An Extensive Study of the Production of Hydrogen by Cellulose and Lignin Pyrolysis Using Rhenium-Based Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
2.2. Mechanosynthesis and Characterization of Rhenium-Based Catalysts
2.3. Cellulose and Lignin Pyrolysis Using Rhenium-Based Catalysts
2.4. Monitoring of H2, CH4, CO Production and Char Waste Generation
3. Results and Discussion
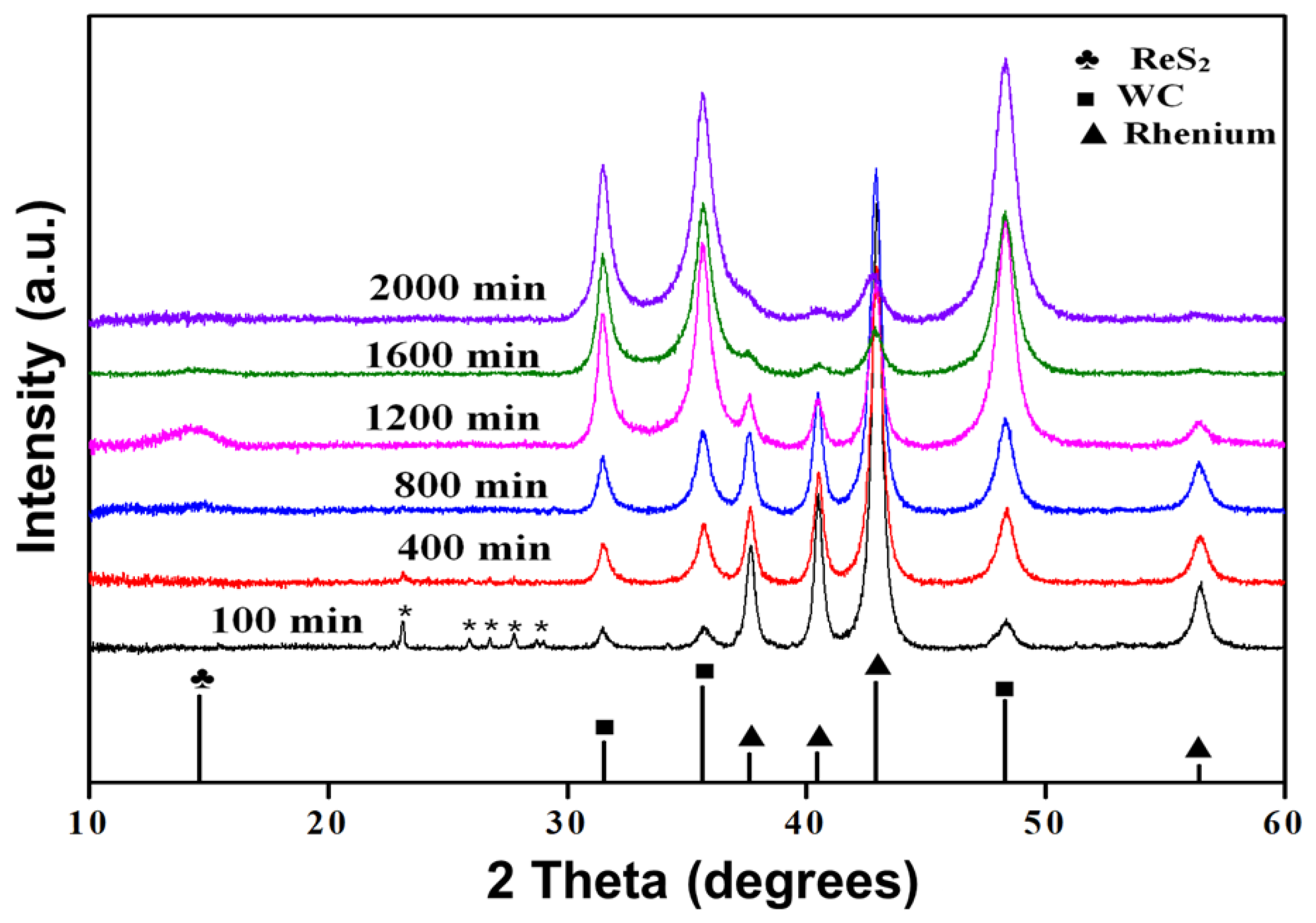
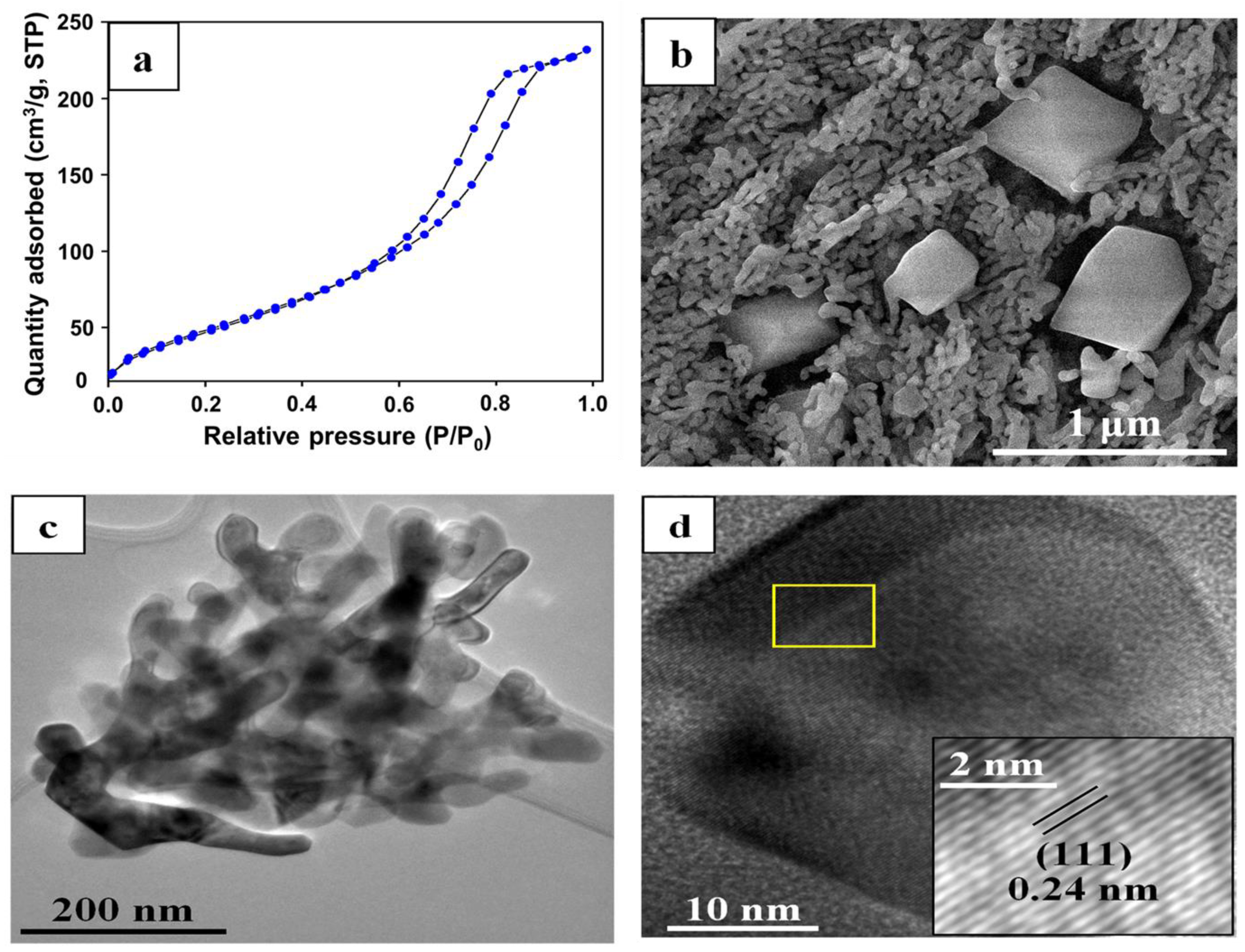
3.1. Characterization of Catalysts
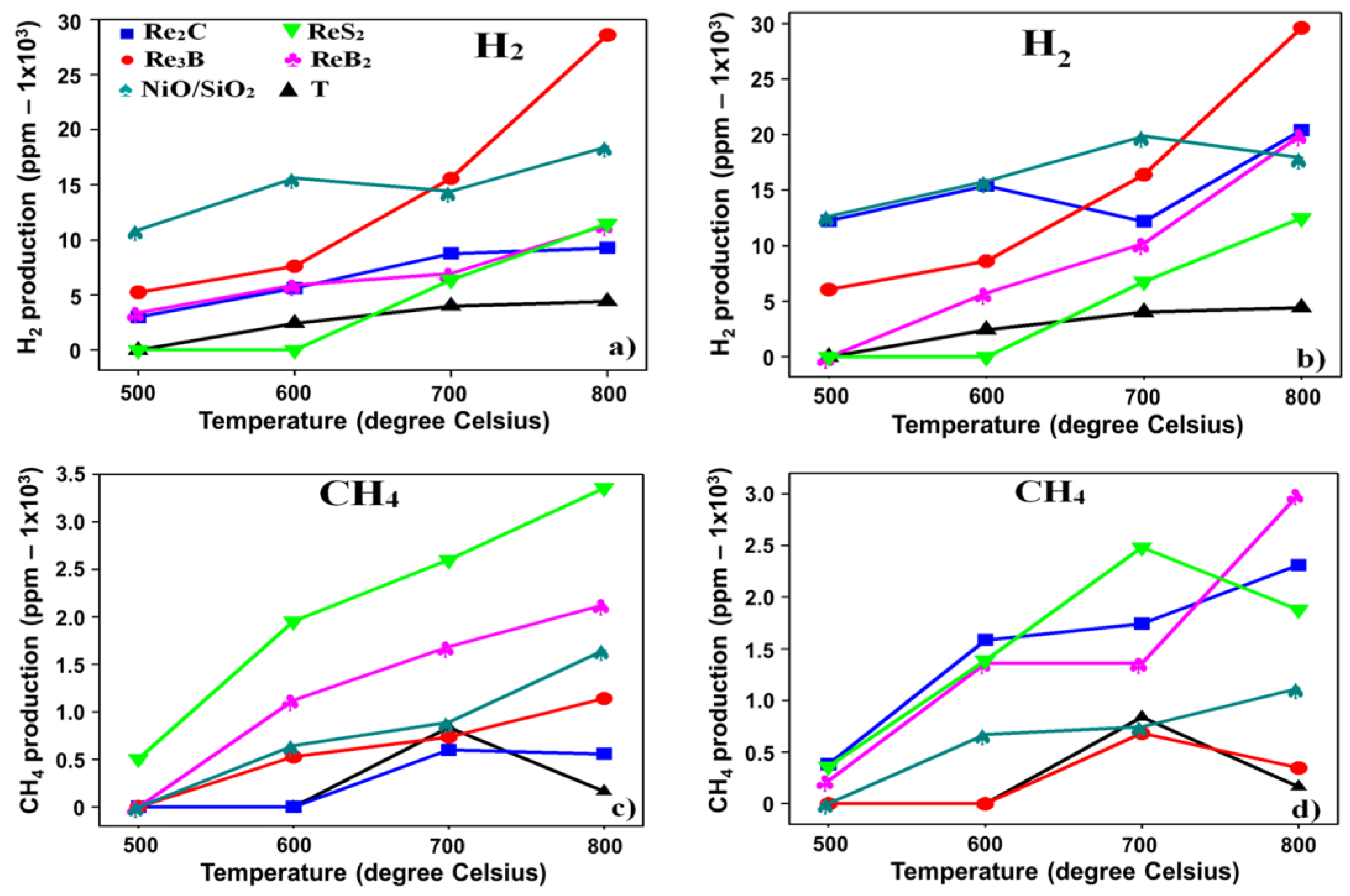
3.2. Cellulose Catalytic Pyrolysis
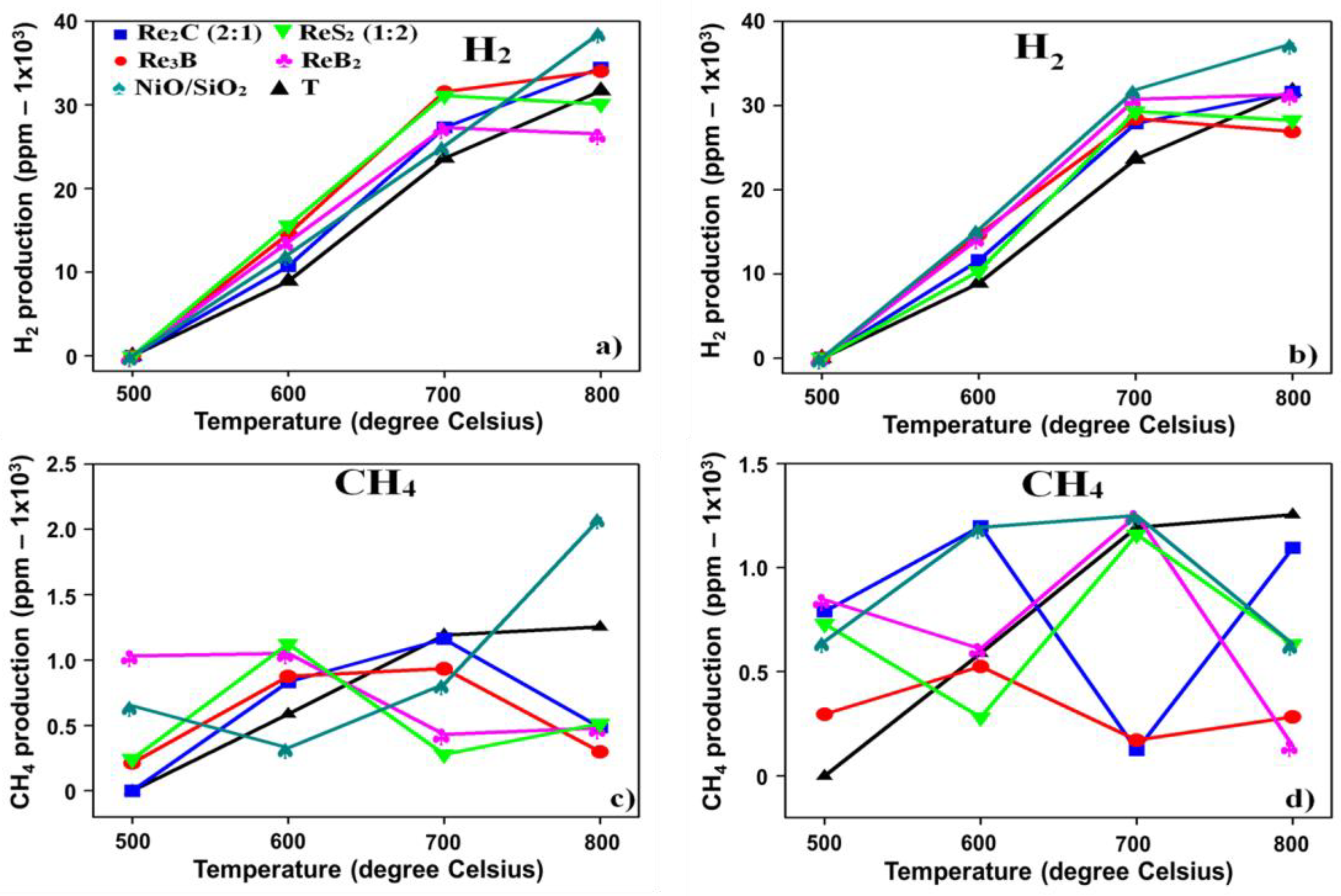
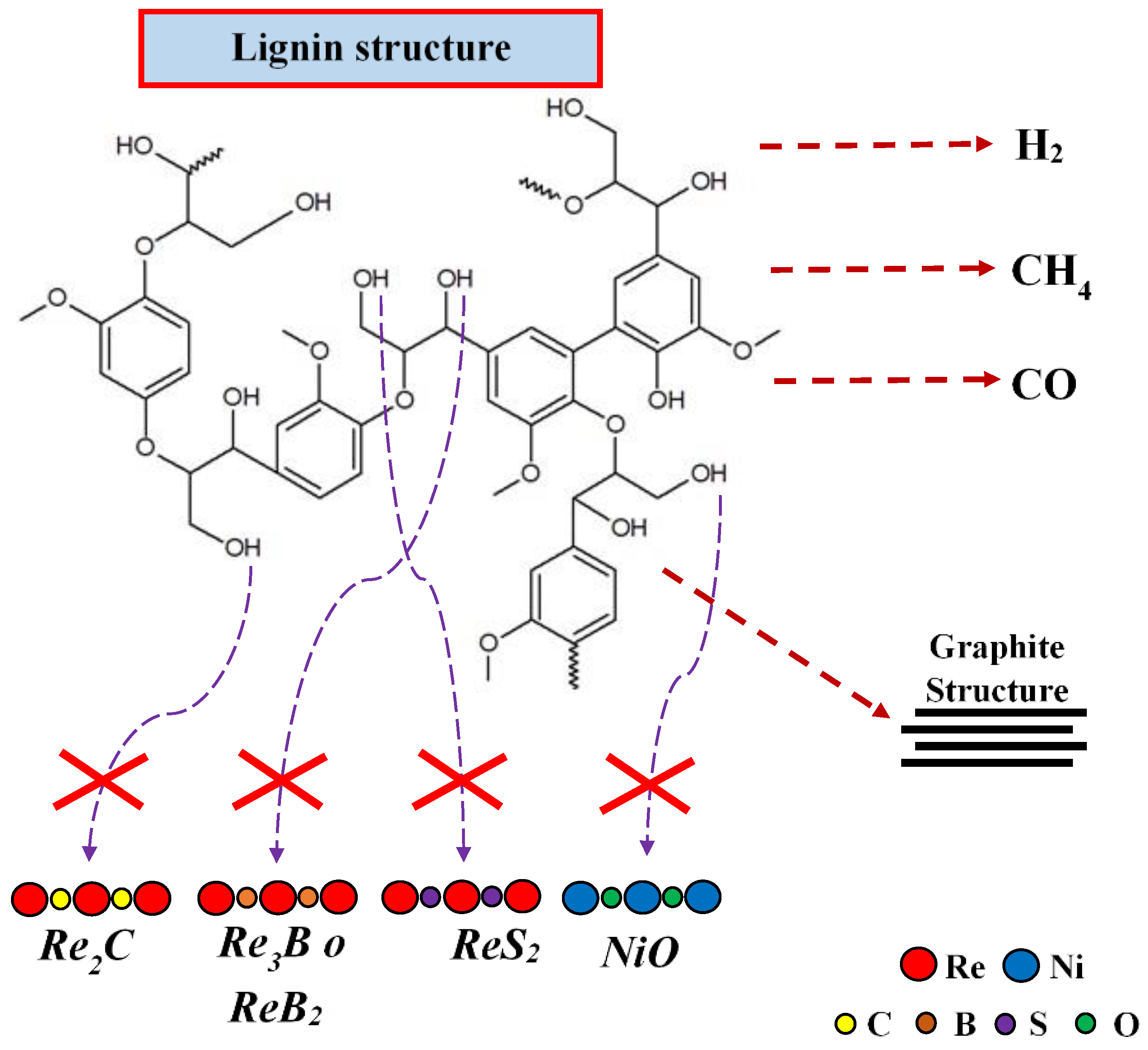
3.3. Lignin Catalytic Pyrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machrafi, H. Green Energy and Technology; Benthan Science Publishers: Sharjah, United Arab Emirates, 2012. [Google Scholar]
- Energy Mix. How Much of Global Energy Comes from Low-Carbon Sources. Available online: https://ourworldindata.org/energy-mix (accessed on 11 December 2024).
