In Situ Encapsulated RhB@Er-MOF with Dual-Emitting Rationmetric Fluorescence for Rapid and Selective Detection of Fe(III) by Dual-Signal Output
Abstract
1. Introduction
2. Results and Discussion
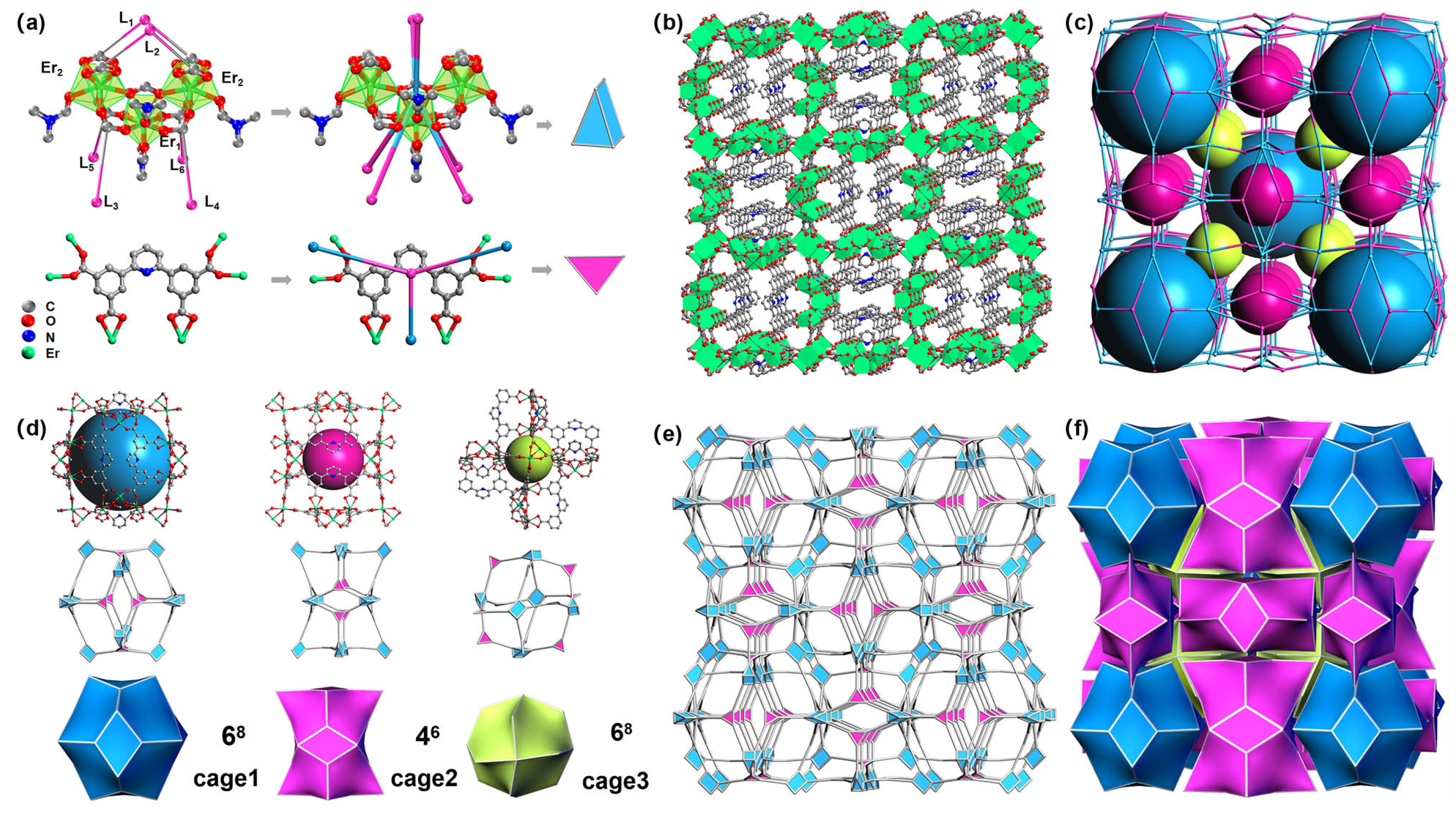
2.1. Crystal Structure
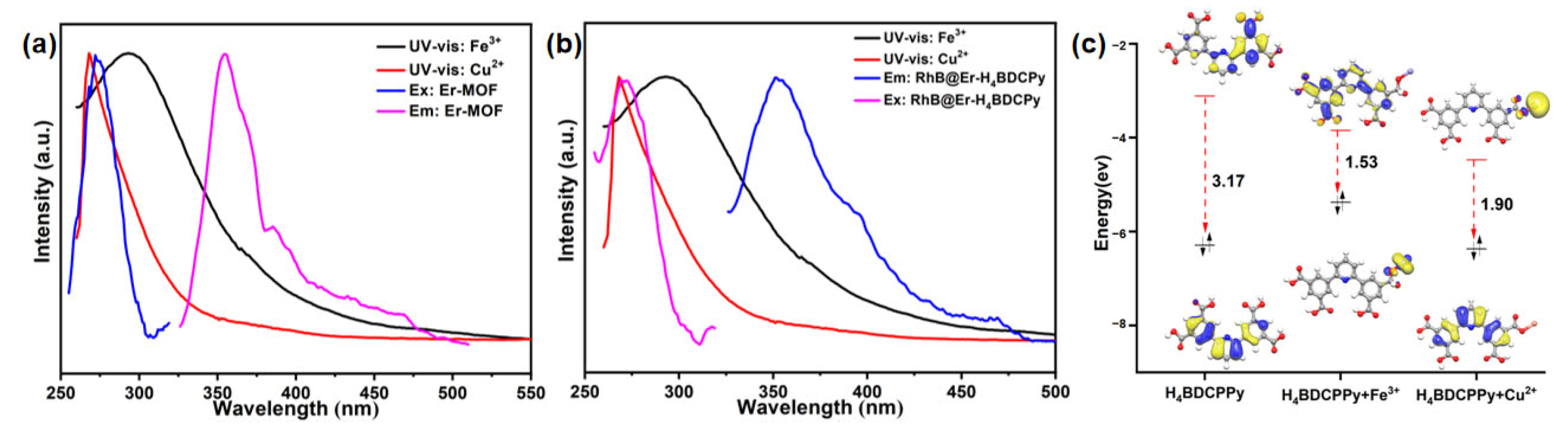
2.2. Fluorescence Property
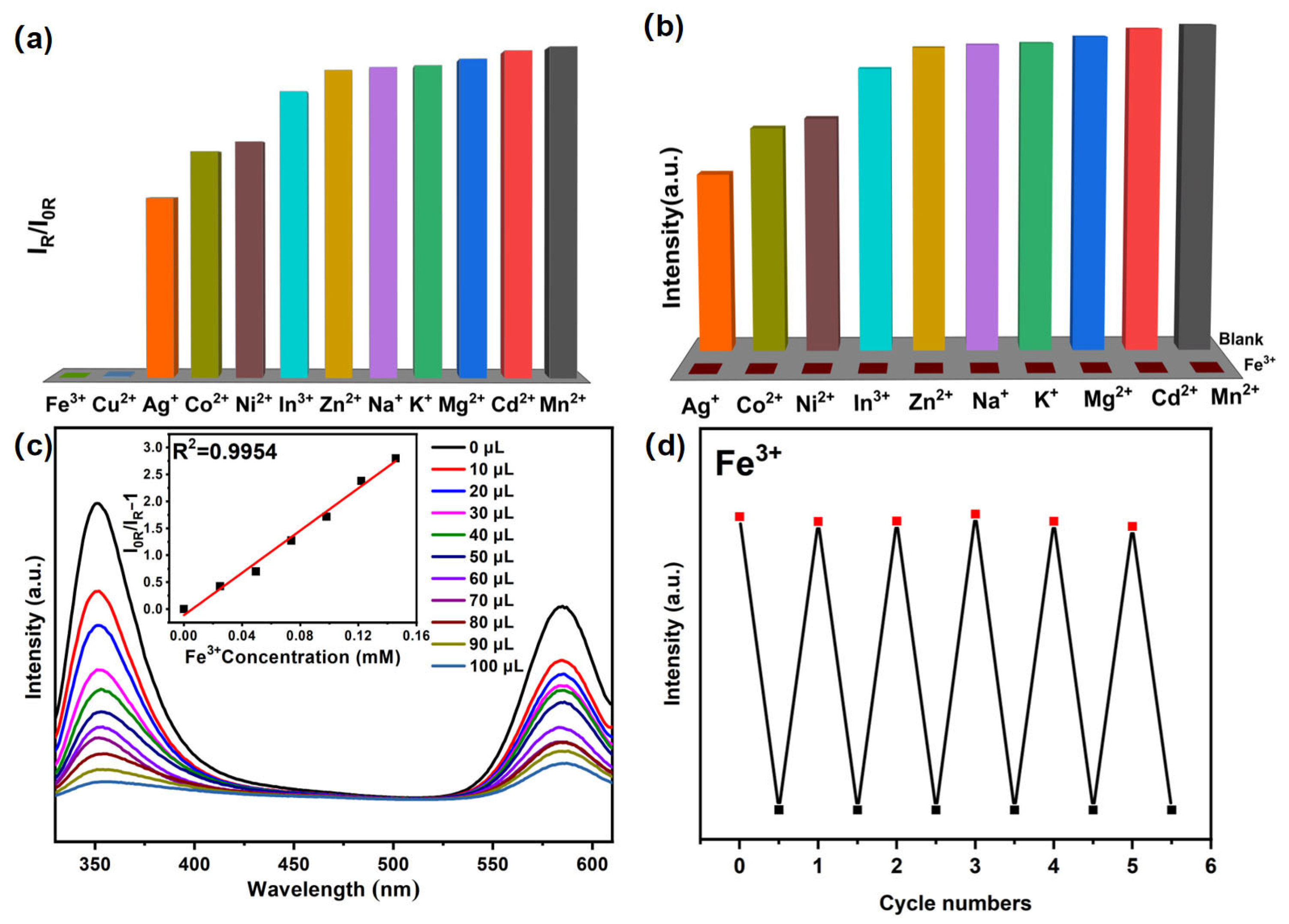
2.3. Er-MOF Fluorescence Sensing of Metal Ions
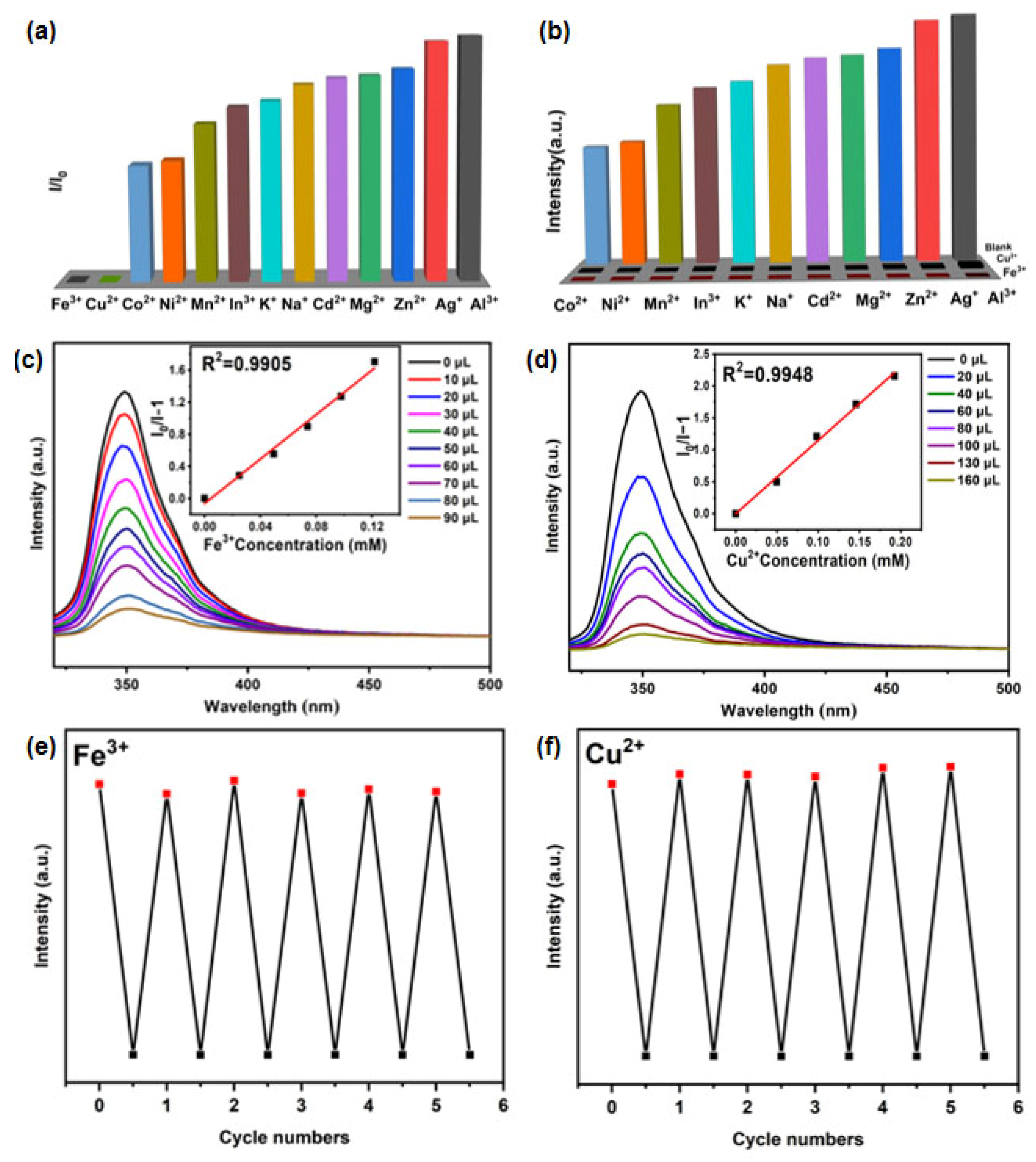
2.4. RhB@Er-MOF Fluorescence Sensing of Metal Ions
2.5. Fluorescence Detection Mechanism
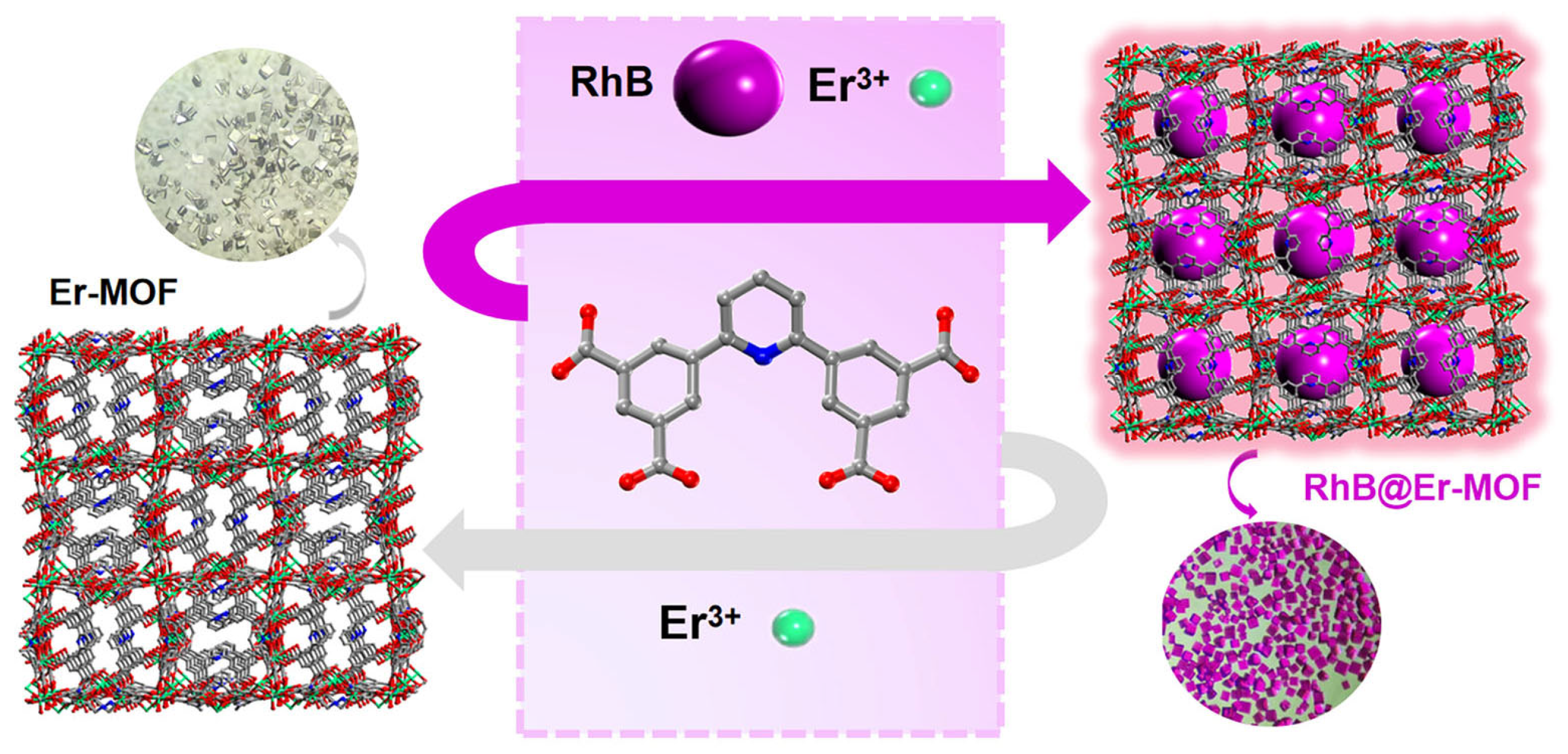
2.6. Portable Fe(III) Sensing Platform Based on RhB@Er-MOF
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Er-MOF
3.3. Synthesis of RhB@Er-MOF
3.4. X-Ray Crystallography
3.5. Fluorescence Sensing Experiments
3.6. Density Functional Theory (DFT) Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, Y.; Peng, Y.; Geng, Z.; Wang, Y.; Ung, C.O.L.; Hu, H. Metal-organic frameworks (MOFs) based analytical techniques for food safety evaluation. eFood 2021, 2, 1–12. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 2016, 188, 206. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Wang, Z.; Zhao, L.; Li, Y. Highly sensitive Fe3+ luminescence detection via single-ion adsorption. Chin. Chem. Lett. 2024, 35, 108532. [Google Scholar] [CrossRef]
- Boudebbouz, A.; Boudalia, S.; Bousbia, A.; Habila, S.; Boussadia, M.I.; Gueroui, Y. Heavy metals levels in raw cow milk and health risk assessment across the globe: A systematic review. Sci. Total Environ. 2021, 751, 141830. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Gomes Júnior, O.; Borges Neto, W.; Machado, A.E.H.; Daniel, D.; Trovó, A.G. Optimization of fipronil degradation by heterogeneous photocatalysis: Identification of transformation products and toxicity assessment. Water Res. 2017, 110, 133–140. [Google Scholar] [CrossRef]
