Application and Suggestions of Morpholine Ring as a Lysosome Targeting Group
Abstract
1. Introduction
2. Morpholine-Modified Molecular Fluorescent Probes
2.1. Lysosome Imaging
2.2. Lysosome-Targeted Photodynamic Therapy (PDT) Agents
2.3. Lysosome pH Detection
2.4. Lysosome Reactive Oxygen Species (ROS) Detection
2.5. Lysosome Metal Ion Detection
2.6. Lysosome Sulfide Detection
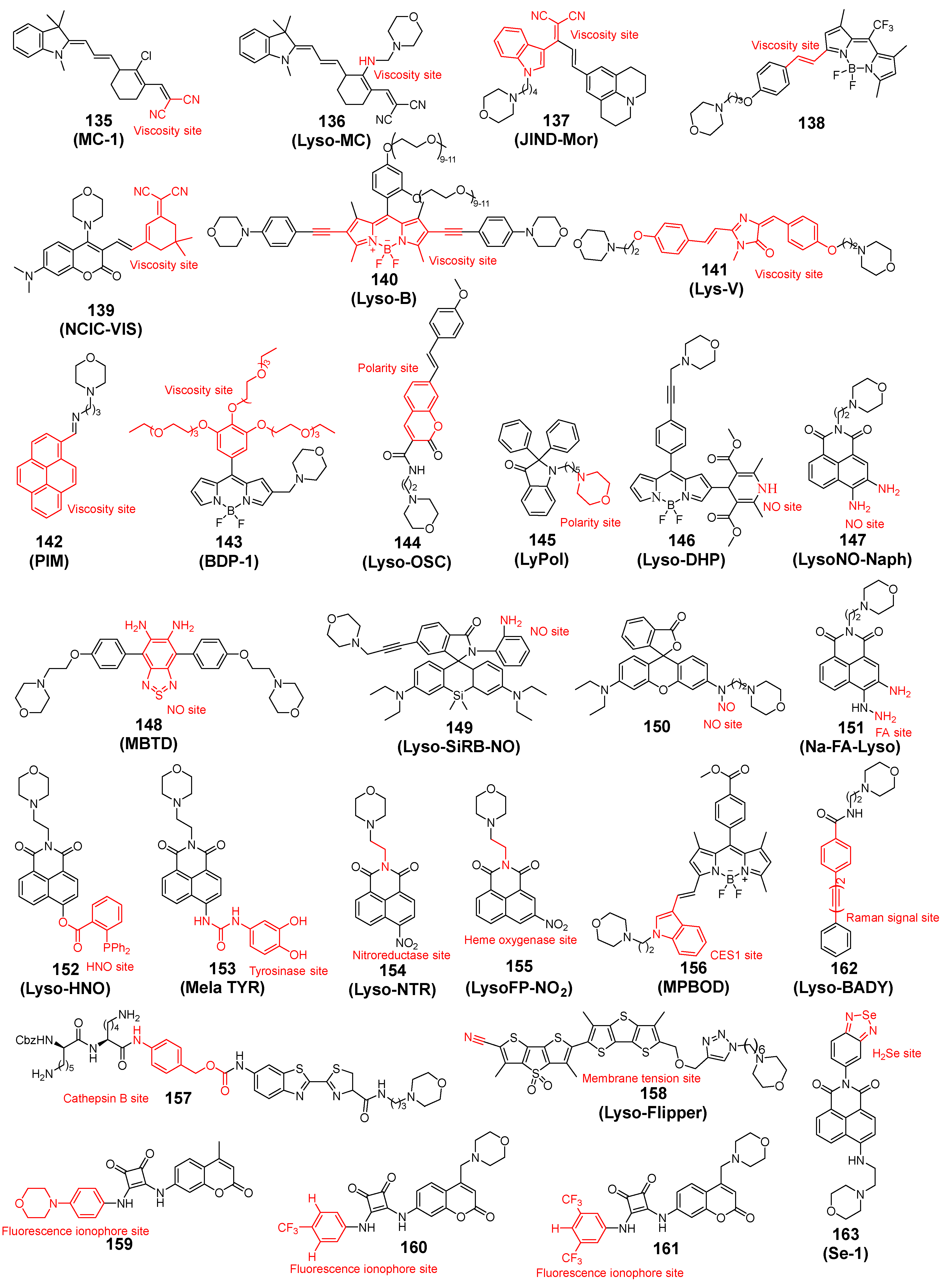
2.7. Lysosome Viscosity Detection
2.8. Lysosome Polarity Detection
2.9. Lysosome Oxidate Detection
2.10. Lysosome Enzyme Detection
2.11. Others
3. Morpholine-Modified Ionic Fluorescent Probes
3.1. Lysosomal Localization
3.2. Dual Localization of Lysosomes and Mitochondrian
3.3. Mitochondria Localization
4. Structure–Activity Relationships Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lou, X.; Zhang, M.; Zhao, Z.; Min, X.; Hakeem, A.; Huang, F.; Gao, P.; Xia, F.; Tang, B.Z. A photostable AIE fluorogen for lysosome-targetable imaging of living cells. J. Mater. Chem. B 2016, 4, 5412–5417. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Curley, M.; Coleman, Z.; Demontis, F. Contribution of proteases to the hallmarks of aging and to age-related neurodegeneration. Aging Cell 2022, 21, e13603. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, X.; Zhang, Y.; Ding, L.; Huo, M.; Li, Q. Fabry disease: Mechanism and therapeutics strategies. Front. Pharmacol. 2022, 13, 1025740. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Wang, C. Lysosomal dysfunction, autophagic defects, and CLN5 accumulation underlie the pathogenesis of KCTD7-mutated neuronal ceroid lipofuscinoses. Autophagy 2023, 19, 1876–1878. [Google Scholar] [CrossRef] [PubMed]
- Kuk, M.U.; Lee, Y.H.; Kim, J.W.; Hwang, S.Y.; Park, J.T.; Park, S.C. Potential Treatment of Lysosomal Storage Disease through Modulation of the Mitochondrial-Lysosomal Axis. Cells 2021, 10, 420. [Google Scholar] [CrossRef]
- Scerra, G.; De Pasquale, V.; Scarcella, M.; Caporaso, M.G.; Pavone, L.M.; D’Agostino, M. Lysosomal positioning diseases: Beyond substrate storage. Open Biol. 2022, 12, 220155. [Google Scholar] [CrossRef]
- Lo, C.H.; Zeng, J. Defective lysosomal acidification: A new prognostic marker and therapeutic target for neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 29. [Google Scholar] [CrossRef]
- Seo, J.; Oh, D.B. Mannose-6-phosphate glycan for lysosomal targeting: Various applications from enzyme replacement therapy to lysosome-targeting chimeras. Anim. Cells Syst. 2022, 26, 84–91. [Google Scholar] [CrossRef]
- Kornfeld, S. Trafficking of lysosomal enzymes. FASEB J. 1987, 1, 462–468. [Google Scholar] [CrossRef]
- Banik, S.M.; Pedram, K.; Wisnovsky, S.; Ahn, G.; Riley, N.M.; Bertozzi, C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020, 584, 291–297. [Google Scholar] [CrossRef]
- Lin, J.; Yang, K.; New, E.J. Strategies for organelle targeting of fluorescent probes. Org. Biomol. Chem. 2021, 19, 9339–9357. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, S.; Poole, B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J. Cell Biol. 1981, 90, 656–664. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C.; De Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef] [PubMed]
- Bertman, K.A.; Abeywickrama, C.S.; Baumann, H.J.; Alexander, N.; McDonald, L.; Shriver, L.P.; Konopka, M.; Pang, Y. A fluorescent flavonoid for lysosome detection in live cells under “wash free” conditions. J. Mater. Chem. B 2018, 6, 5050–5058. [Google Scholar] [CrossRef]
- Liu, S.; Su, H.; Bu, L.; Yan, J.; Li, G.; Huang, J. Fluorogenic probes for mitochondria lysosomes via intramolecular photoclick reaction. Analyst 2021, 146, 1369–1375. [Google Scholar] [CrossRef]
- Wen, Y.; McGarraugh, H.H.; Schreiber, C.L.; Smith, B.D. Cell organelle targeting of near-infrared croconaine dye controls photothermal outcome. Chem. Commun. 2020, 56, 6977–6980. [Google Scholar] [CrossRef]
- Xue, W.-Z.; Wang, B.-B.; Zhao, X.-L.; Wu, W.-N.; Xu, Z.-Q.; Xu, Z.-H.; Wang, Y.; Fan, Y.-C. Rhodamine hydrazone as a lysosome-targetable pH biomarker for the selective differentiation of cancer cells from normal cells. Inorg. Chem. Commun. 2020, 122, 108260. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, G.; Wang, S.; Zhang, X. Boronic acid derived salicylidenehydrazone complexes for wash-free fluorescence imaging of cellular organelles. Dye. Pigm. 2018, 149, 356–362. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, B.; Zheng, G.; Xing, Y.; Wang, C.; Wang, Q. A fluorescent probe for specific lysosome imaging in cells. Anal. Methods 2017, 9, 2788–2790. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Huang, S.; Zhang, X.; Kang, X.; Sun, Y.; Hu, Z.; Han, L.; Du, L.; Liu, Y. A photostable fluorescent probe for long-time imagining of lysosome in cell and nematode. Talanta 2018, 188, 316–324. [Google Scholar] [CrossRef]
- Biswas, S.; Dutta, T.; Silswal, A.; Bhowal, R.; Chopra, D.; Koner, A.L. Strategic engineering of alkyl spacer length for a pH-tolerant lysosome marker and dual organelle localization. Chem. Sci. 2021, 12, 9630–9644. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xiong, C.; Pan, J.; Su, D.; Zeng, L. Highly photostable, lysosome-targeted BODIPYs with green to near-infrared emission for lysosome imaging in living cells. Dye. Pigm. 2018, 155, 30–35. [Google Scholar] [CrossRef]
