A Review for the Design and Optimization of Catalysts: The Use of Statistics as a Powerful Tool for Literature Analysis
Abstract
1. Introduction
2. Materials and Methods
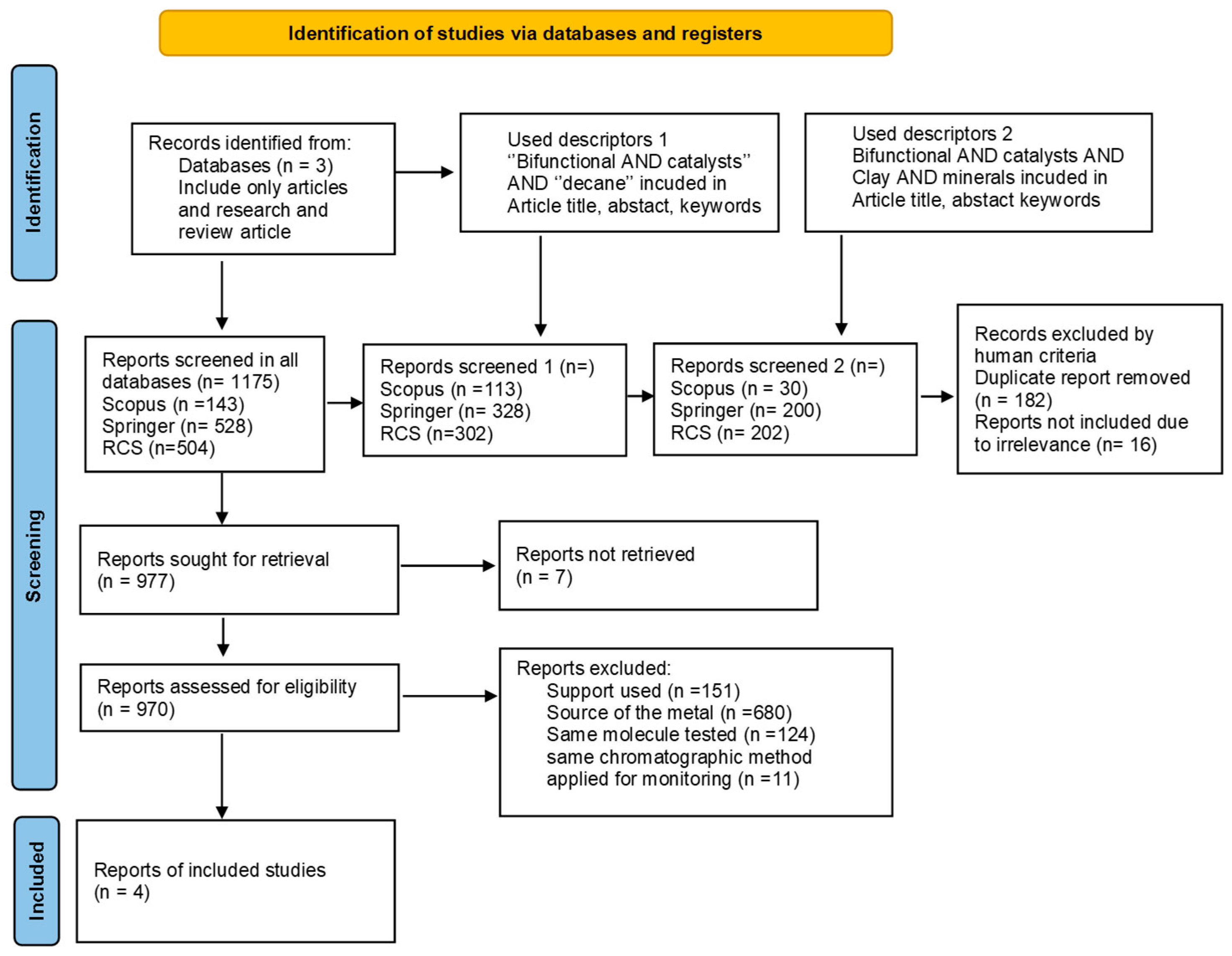
2.1. Systematic Review
2.2. Experimental Design
3. Results and Discussion
3.1. Bifunctional Catalysts with Bimetallic Systems
3.1.1. Textural Properties in Bimetallic Systems
3.1.2. Catalytic Activity of Bimetallic Systems
3.1.3. Optimization of Bimetallic Systems
3.2. Heteropolyacids (HPAs)
3.2.1. Textural Properties with the Incorporation of HPAs
3.2.2. Catalytic Performance with the Incorporation of HPAs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Makondo, C.C. Green growth, sustainability, and decoupling carbon emissions from industrial activities in emerging economies: A focus on Zambia’s energy sector. Extr. Ind. Soc. 2023, 14, 101271. [Google Scholar] [CrossRef]
- Nnene, O.A.; Senshaw, D.; Zuidgeest, M.H.; Hamza, T.; Grafakos, S.; Oberholzer, B. Baseline scenario modelling for low emissions development in Ethiopia’s energy sector. Energy Strat. Rev. 2023, 49, 101166. [Google Scholar] [CrossRef]
- Brutschin, E.; Fleig, A. Innovation in the energy sector—The role of fossil fuels and developing economies. Energy Policy 2016, 97, 27–38. [Google Scholar] [CrossRef]
- Sekhar, S.J.; Samuel, M.S.; Glivin, G.; Le, T.; Mathimani, T. Production and utilization of green ammonia for decarbonizing the energy sector with a discrete focus on Sustainable Development Goals and environmental impact and technical hurdles. Fuel 2023, 360, 130626. [Google Scholar] [CrossRef]
- Khan, S.; Qureshi, K.M.; Lup, A.N.K.; Patah, M.F.A.; Daud, W.M.A.W. Role of Ni–Fe/ZSM-5/SAPO-11 bifunctional catalyst on hydrodeoxygenation of palm oil and triolein for alternative jet fuel production. Biomass-Bioenergy 2022, 164, 106563. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, Z.; Yu, J.; Mei, J.; Peng, S.; Wu, Z.; Guo, R.; Fang, X.; Zhang, X. Supported CoW bifunctional catalyst with high activity and selectivity for hydrocracking alkane. Chem. Eng. Sci. 2023, 282, 119292. [Google Scholar] [CrossRef]
- Wu, X.; Lei, X.; Wang, S.; Zhang, H.; Cui, Q.; Bao, X.; Wang, T.; Yuan, P. Tuning the acidity and pore structures of NiP based bifunctional catalyst for efficient n-hexane isomerization. Fuel 2022, 324, 124396. [Google Scholar] [CrossRef]
- Zecevic, J.; Vanbutsele, G.; de Jong, K.P.; Martens, J.A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 2015, 528, 245–248. [Google Scholar] [CrossRef]
- Verheyen, E.; Jo, C.; Kurttepeli, M.; Vanbutsele, G.; Gobechiya, E.; Korányi, T.I.; Bals, S.; Van Tendeloo, G.; Ryoo, R.; Kirschhock, C.E.; et al. Molecular shape-selectivity of MFI zeolite nanosheets in n-decane isomerization and hydrocracking. J. Catal. 2013, 300, 70–80. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, W.; Li, Y.; Li, F.; Liu, J.; Fan, L.; Fu, J.; Liu, X.; Lyu, Y. Quantitative synergy between metal and acid centers over the Ni/Beta bifunctional catalyst for methyl laurate hydrodeoxygenation to bio-jet fuel. Fuel Process. Technol. 2023, 241, 107602. [Google Scholar] [CrossRef]
- Claude, M.C.; Vanbutsele, G.; Martens, J.A. Dimethyl Branching of Long n-Alkanes in the Range from Decane to Tetracosane on Pt/H–ZSM-22 Bifunctional Catalyst. J. Catal. 2001, 203, 213–231. [Google Scholar] [CrossRef]
- Kasian, N.; Vanbutsele, G.; Houthoofd, K.; Koranyi, T.I.; Martens, J.A.; Kirschhock, C.E.A. Catalytic activity and extra-large pores of germanosilicate UTL zeolite demonstrated with decane test reaction. Catal. Sci. Technol. 2011, 1, 246–254. [Google Scholar] [CrossRef]
- Pinto, T.; Arquillière, P.; Dufaud, V.; Lefebvre, F. Isomerization of n-hexane over Pt-H3PW12O40/SBA-15 bifunctional catalysts: Effect of the preparation method on catalytic performance. Appl. Catal. A Gen. 2016, 528, 44–51. [Google Scholar] [CrossRef]
- Kouzu, M.; Kuwako, T.; Ohto, Y.; Suzuki, K.; Kojima, M. Single stage upgrading with the help of bifunctional catalysis of Pt supported on solid acid for converting product oil of triglyceride thermal cracking into drop-in fuel. Fuel Process. Technol. 2020, 202, 106364. [Google Scholar] [CrossRef]
- Vedachalam, S.; Boahene, P.; Dalai, A.K. Production of jet fuel by hydrorefining of Fischer-Tropsch wax over Pt/Al-TUD-1 bifunctional catalyst. Fuel 2021, 300, 121008. [Google Scholar] [CrossRef]