- Xu, G.; Song, P.; Xia, L. Examples in the detection of heavy metal ions based on surface-enhanced Raman scattering spectroscopy. Nanophotonics 2021, 10, 4419–4445. [Google Scholar] [CrossRef]
- Han, X.; Wang, D.-E.; Chen, S.; Zhang, L.; Guo, Y.; Wang, J. A new rhodamine-based chemosensor for turn-on fluorescent detection of Fe3+. Anal. Methods 2015, 7, 4231–4236. [Google Scholar] [CrossRef]
- Naik, S.; Jujjavarapu, S.E. Self-powered and reusable microbial fuel cell biosensor for toxicity detection in heavy metal polluted water. J. Environ. Chem. Eng. 2021, 9, 105318. [Google Scholar] [CrossRef]
- He, G.; Peng, H.; Liu, T.; Yang, M.; Zhang, Y.; Fang, Y. A novel picric acid film sensor via combination of the surface enrichment effect of chitosan films and the aggregation-induced emission effect of siloles. J. Mater. Chem. 2009, 19, 7347–7353. [Google Scholar] [CrossRef]
- Hao, J.; Liu, F.; Liu, N.; Zeng, M.; Song, Y.; Wang, L. Ratiometric fluorescent detection of Cu2+ with carbon dots chelated Eu-based metal-organic frameworks. Sens. Actuators B Chem. 2017, 245, 641–647. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Zhang, Y.; Tan, J.C. Confinement of luminescent guests in metal-organic frameworks: Understanding pathways from synthesis and multimodal characterization to potential applications of LG@MOF Systems. Chem. Rev. 2022, 122, 10438–10483. [Google Scholar] [CrossRef]
- Guo, B.B.; Yin, J.C.; Li, N.; Fu, Z.X.; Han, X.; Xu, J.; Bu, X.H. Recent progress in luminous particle-encapsulated host–guest metal-organic frameworks for optical applications. Adv. Opt. Mater. 2021, 9, 2100283. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. Engineering metal-organic frameworks for aqueous phase 2,4,6-trinitrophenol (TNP) sensing. CrystEngComm 2016, 18, 2994–3007. [Google Scholar] [CrossRef]