- Kong, X.; Di, L.; Fan, Y.; Zhou, Z.; Feng, X.; Gai, L.; Tian, J.; Lu, H. Lysosome-targeting turn-on red/NIR BODIPY probes for imaging hypoxic cells. Chem. Commun. 2019, 55, 11567–11570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, W.; Tan, X.; Wang, J.; Zhao, Y.; Hu, J.; Wang, S. A mitochondria-targeted dual-functional aggregation-induced emission luminogen for intracellular mitochondrial imaging and photodynamic therapy. Biomater. Sci. 2021, 9, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhang, P.; Li, H.; Lam, J.W.Y.; Cai, Y.; Kwok, R.T.K.; Qian, J.; Zheng, W.; Tang, B.Z. Ultrabright red AIEgens for two-photon vascular imaging with high resolution and deep penetration. Chem. Sci. 2018, 9, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.T.; Pigge, F.C. A fluorescent turn-on probe for cyanide anion detection based on an AIE active cobalt(II) complex. Dalton Trans. 2018, 47, 2079–2085. [Google Scholar] [CrossRef]
- Dai, Y.; He, F.; Ji, H.; Zhao, X.; Misal, S.; Qi, Z. Dual-Functional NIR AIEgens for High-Fidelity Imaging of Lysosomes in Cells and Photodynamic Therapy. ACS Sens. 2020, 5, 225–233. [Google Scholar] [CrossRef]
- Ouyang, J.; Zang, Q.; Chen, W.; Wang, L.; Li, S.; Liu, R.-Y.; Deng, Y.; Liu, Z.-Q.; Li, J.; Deng, L.; et al. Bright and photostable fluorescent probe with aggregation-induced emission characteristics for specific lysosome imaging and tracking. Talanta 2016, 159, 255–261. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Tan, X.; Wang, N.; Wang, J.; He, M.; Peng, J.; Hu, J.; Zhao, Y.; Wang, S. An AIEgen-based photosensitizer for lysosome imaging and photodynamic therapy in tumor. Sens. Actuators B Chem. 2021, 335, 129698. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, F.; Wang, Z.; Zhao, Z.; Qin, A.; Tang, B.Z. A Photostable AIEgen for Specific and Real-time Monitoring of Lysosomal Processes. Chem.—Asian J. 2019, 14, 1662–1666. [Google Scholar] [CrossRef]
- Leung, C.W.; Wang, Z.; Zhao, E.; Hong, Y.; Chen, S.; Kwok, R.T.; Leung, A.C.; Wen, R.; Li, B.; Lam, J.W.; et al. A Lysosome-Targeting AIEgen for Autophagy Visualization. Adv. Healthc. Mater. 2016, 5, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Hong, Y.; Xie, N.; Chen, S.; Zhao, N.; Zhao, E.; Lam, J.W.Y.; Sung, H.H.Y.; Dong, Y.; Tong, B.; et al. Defect-sensitive crystals based on diaminomaleonitrile-functionalized Schiff base with aggregation-enhanced emission. J. Mater. Chem. C 2013, 1, 7314–7320. [Google Scholar] [CrossRef]
- Ding, S.; Yao, B.; Chen, M.Z.; Liu, C.; Owyong, T.C.; Johnston, A.; Hong, Y. Diaminomaleonitrile-Functionalised Schiff Bases: Synthesis, Solvatochromism, and Lysosome-Specific Imaging. Aust. J. Chem. 2020, 73, 942–947. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Mukhopadhyay, S.K.; Gayathri, S.; Biswas, S.; Barman, S.; Dey, S.; Singh, N.D.P. Fluorene-morpholine-based organic nanoparticles: Lysosome-targeted pH-triggered two-photon photodynamic therapy with fluorescence switch on-off. J. Mater. Chem. B 2016, 4, 1862–1868. [Google Scholar] [CrossRef]
- Wang, Q.; Ng, D.K.P.; Lo, P.-C. Functional aza-boron dipyrromethenes for subcellular imaging and organelle-specific photodynamic therapy. J. Mater. Chem. B 2018, 6, 3285–3296. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, L.; Qian, Y. A near-infrared and lysosomal targeting thiophene-BODIPY photosensitizer: Synthesis and its imaging guided photodynamic therapy of cancer cells. Spectrochim. Acta Part A 2021, 252, 119512. [Google Scholar] [CrossRef]
- Bai, J.; Qian, Y. Construction of an NIR and lysosome-targeted quinoline-BODIPY photosensitizer and its application in photodynamic therapy for human gastric carcinoma cells. Dye. Pigm. 2020, 181, 108615. [Google Scholar] [CrossRef]
- Li, M.; Tian, R.; Fan, J.; Du, J.; Long, S.; Peng, X. A lysosome-targeted BODIPY as potential NIR photosensitizer for photodynamic therapy. Dye. Pigment. 2017, 147, 99–105. [Google Scholar] [CrossRef]
- Ramu, V.; Gautam, S.; Kondaiah, P.; Chakravarty, A.R. Diplatinum(II) Catecholate of Photoactive Boron-Dipyrromethene for Lysosome-Targeted Photodynamic Therapy in Red Light. Inorg. Chem. 2019, 58, 9067–9075. [Google Scholar] [CrossRef]
- Wang, C.; Qian, Y. A novel BODIPY-based photosensitizer with pH-active singlet oxygen generation for photodynamic therapy in lysosomes. Org. Biomol. Chem. 2019, 17, 8001–8007. [Google Scholar] [CrossRef]
- Zhuang, J.; Yang, H.; Li, Y.; Wang, B.; Li, N.; Zhao, N. Efficient photosensitizers with aggregation-induced emission characteristics for lysosome-, Gram-positive bacteria-targeted photodynamic therapy. Chem. Commun. 2020, 56, 2630–2633. [Google Scholar] [CrossRef]
- Settembre, C.; Zoncu, R.; Medina, D.L.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Ferron, M.; Karsenty, G.; Vellard, M.C.; et al. A lysosome-to-nucleus signaling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012, 31, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, N.; Jaeaettelae, M. Lysosomes as Targets for Cancer Therapy. Cancer Res. 2005, 65, 2993–2995. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Dang, Y.; Chen, T.; Zhang, A.; Ding, C.; Xu, Z. Imidazole-fused benzothiadiazole-based red-emissive fluorescence probe for lysosomal pH imaging in living cells. Talanta 2020, 217, 121066. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Song, X.; Kong, X.; Wang, C.; Zhang, N.; Lin, W. A tumor-targeting and lysosome-specific two-photon fluorescent probe for imaging pH changes in living cells. J. Mater. Chem. B 2017, 5, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, W.; Liu, T.; Huo, F.; Yin, C. Pinpoint Diagnostic Kit for Heat Stroke by Monitoring Lysosomal, p.H. Anal. Chem. 2017, 89, 11869–11874. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Yue, M.; Li, P.; Liu, Y.; Ye, F.; Fu, Y. A Multifunctional and Fast-Response Lysosome-Targetable Fluorescent Probe for Monitoring pH and Isoxaflutole. Int. J. Mol. Sci. 2022, 23, 6256. [Google Scholar] [CrossRef]
