality. They can measure key indicators such as sugar content, firmness, color, and internal defects without damaging the fruit. These technologies are rapid, portable, real-time, and efficient. They not only enhance the efficiency and accuracy of fruit quality detection but also provide a scientific basis for fruit grading, harvesting decisions, and post-harvest management, thereby promoting the intelligent and efficient development of the fruit industry.
Prior to discussing specific non-destructive techniques, it is essential to outline common image preprocessing workflows and color space utilizations in fruit SSC detection. Raw fruit images typically undergo standardized preprocessing to enhance feature extraction:
- (1)
Noise reduction (e.g., median/Gaussian filtering) mitigates sensor artifacts.
- (2)
Region of interest (ROI) segmentation isolates fruit from the background via thresholding or edge detection.
- (3)
Illumination correction (e.g., white referencing) ensures spectral consistency.
- (4)
Color model selection directly impacts analytical efficacy.
Numbered lists can be added as follows:
- (1)
RGB (red, green, blue): baseline spatial-color representation, sensitive to lighting variations.
- (2)
HSV/HSL (hue, saturation, value/lightness): decouples color (hue) from intensity, enhancing robustness in segmentation.
- (3)
CIE Lab: L (lightness), a (green-red), b (blue-yellow) enables accurate color grading.
- (4)
HIS (hyperspectral imaging): Extends beyond surface color by capturing spatial-spectral data cubes (400–2500 nm), allowing internal SSC prediction via spectral fingerprinting.
2.1. Optical Spectroscopy-Based Techniques
2.1.1. Visible and Near-Infrared Spectroscopy Technology
Visible and near-infrared (Vis/NIR) spectroscopy is a popular non-destructive technique for detecting fruit sugar content. It uses electromagnetic radiation from 380 to 2500 nm. Vis light (380–700 nm) and NIR light (780–2526 nm) interact with fruit samples through reflection, transmission, or absorption, producing spectra that reveal physical and chemical properties. Vis/NIR spectroscopy is favored for its ability to detect signals from most organic compounds’ structures and functional groups, with stable spectral features [
16]. When incident radiation interacts with a sample, reflection, transmission, or absorption can occur, generating spectra in different modes. The reflection mode is easy to use but can be affected by the fruit skin’s characteristics. The transmission mode can penetrate deep tissues but requires a strong light source. The interaction mode reduces surface scattering interference and shows higher precision in predicting soluble solids content (SSC) in fruits like kiwifruit [
17,
18,
19].
Chemometric methods are crucial in this process. Preprocessing techniques like Savitsky-Golay smoothing and multiplicative scatter correction (MSC) remove noise and baseline drift. Variable selection methods like competitive adaptive reweighted sampling (CARS) and genetic algorithms (GA) identify characteristic wavelengths (e.g., 950–1075 nm in apples). Models like partial least squares regression (PLS) and least squares support vector machines (LS-SVM) build precise prediction equations [
20]. The principle of this technology is based on the interaction between light and the organic components of the sample. Different chemical bonds in organic matter absorb or emit specific wavelengths of light. Factors like molecular configuration and bond strength determine the inherent vibration frequencies of chemical bonds, giving rise to unique spectral fingerprints. Absorption peaks from hydrogen-containing functional groups (e.g., O–H, C–H, C–O, and N–H) are particularly prominent. The coupling vibrations of various chemical bonds in complex molecules form bending and stretching vibration modes. The specific molecular absorption behavior in the near-infrared region effectively reveals the chemical composition of the sample.
Spectral techniques analyze the spectral characteristics of internal fruit chemical components. Combined with chemometric methods to build spectral models, they enable rapid and non-destructive sugar content detection in fruits [
21].
In recent years, spectral techniques have made significant progress in fruit sugar content detection. For example, Guo et al. [
22], aiming to meet the non-destructive detection needs of apple SSC, systematically compared short-wave near-infrared (SWNIR, 500–1000 nm) and long-wave near-infrared (LWNIR, 1000–2500 nm) spectral technologies. They proposed a color-compensation-based optimization strategy. By collecting transmission spectra and skin color parameters (CIE Lab space) of Fuji apples and combining independent component analysis (ICA) with support vector machines (SVM) to build a nonlinear regression model (ICA-SVM), they effectively addressed the interference of fruit-skin pigments in spectral information. Experiments showed that color compensation significantly improved the prediction accuracy of SWNIR models, with the optimal model achieving a prediction correlation coefficient of 0.9398 and a root mean square error of prediction (RMSEP) of 0.387%. The LWNIR model performed slightly better (prediction correlation coefficient of 0.9455 and RMSEP of 0.369%), confirming both technologies’ suitability for efficient SSC detection. This study also highlighted SWNIR technology with color compensation as a promising tool for industrial real-time monitoring, offering theoretical support and methodological innovation for non-destructive fruit-quality detection under complex optical interference.
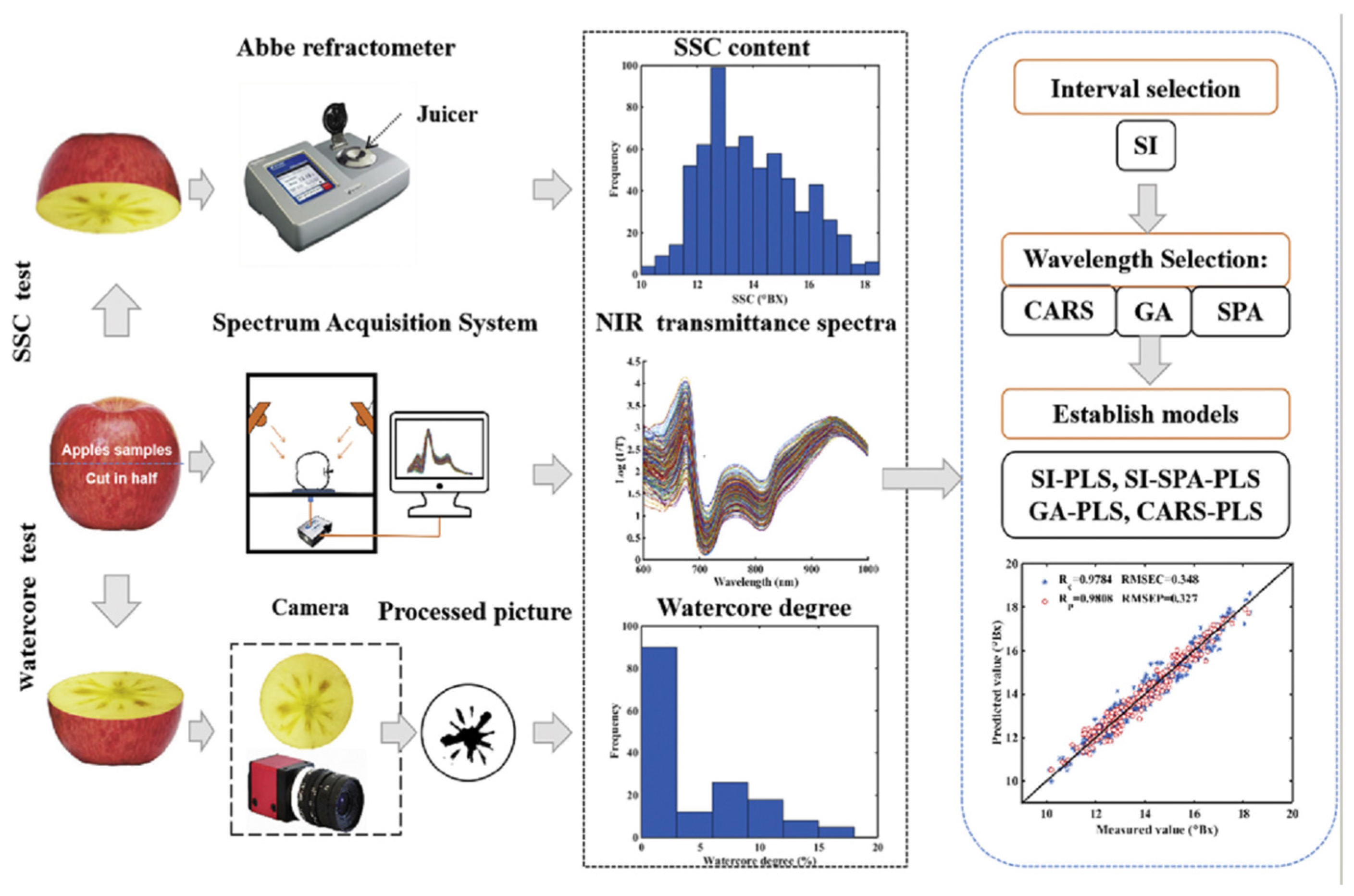
Subsequently, Guo et al. [
13] introduced a non-destructive detection method based on near-infrared transmission spectroscopy (NIR-TS) with chemometric algorithms for precise quantitative analysis of apple SSC (as demonstrated in
Figure 2, which shows the application of variable selection methods such as successive interval (SI), genetic algorithm (GA), competitive adaptive reweighted sampling (CARS), and successive projection algorithm (SPA) in building partial least squares (PLS) models). Using a portable near-infrared spectrometer (590–1200 nm) to collect whole-fruit transmission spectral data and the competitive adaptive reweighted sampling (CARS) algorithm to select characteristic wavelength variables, they built a partial least squares (PLS) prediction model. Results indicated that the CARS-PLS model showed excellent performance in SSC prediction, with a prediction set correlation coefficient of 0.9808, an RMSEP of 0.327 °
Brix, and a residual predictive deviation (RPD) increased to 4.845. This precision was significantly higher than that of traditional physicochemical detection methods and other variable selection strategies (e.g., genetic algorithm (GA) and successive interval (SI)). This research confirmed that near-infrared transmission spectroscopy, by analyzing the optical properties of deep tissues, can effectively obtain the internal quality information of apples, providing reliable technical support for non-destructive fruit-quality detection, online sorting, and the construction of smart agricultural systems.