- Rubab, L.; Anum, A.; Al-Hussain, S.A.; Irfan, A.; Ahmad, S.; Ullah, S.; Al-Mutairi, A.A.; Zaki, M.E.A. Green chemistry in organic synthesis: Recent update on green catalytic approaches in synthesis of 1,2,4-Thiadiazoles. Catalysts 2022, 12, 1329. [Google Scholar] [CrossRef]
- Yan, B. Luminescence response mode and chemical sensing mechanism for lanthanide-functionalized metal-organic framework hybrids. Inorg. Chem. Front. 2021, 8, 201–233. [Google Scholar] [CrossRef]
- Karmakar, A.; Li, J. Luminescent MOFs (LMOFs): Recent advancement towards a greener WLED technology. Chem. Commun. 2022, 58, 10768–10788. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yin, H.Q.; Yin, X.B. MOF@COFs with strong multiemission for differentiation and ratiometric fluorescence detection. ACS Appl. Mater. Interfaces 2020, 12, 20973–20981. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-Functionalized Metal-Organic Framework Hybrid Systems To Create Multiple Luminescent Centers for Chemical Sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef]
- Hu, X.L.; Qin, C.; Wang, X.L.; Shao, K.Z.; Su, Z.M. A luminescent dye@MOF as a dual-emitting platform for sensing explosives. Chem. Commun. 2015, 51, 17521–17524. [Google Scholar] [CrossRef]
- Stîngă, G.; Băran, A.; Iovescu, A.; Maxim, M.E.; Jerca, V.V. A highly selective multifunctional naphthalene-labeled polymer sensor for Cu2+ and Fe3+ ions in aqueous medium. J. Mol. Liq. 2024, 406, 125074. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Y.; Chen, X.; Wang, Y.; Shan, J.; Hou, J. Fluorescent sensors and rapid detection films for Fe3+ and Cu2+ based on naphthalene and cholesterol derivative organogels. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131045. [Google Scholar] [CrossRef]
- Lal, S.; Kumar, S.; Hooda, S.; Kumar, P. A highly selective sensor for Cu2+ and Fe3+ ions in aqueous medium: Spectroscopic, computational and cell imaging studies. J. Photochem. Photobiol. A Chem. 2018, 364, 811–818. [Google Scholar] [CrossRef]