- Gagea, B.; Lorgouilloux, Y.; Altintas, Y.; Jacobs, P.; Martens, J. Bifunctional conversion of n-decane over HPW heteropoly acid incorporated into SBA-15 during synthesis. J. Catal. 2009, 265, 99–108. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, P.; Wang, X.; Wu, T.; Ge, S.; Xie, H.; Liu, B.; Chen, S.; Wu, Z. Selective hydrocracking of 1-methylnaphthalene to benzene/toluene/xylenes (BTX) over NiW/Beta bifunctional catalyst: Effects of metal–acid balance. Fuel 2024, 363, 130947. [Google Scholar] [CrossRef]
- Qiu, S.; Huang, Z.; Sang, X.; Liu, Y.; Zhang, Q.; Chen, Q.; Meng, Q.; Wang, T. Regulating isomerization of gasoline-alkanes in aqueous-phase hydrodeoxygenation of sorbitol using regenerable Ni@MoOx catalysts. Fuel Process. Technol. 2023, 242, 107647. [Google Scholar] [CrossRef]
- Ullah, L.; Zhao, G.; Ma, J.-X.; Usman, M.; Khan, R.; Hedin, N. Pd-promoted heteropolyacid on mesoporous zirconia as a stable and bifunctional catalyst for oxidation of thiophenes. Fuel 2022, 310, 122462. [Google Scholar] [CrossRef]
- Wu, Q.; Yuan, J.; Guo, C.; Liu, Z.; Li, X.; Liu, Z.; Wang, W.; Wu, W. The hydroisomerization of n-hexadecane over Pd/SAPOs bifunctional catalysts with different opening size: Features of the diffusion properties in pore channels and the metal-acid synergistic catalysis. Fuel Process. Technol. 2023, 244, 107692. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, X.; Zheng, D.; Zhao, Q.; Feng, J.; Feng, Y.; Ge, X.; Chen, X. Constructing highly efficient bifunctional catalysts for oxygen reduction and oxygen evolution by modifying MXene with transition metal. J. Colloid Interface Sci. 2024, 660, 628–636. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Gao, G.; Zhao, Z.; Sun, P.; Li, F. Enhanced stability of hierarchical zeolite-metal bifunctional catalysts for conversion of levulinic acid to valeric biofuels. Chem. Eng. J. 2024, 483, 149405. [Google Scholar] [CrossRef]
- Wen, C.; Lu, L.; Zhang, X.; Jin, K.; Chen, L.; Zhang, Q.; Ma, L.; Wang, C. Optimizing zeolitic hierarchical pore structure to boost the direct conversion of aromatics from syngas over the iron-based/zeolite bifunctional catalysts. Fuel 2023, 357, 129791. [Google Scholar] [CrossRef]
- De Waele, B.; Franken, J.; Huybreehts, W.; Vanbutsele, G.; Houthoofd, K.J.; Bertinchamps, F.; Denayer, J.F.; Thybaut, J.W.; Gaigneanx, E.M.; Marin, G.B.; et al. Synthesis of MTT zeolite catalysts with surface Al depletion. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., De Vos, D.E., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 162, pp. 873–880. [Google Scholar] [CrossRef]
- Echevskii, G.V.; Weixin, Q.; Kodenev, E.G.; Toktarev, A.V.; Chunmei, D. Study of Bifunctional Hydrocracking and Hydroisodeparaffinization Catalysts Based on Zeolites and Alumophosphates, Part 2: Effect of the Activity of Hydrogenating and Acidic Components on the Activity and Selectivity of Hydroisodeparaffinization Catalysts. Catal. Ind. 2020, 12, 81–87. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, P.; Wang, J.; Gao, H.; Guo, J.; Wang, J.; Wang, Y.; Tian, Y.; Qiao, Y. Catalytic dehydrogenation cracking of crude oil to light olefins by structure and basicity/acidity adjustment of bifunctional metal/acid catalysts. Fuel 2023, 334, 126808. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, X.; Zhang, Y.; Yuan, B.; Yu, F.; Xie, C.; Yu, S. One-pot conversion of α-pinene into biomass high energy density fuel over a bifunctional zeolite catalyst. Biomass-Bioenergy 2023, 174, 106859. [Google Scholar] [CrossRef]
- Castillo, L.J.R.; Barrera, J.I.B.; Morales-Ortuño, J.C.; Klimova, E.I.; Lozada, V.J.d.I.; Lee, I.P.; Klimova, T.E. Hybrid alumina-1D titania nanotube materials as supports for NiMo catalysts with improved activity in hydrodesulfurization. Chem. Phys. Lett. 2023, 813, 140300. [Google Scholar] [CrossRef]
- Sun, J.; Xiong, S.; Lin, H.; Wu, Q.; Xiao, L.; Liu, Z.; Li, X.; Wang, W.; Wu, W. The effects of growth modifiers on the catalytic performance of hierarchical Pd/ZSM-23 bifunctional catalysts for n-hexadecane hydroisomerization. Fuel 2023, 347, 128406. [Google Scholar] [CrossRef]
- Zou, Q.; He, H.; Xie, J.; Han, S.; Lin, W.; Mondal, A.K.; Huang, F. Study on the mechanism of acid modified H-Beta zeolite acidic sites on the catalytic pyrolysis of Kraft lignin. Chem. Eng. J. 2023, 462, 142029. [Google Scholar] [CrossRef]
- Amaya, J.; Bobadilla, L.; Azancot, L.; Centeno, M.; Moreno, S.; Molina, R. Modulation of the acidity of a vermiculite and its potential use as a catalytic support. J. Mater. Sci. 2020, 55, 6482–6501. [Google Scholar] [CrossRef]