Sheng [
23], Wang [
24], and Fan [
25] have focused on enhancing detection accuracy in spectral modeling by addressing biological and environmental variations. Their studies employ common technical strategies, such as spectral preprocessing (SNV, MSC) to eliminate noise, combined with variable selection algorithms (CARS, SPA) to identify key wavelengths and simplify models. They also propose calibration transfer methods (slope/bias correction, EPO orthogonalization) to enhance model robustness against different interference sources (cultivar, origin, temperature). Additionally, they validate model generalization through cross-condition validation (years, temperatures, multi-origin sample sets) and explore portable device development to transition the technology from labs to orchards or production lines. Collectively, these studies show that algorithm optimization and multi-scenario design enable non-destructive spectroscopic technology to overcome traditional single-model limitations and offer systematic solutions for universal and high-precision fruit-quality detection.
Spectral technology shows advantages in non-destructively detecting fruit SSC but faces multiple challenges in industrial application. First, model generalization is limited by fruit variety, maturity, and environmental conditions (e.g., temperature fluctuations), causing the standard error of prediction (SEP) to rise to 2.55 °
Brix across varieties. Second, while hyperspectral imaging systems can capture spatial-spectral information, their high hardware costs and complex data processing make them unsuitable for real-time field detection. Third, factors such as skin optical properties, internal tissue heterogeneity, and background noise (e.g., surface scattering and water-absorption interference) significantly affect the signal-to-noise ratio of spectral signals [
17].
Future research should focus on three breakthroughs: First, on developing low-cost multispectral devices based on characteristic wavelength selection by optimizing optical path design and miniaturizing sensors [
26]. Second, on building standardized databases across varieties and regions, and combining transfer learning and deep learning algorithms to enhance model robustness. For instance, Guo et al. [
27] proposed a CARS-CNN model that inputs CARS-selected feature variables into a convolutional neural network (CNN). The CNN automatically extracts spectral features through convolutional, pooling, and fully connected layers, addressing the limitations of traditional linear models (e.g., PLS) in modeling complex nonlinear relationships. Third, on promoting the integration of multi-modal technologies, such as combining Raman spectroscopy with near-infrared spectroscopy to visualize sugar distribution at the cellular level, and integrating machine vision to achieve on-site grading detection in orchards [
28]. The ultimate goal is to form an intelligent quality monitoring system covering the entire chain of “harvesting–storage–sales” and to promote the widespread application of this technology in fruit-quality detection.
In addition, spectral technologies have great potential in detecting pesticide residues in agricultural products and in rapidly and non-destructively measuring key indicators (e.g., soluble sugar, phenolic compounds, amino acids, and fermentation quality) in other agricultural products [
29,
30]. Combining near-infrared spectroscopy with chemometrics provides a portable and efficient alternative to traditional time-consuming and destructive chemical analysis, supporting food production and quality control [
31,
32,
33].
2.1.2. Hyperspectral Imaging Technology
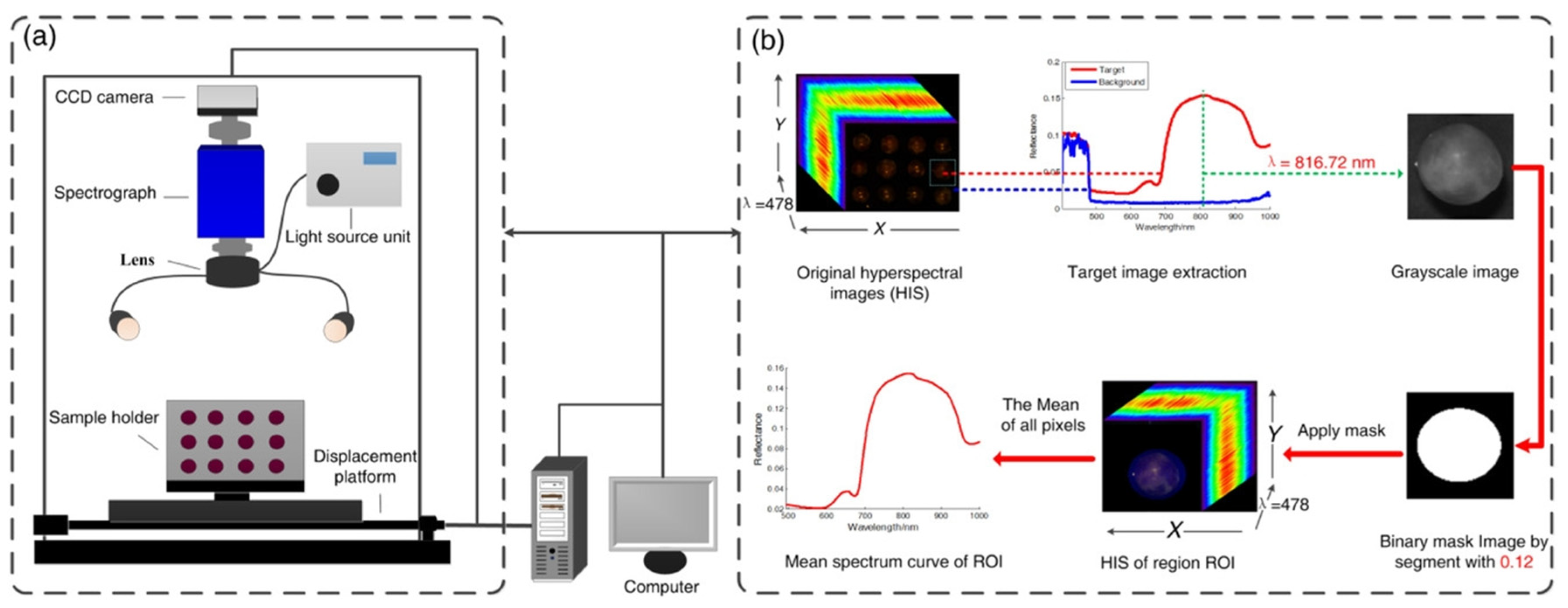
Hyperspectral imaging (HSI) and near-infrared (NIR) spectroscopy, both key in spectral analysis for non-destructive fruit testing, differ significantly in principles, data traits, and uses. HSI, a non-destructive method blending computer imaging and spectroscopy, captures the spatial and spectral data of objects. By splitting light into many contiguous bands via an imaging spectrometer, it forms a 3D data cube where each pixel has a unique spectral curve, like a fingerprint. This reflects internal physical and chemical changes, enabling qualitative and quantitative analysis of fruit traits such as sugar, acidity, and water content.
It is important to note that this section summarizes findings and methodologies from the published literature. In application, HSI has shown great potential in fruit-quality detection. For example, Xu et al. [
34] used HSI (400.68–1001.61 nm) with a new VMD-RC algorithm to non-destructively detect total soluble solids (TSS/SSC) in grapes. After decomposing hyperspectral data into multi-modal feature components via VMD and screening 12 key wavelengths (e.g., 753.6 and 845.14 nm), they built a prediction model (VMD-RC-LSSVM) with a determination coefficient of 0.93 and a root mean square error (RMSEP) of 0.6% on the prediction set, surpassing traditional algorithms. This study confirmed that HSI can accurately analyze internal sugar gradients in fruits by combining spatial distribution and NIR spectral features, offering an efficient solution for online fruit sorting. It also suggested that industrial applications could be advanced through multi-modal data fusion and miniaturized device development. Moreover, HSI has been widely used in food quality and safety as well as waste sorting and recycling, showing its potential in modern processing and distribution.