- Luo, W.; Jiang, H.; Tang, X.; Liu, W. A reversible ratiometric two-photon lysosome-targeted probe for real-time monitoring of pH changes in living cells. J. Mater. Chem. B 2017, 5, 4768–4773. [Google Scholar] [CrossRef]
- Dong, B.; Song, X.; Wang, C.; Kong, X.; Tang, Y.; Lin, W. Dual Site-Controlled and Lysosome-Targeted Intramolecular Charge Transfer-Photoinduced Electron Transfer-Fluorescence Resonance Energy Transfer Fluorescent Probe for Monitoring pH Changes in Living Cells. Anal. Chem. 2016, 88, 4085–4091. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Zhang, X.; Zan, Q.; Dong, W.; Shuang, S.; Dong, C. A red-emission fluorescent probe for visual monitoring of lysosomal pH changes during mitophagy and cell apoptosis. Analyst 2020, 145, 7018–7024. [Google Scholar] [CrossRef]
- Shen, S.L.; Chen, X.P.; Zhang, X.F.; Miao, J.Y.; Zhao, B.X. A rhodamine B-based lysosomal pH probe. J. Mater. Chem. B 2015, 3, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, L.; Qiu, L.; Lu, D.; Wu, Y.; Zhang, X.-B. An efficient ratiometric fluorescent probe for tracking dynamic changes in lysosomal, pH. Analyst 2015, 140, 5563–5569. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.J.; Kong, Z.Z.; Zhang, M.L.; Lv, M.K.; Zhang, G. A structure optimized fluorescent probe for highly sensitive monitoring drug induced lysosomal pH value changes. Talanta 2019, 203, 1–8. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, P.; Liu, W.; Wang, M.; Zhang, H.; Wu, J.; Zhang, L.; Wang, P. Near-Infrared Probe Based on Rhodamine Derivative for Highly Sensitive and Selective Lysosomal pH Tracking. Anal. Chem. 2017, 89, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Wang, T.-R.; Cao, X.-Q.; Shen, S.-L. A near-infrared rhodamine-based lysosomal pH probe and its application in lysosomal pH rise during heat shock. Spectrochim. Acta Part A 2020, 227, 117761. [Google Scholar] [CrossRef]
- Dong, Z.; Han, Q.; Mou, Z.; Li, G.; Liu, W. A reversible frequency upconversion probe for real-time intracellular lysosome-pH detection and subcellular imaging. J. Mater. Chem. B 2018, 6, 1322–1327. [Google Scholar] [CrossRef]
- Chen, T.-H.; Zhang, S.; Jaishi, M.; Adhikari, R.; Bi, J.; Fang, M.; Xia, S.; Zhang, Y.; Luck, R.L.; Pati, R.; et al. New Near-Infrared Fluorescent Probes with Single-Photon Anti-Stokes-Shift Fluorescence for Sensitive Determination of pH Variances in Lysosomes with a Double-Checked Capability. ACS Appl. Bio. Mater. 2018, 1, 549–560. [Google Scholar] [CrossRef]
- Yuan, G.; Ding, H.; Zhou, L. An effective FRET-based two-photon ratiometric fluorescent probe with double well-resolved emission bands for lysosomal pH changes in living cells and zebrafish. Spectrochim. Acta Part A 2020, 224, 117397. [Google Scholar] [CrossRef]
- Srivastava, P.; Srivastava, P.; Patra, A.K. Biological perspectives of a FRET based pH-probe exhibiting molecular logic gate operation with altering pH. New J. Chem. 2018, 42, 9543–9549. [Google Scholar] [CrossRef]
- Xia, S.; Fang, M.; Wang, J.; Bi, J.; Mazi, W.; Zhang, Y.; Luck, R.L.; Liu, H. Near-infrared fluorescent probes with BODIPY donors and rhodamine and merocyanine acceptors for ratiometric determination of lysosomal pH variance. Sens. Actuators B 2019, 294, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, M.; Mazi, W.; Adhikari, K.; Fang, M.; Xie, F.; Valenzano, L.; Tiwari, A.; Luo, F.T.; Liu, H. Unusual Fluorescent Responses of Morpholine-functionalized Fluorescent Probes to pH via Manipulation of BODIPY’s HOMO and LUMO Energy Orbitals for Intracellular pH Detection. ACS Sens. 2016, 1, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xing, P.; Zhou, Y.; Gong, L.; Zhang, J.; Qi, D.; Bian, Y.; Du, H.; Jiang, J. Lysosome-targeting ratiometric fluorescent pH probes based on long-wavelength BODIPY. J. Mater. Chem. B 2018, 6, 4422–4426. [Google Scholar] [CrossRef]
- Xia, S.; Wang, J.; Bi, J.; Wang, X.; Fang, M.; Phillips, T.; May, A.; Conner, N.; Tanasova, M.; Luo, F.-T.; et al. Fluorescent probes based on π-conjugation modulation between hemicyanine and coumarin moieties for ratiometric detection of pH changes in live cells with visible and near-infrared channels. Sens. Actuators B 2018, 265, 699–708. [Google Scholar] [CrossRef]
- Wang, J.; Xia, S.; Bi, J.; Fang, M.; Mazi, W.; Zhang, Y.; Conner, N.; Luo, F.-T.; Lu, H.P.; Liu, H. Ratiometric Near-Infrared Fluorescent Probes Based On Through-Bond Energy Transfer and π-Conjugation Modulation between Tetraphenylethene and Hemicyanine Moieties for Sensitive Detection of pH Changes in Live Cells. Bioconjugate Chem. 2018, 29, 1406–1418. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, W.; Xi, Y.; Yang, T.; Gao, B. Cell membrane permeable fluorescent perylene bisimide derivatives for cell lysosome imaging. RSC Adv. 2016, 6, 83864–83869. [Google Scholar] [CrossRef]
- Hande, P.E.; Mishra, M.; Ali, F.; Kapoor, S.; Datta, A.; Gharpure, S.J. Design and Expeditious Synthesis of Quinoline-Pyrene-Based Ratiometric Fluorescent Probes for Targeting Lysosomal pH. ChemBioChem 2020, 21, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Hou, L.; Feng, Y.; Xu, G.; Bai, Y.; Yu, H.; Meng, X. Real-time visualization of autophagy by monitoring the fluctuation of lysosomal pH with a ratiometric two-photon fluorescent probe. Chem. Commun. 2019, 55, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Su, Y.; Tian, H.; Yang, L.; Zhang, H.; Song, X.; Foley, J.W. Ratiometric Fluorescent Probe for Lysosomal pH Measurement and Imaging in Living Cells Using Single-Wavelength Excitation. Anal. Chem. 2017, 89, 7038–7045. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, P.; Wang, X.; Tian, Y.; Liu, C.; Li, X.; Wang, K.; Li, P.; Zhu, B.; Tang, B. In Situ Observation of Lysosomal Hypobromous Acid Fluctuations in the Brain of Mice with Depression Phenotypes by Two-Photon Fluorescence Imaging. Anal. Chem. 2022, 94, 11783–11790. [Google Scholar] [CrossRef]