- Amaya, J.; Suarez, N.; Moreno, A.; Moreno, S.; Molina, R. Mo or W catalysts promoted with Ni or Co supported on modified bentonite for decane hydroconversion. N. J. Chem. 2020, 44, 2966–2979. [Google Scholar] [CrossRef]
- Torres-Luna, J.A.; Moreno, S.; Molina, R.; Carriazo, J.G. Hydroconversion of n-Decane over Ni–Mo Supported on Modified Halloysite Catalysts. Energy Fuels 2018, 32, 9782–9792. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Structured clay minerals-based nanomaterials for sustainable photo/thermal carbon dioxide conversion to cleaner fuels: A critical review. Sci. Total. Environ. 2022, 845, 157206. [Google Scholar] [CrossRef]
- Kurniawan, A.; Yin, S.T.; Li, D.J.; Li, K.J.; Chen, X.L.; Huang, W.J.; Zhao, P.Y.; Liu, J.H.; Zhou, C.H. Elucidating Metal Composition–Coking Relationships and Coke Formation Pathways of the Gas Phase Oxydehydration of Glycerol over Clay Mineral-Supported Mo-V-O Catalysts. Appl. Catal. B Environ. 2024, 347, 123766. [Google Scholar] [CrossRef]
- Taranu, B.O.; Vlazan, P.; Svera, P.; Poienar, M.; Sfirloaga, P. New functional hybrid materials based on clay minerals for enhanced electrocatalytic activity. J. Alloy. Compd. 2022, 892, 162239. [Google Scholar] [CrossRef]
- Camargo, M.d.O.; Pimenta, J.L.C.W.; Arroyo, P.A. Green diesel production by solvent-free deoxygenation of oleic acid over nickel phosphide bifunctional catalysts: Effect of the support. Fuel 2020, 281, 118719. [Google Scholar] [CrossRef]
- Dehghani, S.; Haghighi, M. Sono-dispersion of MgO over Al-Ce-doped MCM-41 bifunctional nanocatalyst for one-step biodiesel production from acidic oil: Influence of ultrasound irradiation and Si/Ce molar ratio. Ultrason. Sonochem. 2019, 54, 142–152. [Google Scholar] [CrossRef]
- Li, T.; Tao, Z.; Zhang, L.; Yang, Y. Facile and cost-effective synthesis of acidity-enhanced amorphous silica-alumina for high-performance isomerization. J. Solid State Chem. 2021, 300, 122249. [Google Scholar] [CrossRef]
- Housecroft, C.; Sharpe, A. Inorganic Chemistry, 2nd ed.; Pearson Education Limited: Harlow, UK, 2005; Available online: https://books.google.com.co/books?id=_1gFM51qpAMC (accessed on 19 January 2025).
- Zhu, S.; Gao, X.; Zhu, Y.; Cui, J.; Zheng, H.; Li, Y. SiO2 promoted Pt/WOx/ZrO2 catalysts for the selective hydrogenolysis of glycerol to 1,3-propanediol. Appl. Catal. B Environ. 2014, 158–159, 391–399. [Google Scholar] [CrossRef]
- Amaya, J.; Suarez, N.; Moreno, A.; Moreno, S.; Molina, R. Bifunctional catalysts supported on modified vermiculite for the hydroconversion of decane. Effect of the metal phase (Mo or W) and promoters (Ni or Co). Catal. Today 2020, 356, 271–283. [Google Scholar] [CrossRef]
- Hao, J.; Xiao-Dan, S.; Shu, D.; Yi-Zhi, W.; Su-Hua, S.; Jie, L.; Hui-Hong, Z.; Guang, Y.; Xiao-Dong, Y.; Wei-Ping, F. Influence of Cs Substitution on the Structural Properties and Catalytic Performance of Ni-H3PW12O40/SiO2 Catalysts. Acta Phys. Chim. Sin. 2014, 30, 527–534. [Google Scholar] [CrossRef]
- Rabl, S.; Haas, A.; Santi, D.; Flego, C.; Ferrari, M.; Calemma, V.; Weitkamp, J. Ring opening of cis-decalin on bifunctional Ir/- and Pt/La-X zeolite catalysts. Appl. Catal. A Gen. 2011, 400, 131–141. [Google Scholar] [CrossRef]
- Roussel, M.; Norsic, S.; Lemberton, J.-L.; Guisnet, M.; Cseri, T.; Benazzi, E. Hydrocracking of n-decane on a bifunctional sulfided NiW/silica–alumina catalyst: Effect of the operating conditions. Appl. Catal. A Gen. 2005, 279, 53–58. [Google Scholar] [CrossRef]
- Collins, L.M.; Dziak, J.J.; Li, R. Design of Experiments with Multiple Independent Variables: A Resource Management Perspective on Complete and Reduced Factorial Designs. Psychol. Methods 2009, 14, 202–224. [Google Scholar] [CrossRef]
- Amaya, J.; Moreno, S.; Molina, R. Heteropolyacids supported on clay minerals as bifunctional catalysts for the hydroconversion of decane. Appl. Catal. B Environ. 2021, 297, 120464. [Google Scholar] [CrossRef]
- Martinez, T.; Amaya, J. Diseño y Optimización de Catalizadores Bifuncionales: Uso de la Estadística como una Herramienta para el análisis de la Literature, Universidad Antonio Nariño, Bogotá D.C, 2024. Available online: https://repositorio.uan.edu.co/handle/123456789/10301 (accessed on 19 January 2025).
- Valderrama, R.; Ballesteros, L.; Baldovino, V. Presented at the MEMORIAS DEL XIII SIMPOSIO COLOMBIANO DE CATÁLISIS, Bucaramanga, Colombia, 9–13 October 2023. Available online: http://cicat.uis.edu.co/siccat/ (accessed on 19 January 2025).