Figure 3 illustrates the hyperspectral imaging system along with its corresponding data acquisition process. The examples above illustrate the application and potential of HSI for SSC detection.
However, hyperspectral technology faces several practical challenges. First, the high data volume of hyperspectral images requires efficient dimensionality reduction techniques for key feature retention. Second, high equipment costs hinder its large-scale production adoption. Third, the technology’s stability in complex environments needs enhancement to suit diverse application scenarios.
Looking ahead, with the growth of AI and big data tech, hyperspectral imaging can achieve higher accuracy and efficiency in fruit-quality detection. Combined with advanced algorithms like deep learning, it can optimize data processing, cut detection costs, and offer more comprehensive and reliable non-destructive solutions for fruit-quality detection.
For example, Xu et al. [
35] innovatively combined visible/near-infrared hyperspectral imaging (400–1001 nm) with deep learning stacked autoencoders (SAE) to NDT detect total soluble solids (TSS/SSC) and titratable acidity (TA) in grapes. They used a pixel-level spectral analysis (extracting 700 spectra per sample) and an SAE network with a symmetric structure to automatically extract deep spectral features (400 → 200 → 100 → 50 → h → 50 → 100 → 200 → 400). They also introduced fruit size as a compensatory factor to build an SAE-LSSVM hybrid model. Results showed that the compensated model significantly improved TSS prediction accuracy (
R2 = 0.9237, RMSEP = 0.5041%), outperforming traditional feature-wavelength-selection-based models (CARS, SPA;
R2 = 0.8747). This approach, via multimodal data fusion (spectral + morphological parameters) and the feature abstraction of deep learning, overcame nonlinear modeling challenges in high-dimensional data. It also enhanced data robustness with EEMD-DWT denoising and Monte Carlo outlier removal, providing an efficient solution for online fruit sorting and portable device development. This highlights deep learning’s potential in uncovering spatial heterogeneity in spectral data.
2.1.3. Raman Spectroscopy Technology
Raman spectroscopy is a scattering spectroscopy technique. When light is shone on a fruit sample, the molecules in the sample interact with the light to produce scattered light [
36]. Most of the scattered lights have the same frequency as the incident light, and this process is called Rayleigh scattering. However, a small portion of the scattered light undergoes a change in frequency, and this type of scattered light is known as Raman scattering. Raman spectroscopy measures the frequency changes and intensity of Raman scattered light to obtain information about the molecular structure and chemical composition of the sample. In fruit sugar detection, Raman spectroscopy technology can use the characteristic Raman scattering peaks of sugar molecules in fruits to establish a quantitative relationship model between Raman spectra and sugar content, thereby achieving the non-destructive detection of fruit sugar content [
37]. This technology offers advantages such as high sensitivity, high selectivity, and rapid detection, providing chemical information at the molecular level and offering strong support for precise fruit sugar detection. However, Raman spectroscopy signals are relatively weak and prone to fluorescence interference. Additionally, this technique has certain requirements for sample uniformity and surface condition, which can somewhat affect its detection effectiveness and application scope [
38,
39]. As depicted in
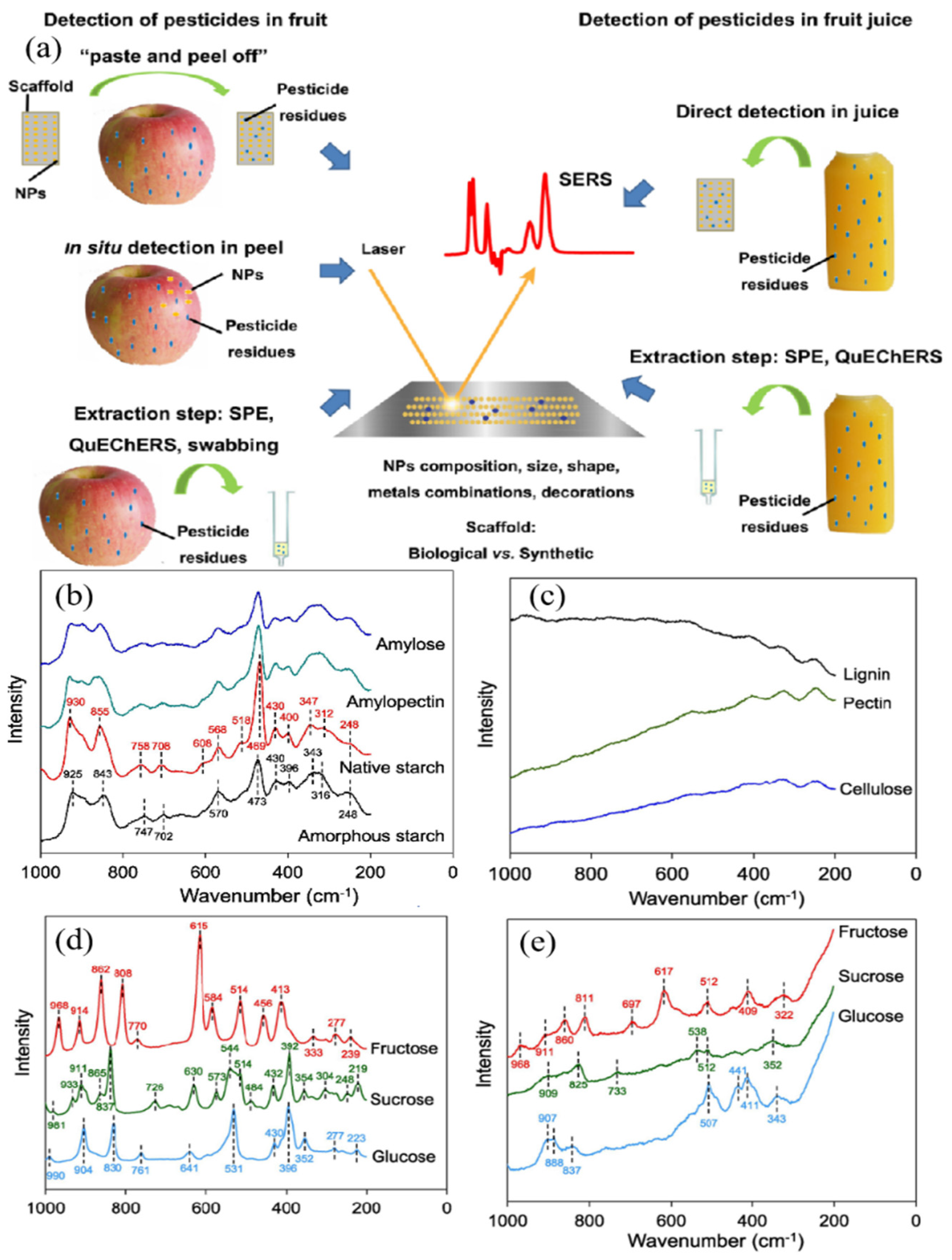
Figure 4a, Wang et al. established a generalized protocol for surface-enhanced Raman spectroscopy (SERS) sample preparation, incorporating sequential steps of nanoparticle synthesis, functionalization, and substrate characterization. Then,
Figure 4b–e demonstrates Nakajima’s systematic investigation of molecular vibrational profiles across diverse carbohydrate systems:
Figure 4b presents vertically offset Raman spectra of starch variants (native amylose, amylopectin, banana starch, and amorphous banana starch) in solid states, enabling the clear visualization of characteristic band patterns.
Figure 4c,d comparatively analyzes structural fingerprints of solid-phase biopolymers (cellulose fibers) and crystalline sugars (sucrose/glucose), while
Figure 4e extends this methodology to liquid-phase sugar systems (fructose solutions), highlighting solvent-induced spectral modifications. This integrated visualization framework bridges nanomaterial engineering (
Figure 4a) with the spectroscopic discrimination of complex food matrices (
Figure 4b–e), providing methodological cross-validation for pesticide residue detection in agricultural products.
2.2. Imaging and Sensing Technologies
2.2.1. X-Ray Technology
X-ray technology is significant for non-destructively detecting fruit soluble sugar content. It works by using X-rays’ penetrability of fruit and the different absorption rates of internal structures. When X-rays pass through fruit, varying quality parts attenuate the rays differently. Capturing these intensity changes from internal defects or foreign objects converts them into 2D images for internal-quality detection [
42].
