Electrochemical Properties of Soluble CuCl·3TU Coordination Compound and Application in Electrolysis for Copper Foils
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cuprous Coordination Compound
2.2. Electrochemical Measurements
2.3. Characterizations
3. Results
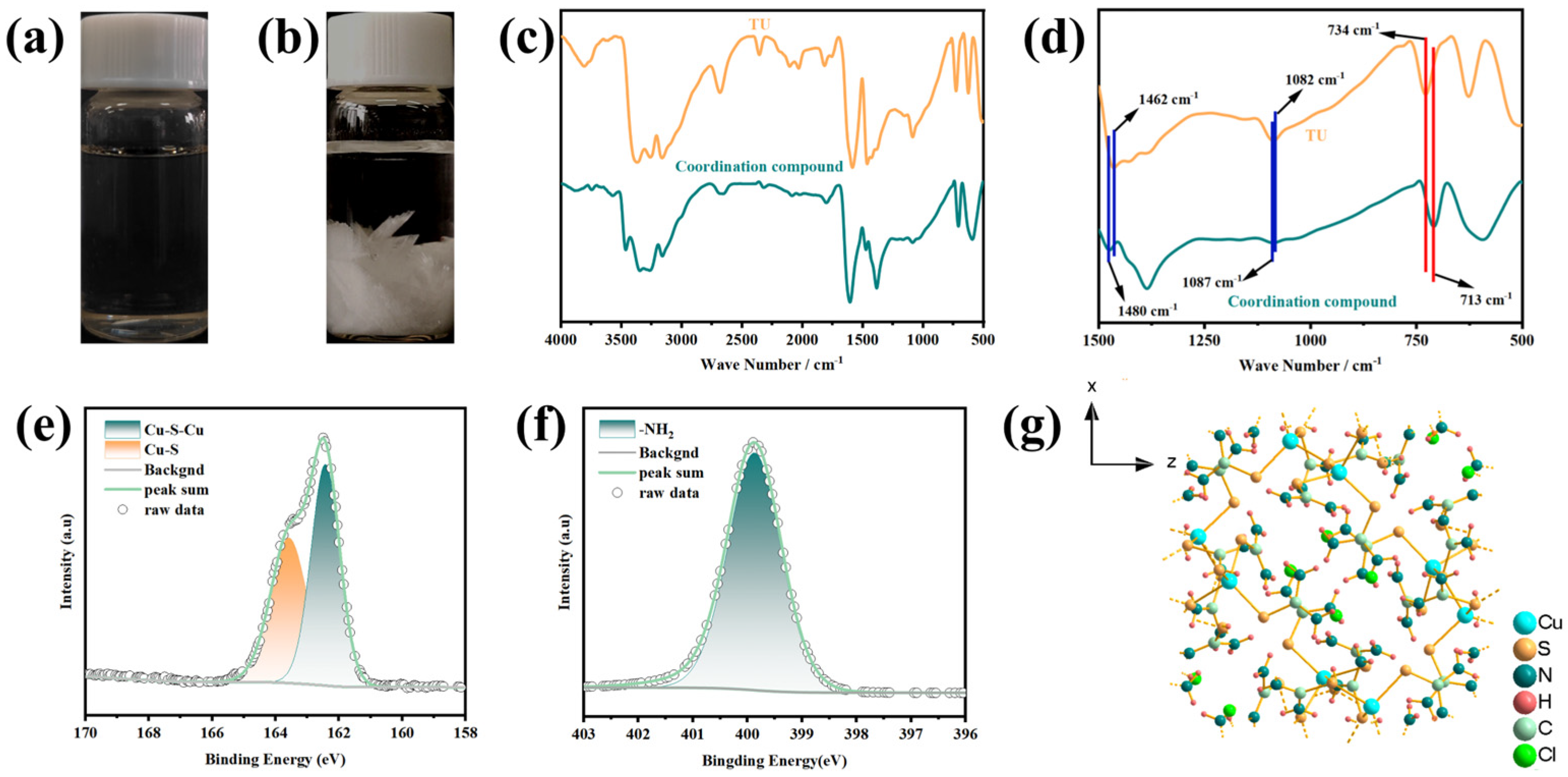
3.1. Preparation of Coordination Compound and Analysis of Coordination Relationships
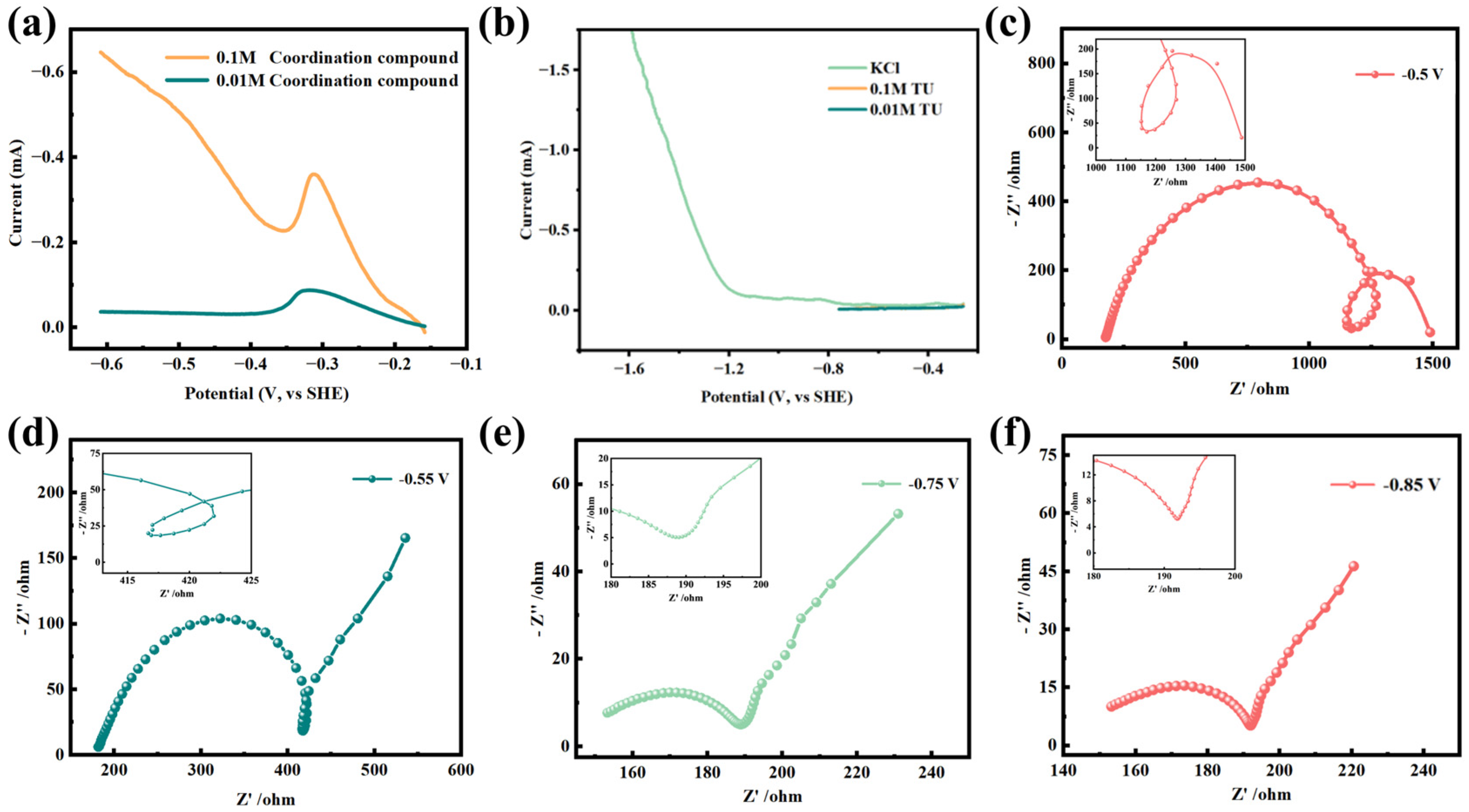
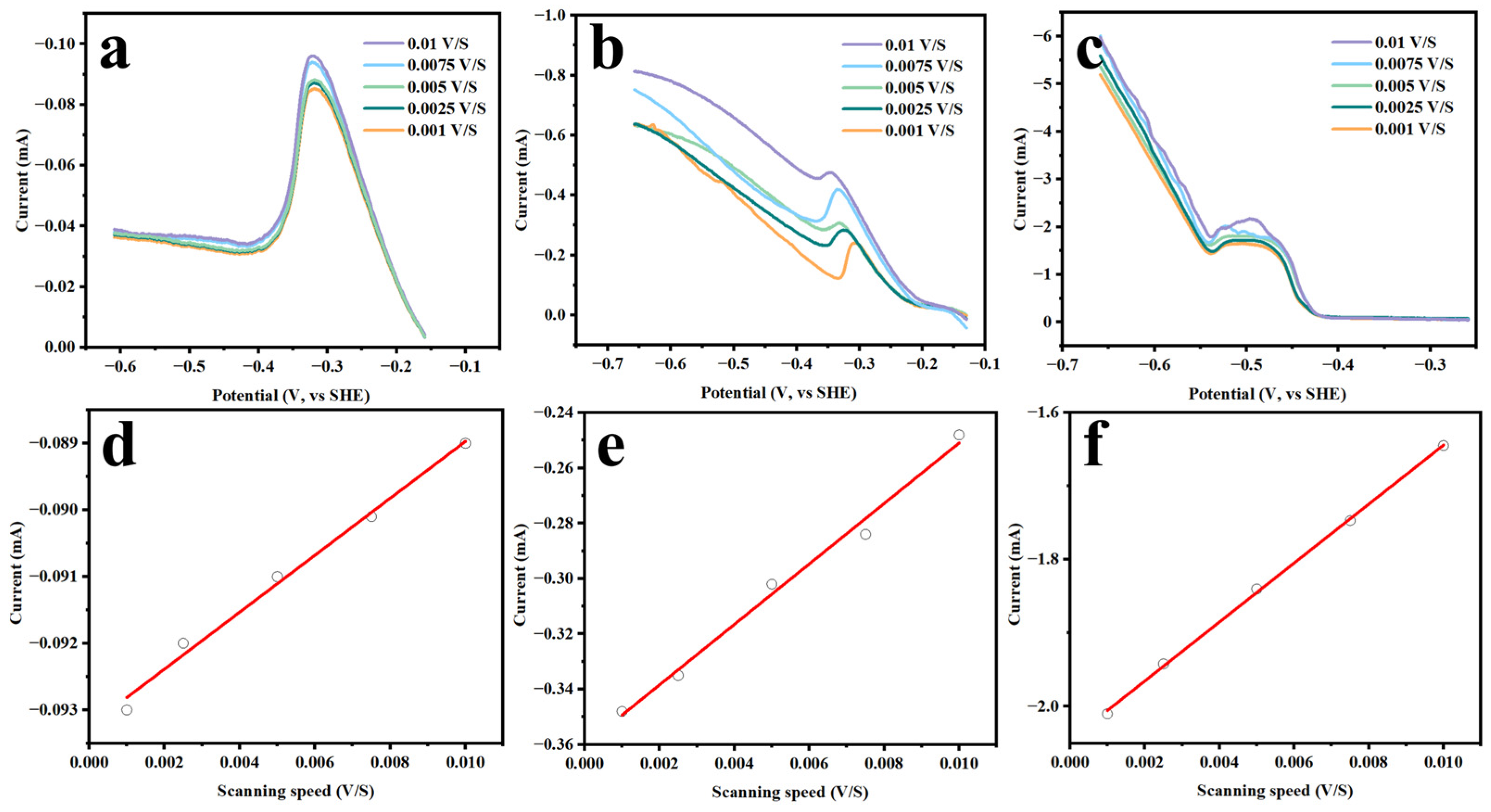
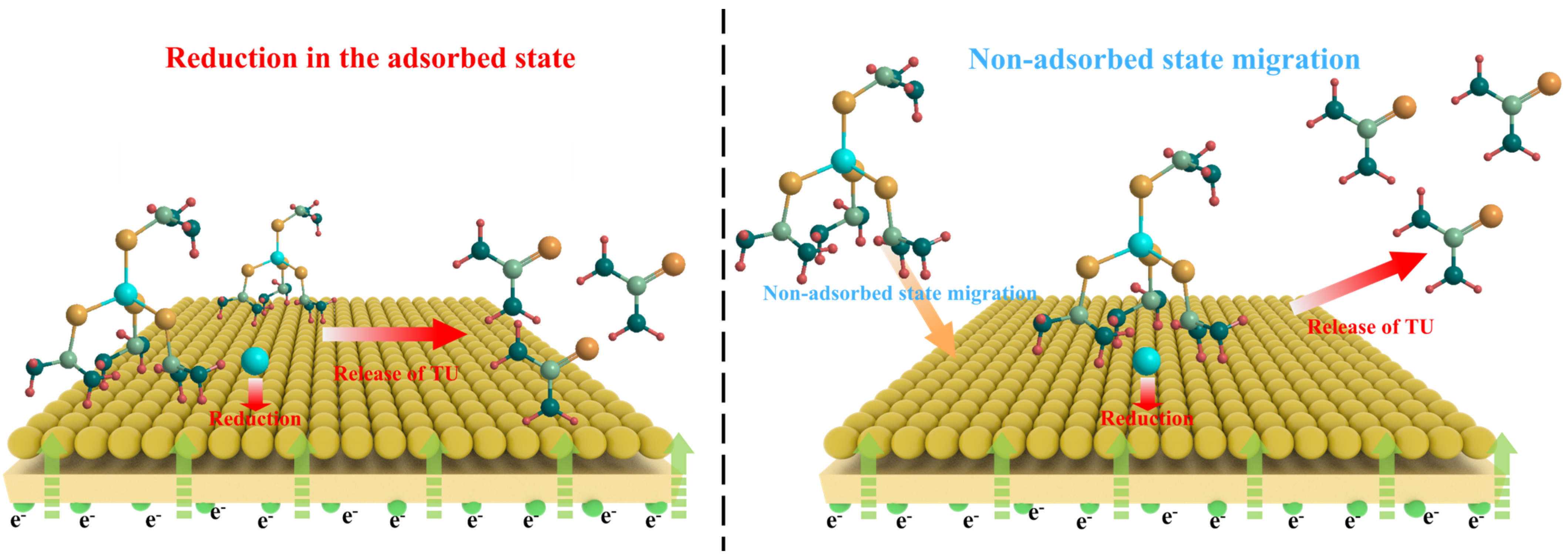
3.2. Electrochemical Behaviors of CuCl·3TU in Aqueous Solution
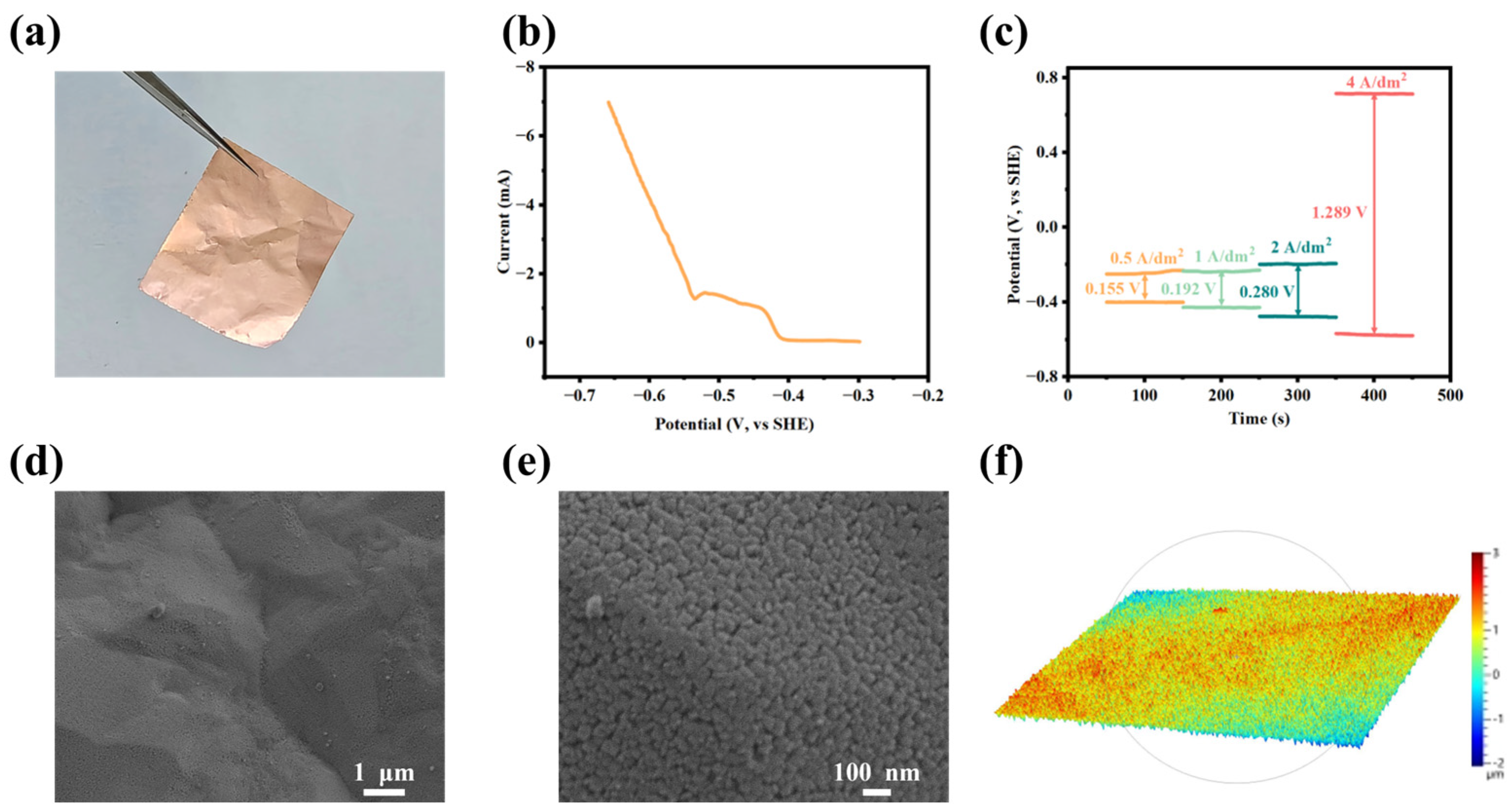
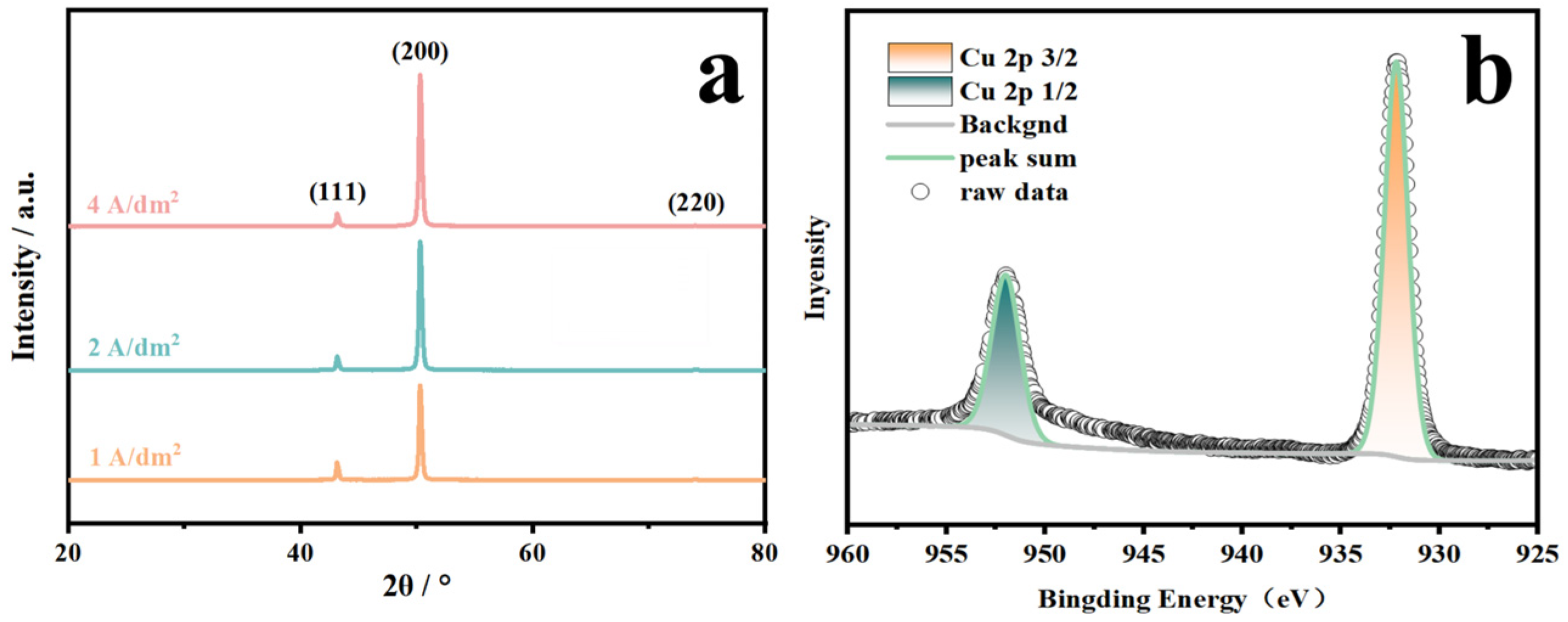
3.3. Applications in Electrolytic Copper Foil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhang, J.; Niu, R.; Bayat, M.; Zhou, Y.; Yin, Y.; Tan, Q.; Liu, S.; Hattel, J.H.; Li, M.; et al. Manufacturing of high strength and high conductivity copper with laser powder bed fusion. Nat. Commun. 2024, 15, 1283. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-S.; Hu, J.-Q.; Mao, T.-T.; Liao, S.-Y.; Feng, R.-M.; Liu, Y.-D.; Min, Y.-G. Copper-coated Porous Polyimide as Ultralight and Safe Current Collectors for Advanced LIBs. Chin. J. Polym. Sci. 2024, 42, 521–531. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Lang, T.; Gao, J.; Zhang, H.; Zhao, Y.; Guo, Z.; Miao, Z. Research Progress on Intrinsically Conductive Polymers and Conductive Polymer-Based Composites for Electromagnetic Shielding. Molecules 2023, 28, 7647. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Gao, J.; Liu, X.; Liu, Y.; Jiao, W.; Zhang, D.; Zheng, R.; Li, L.; Ma, F. High-efficiency and low-consumption preparation of ultra-thin and high-performance nanotwinned copper foils by high-gravity intensified direct current electrodeposition. Chem. Eng. Sci. 2024, 296, 120248. [Google Scholar] [CrossRef]
- Yan, F.; Dai, H.; Wang, Y.; Ruan, M.; Zhao, S.; Yu, X.; Lin, Y. The study on the shortcut approach for cooperative disposal of arsenic-rich copper dust and waste acid to prepare high-purity copper. J. Clean. Prod. 2024, 447, 141534. [Google Scholar] [CrossRef]