- Molina, R.; Moreno, S.; Vieiracoelho, A.; Martens, J.; Jacobs, P.; Poncelet, G. Hydroisomerization-Hydrocracking of Decane over Al- and Ga-Pillared Clays. J. Catal. 1994, 148, 304–314. [Google Scholar] [CrossRef]
- Aerts, A.; van Isacker, A.; Huybrechts, W.; Kremer, S.P.; Kirschhock, C.E.; Collignon, F.; Houthoofd, K.; Denayer, J.F.; Baron, G.V.; Marin, G.B.; et al. Decane hydroconversion on bifunctional Zeogrid and nano-zeolite assembled from aluminosilicate nanoslabs of MFI framework type. Appl. Catal. A Gen. 2004, 257, 7–17. [Google Scholar] [CrossRef]
- Roussel, M.; Lemberton, J.-L.; Guisnet, M.; Cseri, T.; Benazzi, E. Mechanisms of n-decane hydrocracking on a sulfided NiW on silica-alumina catalyst. J. Catal. 2003, 218, 427–437. [Google Scholar] [CrossRef]
- Qiu, B.; Yi, X.; Lin, L.; Fang, W.; Wan, H. The hydrocracking of n-decane over bifunctional Ni-H3PW12O40/SiO2 catalysts. Catal. Today 2008, 131, 464–471. [Google Scholar] [CrossRef]
- Bohre, A.; Modak, A.; Chourasia, V.; Jadhao, P.R.; Sharma, K.; Pant, K.K. Recent advances in supported ionic liquid catalysts for sustainable biomass valorisation to high-value chemicals and fuels. Chem. Eng. J. 2022, 450, 138032. [Google Scholar] [CrossRef]
- Abdullah, R.F.; Rashid, U.; Ibrahim, M.L.; NolHakim, M.A.H.L.; Moser, B.R.; Alharthi, F.A. Bifunctional biomass-based catalyst for biodiesel production via hydrothermal carbonization (HTC) pretreatment—Synthesis, characterization and optimization. Process. Saf. Environ. Prot. 2021, 156, 219–230. [Google Scholar] [CrossRef]
- Shao, S.; Hu, X.; Dong, W.; Li, X.; Zhang, H.; Xiao, R.; Cai, Y. Integrated C–C coupling/hydrogenation of ketones derived from biomass pyrolysis for aviation fuel over Ni/Mg–Al–O/AC bifunctional catalysts. J. Clean. Prod. 2021, 282, 124331. [Google Scholar] [CrossRef]
- Li, P.; Wen, B.; Yu, F.; Zhu, M.; Guo, X.; Han, Y.; Kang, L.; Huang, X.; Dan, J.; Ouyang, F.; et al. High efficient nickel/vermiculite catalyst prepared via microwave irradiation-assisted synthesis for carbon monoxide methanation. Fuel 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Marosz, M.; Kowalczyk, A.; Chmielarz, L. Modified vermiculites as effective catalysts for dehydration of methanol and ethanol. Catal. Today 2020, 355, 466–475. [Google Scholar] [CrossRef]