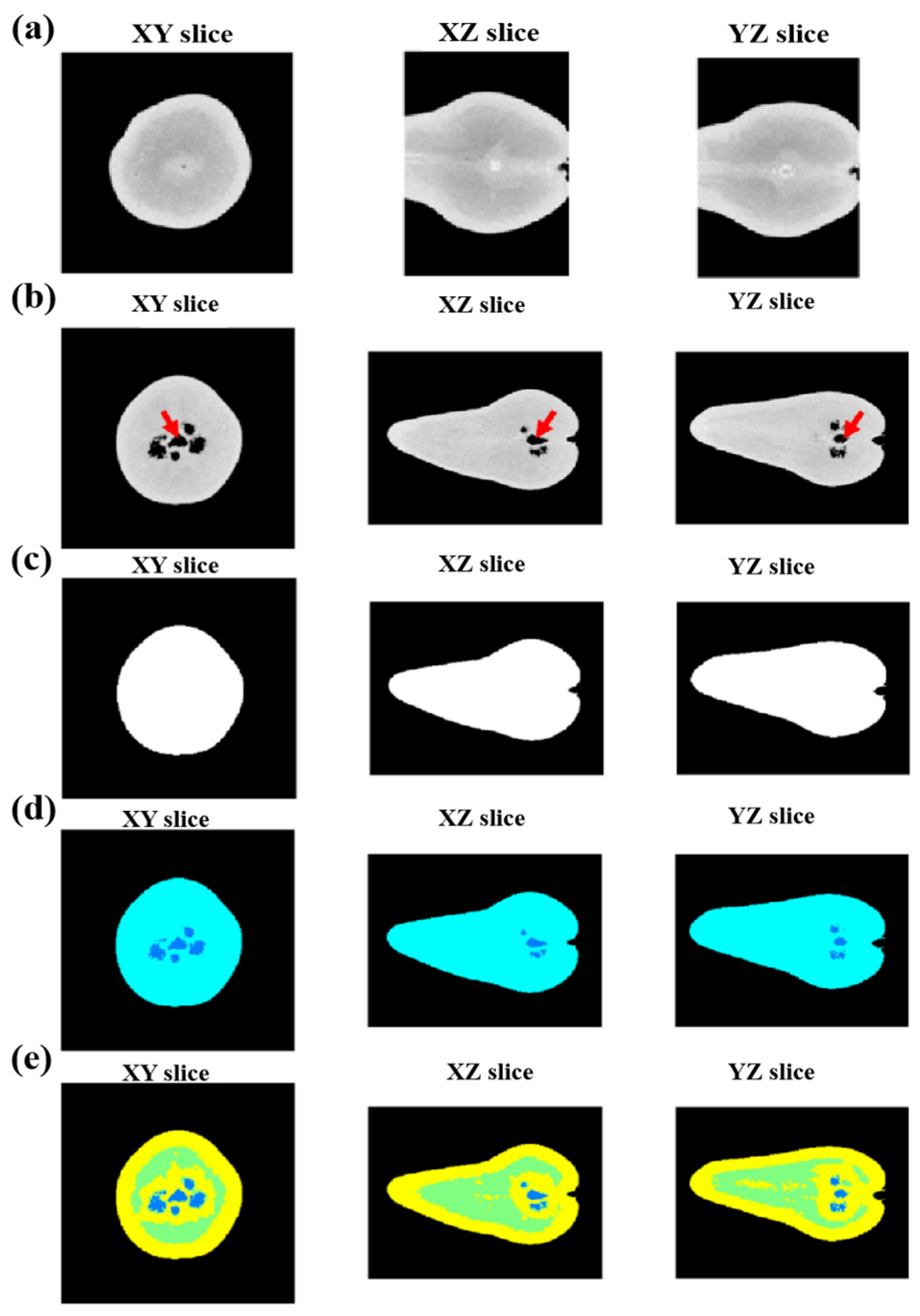
Figure 5 systematically demonstrates the multi-phase segmentation framework through orthogonal CT slices. The XZ and YZ slices are zoomed in on the region affected by internal browning.
Figure 5b–e shows orthogonal slices through the original grayscale CT reconstruction of the sample; cavities can be observed around the core (core indicated by the red arrow), orthogonal slices through the fruit mask (white), orthogonal slices through the tissue (cyan) and internal air masks (dark blue), orthogonal slices through the internal air (dark blue), low-density tissue (green), and high-density tissue (yellow) masks.
With the development of electronic computers, X-ray computed tomography (CT) technology has emerged. CT technology uses X-rays to penetrate a cross-sectional layer of an object. Different materials absorb X-rays differently. The CT detector receives the attenuated X-rays and converts them into electrical signals, which are input into a computer. After data processing, the computer displays an image and generates CT values for corresponding points. By establishing a mathematical model between CT values and target detection values, non-destructive detection can be achieved [
43,
44].
X-ray technology has shown good performance in detecting the internal quality of fruits like apples and pears, especially in identifying internal pests. However, the high manufacturing cost of X-ray imaging systems limits their widespread use in production.
Figure 5.
(
a) Orthogonal slices through the CT volume of the same fruit. The XZ and YZ slices are zoomed in on the region affected by internal browning. (
b) Orthogonal slices through the original grayscale CT reconstruction of the sample; cavities can be observed around the core (core indicated by red arrow); (
c) orthogonal slices through the fruit mask (white); (
d) orthogonal slices through the tissue (cyan) and internal air masks (dark blue); (
e) orthogonal slices through the internal air (dark blue), low-density tissue (green) and high-density tissue (yellow) masks [
45].
Figure 5.
(
a) Orthogonal slices through the CT volume of the same fruit. The XZ and YZ slices are zoomed in on the region affected by internal browning. (
b) Orthogonal slices through the original grayscale CT reconstruction of the sample; cavities can be observed around the core (core indicated by red arrow); (
c) orthogonal slices through the fruit mask (white); (
d) orthogonal slices through the tissue (cyan) and internal air masks (dark blue); (
e) orthogonal slices through the internal air (dark blue), low-density tissue (green) and high-density tissue (yellow) masks [
45].
Currently, X-ray technology faces challenges in the non-destructive detection of fruit soluble sugar content, such as improving detection accuracy and efficiency, and reducing costs for large-scale applications.
In the future, with technological progress and cost reduction, X-ray technology is expected to have broad application prospects in fruit-quality detection. Combining it with other non-destructive techniques (e.g., near-infrared spectroscopy and hyperspectral imaging) can further enhance detection accuracy and efficiency, offering more comprehensive and reliable solutions for non-destructive fruit-quality detection [
46].
X-ray technology shows potential in fruit sugar content detection but faces challenges. It is costly, slow in data collection, and provides limited quality-characteristic information, mainly focusing on internal structure. Additionally, while it is sensitive to fruit density and moisture, its ability to detect chemical components like sugar is limited. Thus, its application in fruit sugar detection is mainly for internal defect detection and structural analysis, rather than direct sugar measurement.
2.2.2. Computer Vision Technology
Computer vision technology, simulating human vision for intelligent image/video analysis, involves image acquisition, preprocessing, feature extraction, pattern recognition, and machine learning. It uses sensors like cameras and spectrometers to capture digital images of objects, then optimizes image quality via filtering, segmentation, and enhancement. Features like color (RGB/HSV parameters), shape (diameter, roundness), and texture (gray-level co-occurrence matrix, wavelet features) are extracted. Models such as multivariate regression, support vector machines, or deep learning establish links between these features and internal quality.
In non-destructive fruit sugar content detection, researchers use hyperspectral imaging to capture reflective spectral information from 400 to 1000 nm and combine it with visible light image features to build sugar prediction models. For example, apple sugar content correlates significantly with skin anthocyanin and NIR spectral absorption peaks. This technology can detect a single fruit in 0.5 s with an accuracy of 85–92%, and it can simultaneously sort multiple indicators like sugar, acidity, and defects online, which is faster and more efficient than traditional refractometer methods.