- Yu, H.; Fan, M.; Liu, Q.; Su, Z.; Li, X.; Pan, Q.; Hu, X. Two Highly water-stable imidazole-based Ln-MOFs for sensing Fe3+,Cr2O72–/CrO42– in a water environment. Inorg. Chem. 2020, 59, 2005–2010. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Kirillov, A.M.; Dou, W.; Xu, C.; Xu, C.; Yang, L.; Fang, R.; Liu, W. Multifunctional Ln–MOF luminescent probe for efficient sensing of Fe3+, Ce3+, and acetone. ACS Appl. Mater. Interfaces 2018, 10, 23976–23986. [Google Scholar] [CrossRef]
- Zhao, J.J.; Liu, P.Y.; Dong, Z.P.; Liu, Z.L.; Wang, Y.Q. Eu(III)-organic framework as a multi-responsive photoluminescence sensor for efficient detection of 1-naphthol, Fe3+ and MnO4− in water. Inorg. Chim. Acta 2020, 511, 119843. [Google Scholar] [CrossRef]
- Let, S.; Samanta, P.; Dutta, S.; Ghosh, S.K. A Dye@MOF composite as luminescent sensory material for selective and sensitive recognition of Fe(III) ions in water. Inorg. Chim. Acta 2020, 500, 119205. [Google Scholar] [CrossRef]
- Shi, H.; Yu, X.; Liu, Y.; Shi, Y.; Liu, H.; Wang, H. Construction of luminescent dye@MOF platforms for sensing antibiotics with enhanced selectivity and sensitivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 322, 124804. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, S.; Wang, Y.; Xue, Y.D.; Chen, M. Preparation of dual-ligands Eu-MOF nanorods with dual fluorescence emissions for highly sensitive and selective ratiometric/visual fluorescence sensing phosphate. Sens. Actuators B Chem. 2022, 367, 132008. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Meng, F.; Su, J.; Chen, D.; Guo, Z.; Xing, H. RhB-embedded zirconium–naphthalene-based metal-organic framework composite as a luminescent self-calibrating platform for the selective detection of inorganic ions. Chem. Eur. J. 2020, 26, 1661–1667. [Google Scholar] [CrossRef]