- Wang, A.-Y.; Chen, B.; Fang, L.; Yu, J.-J.; Wang, L.-M. Influence of branched quaternary ammonium surfactant molecules as levelers for copper electroplating from acidic sulfate bath. Electrochim. Acta 2013, 108, 698–706. [Google Scholar] [CrossRef]
- Bonou, L.; Eyraud, M.; Denoyel, R.; Massiani, Y. Influence of additives on Cu electrodeposition mechanisms in acid solution: Direct current study supported by non-electrochemical measurements. Electrochim. Acta 2002, 47, 4139–4148. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, H.J.; Yong, S.H.; Kwon, O.J.; Kim, S.-K.; Kim, J.J. Facile Formation of Cu–Ag Film by Electrodeposition for the Oxidation-Resistive Metal Interconnect. J. Electrochem. Soc. 2012, 159, D253. [Google Scholar] [CrossRef]
- Popescu, A.-M.; Cojocaru, A.; Donath, C.; Constantin, V. Electrochemical study and electrodeposition of copper(I) in ionic liquid-reline. Chem. Res. Chin. Univ. 2013, 29, 991–997. [Google Scholar] [CrossRef]
- Aravinda, C.L.; Mayanna, S.M.; Muralidharan, V.S. Electrochemical behaviour of alkaline copper complexes. J. Chem. Sci. 2000, 112, 543–550. [Google Scholar] [CrossRef]
- Brooks, N.R.; Schaltin, S.; Van Hecke, K.; Van Meervelt, L.; Binnemans, K.; Fransaer, J. Copper(I)-Containing Ionic Liquids for High-Rate Electrodeposition. Chem. A Eur. J. 2011, 17, 5054–5059. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Deng, L.; Liu, Y.; Hao, M.; Wang, Z.; Dong, L.-T.; Yang, Y.; Song, K.; Qi, D.; Wang, J.; et al. Self-Selective (220) Directional Grown Copper Current Collector Design for Cycling-Stable Anode-Less Lithium Metal Batteries. Adv. Mater. 2025, 37, 2413420. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Hu, J.; Wu, Z.; Yang, X.; Xue, Z.; Chen, Q.; Zhang, J.; Hou, G.; Wu, B.; Tang, Y.; et al. Effect of pulse electrodeposition process on the microstructure and properties of electrolytic copper foil as anode current collectors. Electrochim. Acta 2025, 528, 146278. [Google Scholar] [CrossRef]
- Xu, P.; Lu, W.; Song, K.; Cheng, H.; Hu, H.; Zhu, Q.; Liu, H.; Yang, X. Preparation of electrodeposited copper foils with ultrahigh tensile strength and elongation: A functionalized ionic liquid as the unique additive. Chem. Eng. J. 2024, 484, 149557. [Google Scholar] [CrossRef]
