Pyrrolizine- and Indolizine-Derived Spirooxindoles: Synthesis, Antibacterial Activity and Inverse Docking Analysis
Abstract
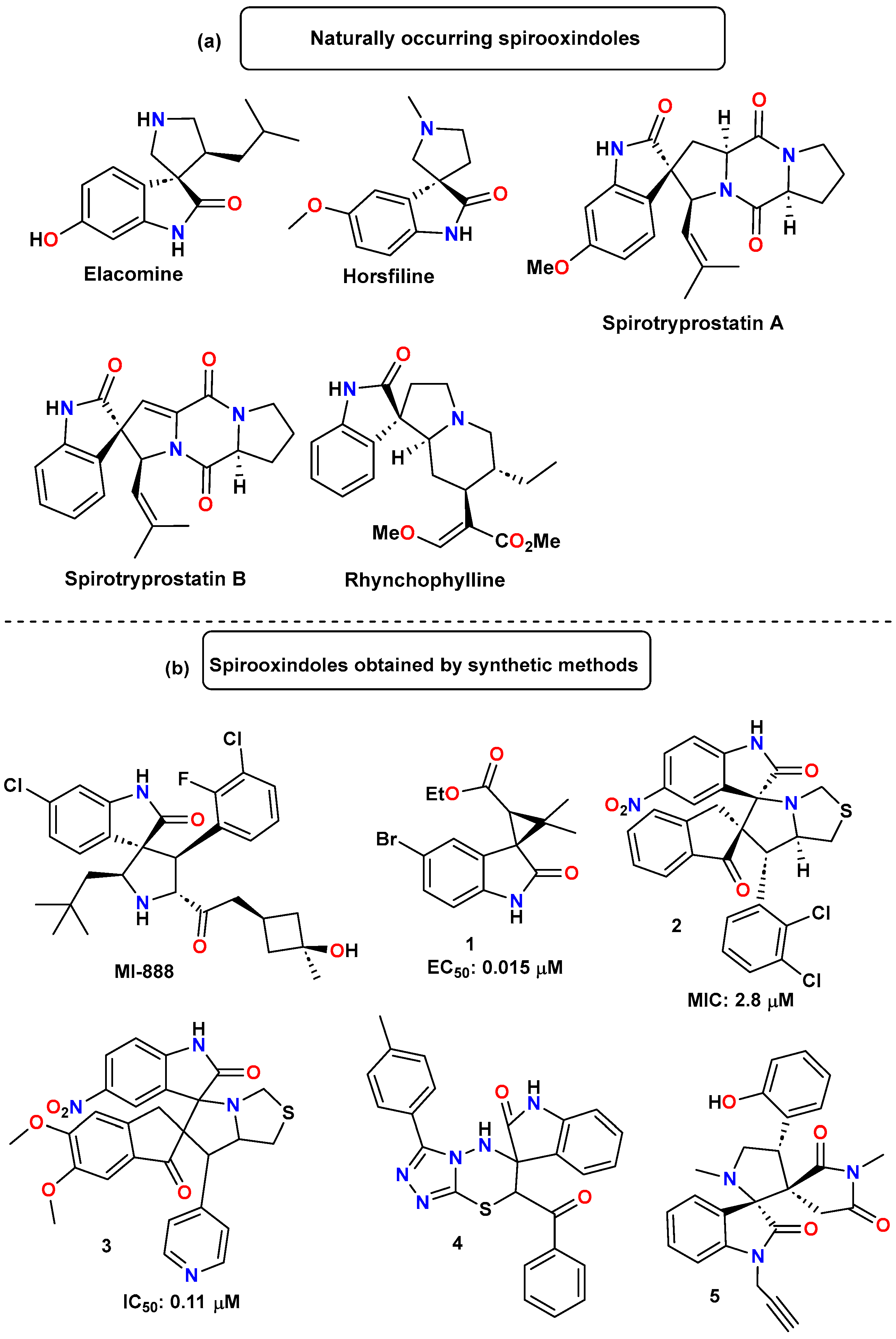
1. Introduction
2. Materials and Methods
2.1. Chemistry
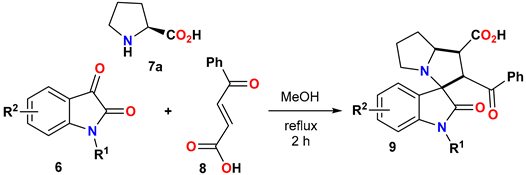
2.1.1. General Procedure for the Synthesis of Spirooxindoles 9a–m
2.1.2. General Procedure for the Synthesis of Spirooxindoles 11a–j
2.2. Biology
2.2.1. Antibacterial Activity
2.2.2. Toxicity Studies Using the In Vivo Model
2.2.3. Hemolytic Activity
2.3. Molecular Modeling
In Silico Analysis of Toxicity and Inverse Docking Analysis
3. Results and Discussion
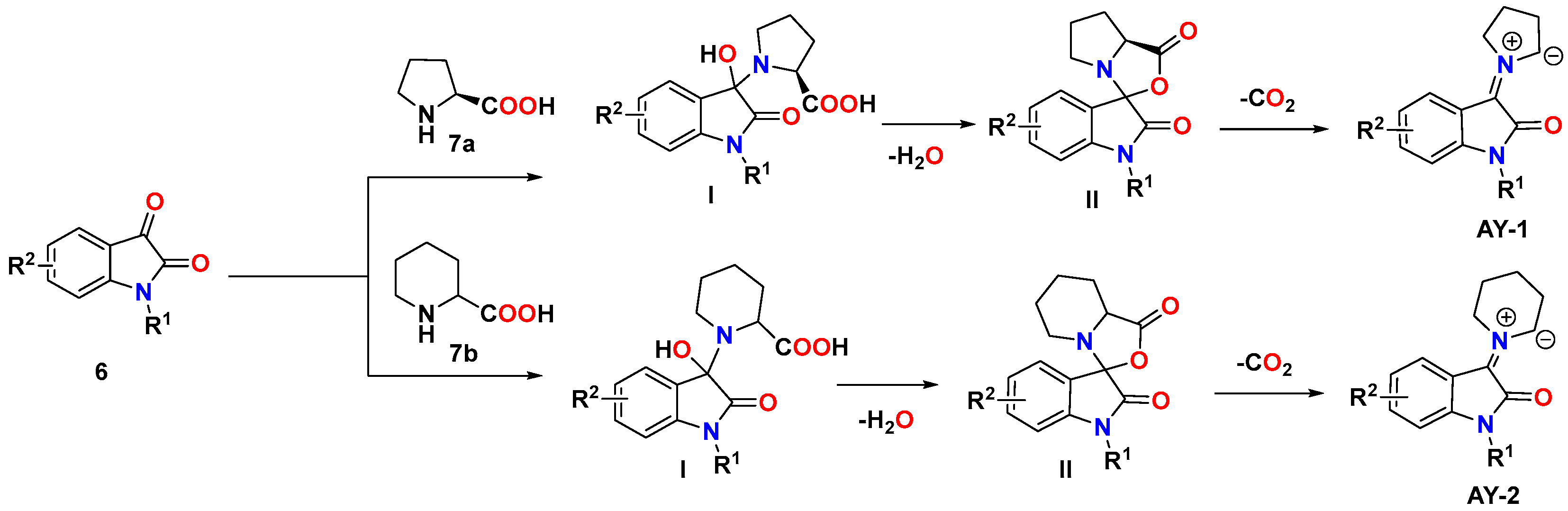
3.1. Synthetic Approaches
3.2. Biological Evaluation
3.2.1. Antibacterial Activity
3.2.2. Toxicological Activity
3.3. Inverse Docking Analysis and Computing the Physicochemical Descriptors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G.S.; Desta, Z.Y. Isatins as Privileged Molecules in Design and Synthesis of Spiro-Fused Cyclic Frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Weher, M.; Borschberg, H. Total Synthesis of (+)-Elacomina and (-)-Isoelacomine, Two Hitherto Unnamed Oxindole Alkaloids from Elueagnus cornrnutata. Helv. Chim. Acta 1996, 79, 151–168. [Google Scholar] [CrossRef]
- James, M.N.G.; Williams, G.J.B. The Molecular and Crystal Structure of an Oxindole Alkaloid (6-Hydroxy-2′-(2-Methylpropyl)-3,3′-Spirotetrahydropyrrolidino-Oxindole). Can. J. Chem. 1972, 50, 2407–2412. [Google Scholar] [CrossRef]