- Renewable Energy Market Outlook—2025. Available online: https://www2.deloitte.com/us/en/insights/industry/renewable-energy/renewable-energy-industry-outlook.html (accessed on 11 December 2024).
- Huggins, R.A. Energy Storage: Fundamentals, Materials and Applications, 2nd ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Gupta, R.B. Hydrogen Fuel: Production, Transport and Storage; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- McKendry, P. Energy production from biomass (Part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.M.; Gentry, M. Kinetic formation of CO, CO2, H2, and light hydrocarbon gases from cellulose pyrolysis. J. Anal. Appl. Pyrolysis 1986, 10, 129–138. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Huang, J.; Williams, P.T. Pyrolysis/gasification of cellulose, hemicellulose and lignin for hydrogen production in the presence of various nickel-based catalysts. Fuel 2013, 106, 697–706. [Google Scholar] [CrossRef]
- Lanza, R.; Nogare, D.D.; Canu, P. Gas phase chemistry in cellulose fast pyrolysis. Ind. Eng. Chem. Res. 2009, 48, 1391–1399. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, X.; Church, T.L.; Harris, A.T. Interaction between a bimetallic Ni-Co catalyst and micrometer-sized CaO for enhanced H2 production during cellulose decomposition. Int. J. Hydrogen Energy 2011, 36, 421–431. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, X.; Church, T.L.; Harris, A.T. Novel CaO-SiO2 sorbent and bifunctional Ni/Co-CaO/SiO2 complex for selective H2 synthesis from cellulose. Environ. Sci. Technol. 2012, 46, 2976–2983. [Google Scholar] [CrossRef]
- Zhao, M.; Cui, X.; Ji, G.; Zhou, H.; Vuppaladadiyam, A.K.; Zhao, X. Alkaline Thermal Treatment of Cellulosic Biomass for H2 Production Using Ca-Based Bifunctional Materials. ACS Sustain. Chem. Eng. 2019, 7, 1202–1209. [Google Scholar] [CrossRef]
- Widyaningrum, R.N.; Church, T.L.; Zhao, M.; Harris, A.T. Mesocellular-foam-silica supported Ni catalyst: Effect of pore size on H2 production from cellulose pyrolysis. Int. J. Hydrogen Energy 2012, 37, 9590–9601. [Google Scholar] [CrossRef]
- Widyaningrum, R.N.; Church, T.L.; Harris, A.T. Promoting effect of Pd on H2 production from cellulose pyrolysis over mesocellular-foam-supported Ni catalysts. Catal. Commun. 2013, 35, 45–50. [Google Scholar] [CrossRef]
- Matras, J.; Niewiadomski, M.; Ruppert, A.; Grams, J. Activity of Ni catalysts for hydrogen production via biomass pyrolysis. Kinet. Catal. 2012, 53, 565–569. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Niewiadomski, M.; Grams, J.; Kwapiński, W. Optimization of Ni/ZrO2 catalytic performance in thermochemical cellulose conversion for enhanced hydrogen production. Appl. Catal. B Environ. 2014, 145, 85–90. [Google Scholar] [CrossRef]
- Donar, Y.O.; Sinaǧ, A. Catalytic effect of tin oxide nanoparticles on cellulose pyrolysis. J. Anal. Appl. Pyrolysis 2016, 119, 69–74. [Google Scholar] [CrossRef]
- Grams, J.; Niewiadomski, M.; Ryczkowski, R.; Ruppert, A.M.; Kwapiński, W. Activity and characterization of Ni catalyst supported on CeO2-ZrO2 for thermo-chemical conversion of cellulose. Int. J. Hydrogen Energy 2016, 41, 8679–8687. [Google Scholar] [CrossRef]
- Grams, J.; Potrzebowska, N.; Goscianska, J.; Michalkiewicz, B.; Ruppert, A.M. Mesoporous silicas as supports for Ni catalyst used in cellulose conversion to hydrogen rich gas. Int. J. Hydrogen Energy 2016, 41, 8656–8667. [Google Scholar] [CrossRef]
- Grams, J.; Goscianska, J.; Potrzebowska, N.; Ryczkowski, R.; Michalkiewicz, B.; Ruppert, A.M. Impact of Zr Incorporation into the Ni/AlSBA-15 Catalyst on Its Activity in Cellulose Conversion to Hydrogen-Rich Gas. Energy Fuels 2017, 31, 14089–14096. [Google Scholar] [CrossRef]
- Ryczkowski, R.; Niewiadomski, M.; Michalkiewicz, B.; Skiba, E.; Ruppert, A.M.; Grams, J. Effect of alkali and alkaline earth metals addition on Ni/ZrO2 catalyst activity in cellulose conversion. J. Therm. Anal. Calorim. 2016, 126, 103–110. [Google Scholar] [CrossRef]
- Ryczkowski, R.; Ruppert, A.M.; Przybysz, P.; Chałupka, K.; Grams, J. Hydrogen production from biomass woodchips using Ni/CaO–ZrO2 catalysts. React. Kinet. Mech. Catal. 2017, 121, 97–107. [Google Scholar] [CrossRef]
