Guava (Psidium guajava) Fruit Extract Ameliorates Monosodium Urate-Induced Inflammatory Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Plant Material
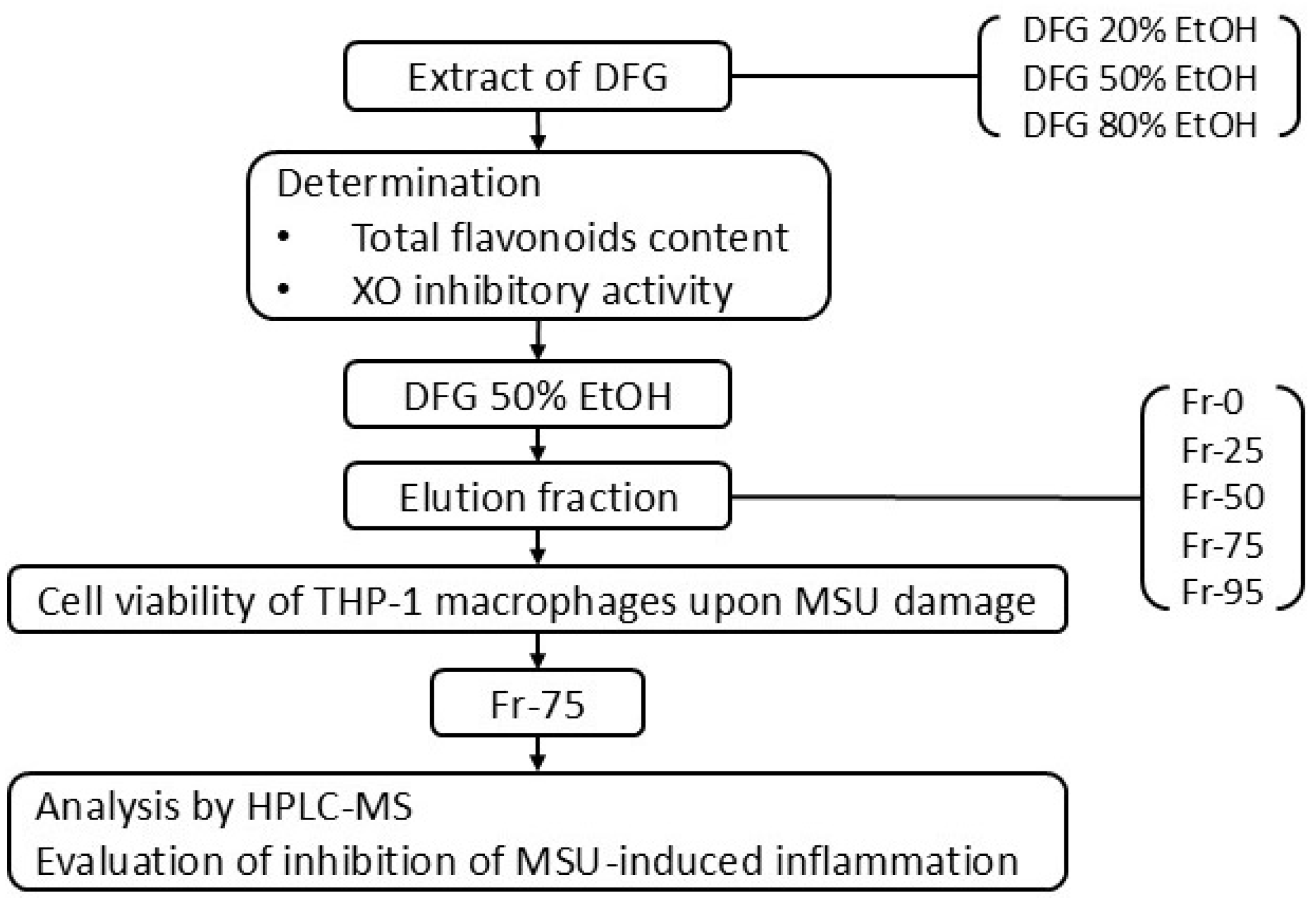
2.3. Preparation of Sample Extract
2.4. Determination of Total Flavonoids Content
2.5. Analysis of Guava Extract by HPLC and HPLC/ESI–MS/MS
2.6. XO Inhibitory Activity
2.7. Macroporous Resin Column Chromatography
2.8. Cell Culture
2.9. Cell Treatment
2.10. Total RNA Extraction
2.11. Quantitative Analysis of mRNA Levels
2.12. Statistical Analysis
3. Results
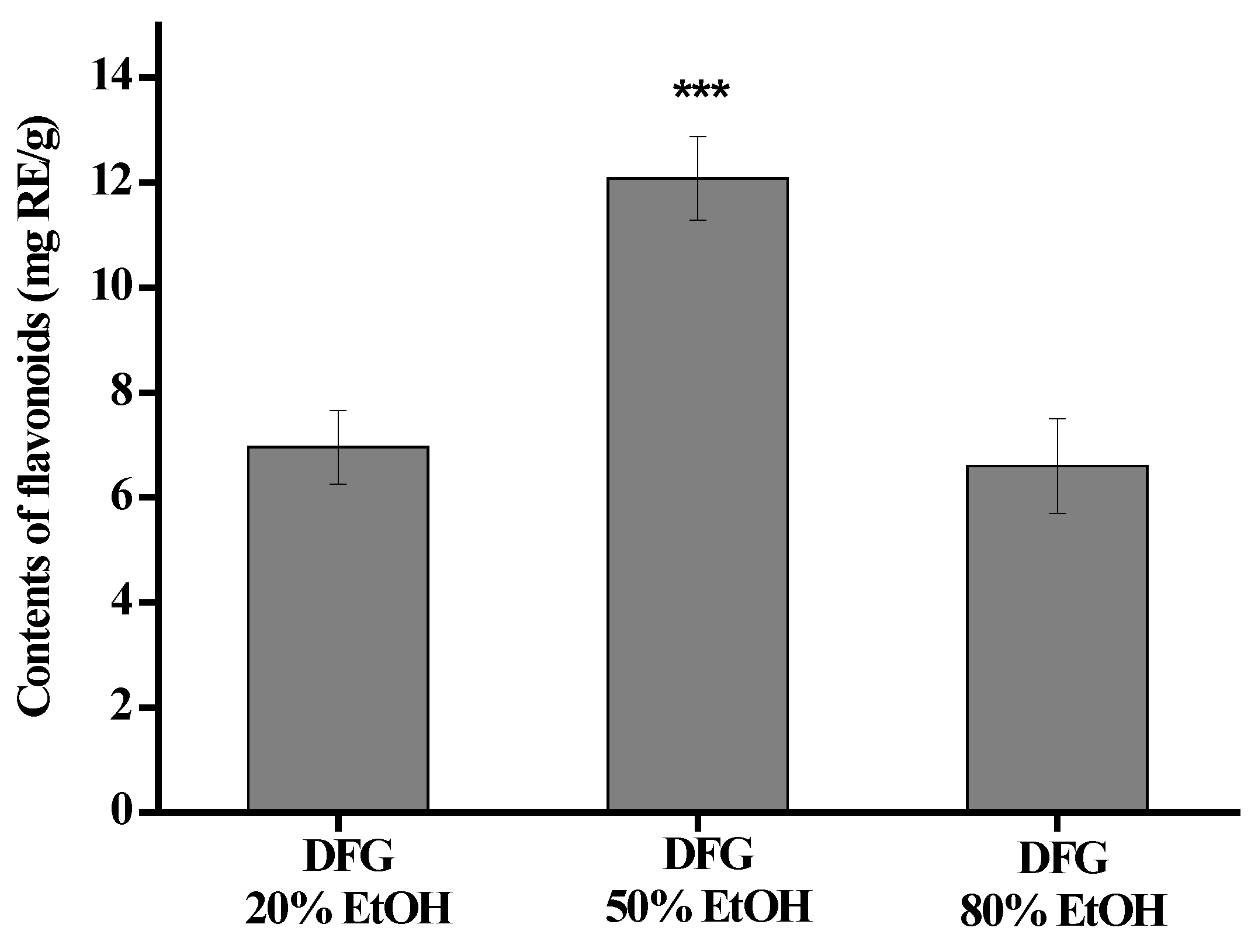
3.1. TFC of Ethanol Extracts from DFG
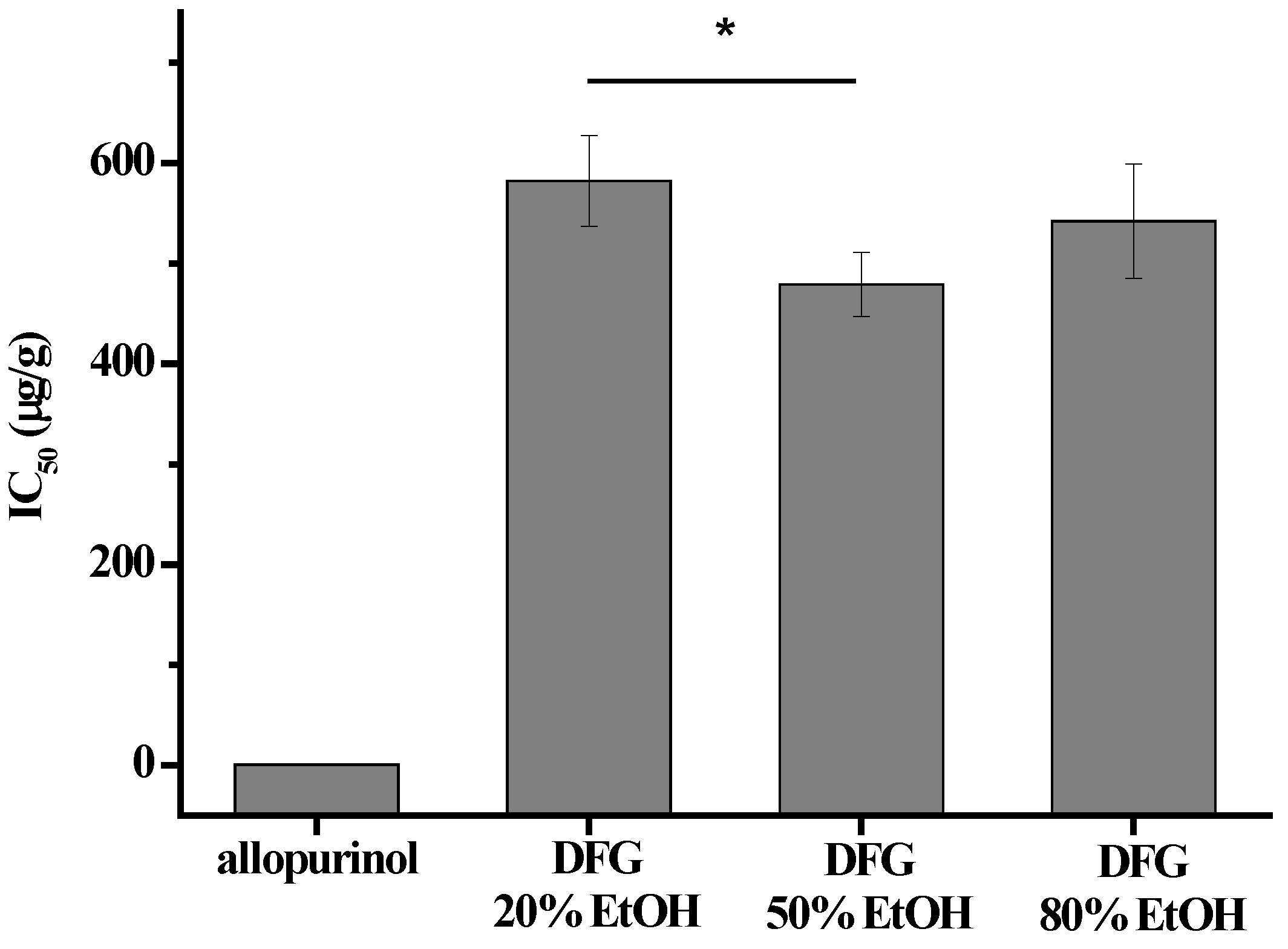
3.2. XOI Activity
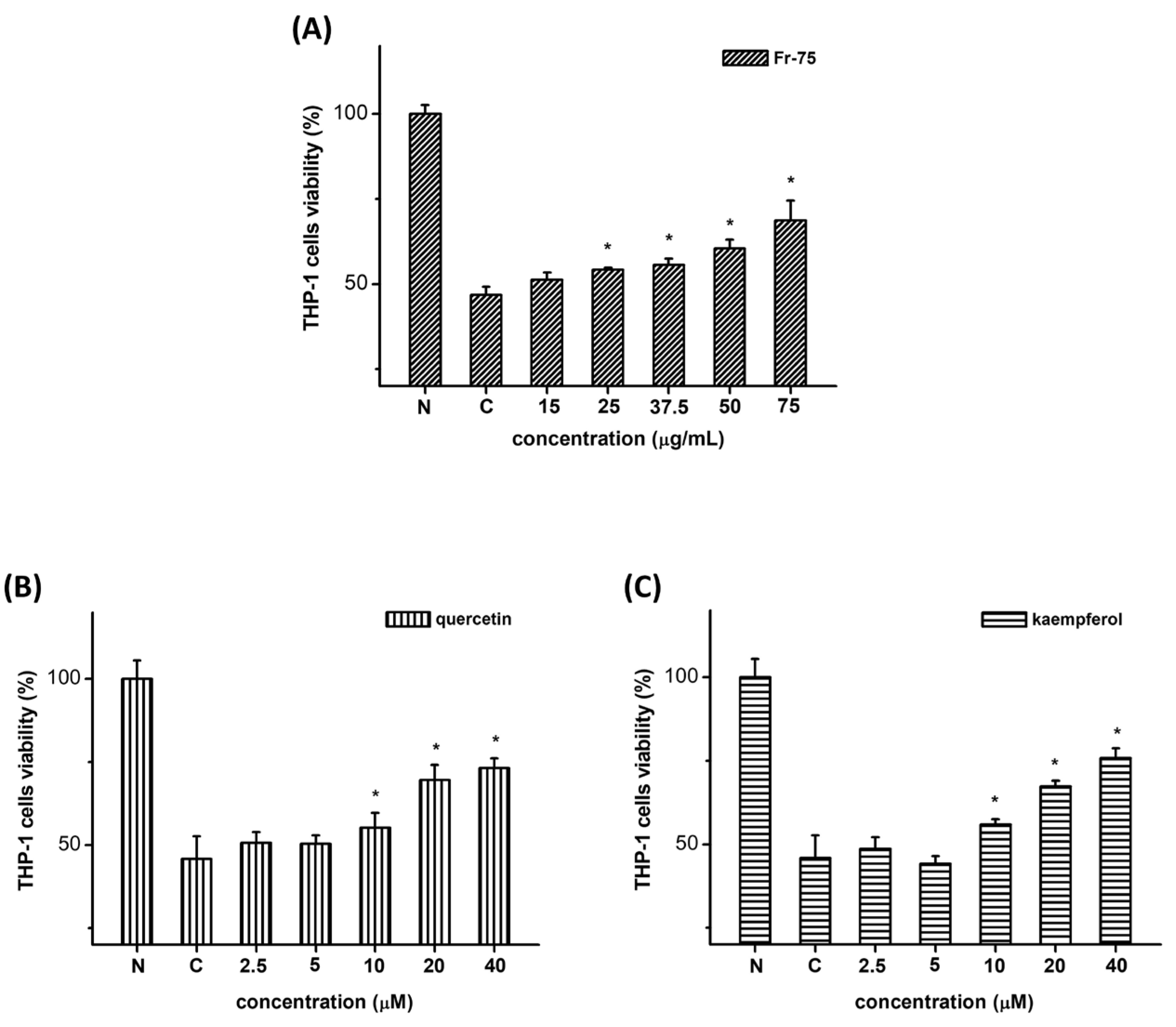
3.3. Effect of DFG Extract on Cell Proliferation of THP-1 Macrophages
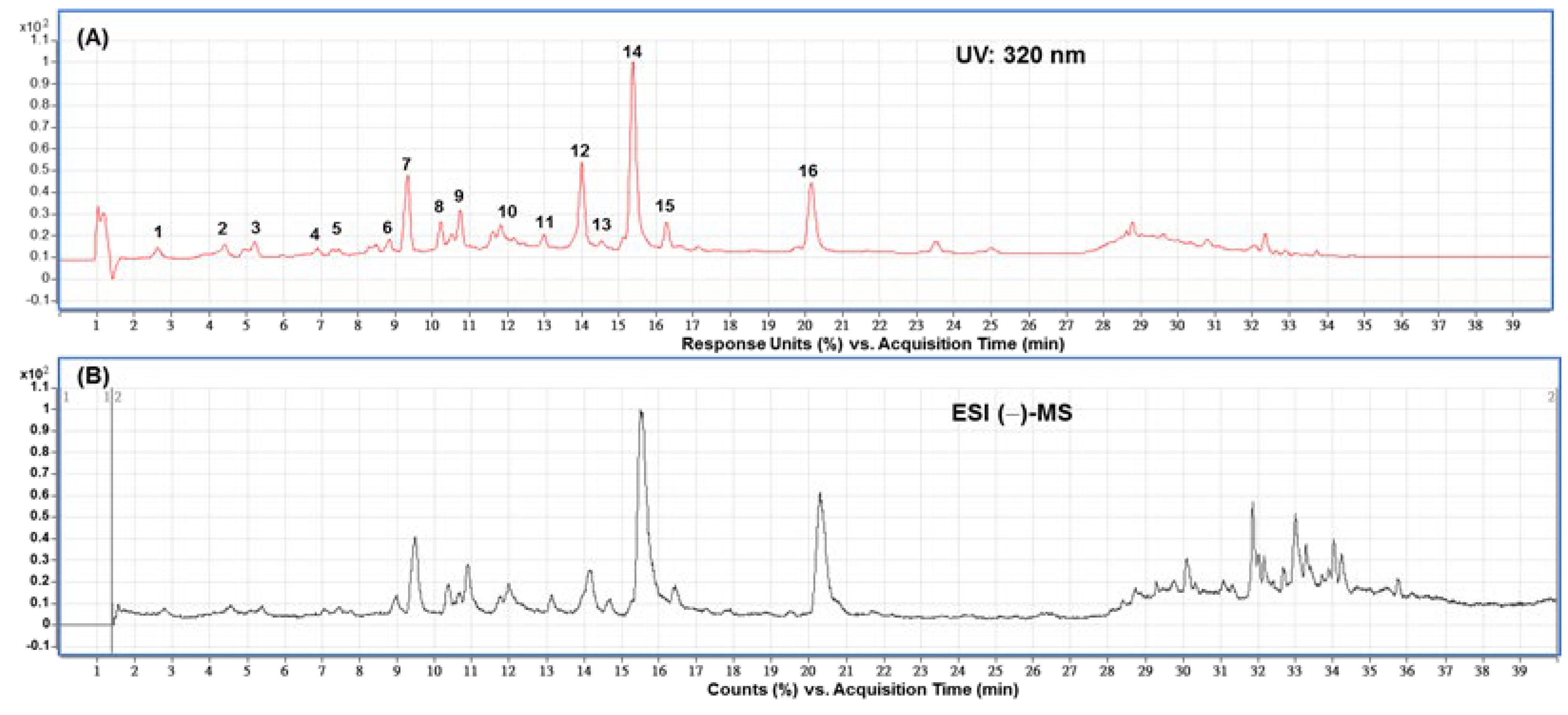
3.4. Main Compounds in Fr-75 of DFG by HPLC and HPLC-MS Analysis
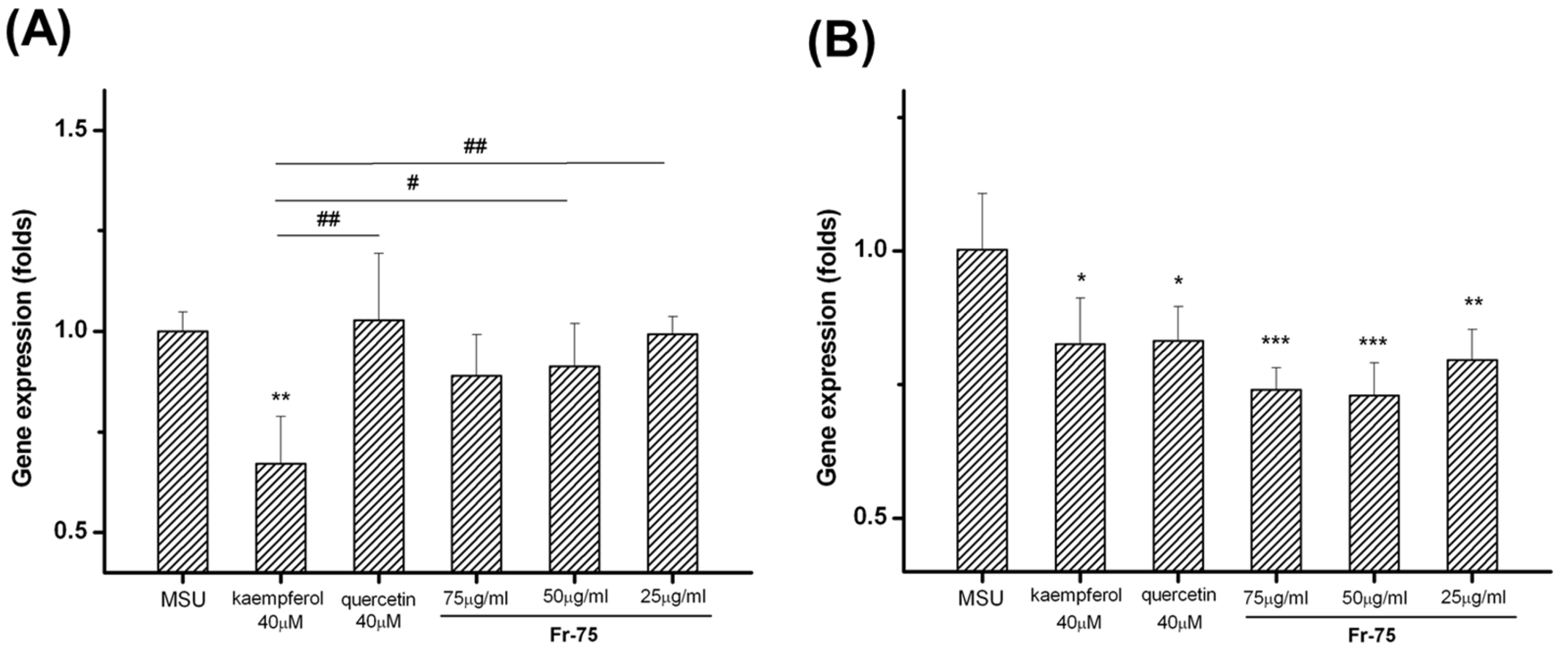
3.5. Effect of Main Compounds in Fr-75 on MSU-Induced Inflammatory Factor
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hainer, B.L.; Matheson, E.; Wilkes, R.T. Diagnosis, treatment, and prevention of gout. Am. Fam. Physician 2014, 90, 831–836. [Google Scholar] [PubMed]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, G.; Edwards, N.L. Overview of serum uric acid treatment targets in gout: Why less than 6 mg/dL? Postgrad. Med. 2016, 128, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Atakul, N.; Demir, H. Gut tedavisinde kullanılan ilaçlar. Türk. Klin. Spec. Top. 2014, 7, 42–47. [Google Scholar]