- Jossang, A.; Jossang, P.; Hadi, H.A.; Sévenet, T.; Bodo, B. Horsfiline, an Oxindole Alkaloid from Horsfieldia Superba. J. Org. Chem. 1991, 56, 6527–6530. [Google Scholar] [CrossRef]
- Cui, C.-B.; Kakeya, H.; Osada, H. Novel Mammalian Cell Cycle Inhibitors, Spirotryprostatins A and B, Produced by Aspergillus fumigatus, Which Inhibit Mammalian Cell Cycle at G2/M Phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Shi, J.-S.; Yu, J.-X.; Chen, X.-P.; Xu, R.-X. Pharmacological Actions of Uncaria Alkaloids, Rhynchophylline and Isorhynchophylline. Acta Pharmacol. Sin. 2003, 24, 97–101. [Google Scholar]
- Chou, C.H.; Gong, C.L.; Chao, C.C.; Lin, C.H.; Kwan, C.Y.; Hsieh, C.L.; Leung, Y.M. Rhynchophylline from Uncaria Rhynchophylla Functionally Turns Delayed Rectifiers into A-Type K+ Channels. J. Nat. Prod. 2009, 72, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Murakami, Y.; Matsumoto, K.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Rhynchophylline and Isorhynchophylline Inhibit NMDA Receptors Expressed in Xenopus Oocytes. Eur. J. Pharmacol. 2002, 455, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Murakami, Y.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H.; Matsumoto, K. Protective Effect of Rhynchophylline and Isorhynchophylline on in vitro Ischemia-Induced Neuronal Damage in the Hippocampus: Putative Neurotransmitter Receptors Involved in Their Action. Life Sci. 2004, 76, 331–343. [Google Scholar] [CrossRef]
- Panda, S.S.; Girgis, A.S.; Aziz, M.N.; Bekheit, M.S. Spirooxindole: A Versatile Biologically Active Heterocyclic Scaffold. Molecules 2023, 28, 618. [Google Scholar] [CrossRef]