- Ma, C.; Ma, M.; Zhang, Y.; Zhu, X.; Zhou, L.; Fang, R.; Liu, X.; Zhang, H. Lysosome-targeted two-photon fluorescent probe for detection of hypobromous acid in vitro and in vivo. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 48–54. [Google Scholar] [CrossRef]
- Ma, S.; Ma, Y.; Liu, Q.; Lin, W. A two-photon fluorescent probe with lysosome targetability for imaging endogenous superoxide anion in living cells, zebrafish and pneumonia tissue. Sens. Actuators B Chem. 2021, 332, 129523. [Google Scholar] [CrossRef]
- Qian, J.; Gong, D.; Ru, J.; Guo, Y.; Cao, T.; Liu, W.; Iqbal, A.; Iqbal, K.; Qin, W.; Guo, H. A naphthalimide-based lysosome-targeting fluorescent probe for the selective detection and imaging of endogenous peroxynitrite in living cells. Anal. Bioanal. Chem. 2019, 411, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Reja, S.I.; Gupta, M.; Gupta, N.; Bhalla, V.; Ohri, P.; Kaur, G.; Kumar, M. A lysosome targetable fluorescent probe for endogenous imaging of hydrogen peroxide in living cells. Chem. Commun. 2017, 53, 3701–3704. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, G.; Nam, S.J.; Yin, J.; Yoon, J. Visualization of endogenous and exogenous hydrogen peroxide using a lysosome-targetable fluorescent probe. Sci. Rep. 2015, 5, 8488. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, S.; Ren, J.; Wu, C.; Zhao, Y. A lysosome-locating acidic pH-activatable fluorescent probe for visualizing endogenous H2O2 in lysosomes. Analyst 2017, 142, 4522–4528. [Google Scholar] [CrossRef]
- Liu, C.; Jiao, X.; He, S.; Zhao, L.; Zeng, X. A highly selective and sensitive fluorescent probe for hypochlorous acid and its lysosome-targetable biological applications. Talanta 2017, 174, 234–242. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, C.; Bai, Y.; Xu, J.; Zhang, J.; Li, Z.; Guo, X. A lysosome-targetable and ratiometric fluorescent probe for hypochlorous acid in living cells based on a 1,8-naphthalimide derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117334. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, H.; Zhang, D.; Zeng, X.; Zeng, R.; Xiao, L.; Tao, H.; Long, Y.; Yi, P.; Chen, J. Two-photon fluorescent probe for lysosome-targetable hypochlorous acid detection within living cells. Sens. Actuators B 2018, 255, 2223–2231. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Zhang, R.; Liu, Y.; Ren, X.; Xian, M.; Ye, Y.; Zhao, Y. Lysosomal-Targeted Two-Photon Fluorescent Probe to Sense Hypochlorous Acid in Live Cells. Anal. Chem. 2017, 89, 10384–10390. [Google Scholar] [CrossRef]
- Xing, Y.; Zhu, Y.; Tang, Y.; Mao, Y.; Han, J.; Wang, Y.; Ni, L. Construction application of a ratiometric fluorescent probe for detecting endogenous ClO. Fenxi Shiyanshi 2020, 39, 772–776. [Google Scholar]
- Jiao, X.; Liu, C.; Wang, Q.; Huang, K.; He, S.; Zhao, L.; Zeng, X. Fluorescence probe for hypochlorous acid in water and its applications for highly lysosome-targetable live cell imaging. Anal. Chim. Acta 2017, 969, 49–56. [Google Scholar] [CrossRef]
- Gong, Y.-J.; Zhang, M.-L.; Wang, B.-X.; Lv, Q.; Wang, Y.; Dong, W. A smart approach toward rhodamine spiro-ring derivatives sensing platform for lysosome-targetable imaging applications. Sens. Actuators B Chem. 2019, 283, 239–246. [Google Scholar] [CrossRef]
- Huang, X.-Q.; Wang, Z.-Y.; Lv, Y.-J.; Shen, S.-L.; Zhu, Y.; Wang, J.; Zhang, Y.-R.; Wang, J.-M.; Ge, Y.-Q.; Cao, X.-Q. A fluorescent probe for the detection of HOCl in lysosomes. New J. Chem. 2018, 42, 11480–11484. [Google Scholar] [CrossRef]
- Ren, M.; Nie, J.; Deng, B.; Zhou, K.; Wang, J.-Y.; Lin, W. A fluorescent probe for ratiometric imaging of exogenous and intracellular formed hypochlorous acid in lysosomes. New J. Chem. 2017, 41, 5259–5262. [Google Scholar] [CrossRef]
- Shen, S.-L.; Huang, X.-Q.; Lin, X.-H.; Cao, X.-Q. A ratiometric fluorescent probe for lysosomal hypochlorous acid based on through-bond energy transfer strategy. Anal. Chim. Acta 2019, 1052, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-L.; Huang, X.-Q.; Jiang, H.-L.; Lin, X.-H.; Cao, X.-Q. A rhodamine B-based probe for the detection of HOCl in lysosomes. Anal. Chim. Acta 2019, 1046, 185–191. [Google Scholar] [CrossRef]
- Shen, S.-L.; Huang, X.-Q.; Zhang, Y.-Y.; Zhu, Y.; Hou, C.; Ge, Y.-Q.; Cao, X.-Q. Ratiometric fluorescent probe for the detection of HOCl in lysosomes based on FRET strategy. Sens. Actuators B 2018, 263, 252–257. [Google Scholar] [CrossRef]
- Mao, G.-J.; Liang, Z.-Z.; Bi, J.; Zhang, H.; Meng, H.-M.; Su, L.; Gong, Y.-J.; Feng, S.; Zhang, G. A near-infrared fluorescent probe based on photostable Si-rhodamine for imaging hypochlorous acid during lysosome-involved inflammatory response. Anal. Chim. Acta 2019, 1048, 143–153. [Google Scholar] [CrossRef]
- Meng, H.; Huang, X.Q.; Lin, Y.; Yang, D.Y.; Lv, Y.J.; Cao, X.Q.; Zhang, G.X.; Dong, J.; Shen, S.L. A new ratiometric fluorescent probe for sensing lysosomal HOCl based on fluorescence resonance energy transfer strategy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117355. [Google Scholar] [CrossRef]
- Ren, M.; Li, Z.; Deng, B.; Wang, L.; Lin, W. Single Fluorescent Probe Separately and Continuously Visualize H2S and HClO in Lysosomes with Different Fluorescence Signals. Anal. Chem. 2019, 91, 2932–2938. [Google Scholar] [CrossRef]
- Huang, C.; Qian, Y. A fast-responsed lysosomal-targeted fluorescent probe based on BODIPY with low limit detection for hypochlorous acid and its application of intracellular hypochlorous acid bioimaging. Opt. Mater. 2019, 92, 53–59. [Google Scholar] [CrossRef]