- Bayraktar, A. Hiperürisemi ve gut. TEB Haberler 2010, 2, 22–25. [Google Scholar]
- Conaghan, P.G.; Day, R.O. Risks and benefits of drugs used in the management and prevention of gout. Drug Saf. 1994, 11, 252–258. [Google Scholar] [CrossRef]
- Strilchuk, L.; Fogacci, F.; Cicero, A.F. Safety and tolerability of available urate-lowering drugs: A critical review. Expert. Opin. Drug Saf. 2019, 18, 261–271. [Google Scholar] [CrossRef]
- Laily, N.; Kusumaningtyas, R.W.; Sukarti, I.; Rini, M.R.D.K. The potency of guava Psidium guajava (L.) leaves as a functional immunostimulatory ingredient. Procedia Chem. 2015, 14, 301–307. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yen, G.C. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007, 101, 686–694. [Google Scholar] [CrossRef]
- Chang, C.H.; Hsieh, C.L.; Wang, H.E.; Peng, C.C.; Chyau, C.C.; Peng, R.Y. Unique bioactive polyphenolic profile of guava (Psidium guajava) budding leaf tea is related to plant biochemistry of budding leaves in early dawn. J. Sci. Food Agric. 2013, 93, 944–954. [Google Scholar] [CrossRef]