- Xu, J.X.; Li, R.H.; Zhang, P.; Zhang, Z.F. Crack propagation behavior and mechanism of coarse-grained copper in cyclic torsion with axial static tension. Int. J. Fatigue 2020, 131, 105304. [Google Scholar] [CrossRef]
- Chlupová, A.; Šulák, I.; Babinský, T.; Polák, J. Intergranular fatigue crack initiation in polycrystalline copper. Mater. Sci. Eng. A 2022, 848, 143357. [Google Scholar] [CrossRef]
- Kondo, T.; Hirakata, H.; Minoshima, K. Thickness effects on fatigue crack propagation in submicrometer-thick freestanding copper films. Int. J. Fatigue 2017, 103, 444–455. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Gao, H.; Yan, S.; Chen, S.; Liu, X.; Hu, X. Rolled electrodeposited copper foil with modified surface morphology as anode current collector for high performance lithium-ion batteries. Surf. Coat. Technol. 2021, 410, 126881. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Han, H.; Li, L.; Lai, Z.; Hong, Y.; Wang, S.; Zhou, G.; He, W.; Chen, Y.; et al. Bromine-enhanced polarization for strengthening ultra-thin copper foil in lithium-ion battery. J. Mater. Res. Technol. 2024, 30, 3831–3839. [Google Scholar] [CrossRef]
- Li, Y.; Ren, P.; Li, R.; Zhang, Y.; Zhang, J.; Yang, P.; An, M. A novel bright additive for copper electroplating: Electrochemical and theoretical study. Ionics 2023, 29, 363–375. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Li, Y.-H.; Du, H.-Y.; Zhong, Z.-Q.; Peng, Y. The impact of gelatin on the electrodeposition behavior and microstructure of copper in industrial electrolyte solutions. J. Appl. Electrochem. 2025, 55, 769–783. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, Z.; Zhang, J. Research on the optimization of microstructure and enhancement of properties in ultra-thin electrolytic copper foil by rare-earth ions Ce3+ and La3+ additions. J. Mater. Res. Technol. 2025, 36, 580–591. [Google Scholar] [CrossRef]