- Motak, M.; Kuterasiński, Ł.; Da Costa, P.; Samojeden, B. Catalytic activity of layered aluminosilicates for VOC oxidation in the presence of NOx. Comptes Rendus Chim. 2015, 18, 1106–1113. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Liu, S.; Zhao, N.; Jiang, X.; Su, M.; Li, Z. Enhanced gasoline selectivity through Fischer-Tropsch synthesis on a bifunctional catalyst: Effects of active sites proximity and reaction temperature. Chem. Eng. J. 2021, 416, 129180. [Google Scholar] [CrossRef]
- Gómez-Cortés, A.; González-Vigi, F.; Arenas-Alatorre, J.; Díaz, G. Propiedades Texturales, Redox y Superficiales De Catalizadores CuO/SiO2, CuO/CeO2-SiO2 y CuO/CeO2. Rev. Mex. Ing. Quim. 2006, 5, 253–261. Available online: http://www.redalyc.org/articulo.oa?id=62050311 (accessed on 19 January 2025).
- Ávalos, V.; Vladimir, A. Diseño, Síntesis y Evaluación de Catalizadores de CeO2/Grafeno Modificados con ZrO2 y/o NiO para la Reacción de Water Gas Shift; Instituto Potosino de Investigación Científica y Tecnológica: San Luis Potosí, México, 2020. [Google Scholar]
- Wu, H.; Zheng, J.; Wang, G. Catalytic liquefaction of switchgrass in isobutanol/water system for bio-oil development over bifunctional Ni-HPMo/Fe3O4@Al-MCM-41 catalysts. Renew. Energy 2019, 141, 96–106. [Google Scholar] [CrossRef]
- Naeem, M.M.; Al-Sakkari, E.G.; Boffito, D.C.; Rene, E.R.; Gadalla, M.A.; Ashour, F.H. Single-stage waste oil conversion into biodiesel via sonication over bio-based bifunctional catalyst: Optimization, preliminary techno-economic and environmental analysis. Fuel 2023, 341, 127587. [Google Scholar] [CrossRef]
- Oh, S.; Gu, S.; Choi, J.-W.; Suh, D.J.; Lee, H.; Kim, C.S.; Kim, K.H.; Yoo, C.-J.; Choi, J.; Ha, J.-M. Metal/acid bifunctional catalysts for the reductive catalytic fractionation of lignocellulose into phenols and holocellulose. J. Environ. Chem. Eng. 2022, 10, 108085. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Pan, H.; Dong, Y.; Li, X.; Tao, S. Construction of hierarchical core-shell Pt/USY@MSA bifunctional catalysts for hydroisomerization of n-hexadecane. Appl. Catal. A Gen. 2023, 653, 119011. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, G.; Zhang, Y.; Liu, Z.; Li, X.; Wang, W.; Wu, W. Bifunctional catalysts based on hierarchical SAPO-41 nanosheet for Highly-efficient hydroisomerization of n-Hexadecane. Fuel 2023, 352, 129066. [Google Scholar] [CrossRef]
- Liu, J.; Wei, J.; Feng, X.; Song, M.; Shi, S.; Liu, S.; Liu, G. Ni/HZSM-5 catalysts for hydrodeoxygenation of polycarbonate plastic wastes into cycloalkanes for sustainable aviation fuels. Appl. Catal. B Environ. 2023, 338, 123050. [Google Scholar] [CrossRef]