Applications of computer vision in fruit sugar detection rely on the correlation between external fruit features and internal sugar content. For instance, Huang et al. [
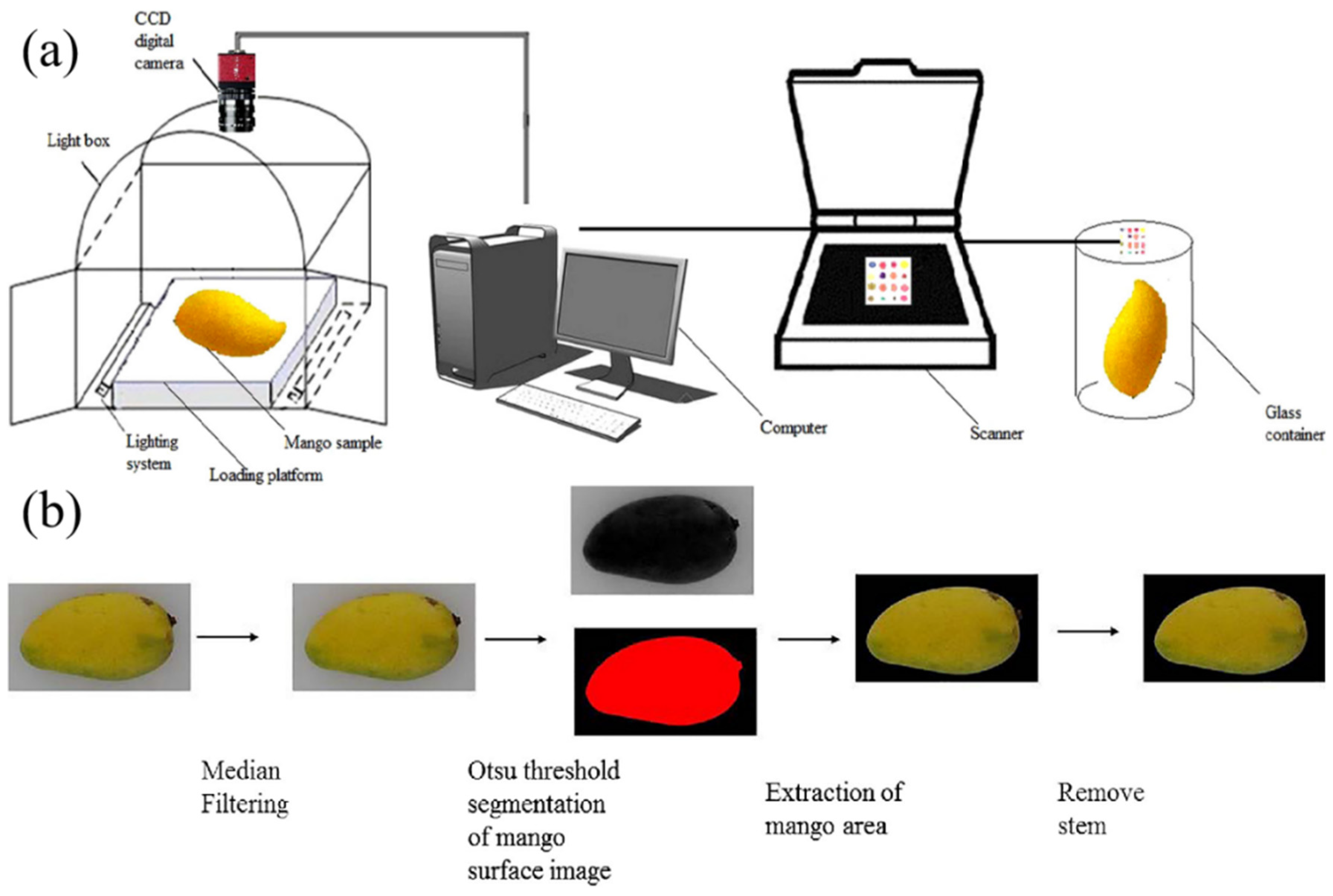
47] explored computer vision for non-destructively detecting total soluble solids (TSS) in fruits, using mangoes as a case study. By capturing surface images and extracting color features (hue, saturation, Lab parameters) and texture features (homogeneity, entropy, correlation) and using principal component analysis (PCA) for dimensionality reduction, they built a support vector regression (SVR) model to link external features with internal TSS. The model achieved correlation coefficients of 0.9515 (training set) and 0.9241 (prediction set), highlighting the significant correlation between color/texture and sugar content. The study innovatively combined computer vision with a colorimetric sensor array (CSA) to capture the chemical information of mango volatiles, enhancing classification accuracy to 98.75% (training set) and 97.5% (prediction set). This method overcame traditional chemical detection limitations, enabling rapid, non-destructive sugar assessment and offering a technical reference for intelligent fruit-quality grading.
Figure 6 illustrates the integrated detection system developed by Huang et al., which synergizes computer vision with a Cross-Stitch Architecture (CSA) model to analyze mango samples, encompassing image acquisition, feature extraction, and quality grading through multimodal data fusion. Additionally, deep-learning-based regression modeling has been used for fruit sugar detection, improving accuracy and efficiency and advancing Vis/NIR spectroscopic non-destructive detection. Guo et al. [
48] further confirmed the advantages of deep learning models (e.g., ResNet-CBAM) in complex texture analysis for tea grading. Data enhancement (brightness transformation, rotation) and multi-scale image segmentation improved model robustness, reducing the blended proportion evaluation error to 2.26%. This approach overcame traditional chemical detection limitations, enabling rapid, high-precision sugar assessment through multi-source data fusion and nonlinear modeling, providing an efficient technical path for intelligent fruit-quality grading [
49].
Computer vision technology has some limitations in fruit sugar content detection. Reliant on the correlation between external fruit features and internal sugar content, it may be less accurate for fruits with uneven internal sugar distribution. Moreover, ambient lighting and fruit-surface reflections can interfere with image acquisition and analysis.
Future development directions may involve combining computer vision with other non-destructive techniques like near-infrared spectroscopy and electronic nose technology for multimodal data fusion, enhancing detection accuracy and reliability. Huang et al. [
50] proposed a non-destructive method fusing computer vision and electronic nose data to assess tomato quality (maturity and firmness) during storage. Computer vision captured surface color information (RGB, HSV, CIE Lab* space), and after preprocessing and PCA, it extracted key color features (e.g., mean gray values of G, B, H, S, a* channels). The electronic nose, with semiconductor sensor arrays, captured volatile flavor features, and PCA derived the main odor components. By fusing the color and odor principal components, they built SVC and SVR models. The fused model showed superior performance in tomato maturity classification (94.20% prediction accuracy) and firmness prediction (
R2 = 95.14%,
RMSEP = 0.03 N), proving multimodal fusion’s advantage in boosting model robustness and information complementarity. Though not directly involving soluble sugar detection, this fusion framework offers a methodological reference for non-destructively assessing other quality parameters like sugar content. For example, cooperative analysis of color and volatile substances can indirectly link sugar metabolism, showing the potential of multi-source information fusion in agricultural product quality evaluation.
Additionally, further optimizing algorithms and models to adapt to the detection requirements of different fruit varieties and ripeness levels is also a key focus of research. Hu et al. [
51] proposed improvements to the YOLOX network for efficient and precise model optimization in apple detection and localization tasks: Firstly, structural reparameterization techniques (DBB and RepBlock modules) were employed to enhance feature extraction during training through multi-branch structures, while converting to a single-branch structure during inference to reduce computational complexity, thereby significantly improving real-time performance (detection speed reached 167.43 F/s). Secondly, the traditional spatial pyramid pooling (SPP) was replaced with a serially structured SPPF layer, combined with ReLU activation functions to accelerate computation and optimize feature fusion. Furthermore, a dynamic weight loss adjustment strategy was introduced, which dynamically optimized the training process by monitoring the proportions of regression, confidence, and classification losses, thereby accelerating model convergence (mAP improved to 94.09%). Additionally, 3D localization was achieved through RGB-D multimodal data fusion (combining RGB images with depth information), with a localization error below 7 mm, validating the robust improvement of multisource information fusion in complex scenarios. These optimization strategies (e.g., lightweight network design, dynamic loss optimization, and multimodal data collaboration) can be transferred to sugar content detection scenarios in fruits, such as constructing more accurate sugar prediction models by integrating hyperspectral imaging (sugar spectral features) with depth information or enabling rapid online sugar sorting using lightweight models. These approaches provide methodological support for algorithm optimization and engineering applications in non-destructive agricultural quality detection.
2.2.3. Electronic Nose Technology
With the breakthrough development of biosensing and bionic technologies, detection technologies based on biomolecular recognition mechanisms and artificial olfactory systems have provided innovative solutions for non-destructive fruit quality detection, with typical examples being biosensors and bionic sensors (electronic tongues and electronic noses) [
52]. Biosensors consist of a bioactive component and a signal conversion system, forming a precision detection system. Their core mechanism lies in the specific molecular recognition of biological components (such as enzymes, antibodies, or aptamers) with target substances, which trigger enzymatic reactions or conformational changes. The resulting chemical signals are converted into quantifiable optical/electrical signals by a transducer. Systematic analysis of signal intensity and characteristic parameters enables precise detection of endogenous substance concentrations in fruits. Among these, electronic nose (EN) technology mimics the olfactory perception mechanisms of mammals, constructing a three-tier detection architecture that includes a gas sensor array, a signal processing unit, and a pattern recognition system, with the sensor array at its core being capable of identifying multiple gases [
53].