- Araújo, H.M.; Rodrigues, F.F.; Costa, W.D.; Nonato Cde, F.; Rodrigues, F.F.; Boligon, A.A.; Athayde, M.L.; Costa, J.G. Chemical profile and antioxidant capacity verification of Psidium guajava (Myrtaceae) fruits at different stages of maturation. EXCLI J. 2015, 14, 1020–1030. [Google Scholar] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Irondi, E.A.; Agboola, S.O.; Oboh, G.; Boligon, A.A.; Athayde, M.L.; Shode, F.O. Guava leaves polyphenolics-rich extract inhibits vital enzymes implicated in gout and hypertension in vitro. J. Intercult. Ethnopharmacol. 2016, 5, 122–130. [Google Scholar] [CrossRef]
- Mahomoodally, F.; Aumeeruddy-Elalfi, Z.; Venugopala, K.N.; Hosenally, M. Antiglycation, comparative antioxidant potential, phenolic content and yield variation of essential oils from 19 exotic and endemic medicinal plants. Saudi J. Biol. Sci. 2019, 26, 1779–1788. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Seo, J.W.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Comparative Study on Seed Characteristics, Antioxidant Activity, and Total Phenolic and Flavonoid Contents in Accessions of Sorghum bicolor (L.) Moench. Molecules 2021, 26, 3964. [Google Scholar] [CrossRef]
- Lien, H.M.; Lin, H.T.; Huang, S.H.; Chen, Y.R.; Huang, C.L.; Chen, C.C.; Chyau, C.C. Protective Effect of Hawthorn Fruit Extract against High Fructose-Induced Oxidative Stress and Endoplasmic Reticulum Stress in Pancreatic β-Cells. Foods 2023, 8, 1130. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, Q.; Wu, H.; Wang, S.; Zou, M. A comparative metabolomics analysis of guava (Psidium guajava L.) fruit with different colors. ACS Food Sci. Technol. 2020, 1, 96–106. [Google Scholar] [CrossRef]