- Memon, M.Z.; Ji, G.; Li, J.; Zhao, M. Na2ZrO3 as an Effective Bifunctional Catalyst Sorbent during Cellulose Pyrolysis. Ind. Eng. Chem. Res. 2017, 56, 3223–3230. [Google Scholar] [CrossRef]
- Ferdous, D.; Dalai, A.K.; Bej, S.K.; Thring, R.W.; Bakhshi, N.N. Production of H2 and medium Btu gas via pyrolysis of lignins in a fixed-bed reactor. Fuel Process. Technol. 2001, 70, 9–26. [Google Scholar] [CrossRef]
- Ferdous, D.; Dalai, A.K.; Bej, S.K.; Thring, R.W. Pyrolysis of lignins: Experimental and kinetics studies. Energy Fuels 2002, 16, 1405–1412. [Google Scholar] [CrossRef]
- Baumlin, S.; Broust, F.; Bachi, F.B.; Bourdeaux, T.; Herbinet, O.; Ndiaye, F.T.; Ferrer, M.; Lédé, J. Production of hydrogen by lignins fast pyrolysis. Int. J. Hydrogen Energy 2006, 31, 2179–2192. [Google Scholar] [CrossRef]
- Widyawati, M.; Church, T.L.; Florin, N.H.; Harris, A.T. Hydrogen synthesis from biomass pyrolysis with in situ carbon dioxide capture using calcium oxide. Int. J. Hydrogen Energy 2011, 36, 4800–4813. [Google Scholar] [CrossRef]
- Trinh, T.N.; Jensen, P.A.; Sárossy, Z.; Johansen, K.D.; Knudsen, N.O.; Sørensen, H.R.; Egsgaard, H. Fast pyrolysis of lignin using a pyrolysis centrifuge reactor. Energy Fuels 2013, 27, 3802–3810. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic aromatic hydrocarbon formation from the pyrolysis/gasification of lignin at different reaction conditions. Energy Fuels 2014, 28, 6371–6379. [Google Scholar] [CrossRef]
- Jung, K.A.; Nam, C.W.; Woo, S.H.; Park, J.M. Response surface method for optimization of phenolic compounds production by lignin pyrolysis. J. Anal. Appl. Pyrolysis 2016, 120, 409–415. [Google Scholar] [CrossRef]
- Volpe, R.; Zabaniotou, A.A.; Skoulou, V. Synergistic Effects between Lignin and Cellulose during Pyrolysis of Agricultural Waste. Energy Fuels 2018, 32, 8420–8430. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Lv, P.; Chang, J.; Wang, T.; Wu, C.; Tsubaki, N. A kinetic study on biomass fast catalytic pyrolysis. Energy Fuels 2004, 18, 1865–1869. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J.; Bensakhria, A.; Valette, J. Influence of impregnated metal on the pyrolysis conversion of biomass constituents. J. Anal. Appl. Pyrolysis 2012, 95, 213–226. [Google Scholar] [CrossRef]
- Milovanović, J.; Luque, R.; Tschentscher, R.; Romero, A.A.; Li, H.; Shih, K.; Rajić, N. Study on the pyrolysis products of two different hardwood lignins in the presence of NiO contained-zeolites. Biomass Bioenergy 2017, 103, 29–34. [Google Scholar] [CrossRef]
- Yang, H.; Norinaga, K.; Li, J.; Zhu, W.; Wang, H. Effects of HZSM-5 on volatile products obtained from the fast pyrolysis of lignin and model compounds. Fuel Process. Technol. 2018, 181, 207–214. [Google Scholar] [CrossRef]
- Simonetti, D.A.; Kunkes, E.L.; Dumesic, J.A. Gas-phase conversion of glycerol to synthesis gas over carbon-supported platinum and platinum-rhenium catalysts. J. Catal. 2007, 247, 298–306. [Google Scholar] [CrossRef]
- Granados-Fitch, M.G.; Quintana-Melgoza, J.M.; Juarez-Arellano, E.A.; Avalos-Borja, M. Mechanism to H2 production on rhenium carbide from pyrolysis of coconut shell. Int. J. Hydrogen Energy 2019, 44, 2784–2796. [Google Scholar] [CrossRef]
- Granados-Fitch, M.G.; Quintana-Melgoza, J.M.; Juarez-Arellano, E.A.; Avalos-Borja, M. Rhenium borides (Re3B and ReB2) mechanosynthesis and their use as a catalyst for H2 production from biomass pyrolysis. Mater. Res. Bull. 2021, 137, 111180. [Google Scholar] [CrossRef]
- Granados-Fitch, M.G.; Juarez-Arellano, E.A.; Quintana-Melgoza, J.M.; Avalos-Borja, M. Mechanosynthesis of rhenium carbide at ambient pressure and temperature. Int. J. Refract. Met. Hard Mat. 2016, 55, 11–15. [Google Scholar] [CrossRef]
- Granados-Fitch, M.G.; Quintana-Melgoza, J.M.; Juarez-Arellano, E.A.; Avalos-Borja, M. Chemical stability of ReB2 at oxygen and moisture ambient environmental conditions prepared by mechanical milling. J. Am. Ceram. Soc. 2018, 101, 3148–3155. [Google Scholar] [CrossRef]
- Murray, H.H.; Kelty, S.P.; Chianelli, R.R.; Day, C.S. Structure of Rhenium Disulfide. Inorg. Chem. 1994, 33, 4418–4420. [Google Scholar] [CrossRef]
- Coleman, K.S.; Sloan, J.; Hanson, N.A.; Brown, G.; Clancy, G.P.; Terrones, M.; Terrones, H.; Green, M.L.H. The Formation of ReS2 Inorganic Fullerene-like Structures Containing Re4 Parallelogram Units and Metal–Metal Bonds. Am. Chem. Soc. 2002, 124, 11580–11581. [Google Scholar] [CrossRef]