- Liu, M.; Yu, X.; Zhong, K.; Chen, X.; Feng, L.; Yao, S. Dye-encapsulated nanocage-based metal-organic frameworks as luminescent dual-emitting sensors for selective detection of inorganic ions. Appl. Organomet. Chem. 2022, 36, e6692. [Google Scholar] [CrossRef]
- Mohan, B.; Priyanka; Singh, G.; Chauhan, A.; Pombeiro, A.J.L.; Ren, P. Metal-organic frameworks (MOFs) based luminescent and electrochemical sensors for food contaminant detection. J. Hazard. Mater. 2023, 453, 131324. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, J.; Gu, J.; Liu, Y. Hydrogen-bond-connected 2D Zn-LMOF with fluorescent sensing for inorganic pollutants and nitro aromatic explosives in the aqueous phase. Inorg. Chem. 2023, 62, 1272–1278. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Ryder, M.R.; Tan, J.C. Photonic hybrid crystals constructed from in situ host–guest nanoconfinement of a light-emitting complex in metal-organic framework pores. Nanoscale 2016, 8, 6851–6859. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable metal-organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, e1704303. [Google Scholar] [CrossRef]
- Juan-Alcañiz, J.; Gascon, J.; Kapteijn, F. Metal-organic frameworks as scaffolds for the encapsulation of active species: State of the art and future perspectives. J. Mater. Chem. 2012, 22, 10102–10118. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, P.-L.; Yue, Y.-N.; Song, X.Q.; Wang, L. A multifunctional Er-MOF for methylene blue adsorption and CO2 cycloaddition catalysis. J. Mol. Struct. 2024, 1316, 138943. [Google Scholar] [CrossRef]
- Han, L.; Zhang, S.; Wang, Y.; Yan, X.; Lu, X. A strategy for synthesis of ionic metal-organic frameworks. Inorg. Chem. 2009, 48, 786–788. [Google Scholar] [CrossRef]
- Yin, L.; Huang, J.-B.; Yue, T.C.; Wang, L.L.; Wang, D.Z. Two 2D metal-organic frameworks based on purine carboxylic acid ligands for photocatalytic oxidation of sulfides and CO2 chemical fixation. Inorg. Chem. 2024, 63, 9109–9118. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, T.; Gu, J.; Zhang, L.; Liu, Y. A series of isostructural lanthanide metal-organic frameworks: Effective fluorescence sensing for Fe3+, 2,4-DNP and 4-NP. CrystEngComm 2022, 24, 2759–2766. [Google Scholar] [CrossRef]