- Shi, L.; Yang, S.; Hong, H.-J.; Li, Y.; Yu, H.-J.; Shao, G.; Zhang, K.; Gong, S.-Z. A novel target and pH dual-activatable fluorescent probe for precisely detecting hypochlorite in lysosomes. Anal. Chim. Acta 2020, 1094, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.F.; Wu, W.N.; Zhao, X.L.; Wang, Y.; Fan, Y.C.; Zhang, C.X.; Xu, Z.H. A deep-red lysosome-targetable fluorescent probe for detection of hypochlorous acid in pure water and its imaging application in living cells and zebrafish. Spectrochim Acta A Mol. Biomol. Spectrosc. 2022, 264, 120270. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, F.; Niu, H.; Liu, Y.; Zhang, D.; Ye, Y. A highly sensitive and fast responsive naphthalimide-based fluorescent probe for Cu2+, its application. New J. Chem. 2017, 41, 14683–14688. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Polishchuk, R.S. The emerging role of lysosomes in copper homeostasis. Metallomics 2016, 8, 853–862. [Google Scholar] [CrossRef]
- Song, Y.-F.; Fan, Y.-C.; Cai, H.-X.; Zong, H.-T.; Li, M.; Wu, W.-N.; Wang, Y.; Xu, Z.-H. A simple hydrazone probe for recognition of Al(3+) and PPi and its applicability in lysosomal imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 268, 120680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.D.; Banasiewicz, M.; Wrzosek, A.; O’Mari, O.; Zochowska, M.; Vullev, V.I.; Jacquemin, D.; Szewczyk, A.; Gryko, D.T. A sensitive zinc probe operating via enhancement of excited-state intramolecular charge transfer. Org. Biomol. Chem. 2022, 20, 7439–7447. [Google Scholar] [CrossRef]
- Du, C.; Fu, S.; Wang, X.; Sedgwick, A.C.; Zhen, W.; Li, M.; Li, X.; Zhou, J.; Wang, Z.; Wang, H.; et al. Diketopyrrolopyrrole-based fluorescence probes for the imaging of lysosomal Zn2+ and identification of prostate cancer in human tissue. Chem. Sci. 2019, 10, 5699–5704. [Google Scholar] [CrossRef]
- Duan, H.; Ding, Y.; Huang, C.; Zhu, W.; Wang, R.; Xu, Y. A lysosomal targeting fluorescent probe and its zinc imaging in SH-SY5Y human neuroblastoma cells. Chin. Chem. Lett. 2019, 30, 55–57. [Google Scholar] [CrossRef]
- Sudheesh, K.V.; Joseph, M.M.; Philips, D.S.; Samanta, A.; Kumar Maiti, K.; Ajayaghosh, A. A pH-Controlled Nanoparticles Formation and Tracking of Lysosomal Zinc Ions in Cancer Cells by Fluorescent Carbazole-Bipyridine Conjugates. ChemistrySelect 2018, 3, 2416–2422. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, G.; Hu, J.; Wang, H.; Eriksson, P.; Zhang, R.; Zhang, Z.; Uvdal, K. Real-time visualizing the regulation of reactive oxygen species on Zn2+ release in cellular lysosome by a specific fluorescent probe. Sens. Actuators B 2018, 264, 419–425. [Google Scholar] [CrossRef]
- Li, X.; Qin, W. A novel dual-capability naphthalimide-based fluorescent probe for Fe3+ ion detection and lysosomal tracking in living cells. RSC Adv. 2022, 12, 24252–24259. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Yi, Q.; Wang, M.; Wang, J. Design, synthesis and biological evaluation of novel dual-targeting fluorescent probes for detection of Fe(3+) in the lysosomes of hepatocytes mediated by galactose-morpholine moieties. Talanta 2022, 243, 123362. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.L.; Trusso Sfrazzetto, G.; Satriano, C.; Zimbone, S.; Tomaselli, G.A.; Copani, A.; Rizzarelli, E. A New Ratiometric Lysosomal Copper(II) Fluorescent Probe To Map a Dynamic Metallome in Live Cells. Inorg. Chem. 2018, 57, 2365–2368. [Google Scholar] [CrossRef]
- Ahmed, N.; Zareen, W.; Zhang, D.; Yang, X.; Ye, Y. Irreversible coumarin based fluorescent probe for selective detection of Cu2+ in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120313. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.N.; Wu, H.; Zhong, R.B.; Wang, Y.; Xu, Z.H.; Zhao, X.L.; Xu, Z.Q.; Fan, Y.C. Ratiometric fluorescent probe based on pyrrole-modified rhodamine 6G hydrazone for the imaging of Cu2+ in lysosomes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 121–127. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakraborty, S.; Lohar, S.; Ahmmed, E.; Saha, N.C.; Mandal, S.K.; Dhara, K.; Chattopadhyay, P. A Lysosome-Targetable Fluorescence Sensor for Ultrasensitive Detection of Hg2+ in Living Cells and Real Samples. Chem. Res. Toxicol. 2019, 32, 1144–1150. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Chen, D.; Wu, D.; Chen, Z.; Zhang, J.; Chen, X.; Liu, S.; Yin, J. A colorimetric and ratiometric fluorescent probe for mercury (II) in lysosome. Sens. Actuators B Chem. 2016, 224, 907–914. [Google Scholar] [CrossRef]
- Cao, X.J.; Chen, L.N.; Zhang, X.; Liu, J.T.; Chen, M.Y.; Wu, Q.R.; Miao, J.Y.; Zhao, B.X. A NBD-based simple but effective fluorescent pH probe for imaging of lysosomes in living cells. Anal. Chim. Acta 2016, 920, 86–93. [Google Scholar] [CrossRef]
- Kong, L.; Jiao, C.; Luan, L.; Li, S.; Ma, X.; Wang, Y. Reversible Ni2+ fluorescent probe based on ICT mechanism and its application in bio-imaging of Zebrafish. J. Photochem. Photobiol. A Chem. 2021, 422, 113555. [Google Scholar] [CrossRef]
- Ye, F.; Wu, N.; Li, P.; Liu, Y.L.; Li, S.J.; Fu, Y. A lysosome-targetable fluorescent probe for imaging trivalent cations Fe3+, Al3+ and Cr3+ in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117242. [Google Scholar] [CrossRef]
- Xie, Q.-L.; Liu, W.; Liu, X.-J.; Ouyang, F.; Kuang, Y.-Q.; Jiang, J.-H. An azidocoumarin-based fluorescent probe for imaging lysosomal hydrogen sulfide in living cells. Anal. Methods 2017, 9, 2859–2864. [Google Scholar] [CrossRef]