- Pavithra, C.L.P.; Sarada, B.V.; Rajulapati, K.V.; Ramakrishna, M.; Gundakaram, R.C.; Rao, T.N.; Sundararajan, G. Controllable Crystallographic Texture in Copper Foils Exhibiting Enhanced Mechanical and Electrical Properties by Pulse Reverse Electrodeposition. Cryst. Growth Des. 2015, 15, 4448–4458. [Google Scholar] [CrossRef]
- Golub, G.; Cohen, H.; Meyerstein, D. The stabilization of monovalent copper ions by complexation with saturated tertiary amine ligands in aqueous solutions. The case of 2,5,9,12-tetramethyl-2,5,9,12-tetraazatridecane. J. Chem. Soc. Chem. Commun. 1992, 5, 397–398. [Google Scholar] [CrossRef]
- Liang, S.-W.; Li, M.-X.; Shao, M.; Miao, Z.-X. Hydrothermal synthesis and crystal structure of a novel cyanide-bridged double helical copper(I) coordination polymer [Cu3(CN)3(phen)]n. Inorg. Chem. Commun. 2006, 9, 1312–1314. [Google Scholar] [CrossRef]
- Qin, Y.-L.; Wu, Y.-Q.; Hou, J.-J.; Zhang, X.-M. Cyanide-bridged mixed-valence copper(II/I) coordination polymers: Unique 7-connected sev-type 3D network versus anionic 2D host network encapsulated with cationic complex. Inorg. Chem. Commun. 2016, 63, 101–106. [Google Scholar] [CrossRef]
- Meng, S.; Xu, Z.; Cao, T.; Xin, Y.; Chen, Y.; Wang, C.; Zhou, Z.; Liu, H.; Zhang, D. A serial of cyanide-bridged CrIII/ICuII complexes from 0D cluster to 2D network: Synthesis, crystal structure and magnetic property. J. Mol. Struct. 2024, 1296, 136897. [Google Scholar] [CrossRef]
- Dudek, D.A.; Fedkiw, P.S. Electrodeposition of copper from cuprous cyanide electrolyte: I. Current distribution on a stationary disk. J. Electroanal. Chem. 1999, 474, 16–30. [Google Scholar] [CrossRef]
- Guo, B. Electrodeposition on pyrite from copper(i) cyanide electrolyte. RSC Adv. 2016, 6, 2183–2190. [Google Scholar] [CrossRef]
- Litvinov, I.A.; Lodochnikova, O.A.; Karamov, F.A. Structure of “coordination oligomers” based on complexes of thiophosphorylthiourea compounds with monovalent copper and silver cations. J. Struct. Chem. 2020, 61, 1786–1793. [Google Scholar] [CrossRef]