- Yella, A.; Therese, H.A.; Zink, N.; Panthöfer, M.; Tremel, W. Large Scale MOCVD Synthesis of Hollow ReS2 Nanoparticles with Nested Fullerene-Like Structure. Chem. Mater. 2008, 20, 3587–3593. [Google Scholar] [CrossRef]
- Ho, T.C.; Shen, Q.; McConnachie, J.M.; Kliewer, C.E. Kinetic characterization of unsupported ReS2 as hydroprocessing catalyst. J. Catal. 2010, 176, 114–122. [Google Scholar] [CrossRef]
- Tu, W.; Denizot, B. Synthesis of small-sized rhenium sulfide colloidal nanoparticles. J. Colloid Interface Sci. 2007, 30, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charact. 2007, 58, 883–891. [Google Scholar] [CrossRef]
- Garnier, G.; Bouchard, J.; Overend, R.P.; Chornet, E.; Vidal, P.F. High yield production of microcrystalline cellulose by a thermo-mechano-solvolytic treatment. Can. J. Chem. Eng. 1990, 68, 113–118. [Google Scholar] [CrossRef]
- Che, M. Nobel Prize in chemistry 1912 to Sabatier: Organic chemistry or catalysis? Catal. Today 2013, 218–219, 162–171. [Google Scholar] [CrossRef]
- Ferro, Y.; Marinelli, F.; Allouche, A.; Brosset, C. Density functional theory investigation of H adsorption on the basal plane of boron-doped graphite. J. Chem. Phys. 2003, 118, 5650–5657. [Google Scholar] [CrossRef]
- Ferro, Y.; Marinelli, F.; Jelea, A.; Allouche, A. Adsorption, diffusion, and recombination of hydrogen on pure and boron-doped graphite surfaces. J. Chem. Phys. 2004, 120, 11882–11888. [Google Scholar] [CrossRef]
- Dowling, N.I.; Hyne, J.B.; Brown, D.M.; Brown, D.M. Kinetics of the Reaction between Hydrogen and Sulfur under High-Temperature Claus Furnace Conditions. Ind. Eng. Chem. Res. 1990, 29, 2327–2332. [Google Scholar] [CrossRef]
- Ducros, R.; Ehrhardt, J.J.; Alnot, M.; Cassuto, A. Hydrogen and deuterium adsorption on polycrystalline rhenium surfaces; effect of carbon and oxygen coadsorption. Surf. Sci. 1976, 55, 509–522. [Google Scholar] [CrossRef]
- Pallassana, V.; Neurock, M. Theoretical analysis of hydrogen chemisorption on Pd(111), Re(0001) and PdML/Re(0001), ReML/Pd(111) pseudomorphic overlayers. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 60, 6146–6154. [Google Scholar] [CrossRef]
- Poulter, K.F.; Pryde, J.A. Chemisorption of hydrogen on rhenium. J. Phys. D. Appl. Phys. 1968, 1, 169–172. [Google Scholar] [CrossRef]
- Muschiol, U.; Lenz, J.; Schwarz, E.; Christmann, K. Interaction of hydrogen with Re(10 10): H binding states and ordered phases. Surf. Sci. 1995, 333, 127–132. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, J.; Chen, Z.; Gao, X.; Yan, T.; Wen, B.; Schleyer, P.V.R. Comparative study of hydrogen adsorption on carbon and BN nanotubes. J. Phys. Chem. B 2006, 110, 13363–13369. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Luo, H.; Cai, J.; Dong, C. First-principles study of single atom adsorption on capped single-walled carbon nanotubes. Int. J. Hydrogen Energy 2014, 39, 10161–10168. [Google Scholar] [CrossRef]
- Andreoni, W.; Curioni, A.; Kroes, J.M.H.; Pietrucci, F.; Gröning, O. Exohedral hydrogen chemisorption on a carbon nanotube: The clustering effect. J. Phys. Chem. C 2012, 116, 269–275. [Google Scholar] [CrossRef]
- Kayanuma, M.; Nagashima, U.; Nishihara, H.; Kyotani, T.; Ogawa, H. Adsorption and diffusion of atomic hydrogen on a curved surface of microporous carbon: A theoretical study. Chem. Phys. Lett. 2010, 495, 251–255. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I.; Lichtenstein, A.I. Hydrogen on graphene: Electronic structure, total energy, structural distortions and magnetism from first-principles calculations. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 035427. [Google Scholar] [CrossRef]
- Yang, M.; Nurbawono, A.; Zhang, C.; Wu, R.; Feng, Y. Ariando, Manipulating absorption and diffusion of H atom on graphene by mechanical strain. AIP Adv. 2011, 1, 032109. [Google Scholar] [CrossRef]
- Casolo, S.; Løvvik, O.M.; Martinazzo, R.; Tantardini, G.F. Understanding adsorption of hydrogen atoms on graphene. J. Chem. Phys. 2009, 130, 054704. [Google Scholar] [CrossRef]
- Katin, K.P.; Prudkovskiy, V.S.; Maslov, M.M. Chemisorption of hydrogen atoms and hydroxyl groups on stretched graphene: A coupled QM/QM study. Phys. Lett. A 2017, 381, 2686–2690. [Google Scholar] [CrossRef]