- Jing, X.; Yu, F.; Lin, W. A PET-based lysosome-targeted turn-on fluorescent probe for the detection of H2S and its bioimaging application in living cells and zebrafish. New J. Chem. 2019, 43, 16796–16800. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, H.; Yan, Y.; Hang, Y.; Yu, F.; Qu, X.; Hua, J. Targetable N-annulated perylene-based colorimetric ratiometric near-infrared fluorescent probes for the selective detection of hydrogen sulfide in mitochondria lysosomes serum. J. Mater. Chem. B 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Liao, L.; Li, Z.; Hu, W.; Huang, Y.; Liu, B.-M.; Wang, L.; Wang, M.; Wang, J. Design, synthesis and evaluation of a novel fluorescent probe to accurately detect H2S in lysosomes. Tetrahedron Lett. 2018, 59, 2683–2687. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhang, X.-B. Engineering of a dual-site molecular probe for logical bioimaging of lysosomal H2S p.H. Talanta 2020, 219, 121286. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Pan, T.; Ou, P.; Zhou, R.; Ouyang, Z.; Luo, L.; Xiao, Z.; Peng, Y. Construct a lysosome-targeting and highly selective fluorescent probe for imaging of hydrogen sulfide in living cells and inflamed tissues. Spectrochim. Acta Part A 2021, 249, 119311. [Google Scholar] [CrossRef]
- Luo, W.; Xue, H.; Ma, J.; Wang, L.; Liu, W. Molecular engineering of a colorimetric two-photon fluorescent probe for visualizing H2S level in lysosome and tumor. Anal. Chim. Acta 2019, 1077, 273–280. [Google Scholar] [CrossRef]
- Gao, C.; Liu, X.; Chen, W.; Wang, F.; Jiang, J.H. A naphthalene-based fluorescent probe for ratiometric imaging of lysosomal hydrogen sulfide in living cells. Methods Appl. Fluoresc. 2018, 7, 014002. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Lin, W. A lysosome-targeted two-photon fluorescence probe for imaging of sulfur dioxide derivatives in living cells and zebrafish. Sens. Actuators B 2018, 268, 157–163. [Google Scholar] [CrossRef]
- Huo, F.; Wu, Q.; Yin, C.; Zhang, W.; Zhang, Y. A high efficient and lysosome targeted “off-on” probe for sulfite based on nucleophilic addition and ESIPT. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xu, J.; Ma, Q.; Li, L.; Yuan, H.; Sun, J.; Zhu, N.; Liu, S. A novel lysosome-located fluorescent probe for highly selective determination of hydrogen polysulfides based on a naphthalimide derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 268, 120708. [Google Scholar] [CrossRef]
- Han, Q.; Liu, X.; Wang, X.; Yin, R.; Jiang, H.; Ru, J.; Liu, W. Rational design of a lysosomal-targeted ratiometric two-photon fluorescent probe for imaging hydrogen polysulfides in live cells. Dye. Pigm. 2020, 173, 107877. [Google Scholar] [CrossRef]
- Feng, W.; Mao, Z.; Liu, L.; Liu, Z. A ratiometric two-photon fluorescent probe for imaging hydrogen sulfide in lysosomes. Talanta 2017, 167, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, Q.; Li, S.; Feng, Y.; Zhang, W.; Fang, G.; Li, L.; Sun, C.; Wang, X.; Meng, X. A dual-emission two-photon fluorescent probe for specific-cysteine imaging in lysosomes and in vivo. Sens. Actuators B Chem. 2019, 293, 247–255. [Google Scholar] [CrossRef]
- Liang, B.; Wang, B.; Ma, Q.; Xie, C.; Li, X.; Wang, S. A lysosome-targetable turn-on fluorescent probe for the detection of thiols in living cells based on a 1,8-naphthalimide derivative. Spectrochim. Acta Part A 2018, 192, 67–74. [Google Scholar] [CrossRef]
- Tan, H.; Zou, Y.; Guo, J.; Chen, J.; Zhou, L. A simple lysosome-targeted fluorescent probe based on flavonoid for detection of cysteine in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121552. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yue, Y.; Chao, J.; Huo, F.; Yin, C. Based on morpholine as luminescence mechanism regulation and organelle targeting dual function Cys NIR specific biological imaging probe. Sens. Actuators B 2020, 320, 128348. [Google Scholar] [CrossRef]
- Yue, Y.; Huo, F.; Yue, P.; Meng, X.; Salamanca, J.C.; Escobedo, J.O.; Strongin, R.M.; Yin, C. In Situ Lysosomal Cysteine-Specific Targeting and Imaging during Dexamethasone-Induced Apoptosis. Anal. Chem. 2018, 90, 7018–7024. [Google Scholar] [CrossRef]
- Long, Z.; Chen, L.; Dang, Y.; Chen, D.; Lou, X.; Xia, F. An ultralow concentration of two-photon fluorescent probe for rapid and selective detection of lysosomal cysteine in living cells. Talanta 2019, 204, 762–768. [Google Scholar] [CrossRef]
- Kand, D.; Saha, T.; Lahiri, M.; Talukdar, P. Lysosome targeting fluorescence probe for imaging intracellular thiols. Org. Biomol. Chem. 2015, 13, 8163–8168. [Google Scholar] [CrossRef]
- Alqahtani, Y.; Wang, S.; Huang, Y.; Najmi, A.; Guan, X. Design, Synthesis, and Characterization of Bis(7-(N-(2-morpholinoethyl)sulfamoyl)benzo[c][1,2,5]oxadiazol-5-yl)sulfane for Nonprotein Thiol Imaging in Lysosomes in Live Cells. Anal. Chem. 2019, 91, 15300–15307. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Han, Z.; Kang, Y.; Peng, X. A Two-Photon Fluorescent Probe for Lysosomal Thiols in Live Cells and Tissues. Sci. Rep. 2016, 6, 19562. [Google Scholar] [CrossRef]
- Song, X.; Tu, Y.; Wang, R.; Pu, S. A lysosome-targetable fluorescent probe for simultaneous detection and discrimination of Cys/Hcy and GSH by dual channels. Dye. Pigm. 2020, 177, 108270. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Li, N.; Huang, J.; Wang, Q.; Gu, Y. A novel DCM-NBD conjugate fluorescent probe for discrimination of Cys/Hcy from GSH and its bioimaging applications in living cells and animals. Sens. Actuators B 2017, 245, 297–304. [Google Scholar] [CrossRef]
- Xia, X.; Qian, Y. NIR two-photon fluorescent probe for biothiol detection and imaging of living cells in vivo. Analyst 2018, 143, 5218–5224. [Google Scholar] [CrossRef]