- Khan, E.; Sikandar, K.; Zarif, G.; Muhammad, M. Medicinal Importance, Coordination Chemistry with Selected Metals (Cu, Ag, Au) and Chemosensing of Thiourea Derivatives. A Review. Crit. Rev. Anal. Chem. 2021, 51, 812–834. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wen, T.-B.; Liu, Q.-T.; Huang, X.-Y.; Kang, B.-S.; Wu, X.-L.; Huang, Z.-S.; Gu, L.-Q. Syntheses and crystal structures of copper(I) complexes with alkyl-thioallophanate derivatives. Polyhedron 1997, 16, 2605–2611. [Google Scholar] [CrossRef]
- Ahmad, S.; Georgieva, I.; Hanif, M.; Monim-ul-Mehboob, M.; Munir, S.; Sohail, A.; Isab, A.A. Periodic DFT modeling and vibrational analysis of silver(I) cyanide complexes of thioureas. J. Mol. Model. 2019, 25, 90. [Google Scholar] [CrossRef] [PubMed]
- Piro, O.E.; Castellano, E.E.; Piatti, R.C.V.; Bolzan, A.E.; Arvia, A.J. Two thiourea-containing gold(I) complexes. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2002, 58, M252–M255. [Google Scholar] [CrossRef] [PubMed]
- Samadov, A.S.; Mironov, I.V.; Kaziev, G.Z.; Cherednichenko, A.G.; Faizullozoda, E.F.; Stepnova, A.F. Thermodynamic Characteristics of Silver(I) Complex Formation with Selected N- and N,N′-Substituted Thioureas in Aqueous Solution. Russ. J. Inorg. Chem. 2022, 67, 1617–1622. [Google Scholar] [CrossRef]
- Sangeetha, M.K.; Mariappan, M.; Madhurambal, G.; Mojumdar, S.C. TG-DTA, XRD, SEM, EDX, UV, and FT-IR spectroscopic studies of l-valine thiourea mixed crystal. J. Therm. Anal. Calorim. 2015, 119, 907–913. [Google Scholar] [CrossRef]
- de Santana, H.; Paesano, A.; da Costa, A.C.S.; di Mauro, E.; de Souza, I.G.; Ivashita, F.F.; de Souza, C.M.D.; Zaia, C.T.B.V.; Zaia, D.A.M. Cysteine, thiourea and thiocyanate interactions with clays: FT-IR, Mössbauer and EPR spectroscopy and X-ray diffractometry studies. Amino Acids 2010, 38, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, A.; Chandrasekaran, A.; Jayakumar, S.; Manickam, P.; Alwarappan, S. Sulphur Doped Graphitic Carbon Nitride as an Efficient Electrochemical Platform for the Detection of Acetaminophen. J. Electrochem. Soc. 2019, 166, B1461. [Google Scholar] [CrossRef]
- Gumus, I.; Solmaz, U.; Binzet, G.; Keskin, E.; Arslan, B.; Arslan, H. Hirshfeld surface analyses and crystal structures of supramolecular self-assembly thiourea derivatives directed by non-covalent interactions. J. Mol. Struct. 2018, 1157, 78–88. [Google Scholar] [CrossRef]