- Takahama, U.; Koga, Y.; Hirota, S.; Yamauchi, R. Inhibition of Xanthine oxidase activity by an oxathiolanone derivative of quercetin. Food Chem. 2011, 126, 1808–1811. [Google Scholar] [CrossRef]
- Fais, A.; Era, B.; Petrillo, A.D.; Floris, S.; Piano, D.; Montoro, P.; Tuberoso, C.I.G.; Medda, R.; Pintus, F. Selected Enzyme Inhibitory Effects of Euphorbia characias Extracts. BioMed Res. Int. 2018, 2018, 1219367. [Google Scholar] [CrossRef]
- Park, J.S.; Rho, H.S.; Kim, D.H.; Chang, I.S. Enzymatic Preparation of Kaempferol from Green Tea Seed and Its Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 2951–2956. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Isa, S.S.P.; Ablat, A.; Mohamad, J. The Antioxidant and Xanthine oxidase Inhibitory Activity of Plumeria rubra Flowers. Molecules 2017, 23, 400. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Hu, W.; Xiu, Z.; Jiang, A.; Men, L.; Hao, K.; Sun, X.; Cao, D. Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD. Molecules 2019, 24, 3200. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Meng, W.; Bian, H.; Zheng, F.; Gu, H.; Zuo, R.; Miao, X.; Zhou, Z.; Wang, L.; Wen, Z.; et al. Total glucosides of paeony protects THP-1 macrophages against monosodium urate-induced inflammation via MALAT1/miR-876-5p/NLRP3 signaling cascade in gouty arthritis. Biomed. Pharmacother. 