- Tomasek, S.; Lónyi, F.; Valyon, J.; Hancsók, J. Fuel purpose hydrocracking of biomass based Fischer-Tropsch paraffin mixtures on bifunctional catalysts. Energy Convers. Manag. 2020, 213, 112775. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, R.; Wu, W.; Zhang, J.; Cao, Y.; Huang, K.; Jiang, L. Enhancing the Activity of MoS2/SiO2-Al2O3 Bifunctional Catalysts for Suspended-Bed Hydrocracking of Heavy Oils by Doping with Zr Atoms. Chin. J. Chem. Eng. 2021, 39, 126–134. [Google Scholar] [CrossRef]
- Amaya, J.; Bobadilla, L.; Azancot, L.; Centeno, M.; Moreno, S.; Molina, R. Potentialization of bentonite properties as support in acid catalysts. Mater. Res. Bull. 2020, 123, 110728. [Google Scholar] [CrossRef]
- Arias, S.; Vascocelos, D.P.; Libório, D.d.O.; Gonzalez, J.F.; Câmara, A.G.; Barbosa, C.M.; Fréty, R.; Pacheco, J.G.A. Hydrogen-free deoxygenation of industrial vegetable oil waste using Ce, Zr-NiAl catalysts for second-generation biofuels production. Mol. Catal. 2022, 529, 112554. [Google Scholar] [CrossRef]
- Chen, M.; Shi, J.; Wang, Y.; Tang, Z.; Yang, Z.; Wang, J.; Zhang, H. Conversion of Kraft lignin to phenol monomers and liquid fuel over trimetallic catalyst W-Ni-Mo/sepiolite under supercritical ethanol. Fuel 2021, 303, 121332. [Google Scholar] [CrossRef]
- Chowdari, R.K.; Agarwal, S.; Heeres, H.J. Hydrotreatment of Kraft Lignin to Alkylphenolics and Aromatics Using Ni, Mo, and W Phosphides Supported on Activated Carbon. ACS Sustain. Chem. Eng. 2018, 7, 2044–2055. [Google Scholar] [CrossRef]
- Costa, J.M.; Clark, M.P.; Neto, A.F.d.A.; Ivey, D.G. In-situ transformation of electrodeposited W–Co oxide to ZnCo2O4 nanoparticles as an effective bifunctional catalysts in Zn-air batteries. Int. J. Hydrogen Energy 2020, 45, 16122–16132. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Alvarez-Rodríguez, J.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Support effects on Ru–HPA bifunctional catalysts: Surface characterization and catalytic performance. Appl. Catal. A Gen. 2007, 333, 281–289. [Google Scholar] [CrossRef]
- Abolghasemi, M.M.; Hassani, S.; Rafiee, E.; Yousefi, V. Nanoscale-supported heteropoly acid as a new fiber coating for solid-phase microextraction coupled with gas chromatography–mass spectrometry. J. Chromatogr. A 2015, 1381, 48–53. [Google Scholar] [CrossRef]