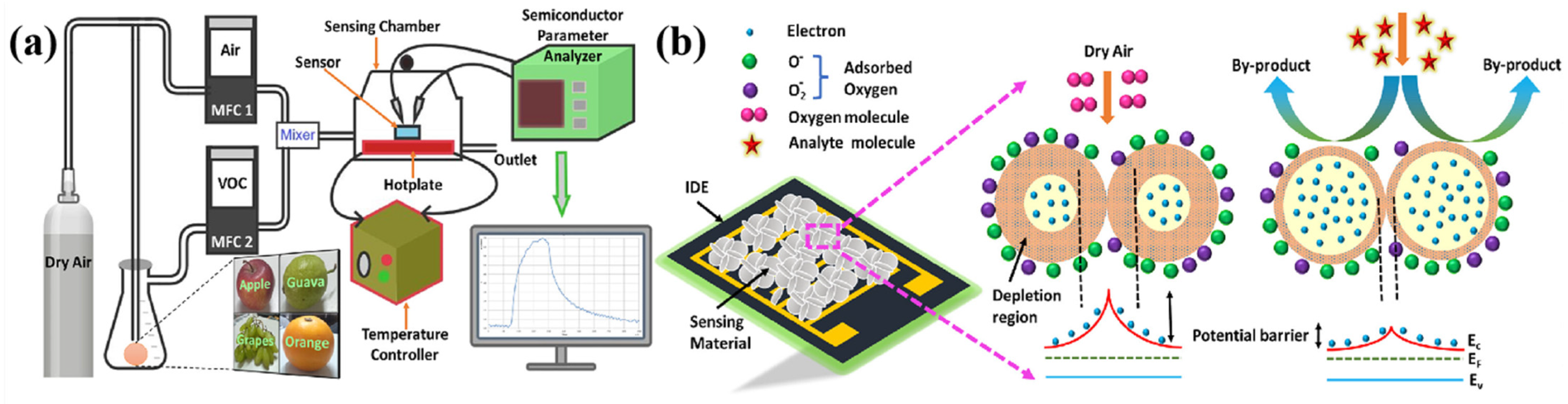
Figure 7 schematically illustrates the electronic nose (e-nose) device architecture.
During operation, the gas sensor array detects volatile organic compounds (VOCs) released by fruits. When these VOCs come into contact with the active materials on the sensor surface, they generate transient response signals. These signals are converted into digital signals via a conversion circuit and transmitted to a computer signal processing unit for analysis. The signals are compared and identified against a database of volatile compound information to determine the type of gas and, consequently, the corresponding substance [
54]. The core principle lies in the gas sensor array’s sensitivity to different gases. When VOCs released by fruits interact with the active materials in the sensor array, the sensors convert chemical signals into electrical signals, forming a characteristic response spectrum for the gas. By analyzing these response spectra using pattern recognition algorithms, qualitative and quantitative detection of fruit quality indicators such as sugar content can be achieved.
Figure 7.
Electronic-nose device schematic diagram [
55].
Figure 7.
Electronic-nose device schematic diagram [
55].
In recent years, electronic nose technology has demonstrated broad application prospects in the field of fruit quality detection. Compared to traditional detection methods, electronic nose technology offers three core advantages: First, the ppb-level detection limit of MOS sensor arrays for C6–C9 aldehydes and ketones, which are markers of sugar metabolism, ensures detection sensitivity. Second, the collaborative response mode of multiple sensors effectively overcomes the cross-sensitivity defects of single sensors, enhancing detection specificity. Third, nonlinear prediction models constructed using machine learning algorithms can reduce detection time to the minute level while maintaining detection accuracy comparable to high-performance liquid chromatography (R2 > 0.92). These characteristics make it highly valuable for on-site orchard detection and post-harvest grading processes.
Significant progress has been made in the application of electronic nose technology for fruit sugar detection. Studies have shown that electronic noses can effectively detect quality indicators of fruits, such as freshness, ripeness, and sugar content, providing a rapid and non-destructive solution for fruit quality evaluation. Huang et al. [
56] demonstrated that electronic noses, by optimizing sensor arrays (e.g., TGS825, TGS831) and combining them with pattern recognition algorithms, can effectively capture changes in volatile organic compounds (VOCs) generated by physiological metabolism and microbial activity during storage, thereby assessing sample freshness. In spinach freshness detection, the single-modality classification accuracy of the electronic nose reached 81.25%, while multi-sensor data fusion (combined with machine vision) improved the accuracy to 93.75%, validating its advantages in integrating complex information. Although this study focused on vegetables, its methodological framework (such as principal component analysis and backpropagation neural networks) is universally applicable and can be extended to the field of fruit quality detection. For instance, by optimizing sensor configurations for sugar-related metabolites (such as ethanol and esters) and combining them with chemometric modeling, electronic noses can achieve non-destructive prediction of fruit sugar content. This study provides theoretical support and technical references for the application of electronic nose technology in fruit freshness and sugar detection, highlighting its practical value in intelligent agricultural detection.
Currently, electronic nose technology has been widely applied in the quality detection of various agricultural products, such as potatoes [
57] and pears [
38], providing a scientific basis for fruit grading and post-harvest management. Additionally, as a novel sensor technology, electronic noses have also been widely used in non-destructive detection in other fields, such as component analysis of white liquor [
58], variety classification of vinegar [
59], and storage time analysis of oil [
60].
However, there are still several challenges in the application of electronic nose technology for fruit sugar detection. First, the electronic nose has limited recognition ability for complex odor mixtures, especially in low-concentration gas detection. Second, environmental factors (such as temperature and humidity) may affect the accuracy of detection results. In addition, the high cost of electronic nose equipment and research and development limits its promotion and application in some fields. For example, Han et al. found a low-cost electronic nose integrated with voltametric electronic tongue technology [
61]. Finally, the standardization and accuracy of the electronic nose still needs to be further improved to ensure the reliability of detection results.
Future research directions include optimizing sensor arrays and pattern recognition algorithms to improve detection accuracy and reliability; developing portable [
62], low-cost [
63], and fast-response [
64] electronic nose devices to adapt to different application scenarios; and promoting the integration of electronic nose technology with other non-destructive detection technologies (such as near-infrared spectroscopy) to achieve multimodal data fusion and further enhance the efficiency and accuracy of fruit quality detection. These advances will help promote the widespread application of electronic nose technology in fruit sugar detection and provide strong support for the intelligent and efficient development of the fruit industry.
2.3. Emerging Physical Detection Methods
In addition, the following non-destructive testing techniques have been applied for the detection of soluble sugar content in fruits:
2.3.1. Neutron Activation Analysis (NAA)
Neutron activation analysis (NAA) is a highly sensitive, multi-element analytical technique [
65]. Its principle involves irradiating fruit samples with neutrons, causing the elements in the samples to undergo nuclear reactions and produce radioactive isotopes. By measuring the energy and intensity of the γ-rays emitted by these radioactive isotopes, the content of various elements in the samples can be determined [
66]. In fruit sugar detection, NAA can indirectly reflect changes in the content of elements related to sugar content, thereby estimating the sugar level of the fruit [
67]. This technique offers advantages such as high sensitivity and the ability to simultaneously determine multiple elements. However, its application is limited due to the high cost of equipment, complex operation, and the requirement for specialized technical personnel for operation and maintenance.
2.3.2. Terahertz Spectroscopy Technology
Terahertz waves, located between microwaves and infrared light, possess unique spectral characteristics. They can penetrate many non-polar materials, such as paper and plastic, and exhibit special spectral responses to some polar substances [
68]. In fruit sugar detection, terahertz spectroscopy technology leverages the absorption and refraction properties of terahertz waves by different components in fruits. By analyzing terahertz spectral data and establishing corresponding mathematical models, non-destructive detection of fruit sugar content can be achieved [
69]. This technology offers advantages such as high resolution, high sensitivity, and non-contact detection, providing rich information on molecular vibrations and rotations, which offers a new approach for precise fruit sugar detection. However, terahertz spectroscopy technology is still in its developmental stage, with high equipment costs and requirements for sample uniformity and surface condition, factors that currently limit its large-scale application.
2.3.3. Nuclear Magnetic Resonance (NMR) Technology
Nuclear magnetic resonance (NMR) technology is an analytical method based on the resonance phenomenon of atomic nuclei in a magnetic field. When fruit samples are placed in a magnetic field and excited by radio frequency pulses, hydrogen nuclei and other atomic nuclei in the samples resonate, generating NMR signals. By analyzing these signals, information on components such as water and sugar in the fruit can be obtained, thereby achieving non-destructive detection of fruit sugar content [
70]. NMR technology offers advantages such as being non-destructive, rapid, and accurate, providing abundant chemical information and being suitable for sugar detection in various fruits [
71]. However, the high equipment costs, requirements for sample size and shape, and relatively long detection time limit its widespread application in fruit sugar detection.