- Solmaz, U.; Ince, S.; Yilmaz, M.K.; Arslan, H. Conversion of monodentate benzoylthiourea palladium(II) complex to bidentate coordination mode: Synthesis, crystal structure and catalytic activity in the Suzuki-Miyaura cross-coupling reaction. J. Organomet. Chem. 2022, 973–974, 122374. [Google Scholar] [CrossRef]
- Krunks, M.; Leskelä, T.; Mutikainen, I.; Niinistö, L. A Thermoanalytical Study of Copper(I) Thiocarbamide Compounds. J. Therm. Anal. Calorim. 1999, 56, 479–484. [Google Scholar] [CrossRef]
- Krunks, M.; Leskelä, T.; Mannonen, R.; Niinistö, L. Thermal Decomposition of Copper(I) Thiocarbamide Chloride Hemihydrate. J. Therm. Anal. Calorim. 1998, 53, 355–364. [Google Scholar] [CrossRef]
- Sarkar, S.; Dutta, S.; Chakrabarti, S.; Bairi, P.; Pal, T. Redox-Switchable Copper(I) Metallogel: A Metal–Organic Material for Selective and Naked-Eye Sensing of Picric Acid. ACS Appl. Mater. Interfaces 2014, 6, 6308–6316. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, P.; Mutikainen, I.; Krunks, M.; Leskelä, T.; Madarász, J.; Niinistö, L. Synthesis, vibrational spectra and X-ray structures of copper(I) thiourea complexes. Inorg. Chim. Acta 2004, 357, 513–525. [Google Scholar] [CrossRef]
- Murray, S.G.; Hartley, F.R. Coordination chemistry of thioethers, selenoethers, and telluroethers in transition-metal complexes. Chem. Rev. 1981, 81, 365–414. [Google Scholar] [CrossRef]
- Tian, D.; Li, D.Y.; Wang, F.F.; Xiao, N.; Liu, R.Q.; Li, N.; Li, Q.; Gao, W.; Wu, G. A Pd-free activation method for electroless nickel deposition on copper. Surf. Coat. Technol. 2013, 228, 27–33. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Hanna, J.V.; Pakawatchai, C.; Skelton, B.W.; Thanyasirikul, Y.; White, A.H. Crystal Structures and Vibrational Spectroscopy of Copper(I) Thiourea Complexes. Inorg. Chem. 2009, 48, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Okaya, Y.; Knobler, C.B. Refinement of the crystal structure of tris(thiourea)-copper(I) chloride. Acta Cryst. 1964, 17, 928–930. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khatun, N.; Jena, H.S.; Patel, B.K. Stable Cu(I) Complexes with Thioamidoguanidine Possessing Halide-Bridge Structure. Inorg. Chem. 2012, 51, 10800–10807. [Google Scholar] [CrossRef] [PubMed]