2021, 138, 111413. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Li, G.; Zeng, X.; Xie, Y.; Cai, Z.; Moore, J.C.; Yuan, X.; Cheng, Z.; Ji, G. Pharmacokinetic properties of isorhamnetin, kaempferol and quercetin after oral gavage of total flavones of Hippophae rhamnoides L. in rats using a UPLC–MS method. Fitoterapia 2012, 83, 182–191. [Google Scholar] [CrossRef]
- Irondi, A.E.; Oboh, G.; Agboola, S.O.; Boligon, A.A.; Athayde, M.L. Phenolics extract of Tetrapleura tetraptera fruit inhibits Xanthine oxidase and Fe2+-induced lipid peroxidation in the kidney, liver, and lungs tissues of rats in vitro. Food Sci. Hum. Wellness 2016, 5, 17–23. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Passamonti, S.; Tramer, F.; Ziberna, L.; Boligon, A.A.; Athayde, M.L. Phenolics from grapefruit peels inhibit HMGCoA reductase and angiotensin-I converting enzyme and show antioxidative properties in endothelial EA. Hy 926 cells. Food Sci. Hum. Wellness 2015, 4, 80–85. [Google Scholar] [CrossRef]
- Irondi, E.A.; Oboh, G.; Akindahunsi, A.A.; Boligon, A.A.; Athayde, M.L. Phenolic composition and inhibitory activity of Mangifera indica and Mucuna urens seeds extracts against key enzymes linked to the pathology and complications of Type 2 diabetes. Asian Pac. J. Trop. Biomed. 2014, 4, 903–910. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Khoi, N.M.; Ha, D.T.; Nhiem, N.X.; Tai, B.H.; Don, D.V.; Luong, H.V.; Son, D.C.; Bae, K. Xanthine oxidase inhibitory activity of constituents of Cinnamomum cassia twigs. Bioorg. Med. Chem. Lett. 2012, 22, 4625–4628. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 15, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of Xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of Xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef]
- Thuaytong, W.; Anprung, P. Bioactive compounds and prebiotic activity in Thailand-grown red and white guava fruit (Psidium guajava L.). Food Sci. Technol. Int. 2011, 17, 205–212. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
 | ||
| Compound | R1 | R2 |
| Quercetin | OH | OH |
| Kaempferol | H | OH |
| Name (Accession No.) | Sequence (5′ to 3′) | Product Length (bp) |
|---|---|---|
| NFκB (XM_054350114.1) | F: aacagcagatggcccatacc R: aacctttgctggtcccacat | 175 |
| NLRP3 (NM_001079821.3) | F: attcggagattgtggttggg R: gagggcgttgtcactcaggt | 117 |
| βactin (NM_001101.5) | F: attggcaatgagcggttc R: ggatgccacaggactccat | 186 |
| % of Cell Viability at Various Concentrations | |||||
|---|---|---|---|---|---|
| Group | 15 μg/mL | 25 μg/mL | 37.5 μg/mL | 50 μg/mL | 75 μg/mL |
| Fr-0 | 51.11 ± 1.73 | 50.20 ± 3.71 | 51.09 ± 4.33 | 50.63 ± 1.03 | 49.33 ± 2.02 |
| Fr-25 | 48.12 ± 3.70 | 46.07 ± 7.16 | 45.26 ± 3.78 | 48.94 ± 5.80 | 49.41 ± 2.36 |
| Fr-50 | 47.49 ± 1.57 | 50.84 ± 4.60 | 50.04 ± 3.16 | 58.48 ± 6.35 * | 60.44 ± 2.73 * |