- Ko, S.; Tang, X.; Gao, F.; Yi, H.; Liu, H.; Luo, N. Remarkable N2-selectivity enhancement of NH3-SCR over HPMo modified MnCo-BTC@SiO2 catalyst. J. Environ. Sci. 2024, 138, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Almohalla, M.; Rodríguez-Ramos, I.; Ribeiro, L.S.; Órfão, J.J.; Pereira, M.F.R.; Guerrero-Ruiz, A. Cooperative action of heteropolyacids and carbon supported Ru catalysts for the conversion of cellulose. Catal. Today 2018, 301, 65–71. [Google Scholar] [CrossRef]
- Bryzhin, A.; Gantman, M.; Buryak, A.; Tarkhanova, I. Brønsted acidic SILP-based catalysts with H3PMo12O40 or H3PW12O40 in the oxidative desulfurization of fuels. Appl. Catal. B 2019, 257, 117938. [Google Scholar] [CrossRef]
- Zhu, S.; Qiu, Y.; Zhu, Y.; Hao, S.; Zheng, H.; Li, Y. Hydrogenolysis of glycerol to 1,3-propanediol over bifunctional catalysts containing Pt and heteropolyacids. Catal. Today 2013, 212, 120–126. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, S.; Jin, Q.; Zhao, J. Review on oxidative desulfurization of fuel by supported heteropolyacid catalysts. J. Ind. Eng. Chem. 2020, 82, 1–16. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Liu, J.; Rong, L. Ni/phosphomolybdic acid immobilized on carbon nanotubes for catalytic cracking of Jatropha oil. Chem. Phys. Lett. 2019, 720, 42–51. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.-Y.; Hu, S.; Li, L.; Lim, H.M.; Yang, Y. Mesostructured TUD-1 supported molybdophosphoric acid (HPMo/TUD-1) catalysts for n-heptane hydroisomerization. Catal. Today 2009, 147, S51–S57. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Mu, C.; Wei, J.; Zhao, Y.; Ma, X.; Wang, S. Supported heteropolyacids catalysts for the selective hydrocracking and isomerization of n-C16 to produce jet fuel. Appl. Catal. A Gen. 2020, 598, 117556. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Muráth, S.; Varga, T.; Kukovecz, Á.; Kónya, Z.; Sipos, P.; Pálinkó, I.; Varga, G. Morphological aspects determine the catalytic activity of porous hydrocalumites: The role of the sacrificial templates. Mater. Today Chem. 2022, 23, 100682. [Google Scholar] [CrossRef]
- Kannan, S.; Arumugam, P.; Govindasamy, G. SBA-15 and carbon supported nickel, heteropoly acid catalysts for the hydrodeoxygenation of lignin derived trans-anethole to sustainable aviation fuel. J. Porous Mater. 2023, 30, 639–653. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, J.; Zhu, Y.; Guo, H.; Yang, W. Jet fuel range hydrocarbons production through competitive pathways of hydrocracking and isomerization over HPW-Ni/MCM-41 catalyst. Fuel 2020, 269, 117465. [Google Scholar] [CrossRef]