- Sun, Y.-H.; Han, H.-H.; Huang, J.-M.; Li, J.; Zang, Y.; Wang, C.-Y. Long-wavelength fluorescent probe with a large Stokes shift for lysosome-targeted imaging of Cys and GSH. Spectrochim. Acta Part A 2021, 261, 120055. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Chen, W.; Huang, J.; Huang, C.; Sheng, J.; Song, X. A Lysosome-Targetable Fluorescent Probe for Simultaneously Sensing Cys/Hcy, GSH, and H2S from Different Signal Patterns. ACS Sens. 2018, 3, 2513–2517. [Google Scholar] [CrossRef]
- Yan, J.-W.; Zhu, J.-Y.; Zhou, K.-X.; Wang, J.-S.; Tan, H.-Y.; Xu, Z.-Y.; Chen, S.-B.; Lu, Y.-T.; Cui, M.-C.; Zhang, L. Neutral merocyanine dyes: For in vivo NIR fluorescence imaging of amyloid-β plaques. Chem. Commun. 2017, 53, 9910–9913. [Google Scholar] [CrossRef]
- Tan, H.Y.; Qiu, Y.T.; Sun, H.; Yan, J.W.; Zhang, L. A lysosome-targeting dual-functional fluorescent probe for imaging intracellular viscosity and beta-amyloid. Chem. Commun. 2019, 55, 2688–2691. [Google Scholar] [CrossRef]
- Silswal, A.; Kanojiya, A.; Koner, A.L. A fluorogenic far red-emitting molecular viscometer for ascertaining lysosomal stress in live cells and Caenorhabditis elegans. Front. Chem. 2022, 10, 840297. [Google Scholar] [CrossRef]
- Shi, W.-J.; Wei, Y.-F.; Yang, J.; Li, H.-Z.; Wan, Q.-H.; Wang, Y.; Leng, H.; Chen, K.; Yan, J.-W. Novel meso-trifluoromethyl BODIPY-based near-infrared-emitting fluorescent probes for organelle-specific imaging of cellular viscosity. Sens. Actuators B 2022, 359, 131594. [Google Scholar] [CrossRef]
- Chai, L.; Liang, T.; An, Q.; Hu, W.; Wang, Y.; Wang, B.; Su, S.; Li, C. Near-Infrared in and out: Observation of Autophagy during Stroke via a Lysosome-Targeting Two-Photon Viscosity-Dependent Probe. Anal. Chem. 2022, 94, 5797–5804. [Google Scholar] [CrossRef]
- Li, L.L.; Li, K.; Li, M.Y.; Shi, L.; Liu, Y.H.; Zhang, H.; Pan, S.L.; Wang, N.; Zhou, Q.; Yu, X.Q. BODIPY-Based Two-Photon Fluorescent Probe for Real-Time Monitoring of Lysosomal Viscosity with Fluorescence Lifetime Imaging Microscopy. Anal. Chem. 2018, 90, 5873–5878. [Google Scholar] [CrossRef]
- Li, X.; Zhao, R.; Wang, Y.; Huang, C. A new GFP fluorophore-based probe for lysosome labelling tracing lysosomal viscosity in live cells. J. Mater. Chem. B 2018, 6, 6592–6598. [Google Scholar] [CrossRef]
- Mu, Y.-L.; Pan, L.; Lu, Q.; Liu, K.-Y.; Zhang, X.; Xing, S. A bifunctional sensitive fluorescence probe based on pyrene for the detection of pH and viscosity in lysosome. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120228. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Shi, Y.; Tao, P.; Fan, X.; Su, X.; Kuang, G.C. BODIPY-based fluorescent thermometer as a lysosome-targetable probe: How the oligo(ethylene glycols) compete photoinduced electron transfer. Chemistry 2015, 21, 3219–3223. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, L.; Jiang, J.; Hou, L.; Fang, G.; Haizhu, Y.; Meng, X. A bright two-photon fluorescent probe for real-time monitoring autophagy in living cells. Chin. Chem. Lett. 2020, 31, 447–450. [Google Scholar] [CrossRef]
- Pal, K.; Kumar, P.; Koner, A.L. Deciphering interior polarity of lysosome in live cancer and normal cells using spectral scanning microscopy. J. Photochem. Photobiol. B 2020, 206, 111848. [Google Scholar] [CrossRef]
- Tang, F.; Gao, C.; Liu, J.-Y.; Lu, Z.-L.; He, L.; Ding, A.-X. Lysosome-targeting BODIPY-derived Hantzsch ester for nitric oxide detection and imaging in live cells. Sens. Actuators B Chem. 2021, 339, 129880. [Google Scholar] [CrossRef]
- Feng, W.; Qiao, Q.-L.; Leng, S.; Miao, L.; Yin, W.-T.; Wang, L.-Q.; Xu, Z.-C. A 1,8-naphthalimide-derived turn-on fluorescent probe for imaging lysosomal nitric oxide in living cells. Chin. Chem. Lett. 2016, 27, 1554–1558. [Google Scholar] [CrossRef]
- Wang, F.; Yu, S.; Xu, Z.; Li, L.; Dang, Y.; Xu, X.; Luo, Y.; Cheng, Z.; Yu, H.; Zhang, W.; et al. Acid-Promoted D-A-D Type Far-Red Fluorescent Probe with High Photostability for Lysosomal Nitric Oxide Imaging. Anal. Chem. 2018, 90, 7953–7962. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, S.; Chai, X.; Li, T.; Wu, Q.; Wang, T. A Lysosome-Compatible Near-Infrared Fluorescent Probe for Targeted Monitoring of Nitric Oxide. Chemistry 2016, 22, 5649–5656. [Google Scholar] [CrossRef]
- Shen, R.; Qian, Y. A turn-on and lysosome-targeted fluorescent NO releaser in water media and its application in living cells and zebrafishes. Spectrochim. Acta Part A 2020, 230, 118024. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Kong, X.; Liu, Z.R.; Xu, A.; Lin, W. Lysosome-Targeted Turn-On Fluorescent Probe for Endogenous Formaldehyde in Living Cells. Anal. Chem. 2016, 88, 9359–9363. [Google Scholar] [CrossRef]
- Wang, X.; Wei, C.; Li, X.; Zheng, X.; Geng, X.; Zhang, P.; Li, X. Lysosome-Targeted Dual-Photon Nitroxyl Fluorescent Probe: Synthesis and Application in Living Cell Imaging. Chin. J. Org. Chem. 2019, 39, 469–474. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, W.; Li, L.; Gong, Q.; Wu, X.; Li, X.; Ma, H. Detection of Misdistribution of Tyrosinase from Melanosomes to Lysosomes Its Upregulation under Psoralen/Ultraviolet A with a Melanosome-Targeting Tyrosinase Fluorescent Probe. Anal. Chem. 2016, 88, 4557–4564. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, W.; Li, L.H.; Gong, Q.Y.; Wu, X.F.; Li, X.H.; Ma, H.M. A Lysosome-Targeting Fluorescence Off-On Probe for Imaging of Nitroreductase and Hypoxia in Live Cells. Chem. Asian J. 2016, 11, 2719–2724. [Google Scholar] [CrossRef]
- Dhara, K.; Lohar, S.; Patra, A.; Roy, P.; Saha, S.K.; Sadhukhan, G.C.; Chattopadhyay, P. A New Lysosome-Targetable Turn-On Fluorogenic Probe for Carbon Monoxide Imaging in Living Cells. Anal. Chem. 2018, 90, 2933–2938. [Google Scholar] [CrossRef]