| Fr-75 | 51.29 ± 2.03 | 54.24 ± 0.61 * | 55.59 ± 1.79 * | 60.39 ± 2.61 * | 68.66 ± 5.96 * |
| Fr-95 | 38.91 ± 3.13 | 40.07 ± 4.87 | 40.87 ± 2.67 | 45.00 ± 2.42 | 46.50 ± 2.42 |
| 1.18 μM | 2.36 μM | 4.72 μM | 9.43 μM | 18.87 μM | |
| Benzbromarone b | 46.44 ± 1.98 | 45.37 ± 3.53 | 46.56 ± 4.23 | 57.26 ± 1.35 * | 64.14 ± 3.88 * |
| Peak No. | Rt (min) | Compound | λmax (nm) | [M−H]− | MS/MS | Area% |
|---|---|---|---|---|---|---|
| 1 | 2.63 | 3-Caffeoylquinic acid a | 325 | 353 | 191 | 1.90 |
| 2 | 4.42 | 5-Caffeoylquinic acid a | 325 | 353 | 191 | 2.07 |
| 3 | 5.23 | Caffeic acid | 220, 324 | 179 | 135 | 2.56 |
| 4 | 6.91 | Unidentified | 220, 270 | 643 | -- | 1.06 |
| 5 | 7.31 | Unidentified | 220, 300 | 335 | -- | 0.27 |
| 6 | 8.83 | Quercetin-3-O-rutinoside b | 220, 254, 350 | 609 | 300, 301 | 1.64 |
| 7 | 9.32 | Quercetin-3-O-glucoside a | 220, 254, 350 | 463 | 300, 301, 271, 255 | 10.78 |
| 8 | 10.22 | Kaempferol-3-O-rutinoside a | 220, 265, 345 | 593 | 285, 284, 326 | 2.65 |
| 9 | 10.75 | Kaempferol-3-O-glucoside a | 220, 265, 345 | 447 | 284, 285, 255 | 3.49 |
| 10 | 11.83 | Quercetin derivatives a | 220, 265, 350 | 601 | 285, 300, 301, 593, 463 | 1.51 |
| 11 | 12.98 | Quercetin derivatives a | 220, 270, 350 | 593 | 463, 301, 300 | 1.29 |
| 12 | 14.00 | Quercetin-3-O-coumaroyl-galactoside a | 220, 315, 265 | 609 | 463, 301, 300 | 13.30 |
| 13 | 14.52 | Quercetin-3-O-coumaroyl-glucoside a | 220, 270 | 609 | 300, 463, 301 | 0.86 |