- Winoto, H.P.; Fikri, Z.A.; Ha, J.-M.; Park, Y.-K.; Lee, H.; Suh, D.J.; Jae, J. Heteropolyacid supported on Zr-Beta zeolite as an active catalyst for one-pot transformation of furfural to γ-valerolactone. Appl. Catal. B 2019, 241, 588–597. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Song, J.; Wang, Q.; Shi, L.; Wang, X.; Huo, M. Synthesis of heteropolyacid (HPA) functionalized graphitic carbon nitride as effective catalysts for converting polysaccharides into high-value chemicals. Resour. Conserv. Recycl. 2022, 185, 106473. [Google Scholar] [CrossRef]
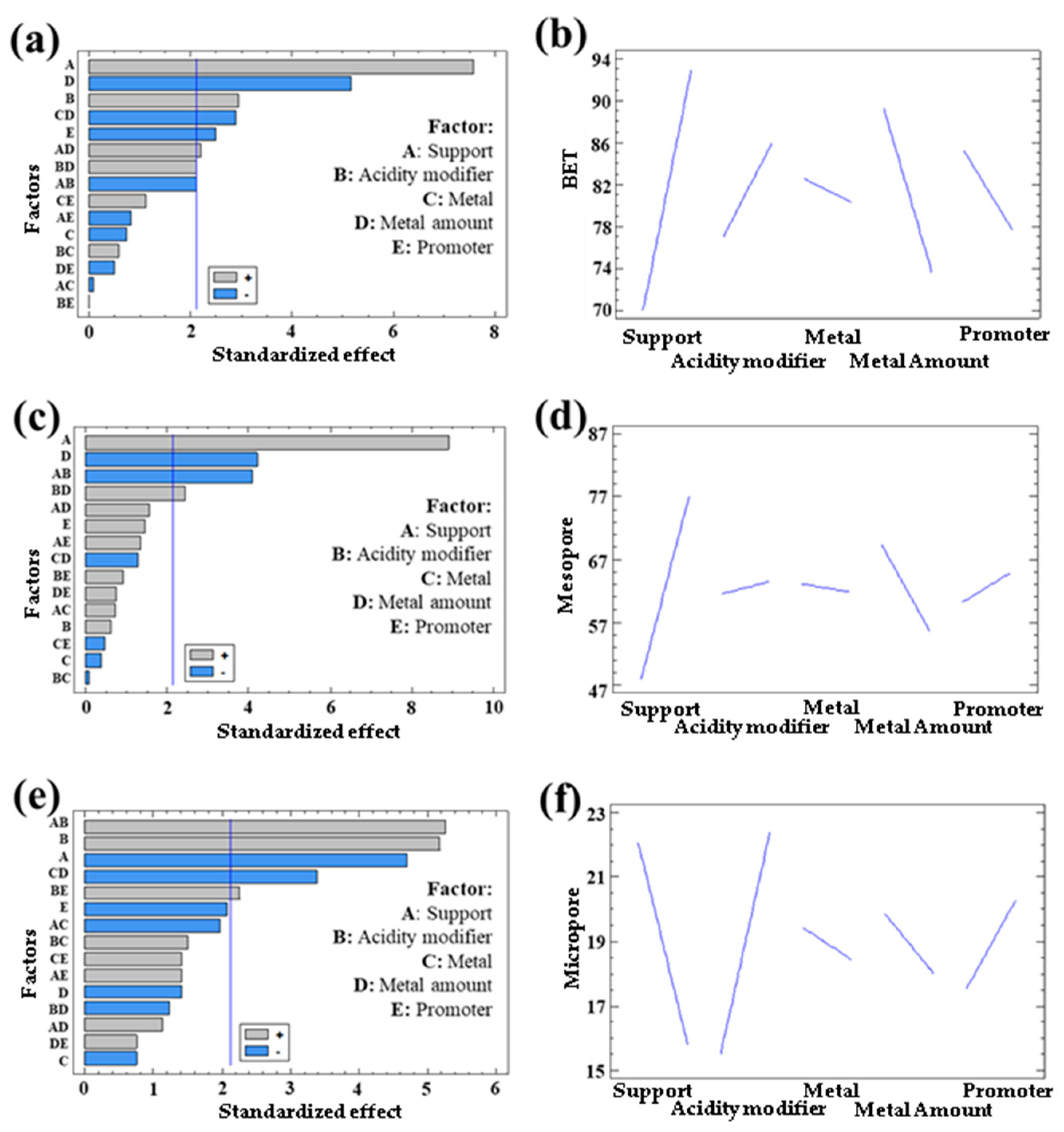
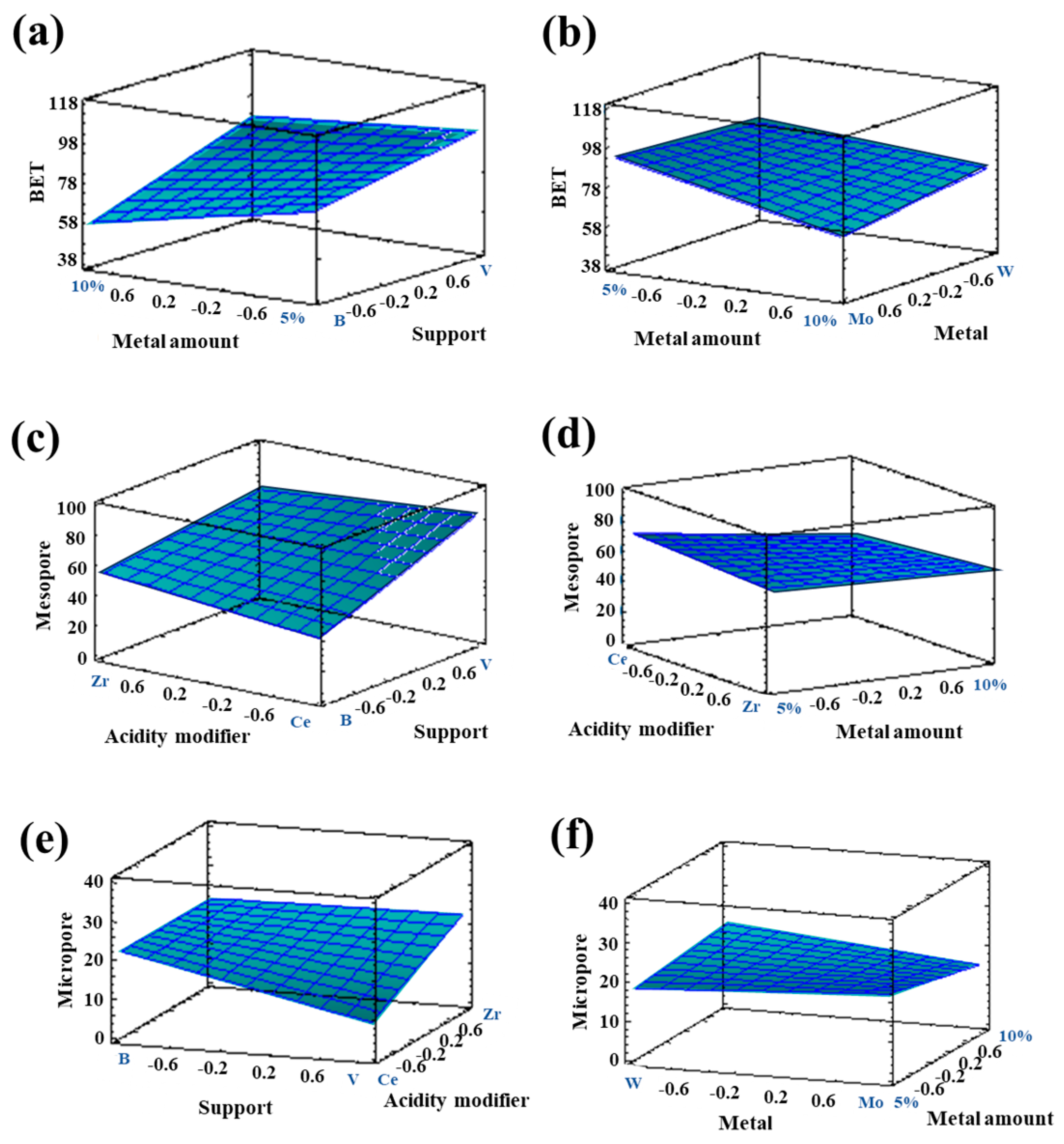
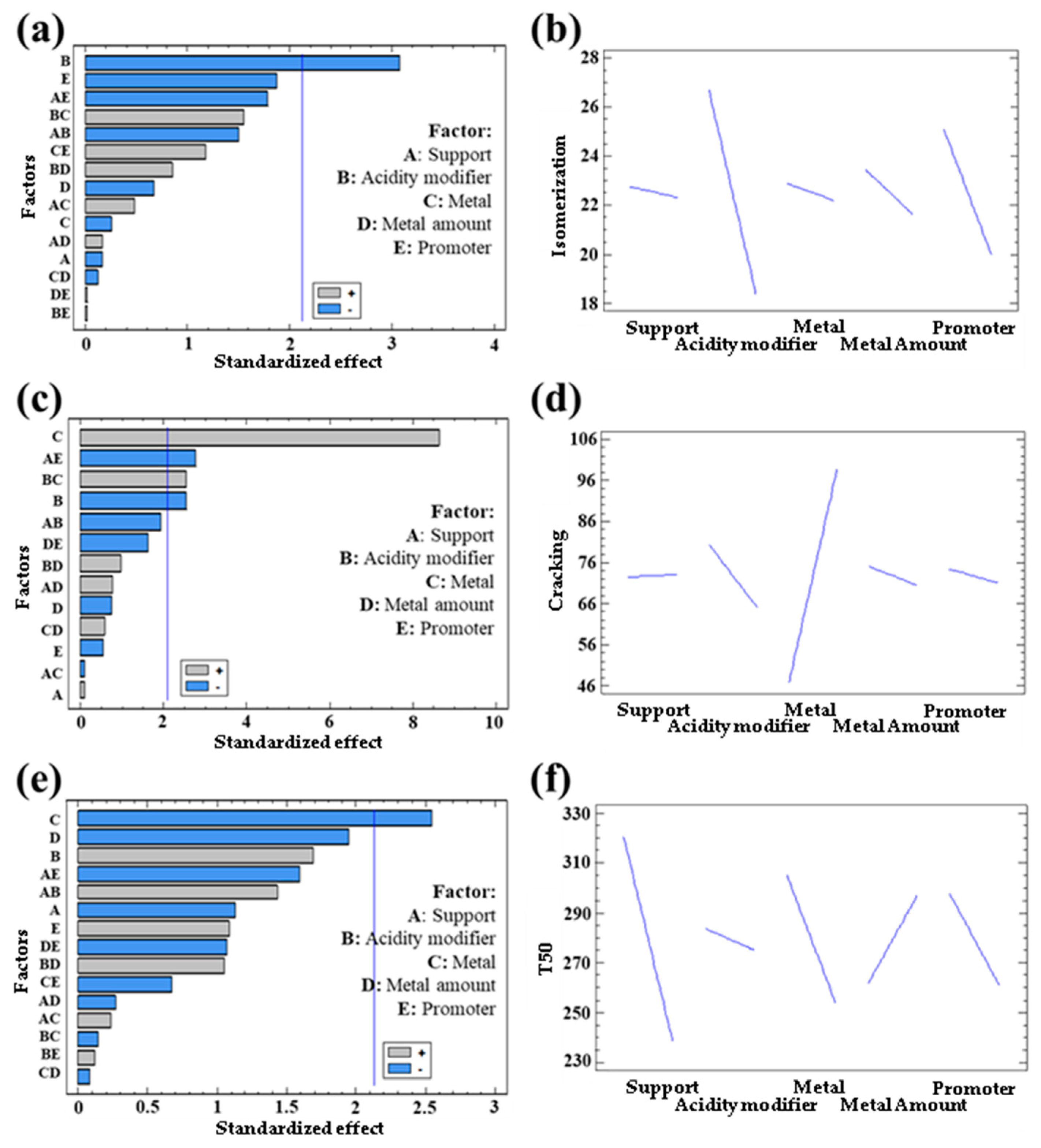
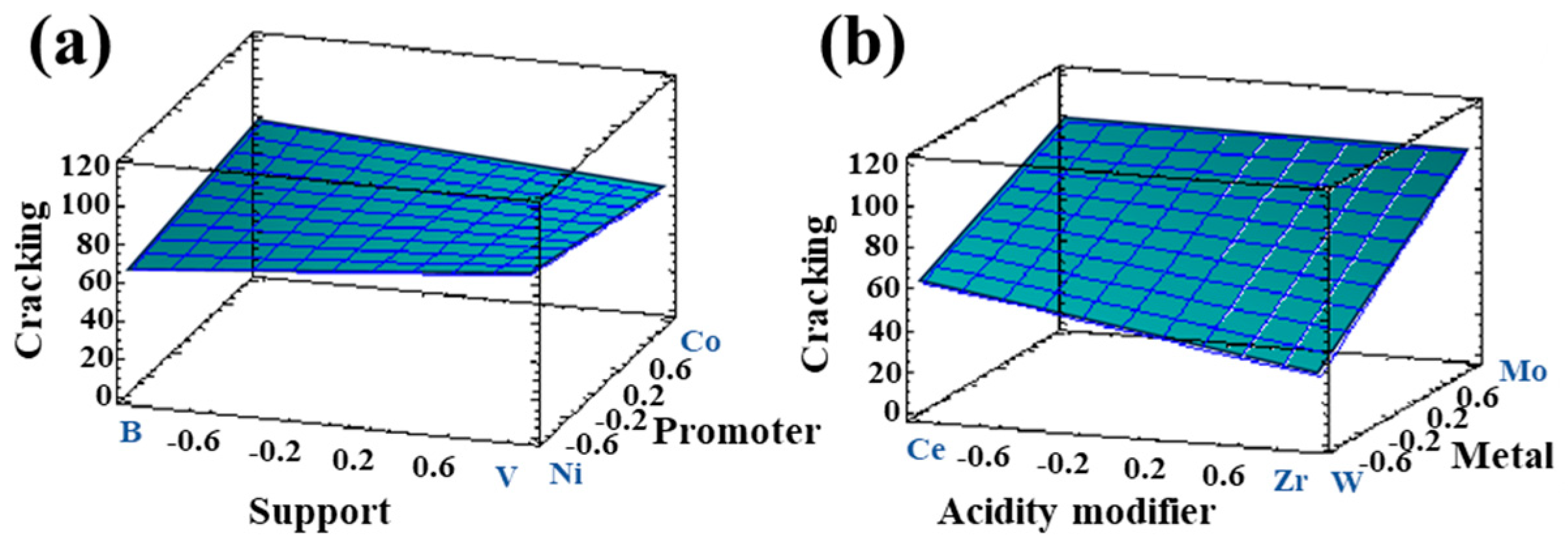
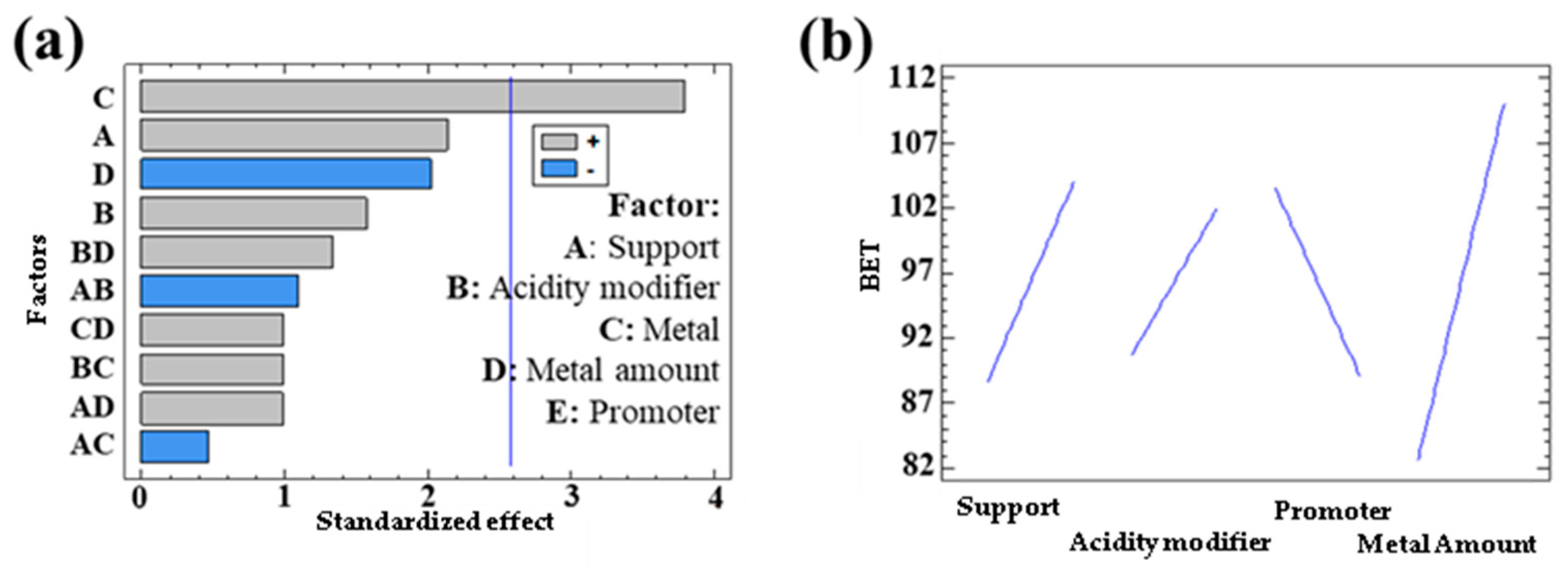
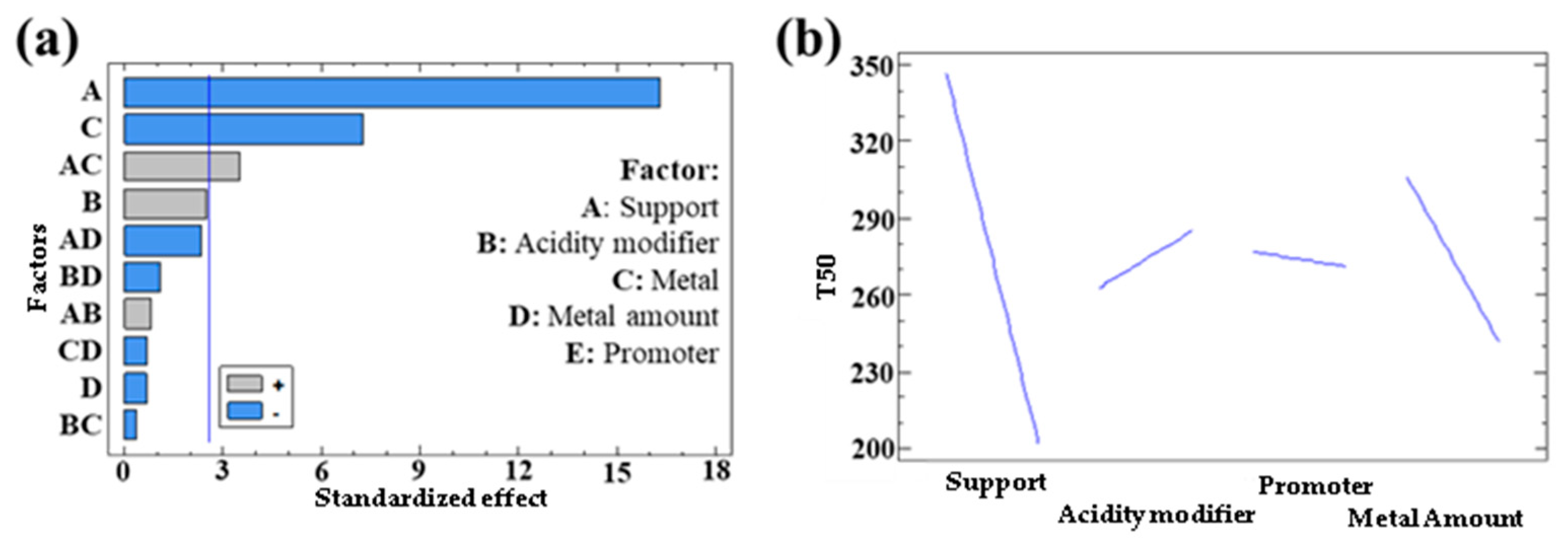
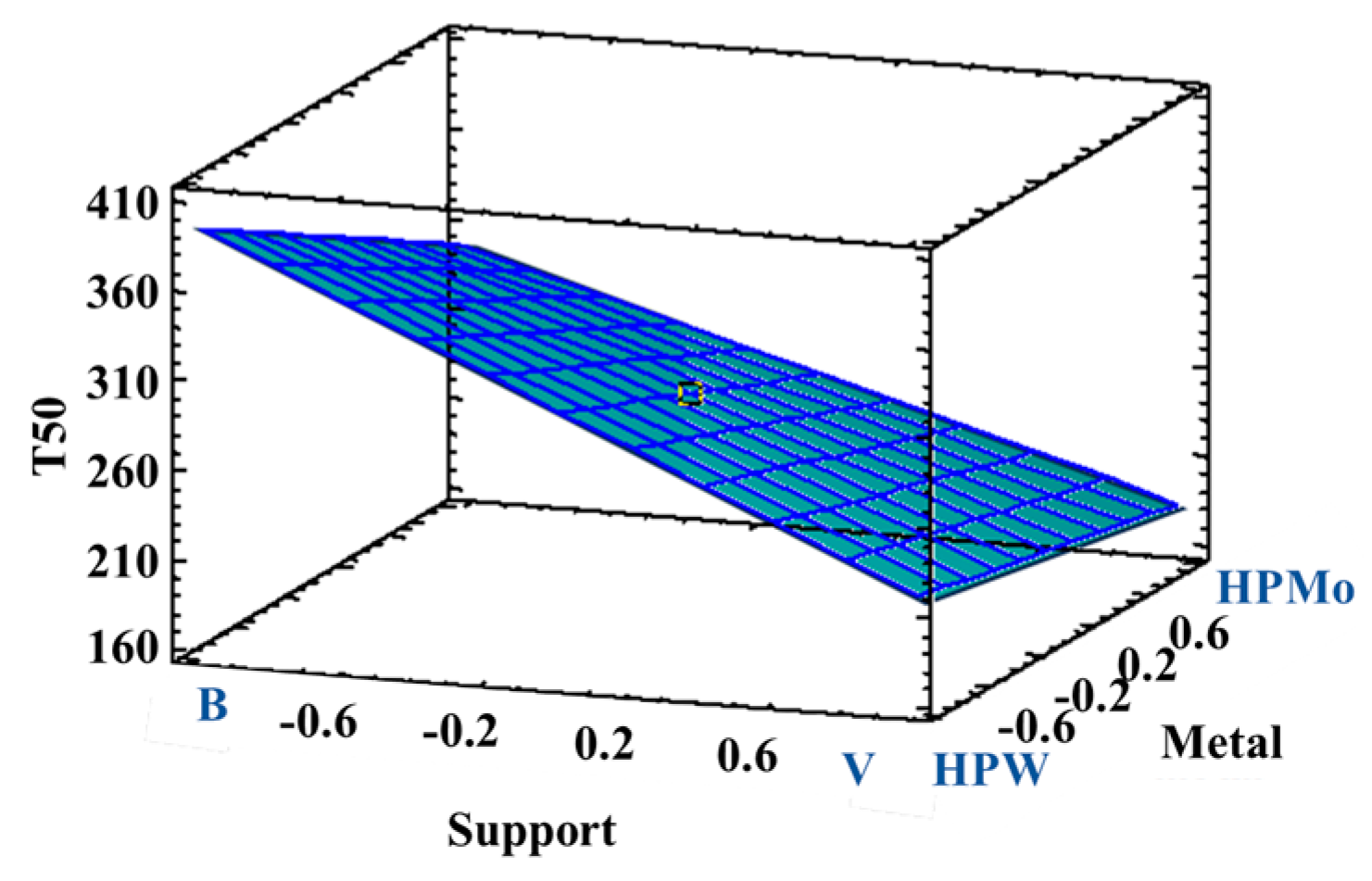
| Response | Support | Acidity Modifier | Metal | Metal Quantity | Promoter |
|---|---|---|---|---|---|
| BET | Vermiculite | Zr | Mo | 5% | Ni |
| Mesopores | Vermiculite | Ce | Mo | 5% | Ni |
| Micropores | Bentonite | Zr | Mo | 5% | Ni |
| Isomerization | Vermiculite | Ce | W | 5% | Ni |
| Cracking | Vermiculite | Ce | Mo | 10% | Ni |
| T50 | Vermiculite | Ce | Mo | 5% | Ni |
| Optimal | Vermiculite | Ce | Mo | 5% | Ni |
| Response | Support | Acidity Modifier | Metal | Promoter |
|---|---|---|---|---|
| BET | Vermiculite | Ce | HPMo | Ni |
| T50 | Vermiculite | Ce | HPMo | Ni |
| Optimal | Vermiculite | Ce | HPMo | Ni |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, T.; Romero, L.S.L.; Rodriguez, D.E.; Amaya, J. A Review for the Design and Optimization of Catalysts: The Use of Statistics as a Powerful Tool for Literature Analysis. Chemistry 2025, 7, 74. https://doi.org/10.3390/chemistry7030074
Martinez T, Romero LSL, Rodriguez DE, Amaya J. A Review for the Design and Optimization of Catalysts: The Use of Statistics as a Powerful Tool for Literature Analysis. Chemistry. 2025; 7(3):74. https://doi.org/10.3390/chemistry7030074
Chicago/Turabian StyleMartinez, Tatiana, Laura Stephania Lavado Romero, D. Estefania Rodriguez, and Jahaziel Amaya. 2025. "A Review for the Design and Optimization of Catalysts: The Use of Statistics as a Powerful Tool for Literature Analysis" Chemistry 7, no. 3: 74. https://doi.org/10.3390/chemistry7030074
APA StyleMartinez, T., Romero, L. S. L., Rodriguez, D. E., & Amaya, J. (2025). A Review for the Design and Optimization of Catalysts: The Use of Statistics as a Powerful Tool for Literature Analysis. Chemistry, 7(3), 74. https://doi.org/10.3390/chemistry7030074