- Xu, H.; Fang, M.; Cao, C.S.; Qiao, W.Z.; Zhao, B. Unique (3,4,10)-connected lanthanide-organic framework as a recyclable chemical sensor for detecting Al3+. Inorg. Chem. 2016, 55, 4790–4794. [Google Scholar] [CrossRef]
- Liu, J.Q.; Li, X.F.; Gu, C.Y.; da Silva, J.C.; Barros, A.L.; Alves-Jr, S.; Li, B.H.; Ren, F.; Batten, S.R.; Soares, T.A. A combined experimental and computational study of novel nanocage-based metal–organic frameworks for drug delivery. Dalton Trans. 2015, 44, 19370–19382. [Google Scholar] [CrossRef]
- Liu, B.; Wu, W.P.; Hou, L.; Wang, Y.Y. Four uncommon nanocage-based Ln-MOFs: Highly selective luminescent sensing for Cu2+ ions and selective CO2 capture. Chem. Commun. 2014, 50, 8731–8734. [Google Scholar] [CrossRef]
- Yin, H.Q.; Wang, X.Y.; Yin, X.B. Rotation restricted emission and antenna effect in single metal-organic frameworks. J. Am. Chem. Soc. 2019, 141, 15166–15173. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. metal ion detection using luminescent-mofs: Principles, strategies and roadmap. Coord. Chem. Rev. 2020, 415, 213299. [Google Scholar] [CrossRef]
- Dong, M.J.; Zhao, M.; Ou, S.; Zou, C.; Wu, C.D. A luminescent Dye@MOF platform: Emission fingerprint relationships of volatile organic molecules. Angew. Chem. Int. Ed. 2014, 53, 1575–1579. [Google Scholar] [CrossRef]
- Yao, G.; Fang, S.; Yin, P.; Li, A.; Yang, W.; Wang, H.; Tan, W. A colorimetric and fluorometric dual-mode probe for Cu2+detection based on functionalized silver nanoparticles. Environ. Sci. Pollut. Res. 2025, 32, 3466–3474. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhong, L.; Bi, Y.; Liu, Y.; Zhao, J.; Wen, X.; Zhu, Y.; Feng, L.; Geng, L.; Yu, F.; et al. Synthesis of Er(III)-based porphyrin metal-organic frameworks for rapid detection of sulfide ions with triple-signal output. Sens. Actuators B Chem. 2024, 404, 135301. [Google Scholar] [CrossRef]