| 14 | 15.36 | Quercetin b | 254, 370 | 603 | 301, 179, 151 | 28.57 |
| 15 | 16.27 | Kaempferol-3-O-rutinoside a | 220, 315, 265 | 593 | 285, 284, 307 | 3.20 |
| 16 | 20.16 | Kaempferol b | 220, 365, 265 | 285 | 185, 229 | 12.73 |
| Total | 87.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lien, H.-M.; Huang, C.-L.; Lai, C.-H.; Chen, C.-C.; Huang, S.-H.; Tseng, C.-J.; Chyau, C.-C. Guava (Psidium guajava) Fruit Extract Ameliorates Monosodium Urate-Induced Inflammatory Response. Chemistry 2025, 7, 73. https://doi.org/10.3390/chemistry7030073
Lien H-M, Huang C-L, Lai C-H, Chen C-C, Huang S-H, Tseng C-J, Chyau C-C. Guava (Psidium guajava) Fruit Extract Ameliorates Monosodium Urate-Induced Inflammatory Response. Chemistry. 2025; 7(3):73. https://doi.org/10.3390/chemistry7030073
Chicago/Turabian StyleLien, Hsiu-Man, Chao-Lu Huang, Chih-Ho Lai, Chia-Chang Chen, Shiau-Huei Huang, Chin-Jui Tseng, and Charng-Cherng Chyau. 2025. "Guava (Psidium guajava) Fruit Extract Ameliorates Monosodium Urate-Induced Inflammatory Response" Chemistry 7, no. 3: 73. https://doi.org/10.3390/chemistry7030073
APA StyleLien, H.-M., Huang, C.-L., Lai, C.-H., Chen, C.-C., Huang, S.-H., Tseng, C.-J., & Chyau, C.-C. (2025). Guava (Psidium guajava) Fruit Extract Ameliorates Monosodium Urate-Induced Inflammatory Response. Chemistry, 7(3), 73. https://doi.org/10.3390/chemistry7030073