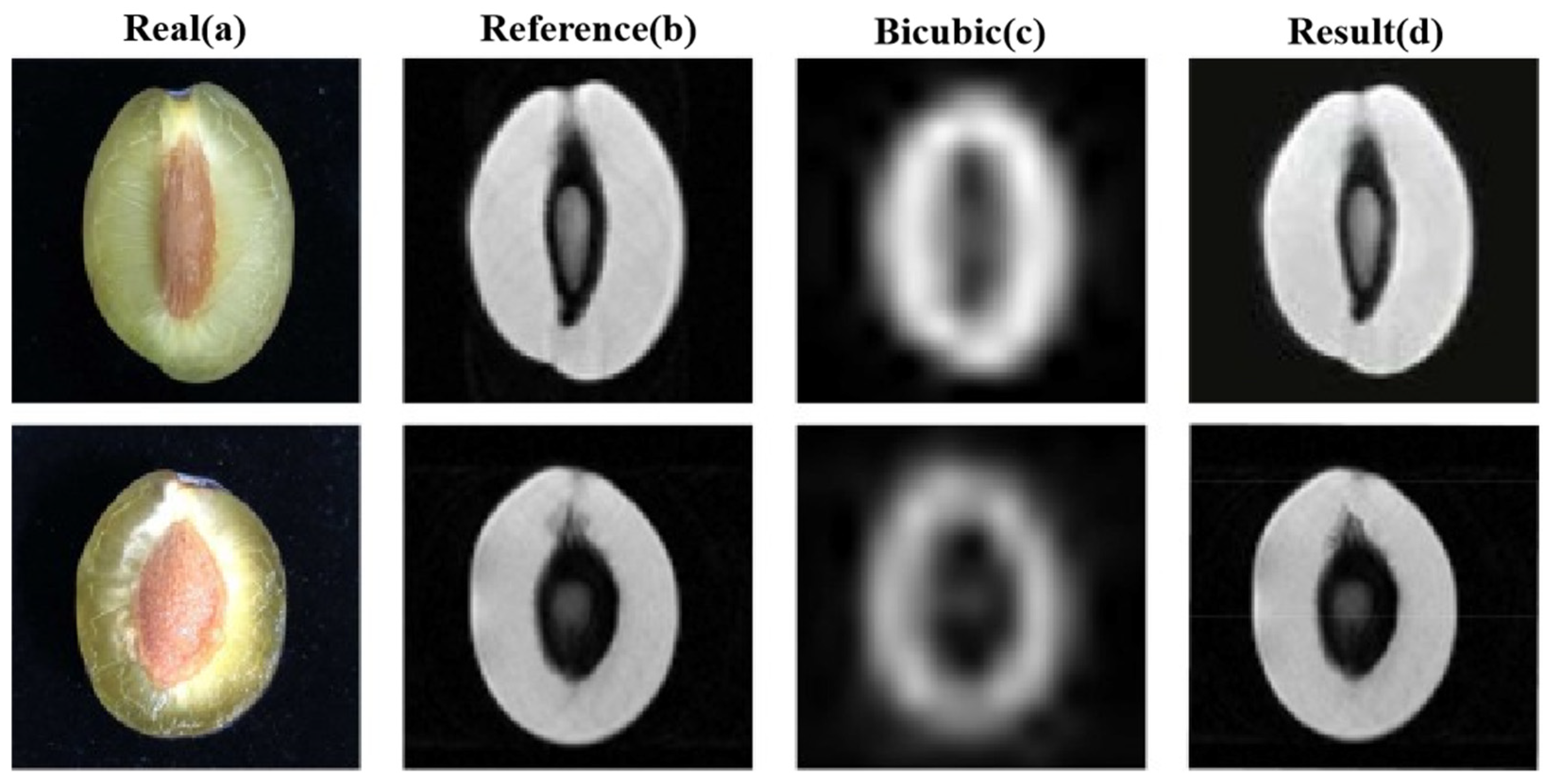
Figure 8 demonstrates the application of Low-Field Nuclear Magnetic Resonance (LF-NMR) technology in prune microstructure analysis.
2.3.4. Ultrasonic Technology
Ultrasonic waves, high-frequency mechanical waves, are influenced by the internal tissue structure and composition of fruits when propagating through them, leading to phenomena such as reflection, refraction, and absorption. By measuring changes in parameters like the propagation velocity, attenuation coefficient, and reflection coefficient of ultrasonic waves in fruits, internal quality information, including sugar content, can be obtained. Ultrasonic technology offers advantages such as being non-destructive, rapid, and easy to operate. It can penetrate fruit peels for internal detection, making it suitable for online detection and real-time monitoring. However, the precision and resolution of ultrasonic detection are relatively low, and this technique has certain requirements for sample size, shape, and surface condition, which somewhat limit its application in fruit sugar detection. Ultrasonic waves, high-frequency mechanical waves, are influenced by the internal tissue structure and composition of fruits when propagating through them, leading to phenomena such as reflection, refraction, and absorption. By measuring changes in parameters like the propagation velocity, attenuation coefficient, and reflection coefficient of ultrasonic waves in fruits, internal quality information, including sugar content, can be obtained. Ultrasonic technology offers advantages such as being non-destructive, rapid, and easy to operate. It can penetrate fruit peels for internal detection, making it suitable for online detection and real-time monitoring. However, the precision and resolution of ultrasonic detection are relatively low, and this technique has certain requirements for sample size, shape, and surface condition, which somewhat limit its application in fruit sugar detection.
2.3.5. Smart Sensor Technology
Smart sensors are intelligent detection devices that integrate functions such as sensing, signal processing, and communication. In fruit sugar detection, smart sensors based on electrochemical, optical, and acoustic principles can be used. These sensors interact with fruit samples to obtain electrical, optical, and acoustic signals related to sugar content. After signal processing and data analysis, non-destructive detection of fruit sugar content can be achieved. Smart sensor technology offers advantages such as high sensitivity, high selectivity, rapid response, and miniaturization. It enables real-time, online, and precise detection of fruit sugar content, providing a new approach for intelligent fruit quality detection [
73]. However, the high research and development costs of smart sensors, along with the need for customized designs for different fruit varieties and detection requirements, somewhat limit their large-scale application.
Figure 9 shows the principles and applications of Smart Sensor Technology.
3. Results
Despite significant advancements in non-destructive detection technologies for soluble solids content (SSC) in fruits, critical challenges persist at both technical and application levels. At the core of technical research limitations lie fundamental constraints in sensor hardware development. The prohibitive acquisition and maintenance costs of high-resolution spectrometers and hyperspectral imaging systems have shifted research focus toward data-driven modeling approaches. However, these models frequently suffer from poor mechanistic interpretability and inadequate cross-cultivar adaptability—limitations exacerbated by spectral library constraints. Limited sample diversity, non-uniform SSC distribution within fruits, and difficulties in standardizing environmental variables compromise model robustness. Furthermore, SSC detection is inherently complicated by background spectral noise and signal overlapping from complex fruit matrices containing water, pigments, and structural components, while physiological confounders such as cultivar characteristics, ripeness stages, and pathological conditions create persistent multicomponent interference that current methodologies struggle to decouple. The absence of universally accepted detection metrics, standardized testing protocols, and comparable evaluation frameworks across different technologies and operational environments further undermines validation consistency and cross-study reproducibility.
Translating these technologies from research to industrial implementation contexts faces equally significant barriers. The interdisciplinary expertise required in optics, computer science, and agricultural engineering establishes substantial technical entry thresholds. Resource intensity presents another major constraint: seasonal fruit availability and the destructiveness of reference methods restrict training data acquisition, while processing high-dimensional spectral data demands expensive computational infrastructure; these cumulative costs prove prohibitively expensive for small-to-medium enterprises. Engineering limitations compound these challenges, as most laboratory systems lack the real-time processing speeds (>5 fruits/s), environmental robustness (vibration/temperature tolerance), and compact form factors necessary for packing-line integration. This mismatch is particularly evident for fruits with challenging morphologies or surface properties, where crop-specific solutions remain underdeveloped. Finally, the absence of internationally harmonized equipment calibration standards, accuracy certification protocols, and legal recognition frameworks for SSC readings creates regulatory uncertainty that obstructs commercial scaling and global market access.
4. Discussion
The evolution of non-destructive SSC detection in fruits will pivot toward multidimensional technological convergence, driven by synergistic advances in multimodal sensing, embedded hardware, intelligent algorithms, and standardized frameworks. These innovations promise to transcend current limitations and unlock new frontiers in precision agriculture.