- Radosinski, L.; Kuchta, B. Hydrogen chemisorption on carbon structure with mixed sp2 sp3 hybridization: Empirical potential studies. Adsorption 2014, 20, 875–882. [Google Scholar] [CrossRef]
- Mårlid, B.; Larsson, K.; Carlsson, J.O. Chemical interaction of H2, Br2, and HBr with α boron surfaces. J. Phys. Chem. B 2001, 105, 12797–12802. [Google Scholar] [CrossRef]
- Oku, T. Hydrogen storage in boron nitride and carbon nanomaterials. Energies 2015, 8, 319–337. [Google Scholar] [CrossRef]
- Koi, N.; Oku, T. Molecular orbital calculations of hydrogen storage in carbon and boron nitride clusters. Sci. Technol. Adv. Mater. 2004, 5, 625–628. [Google Scholar] [CrossRef]
- Manchanda, P.; Enders, A.; Sellmyer, D.J.; Skomski, R. Hydrogen-induced ferromagnetism in two-dimensional Pt dichalcogenides. Phys. Rev. B 2016, 94, 104426. [Google Scholar] [CrossRef]
- Chen, W.; Santos, E.J.G.; Zhu, W.; Kaxiras, E.; Zhang, Z. Tuning the electronic and chemical properties of monolayer MoS2 adsorbed on transition metal substrates. Nano Lett. 2013, 13, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Cha, G.B.; Park, Y.; Hong, S.C. Hydrogen physisorption based on the dissociative hydrogen chemisorption at the sulfur vacancy of MoS2 surface. Sci. Rep. 2017, 7, 7152. [Google Scholar] [CrossRef]
- Mele, F.; Russo, N.; Toscano, M. Hydrogen chemisorption on the Ni(100) surface: Electronic and magnetic properties from a local-spin-density study. Surf. Sci. 1991, 251–252, 46–50. [Google Scholar] [CrossRef]
- Paul, J.F.; Sautet, P. Comparison of the nature of the hydrogen-metal bond on Pd(111) and Ni(111) by a periodic density functional method. Surf. Sci. 1996, 356, L403–L409. [Google Scholar] [CrossRef]
- Klinke, D.J.; Dooling, D.J.; Broadbelt, L.J. Theoretical study of methylidyne chemisorption on Ni(111) and Co(0001) surfaces. Surf. Sci. 1999, 425, 334–342. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Ferreiro, A.I.; Rabaçal, M.; Costa, M.; Giudicianni, P.; Grottola, C.M.; Ragucci, R. Modeling the impact of the presence of KCl on the slow pyrolysis of cellulose. Fuel 2018, 215, 57–65. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Cui, Y.; Li, X. Catalytic fast pyrolysis of cellulose for increasing contents of furans and aromatics in biofuel production. J. Anal. Appl. Pyrolysis 2018, 131, 93–100. [Google Scholar] [CrossRef]
- Watanabe, H.; Shimomura, K.; Okazaki, K. Carbonate formation during lignin pyrolysis under CO2 and its effect on char oxidation. Proc. Combust. Inst. 2015, 35, 2423–2430. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Z.; Chen, G. Catalytic gasification characteristics of cellulose, hemicellulose and lignin. Renew. Energy 2018, 121, 559–567. [Google Scholar] [CrossRef]





| Catalyst | 10 wt.% Catalyst | 20 wt.% Catalyst | ||||||
|---|---|---|---|---|---|---|---|---|
| H2/ppm | CH4/ppm | CO/ppm | Char/mg | H2/ppm | CH4/ppm | CO/ppm | Char/mg | |
| without | 4453 | 116 | 4453 | 8.7 | 4453 | 116 | 4453 | 8.7 |
| ReS2 | 11,512 | 3359 | 20,852 | 5.2 | 12,494 | 1880 | 32,587 | 2.1 |
| ReB2 | 11,389 | 2129 | 19,070 | 9.2 | 20,012 | 2298 | 28,182 | 9.1 |
| Re3B | 28,616 | 1141 | 32,042 | 5.4 | 29,632 | 347 | 36,547 | 2.9 |
| Re2C | 9272 | 557 | 8153 | 9.1 | 20,421 | 2312 | 19,613 | 8.9 |
| NiO/SiO2 | 18,486 | 1654 | 14,813 | 6.8 | 17,949 | 1120 | 17,802 | 3.1 |
| Process | Adsorption Energy (kJ mol−1) | Reference |
|---|---|---|
| Re-H | 280, 272, 127, 125 | [53,54,55,56] |
| C-H (CNT) | 190, 185, 158 | [57,58,59] |
| C-H (graphene) | 140, 138, 73, 72, 66 | [60,61,62,63,64] |
| C-H (graphite) | 31 | [65] |
| B-H | 290, 144, 140 | [66,67,68] |
| S-H | 110 (PtS2), 103 y 63 (MoS2) | [69,70,71] |
| Ni-H | 299, 200, 197 | [72,73,74] |
| Catalyst | SBET (m2g−1) | % H2 Yield | Temperature | Reference |
|---|---|---|---|---|
| 20Ni1Ca/ZrO2 | 2.0 | 56.9 | 700 °C | [21] |
| 20Ni10CaO/ZrO2(CI) a | 32.0 | 52.2 | 700 °C | [22] |
| 20Ni10CaO/ZrO2(I) b | 37.0 | 50.2 | 700 °C | [22] |
| 20Ni/ZrO2 | 127.0 | 48.5 | 700 °C | [22] |
| 20Ni/15CeO2-ZrO2(I) b | 70.0 | 48.0 | 700 °C | [18] |
| 20Ni1Na/ZrO2 | 128.0 | 46.1 | 700 °C | [21] |
| 20Ni/15CeO2-ZrO2(S) c | 30.0 | 46.1 | 700 °C | [18] |
| 20Ni1Mg/ZrO2 | 7.0 | 45.5 | 700 °C | [21] |
| 20Ni/50CeO2-ZrO2(I) b | 47.0 | 44.9 | 700 °C | [18] |
| 20Ni/50CeO2-ZrO2(S) c | 9.0 | 44.9 | 700 °C | [18] |
| Re3B | 0.48 | 44.0 | 800 °C | This study |