- Ma, C.; Wu, J.; Sun, W.; Hou, Y.; Zhong, G.; Gao, R.; Shen, B.; Huang, H. A near infrared BODIPY-based lysosome targeting probe for selectively detection of carboxylesterase 1 in living cells pretreated with pesticides. Sens. Actuators B 2020, 325, 128798. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Feng, L.; Yu, J.; Zhang, Y.; Ye, D.; Chen, H.-Y. Lysosome-Targeting Fluorogenic Probe for Cathepsin B Imaging in Living Cells. Anal. Chem. 2016, 88, 12403–12410. [Google Scholar] [CrossRef]
- Ogasawara, H.; Tanaka, Y.; Taki, M.; Yamaguchi, S. Late-stage functionalisation of alkyne-modified phospha-xanthene dyes: Lysosomal imaging using an off-on-off type of pH probe. Chem. Sci. 2021, 12, 7902–7907. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.-Q.; Yu, X.-H.; Zhang, K.; Chen, W.-H. Synthesis and properties of a lysosome-targeting fluorescent ionophore based on coumarins and squaramides. Org. Biomol. Chem. 2018, 16, 8025–8029. [Google Scholar] [CrossRef]
- Hong, X.-Q.; He, X.-Y.; Tam, K.Y.; Chen, W.-H. Synthesis and biological effect of lysosome-targeting fluorescent anion transporters with enhanced anionophoric activity. Bioorg. Med. Chem. Lett. 2020, 30, 127461. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chen, Y.; Li, H.; Wang, B.; Wei, Q.; Tang, H.; Jia, S.; He, Z.; Wang, P.; Zhou, X. Photostable lysosomal imaging of living cell with hyperspectral stimulated Raman scattering microscopy using a probe based on bisarylbutadiyne. Chin. Chem. Lett. 2019, 30, 1393–1396. [Google Scholar] [CrossRef]
- Tian, Y.; Xin, F.; Jing, J.; Zhang, X. Fluorescence imaging of lysosomal hydrogen selenide under oxygen-controlled conditions. J. Mater. Chem. B 2019, 7, 2829–2834. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, X.J.; Sun, R.; Xu, Y.J.; Ge, J.F. A comparative study of lysosome-targetable pH probes based on phenoxazinium attached with aliphatic and aromatic amines. Analyst 2016, 141, 2962–2969. [Google Scholar] [CrossRef]
- Yingjie, L.; Wu, X.; Yao, Q. A New Hemicyanine-based Fluorophore for Monitoring pH and Lysosome Imaging. J. Anal. Chem. 2019, 74, 940–944. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Huang, C.; Jia, N. Dual-Modal Colorimetric/Fluorescence Molecular Probe for Ratiometric Sensing of pH and Its Application. Anal. Chem. 2016, 88, 8332–8338. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; James, T.D.; Jia, N.; Huang, C. A hemicyanine based ratiometric fluorescence probe for mapping lysosomal pH during heat stroke in living cells. Chem. Commun. 2018, 54, 5518–5521. [Google Scholar] [CrossRef]
- Li, X.; Pan, Y.; Chen, H.; Duan, Y.; Zhou, S.; Wu, W.; Wang, S.; Liu, B. Specific near-infrared probe for ultrafast imaging of lysosomal β-galactosidase in ovarian cancer cells. Anal. Chem. 2020, 92, 5772–5779. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-F.; Wu, W.-N.; Zhao, X.-L.; Wang, Y.; Fan, Y.-C.; Zhang, C.-X.; Xu, Z.-H. A water-soluble lysosome-targetable fluorescent probe for carboxylesterase detection and its application in biological imaging. Dye. Pigment. 2022, 199, 110079. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Xiang, Y.; Pan, W.; Li, N.; Tang, B. An efficient strategy for cancer therapy using a tumor- and lysosome-targeted organic photothermal agent. Nanoscale 2021, 13, 8790–8794. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Saha, P.C.; Das, R.S.; Bera, T.; Guha, S. Acidic pH-Activatable Visible to Near-Infrared Switchable Ratiometric Fluorescent Probe for Live-Cell Lysosome Targeted Imaging. ACS Sens. 2021, 6, 2141–2146. [Google Scholar] [CrossRef]
- Kong, X.; Yin, J.; Li, M.; Zhu, L.; Dong, B.; Ma, Y.; Lin, W. Simultaneously imaging of SO2 in lysosomes and mitochondria based on a dual organelle-targeted fluorescent probe. Sens. Actuators B 2019, 292, 80–87. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Huo, F.; Chao, J.; Yin, C. A dual-targeted organelles SO2 specific probe for bioimaging in related diseases and food analysis. Chem. Eng. J. 2022, 433, 133750. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.-X.; Xu, Y.; Liu, S.H.; Zeng, L.; Chen, H.; Yin, J. The visualization of lysosomal and mitochondrial glutathione via near-infrared fluorophore and in vivo imaging application. Sens. Actuators B 2019, 290, 676–683. [Google Scholar] [CrossRef]
- Xia, S.; Wang, J.; Zhang, Y.; Whisman, N.; Bi, J.; Steenwinkel, T.E.; Wan, S.; Medford, J.; Tajiri, M.; Luck, R.L.; et al. Ratiometric fluorescent probes based on through-bond energy transfer of cyanine donors to near-infrared hemicyanine acceptors for mitochondrial pH detection and monitoring of mitophagy. J. Mater. Chem. B 2020, 8, 1603–1615. [Google Scholar] [CrossRef]
- Munan, S.; Ali, M.; Yadav, R.; Mapa, K.; Samanta, A. PET- and ICT-Based Ratiometric Probe: An Unusual Phenomenon of Morpholine-Conjugated Fluorophore for Mitochondrial pH Mapping during Mitophagy. Anal. Chem. 2022, 94, 11633–11642. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, X.; Han, Y.; Li, X.; Li, J. Application and Suggestions of Morpholine Ring as a Lysosome Targeting Group. Chemistry 2025, 7, 82. https://doi.org/10.3390/chemistry7030082
Liu X, Zhang X, Han Y, Li X, Li J. Application and Suggestions of Morpholine Ring as a Lysosome Targeting Group. Chemistry. 2025; 7(3):82. https://doi.org/10.3390/chemistry7030082
Chicago/Turabian StyleLiu, Xuelian, Ximeng Zhang, Yinghong Han, Xingrui Li, and Jinyao Li. 2025. "Application and Suggestions of Morpholine Ring as a Lysosome Targeting Group" Chemistry 7, no. 3: 82. https://doi.org/10.3390/chemistry7030082
APA StyleLiu, X., Zhang, X., Han, Y., Li, X., & Li, J. (2025). Application and Suggestions of Morpholine Ring as a Lysosome Targeting Group. Chemistry, 7(3), 82. https://doi.org/10.3390/chemistry7030082