- Puškarić, A.; Dunatov, M.; Jerić, I.; Sabljić, I.; Androš Dubraja, L. Room temperature ferroelectric copper(ii) coordination polymers based on amino acid hydrazide ligands. New J. Chem. 2022, 46, 3504–3511. [Google Scholar] [CrossRef]
- Seppälä, P.; Sillanpää, R.; Lehtonen, A. Structural diversity of copper(II) amino alcoholate complexes. Coord. Chem. Rev. 2017, 347, 98–114. [Google Scholar] [CrossRef]
- Calderón, J.A.; Henao, J.E.; Gómez, M.A. Erosion-corrosion resistance of Ni composite coatings with embedded SiC nanoparticles. Electrochim. Acta 2014, 124, 190–198. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy─A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.B.; Yurkovskaya, A.V. Reduction of transient histidine radicals by tryptophan: Influence of the amino group charge. Phys. Chem. Chem. Phys. 2021, 23, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.T.; Singam, S.; Kollu, P.; Bohm, S.; Prasad, M.J.N.V. Achieving exceptional tensile strength in electrodeposited copper through grain refinement and reinforcement effect by co-deposition of few layered graphene. J. Alloys Compd. 2020, 840, 155725. [Google Scholar] [CrossRef]
- Orhan, G.; Gürmen, S.; Timur, S. The behavior of organic components in copper recovery from electroless plating bath effluents using 3D electrode systems. J. Hazard. Mater. 2004, 112, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Li, Z.; Wang, F.; Dou, M.; Liu, J.; Ji, J.; Song, Y. Electrodeposition mechanism of quaternary compounds Cu2ZnSnS4: Effect of the additives. Appl. Surf. Sci. 2018, 427, 267–275. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, R. Separation of Copper and Nickel Metal Ions from Electroplating Wastewater by Ultrafiltration with Tartaric Acid and Sodium Citrate Reinforced Sodium Polyacrylate Complexation. Membranes 2024, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Wang, L.P.; Bai, Z.B.; Peng, X.L.; Feng, B.X.; Liu, E. Study on microstructure and properties of typical copper foils. Kov. Mater. Met. Mater. 2024, 62, 223–234. [Google Scholar] [CrossRef]