- Chen, C.H.; Wang, X.S.; Li, L.; Huang, Y.B.; Cao, R. Highly selective sensing of Fe3+ by an anionic metal-organic framework containing uncoordinated nitrogen and carboxylate oxygen sites. Dalton Trans. 2018, 47, 3452–3458. [Google Scholar] [CrossRef]
- Yu, C.; Sun, X.; Zou, L.; Li, G.; Zhang, L.; Liu, Y. A pillar-layered Zn-LMOF with uncoordinated carboxylic acid sites: High performance for luminescence sensing Fe3+ and TNP. Inorg. Chem. 2019, 58, 4026–4032. [Google Scholar] [CrossRef]
- Mei, D.; Yan, B. Flumequine-mediated fluorescent zeolitic imidazolate framework functionalized by Eu3+ for sensitive and selective detection of UO22+, Ni2+ and Cu2+ in nuclear wastewater. J. Hazard. Mater. 2023, 447, 130822. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, Y.; Wang, M.; Yang, W.; Gao, Z. Density functional theory investigation of As4, As2 and AsH3 adsorption on Ti-doped graphene. Chem. Eng. J. 2021, 421, 129747. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.H.; Yan, B. An Eu3+ post-functionalized nanosized metal-organic framework for cation exchange-based Fe3+-sensing in an aqueous environment. J. Mater. Chem. A 2014, 2, 13691–13697. [Google Scholar] [CrossRef]
- Ji, W.J.; Liu, G.F.; Wang, B.Q.; Lu, W.B.; Zhai, Q.G. Design of a heterometallic Zn/Ca-MOF decorated with alkoxy groups on the pore surface exhibiting high fluorescence sensing performance for Fe3+ and Cr2O72−. CrystEngComm 2020, 22, 4710–4715. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Xu, C.; Chen, C.; Yang, L.; Dou, W.; Chen, W.; Yang, H.; Liu, W. A multi-responsive regenerable europium-organic framework luminescent sensor for Fe(III), Cr(VI) anions, and picric acid. Chem. Eur. J. 2016, 22, 18769–18776. [Google Scholar] [CrossRef]