| 20Ni1K/ZrO2 | 148.0 | 43.5 | 700 °C | [21] |
| 20Ni/15CeO2-ZrO2(P) c | 135.0 | 42.7 | 700 °C | [18] |
| 20Ni/50CeO2-ZrO2(P) c | 140.0 | 42.1 | 700 °C | [18] |
| Ni/ZrAlSBA-15 (I) b | 495.0 | 40.1 | 700 °C | [20] |
| 20Ni/ZrO2 | N.V. | 37.4 | 700 °C | [15] |
| NiO | N.V. | 36.8 | 700 °C | [16] |
| 20Ni/ZrO2 | 164.0 | 34.8 | 700 °C | [21] |
| 20Ni/(10ZrO2+Al2O3) | N.V. | 33.4 | 700 °C | [15] |
| 20Ni/SiO2 | N.V. | 32.8 | 700 °C | [15] |
| 20Ni/SBA-15 | 475.0 | 32.6 | 700 °C | [19] |
| Ni/SiO2 | 219.0 | 32.4 | 700 °C | [20] |
| 20Ni/KIT-6 | 397.0 | 32.0 | 700 °C | [19] |
| 20Ni/CeO2 | N.V. | 31.8 | 700 °C | [15] |
| 20Ni/Al2O3 | N.V. | 31.4 | 700 °C | [15] |
| Re2C | 0.43 | 31.4 | 800 °C | This study |
| ReB2 | 0.52 | 30.7 | 800 °C | This study |
| 20Ni/SiO2 | 232.0 | 29.8 | 700 °C | [19] |
| 20Ni/ZrO2 | 122.0 | 28.9f | 700 °C | [18] |
| Ni/AlSBA-15 | 386.0 | 28.4 | 700 °C | [20] |
| NiO/SiO2 | 189.3 | 28.4 | 800 °C | This study |
| 20Ni/MCM-41 | 493.0 | 26.7 | 700 °C | [19] |
| 20Ni/SBA-16 | 328.0 | 21.2 | 700 °C | [19] |
| N.C. | N.V. | 20.4 | 500 °C | [8] |
| N.C. | N.V. | 20.0 | 700 °C | [15] |
| ReS2 | 41.7 | 19.2 | 800 °C | This study |
| N.C. | N.V. | 18.7 | 700 °C | [18] |
| KIT-6 | 667.0 | 18.4 | 700 °C | [19] |
| SBA-15 | 802.0 | 13.5 | 700 °C | [19] |
| MCM-41 | 852.0 | 11.0 | 700 °C | [19] |
| SnO2 | N.V. | 7.6 | 700 °C | [17] |
| N.C. | N.V. | 6.8 | 800 °C | This study |
| SBA-16 | 743.0 | 5.5 | 700 °C | [19] |
| N.C. | N.V. | 4.3 | 700 °C | [22] |
| Catalyst | 10 wt.% Catalyst | 20 wt.% Catalyst | ||||||
|---|---|---|---|---|---|---|---|---|
| H2/ppm | CH4/ppm | CO/ppm | Char/mg | H2/ppm | CH4/ppm | CO/ppm | Char/mg | |
| without | 31,664 | 1254 | 31,347 | 34.1 | 31,664 | 1254 | 31,347 | 34.1 |
| ReS2 | 30,111 | 517 | 19,244 | 39 | 28,272 | 608 | 20,874 | 39.4 |
| ReB2 | 26,516 | 481 | 20,230 | 37.2 | 31,324 | 187 | 25,640 | 33.6 |
| Re3B | 34,028 | 301 | 26,625 | 33.9 | 26,889 | 294 | 23,974 | 32.8 |
| Re2C | 34,399 | 485 | 35,182 | 32.6 | 31,594 | 1028 | 24,961 | 41.5 |
| NiO/SiO2 | 38,687 | 2089 | 43,477 | 33.5 | 37,393 | 602 | 33,281 | 32.9 |
| Type of Lignin | Catalyst | SBET (m2g−1) | % H2 Yield | Temperature | Reference |
|---|---|---|---|---|---|
| Alkali lignin | NiO/SiO2 | 189.3 | 86.1 | 800 °C | This study |
| Eucalyptus lignin | NiO/H-Y | 642.0 | 81.5 | 500 °C | [35] |
| Alkali lignin | Re2C | 0.43 | 76.6 | 800 °C | This study |
| Alkali lignin | Re3B | 0.48 | 75.7 | 800 °C | This study |
| Kraft lignin | N.C. | N.V. | 73.0 | 800 °C | [25] |
| Alkali lignin | N.C. | N.V. | 70.5 | 800 °C | This study |
| Alkali lignin | ReS2 | 41.7 | 67.7 | 800 °C | This study |
| Eucalyptus lignin | NiO/HSZM-5 | 330.0 | 61.4 | 500 °C | [35] |
| Eucalyptus lignin | NiO/H-BETA | 537.0 | 61.4 | 500 °C | [35] |
| Alkali lignin | ReB2 | 0.52 | 59.0 | 800 °C | This study |
| Alkali lignin | N.C. | N.V. | 40.0 | 527 °C | [26] |
| Dealkaline lignin | N.C. | N.V. | 26.3 | 900 °C | [29] |
| Merck lignin | N.C. | N.V. | 25.8 | 550 °C | [32] |
| Alkali lignin | N.C. | N.V. | 7.0 | 500 °C | [8] |
| Eucalyptus lignin | N.C. | N.V. | 6.2 | 500 °C | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados-Fitch, M.G.; Quintana-Melgoza, J.M.; Juarez-Arellano, E.A.; Avalos-Borja, M. An Extensive Study of the Production of Hydrogen by Cellulose and Lignin Pyrolysis Using Rhenium-Based Catalysts. Chemistry 2025, 7, 33. https://doi.org/10.3390/chemistry7020033
Granados-Fitch MG, Quintana-Melgoza JM, Juarez-Arellano EA, Avalos-Borja M. An Extensive Study of the Production of Hydrogen by Cellulose and Lignin Pyrolysis Using Rhenium-Based Catalysts. Chemistry. 2025; 7(2):33. https://doi.org/10.3390/chemistry7020033
Chicago/Turabian StyleGranados-Fitch, Mizraim Guillermo, Juan Manuel Quintana-Melgoza, Erick Adrian Juarez-Arellano, and Miguel Avalos-Borja. 2025. "An Extensive Study of the Production of Hydrogen by Cellulose and Lignin Pyrolysis Using Rhenium-Based Catalysts" Chemistry 7, no. 2: 33. https://doi.org/10.3390/chemistry7020033
APA StyleGranados-Fitch, M. G., Quintana-Melgoza, J. M., Juarez-Arellano, E. A., & Avalos-Borja, M. (2025). An Extensive Study of the Production of Hydrogen by Cellulose and Lignin Pyrolysis Using Rhenium-Based Catalysts. Chemistry, 7(2), 33. https://doi.org/10.3390/chemistry7020033