- Liu, P.; Hensen, E.J.M. Highly Efficient and Robust Au/MgCuCr2O4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.E.; Chen, J.; Liu, J.; Liang, T.; Xu, Y.; Zhu, C.; Zhong, S. Microcrystalline copper foil as a high performance collector for lithium-ion batteries. J. Power Sources 2019, 438, 226973. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, D.; Pei, X.; Mu, C.; Chen, K.; Chen, Q.; Hou, G.; Tang, Y. Effects of Electrolytic Copper Foil Roughness on Lithium-Ion Battery Performance. Metals 2022, 12, 2110. [Google Scholar] [CrossRef]
- Jeon, H.; Cho, I.; Jo, H.; Kim, K.; Ryou, M.-H.; Lee, Y.M. Highly rough copper current collector: Improving adhesion property between a silicon electrode and current collector for flexible lithium-ion batteries. RSC Adv. 2017, 7, 35681–35686. [Google Scholar] [CrossRef]
- Yang, L.; Weng, W.; Zhu, H.; Chi, X.; Tan, W.; Wang, Z.; Zhong, S. Preparing ultra-thin copper foil as current collector for improving the LIBs performances with reduced carbon footprint. Mater. Today Commun. 2023, 35, 105952. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Xia, F.; Tian, D. Electrochemical Properties of Soluble CuCl·3TU Coordination Compound and Application in Electrolysis for Copper Foils. Chemistry 2025, 7, 114. https://doi.org/10.3390/chemistry7040114
Zhao W, Xia F, Tian D. Electrochemical Properties of Soluble CuCl·3TU Coordination Compound and Application in Electrolysis for Copper Foils. Chemistry. 2025; 7(4):114. https://doi.org/10.3390/chemistry7040114
Chicago/Turabian StyleZhao, Wancheng, Fangquan Xia, and Dong Tian. 2025. "Electrochemical Properties of Soluble CuCl·3TU Coordination Compound and Application in Electrolysis for Copper Foils" Chemistry 7, no. 4: 114. https://doi.org/10.3390/chemistry7040114
APA StyleZhao, W., Xia, F., & Tian, D. (2025). Electrochemical Properties of Soluble CuCl·3TU Coordination Compound and Application in Electrolysis for Copper Foils. Chemistry, 7(4), 114. https://doi.org/10.3390/chemistry7040114