4.1. Multimodal Imaging Integration: Toward Holistic Fruit Phenotyping
Future systems will leverage complementary physical principles to overcome the inherent constraints of single-modality approaches. By orchestrating visible imaging (surface morphology/texture), hyperspectral imaging (spatially resolved chemical mapping), near-infrared spectroscopy (bulk composition), and ultrasound/X-ray (structural integrity), multimodal platforms can establish causal links between fruit physiology and SSC dynamics [
75]. Crucially, data fusion architectures, such as feature-level fusion of infrared spectra with hyperspectral spatial data, will mitigate spectral noise and multi-component interference, enabling sub-0.5 °
Brix prediction errors in complex matrices like apples and grapes [
76]. This paradigm shift toward integrated fruit phenotyping will not only enhance SSC accuracy but also concurrently quantify ripeness, defects, and nutritional traits, transforming quality assessment from univariate to multivariate diagnostics.
4.2. Hardware Democratization and Edge Intelligence
The forthcoming paradigm shift in fruit SSC detection will be catalyzed by synergistic hardware–software co-evolution, fundamentally transforming accessibility and operational efficiency. Silicon photonics stands poised to disrupt traditional cost structures through chip-scale spectrometers and smartphone-integrated multispectral sensors, including nanomaterial-enhanced devices that leverage CMOS compatibility to slash hardware expenditures by >60% while preserving laboratory-grade spectral fidelity [
77,
78]. This miniaturization wave enables truly field-deployable systems, extending high-precision sensing beyond controlled environments. Concurrently, the convergence of edge-native intelligence and distributed computing architectures redefines real-time analytics: lightweight neural networks (e.g., knowledge-distilled CNNs) fused with compressed sensing overcome spectral data bottlenecks, enabling on-device extraction of sugar-distribution signatures without cloud dependency. When embedded within IoT mesh networks [
79,
80], these systems generate prescriptive agricultural insights, from harvest window optimization to irrigation micro-adjustments through sub-100 ms per-fruit decisions [
81,
82]. Such edge-processing capability proves indispensable for industrial packing lines, where throughput demands necessitate millisecond-scale inference while maintaining ±0.3 °
Brix accuracy—a feat unattainable via centralized computing models.
4.3. Embedded Intelligence: Algorithmic Frontiers for Robust Generalization
The quest to resolve model generalizability limitations pivots toward cross-domain adaptive architectures, where transfer learning frameworks [
83,
84,
85] harness knowledge distillation from data-abundant cultivars (e.g., apples) to niche varieties through spectral feature-extractor reparameterization—potentially slashing target-domain data requirements by >50%. Federated learning ecosystems [
86] will concurrently decentralize training across global orchards, preserving data privacy while mitigating sample scarcity and proprietary constraints. This culminates in hierarchical ensemble systems that deploy meta-learning to dynamically weight specialized sub-models for taxonomically grouped fruits (e.g., tropical climacteric vs. temperate non-climacteric varieties), achieving >90% cross-cultivar prediction stability. Parallel innovations confront data scarcity through computational phenotyping synthetics: physics-informed GANs [
87,
88,
89] simulate spectral responses across biological gradients (ripeness, pathology) and environmental regimes, generating physically plausible variance to augment training corpora. Semi-supervised pipelines [
90,
91] exploit spatial-spectral consistency to auto-generate hyperspectral pseudo-labels, reducing manual annotation costs by 70–80%. For industrial deployment, neural architecture search-derived compression (e.g., Mobile Net variants [
92,
93]) enables sub-10 ms edge inference, critical for high-throughput packing lines processing >20 fruits/s while maintaining ±0.4 °
Brix accuracy.
4.4. Standardization and Industrial Integration: Closing the Commercialization Loop
The translation of fruit SSC detection from laboratory validation to industrial scalability necessitates harmonized technical ecosystems, beginning with globally standardized calibration protocols. ISO-certified workflows using Brix-traceable reference materials must be established for reflectance/transmittance modalities, ensuring cross-platform reproducibility with <0.3 °Brix inter-device variance—a critical foundation for technology interoperability. Concurrently, hardware-software co-design will embed chip-based spectrometers directly within robotic sorting end-effectors, enabling real-time SSC mapping (<5 ms latency) synchronized with mechanical grading actions. This integration overcomes throughput bottlenecks while custom ASICs resolve spectral acquisition challenges for irregular-surface fruits (e.g., lychee, mango) through accelerated region-of-interest extraction. Ultimately, international consortia (e.g., ISO/TC 34/SC 5) must establish technology-agnostic certification tiers—such as “Grade A” compliance requiring ±0.4 °Brix accuracy—to confer legal enforceability and market confidence in detection results across global supply chains.
4.5. Sustainable Expansion: Closed-Loop Agri-Technological Ecosystems
Future fruit SSC detection systems will prioritize ecological resilience through energy-autonomous architectures, where perovskite-silicon tandem solar cells [
94,
95] enable >5 years of off-grid operation for field-deployed sensors. This energy sovereignty is further amplified by wake-up radios and compressive sampling techniques that reduce system-level power consumption by 95% compared to conventional platforms. Concurrently, multidimensional quality assessment will transition toward holistic phenotyping frameworks, integrating unified optical-acoustic-electrical sensor arrays to concurrently quantify SSC, titratable acidity [
96,
97], firmness [
98,
99,
100], and early-pathogen biomarkers [
101,
102], generating per-fruit “nutritional passports” that unlock premium market value. Crucially, these advancements will converge within closed-loop value chains: biodegradable sensors fabricated from cellulose-nanocrystal substrates will minimize e-waste, while blockchain-immutable quality data dynamically optimizes supply routing, projected to reduce post-harvest losses by 15–30% through AI-driven logistical recalibration.
Within 5 years, federated learning frameworks and edge-AI hardware will overcome model generalization and speed barriers. By 2030, standardization initiatives and co-designed robotic systems will establish SSC detection as a ubiquitous grading metric across global supply chains. Ultimately, the convergence of zero-waste sensing, multiparameter phenotyping, and certified accuracy will transform fruit quality management from reactive sorting to predictive ecosystem stewardship—increasing market value by USD 12–18 billion annually while reducing food waste [
103].
5. Conclusions
The non-destructive detection technology (NDT) of soluble solids content (SSC) represents a pivotal technological advancement for modernizing fruit quality assessment and postharvest management. This comprehensive review synthesizes critical insights into the principles, capabilities, and limitations of predominant NDT methodologies—including near-infrared spectroscopy (NIR), hyperspectral imaging (HSI), X-ray technology, computer vision (CV), and electronic nose (EN) systems—for SSC quantification. While these technologies demonstrate considerable potential in laboratory settings, our critical analysis identifies three persistent barriers impeding their transition to widespread commercial adoption.
Firstly, effective integration of multi-sensor data streams remains a fundamental challenge. The heterogeneous nature of data derived from optical, structural, and volatile compound analyses necessitate sophisticated fusion algorithms to extract complementary information while mitigating noise and redundancy. Future research must prioritize developing intelligent data fusion frameworks leveraging explainable artificial intelligence (XAI) and multimodal deep learning to enhance prediction robustness beyond single-technology approaches.
Secondly, bridging the gap between laboratory prototypes and industrial-grade systems demands significant engineering innovation. Achieving real-time, high-throughput operation in dynamic packinghouse environments requires concurrent advances in the following: (a) algorithm resilience against biological variability (e.g., cultivar differences, maturity gradients) and environmental interference; (b) hardware optimization for embedded edge-computation deployment; and (c) autonomous calibration protocols to minimize operational complexity. Targeted efforts should focus on lightweight neural network architectures, adaptive sensor systems, and self-diagnostic functionalities to ensure operational reliability under real-world constraints.
Thirdly, economic viability and standardization constitute critical adoption barriers. High capital costs for advanced sensing modalities (notably HSI and X-ray) and the absence of unified performance validation protocols limit accessibility, particularly for small-scale producers. Future work should drive cost reduction through miniaturized optoelectronics and scalable manufacturing, while international consortia must establish standardized metrics for data acquisition, model benchmarking, and cross-technology comparison to foster market confidence.
In summary, realizing the transformative potential of SSC-NDT technologies necessitates a concerted interdisciplinary strategy addressing these interconnected challenges. Key priorities include advancing physics-informed multisensor fusion paradigms, engineering resilient automation platforms for commercial deployment, and promoting cost-optimized hardware through open innovation ecosystems. Success in these domains will not only elevate fruit quality control precision but also significantly contribute to sustainable resource utilization, supply chain transparency, and global food security—propelling the horticultural industry toward intelligent, data-driven modernization.