- Gu, J.Z.; Cai, Y.; Liu, Y.; Liang, X.X.; Kirillov, A.M. New lanthanide 2D coordination polymers constructed from a flexible ether-bridged tricarboxylate block: Synthesis, structures and luminescence sensing. Inorg. Chim. Acta 2018, 469, 98–104. [Google Scholar] [CrossRef]
- Jing, T.; Chen, L.; Jiang, F.; Yang, Y.; Zhou, K.; Yu, M.; Cao, Z.; Li, S.; Hong, M. Fabrication of a robust lanthanide metal-organic framework as a multifunctional material for Fe(III) detection, CO2 capture, and utilization. Cryst. Growth. Des. 2018, 18, 2956–2963. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Lv, X.; Zhang, D.; Zhao, X.; Zhong, K.; Sun, H.; Wang, H.; Liu, L.; Wang, W.; Yao, S. In Situ Encapsulated RhB@Er-MOF with Dual-Emitting Rationmetric Fluorescence for Rapid and Selective Detection of Fe(III) by Dual-Signal Output. Chemistry 2025, 7, 83. https://doi.org/10.3390/chemistry7030083
Yao X, Lv X, Zhang D, Zhao X, Zhong K, Sun H, Wang H, Liu L, Wang W, Yao S. In Situ Encapsulated RhB@Er-MOF with Dual-Emitting Rationmetric Fluorescence for Rapid and Selective Detection of Fe(III) by Dual-Signal Output. Chemistry. 2025; 7(3):83. https://doi.org/10.3390/chemistry7030083
Chicago/Turabian StyleYao, Xiaoyan, Xueyi Lv, Dongmei Zhang, Xiangyu Zhao, Kaixuan Zhong, Hanlei Sun, Hongzhi Wang, Licheng Liu, Wentai Wang, and Shuo Yao. 2025. "In Situ Encapsulated RhB@Er-MOF with Dual-Emitting Rationmetric Fluorescence for Rapid and Selective Detection of Fe(III) by Dual-Signal Output" Chemistry 7, no. 3: 83. https://doi.org/10.3390/chemistry7030083
APA StyleYao, X., Lv, X., Zhang, D., Zhao, X., Zhong, K., Sun, H., Wang, H., Liu, L., Wang, W., & Yao, S. (2025). In Situ Encapsulated RhB@Er-MOF with Dual-Emitting Rationmetric Fluorescence for Rapid and Selective Detection of Fe(III) by Dual-Signal Output. Chemistry, 7(3), 83. https://doi.org/10.3390/chemistry7030083