- Bora, D.; Kaushal, A.; Shankaraiah, N. Anticancer Potential of Spirocompounds in Medicinal Chemistry: A Pentennial Expedition. Eur. J. Med. Chem. 2021, 215, 113263. [Google Scholar] [CrossRef]
- Breedveld, F. Tenidap: A Novel Cytokine-Modulating Antirheumatic Drug for the Treatment of Rheumatoid Arthritis. Scand. J. Rheumatol. 1994, 23, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, R.F.; Robertson, D.W.; Franklin, R.B.; Sandusky, G.E.; Dies, F.; McNay, J.L.; Hayes, J.S. Indolidan: A Potent, Long-Acting Cardiotonic and Inhibitor of Type IV Cyclic AMP Phosphodiesterase. Cardiovasc. Drug Rev. 1990, 8, 303–322. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Sun, W.; Liu, L.; Lu, J.; McEachern, D.; Shargary, S.; Bernard, D.; Li, X.; Zhao, T.; et al. A Potent Small-Molecule Inhibitor of the MDM2-P53 Interaction (MI-888) Achieved Complete and Durable Tumor Regression in Mice. J. Med. Chem. 2013, 56, 5553–5561. [Google Scholar] [CrossRef]
- Jiang, T.; Kuhen, K.L.; Wolff, K.; Yin, H.; Bieza, K.; Caldwell, J.; Bursulaya, B.; Wu, T.Y.H.; He, Y. Design, Synthesis and Biological Evaluations of Novel Oxindoles as HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors. Part I. Bioorg. Med. Chem. Lett. 2006, 16, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Balamurugan, K.; Perumal, S.; Yogeeswari, P.; Sriram, D. A Regio- and Stereoselective 1,3-Dipolar Cycloaddition for the Synthesis of Novel Spiro-Pyrrolothiazolyloxindoles and Their Antitubercular Evaluation. Eur. J. Med. Chem. 2010, 45, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Ismail, R.; Soo, T.; Suresh, R.; Osman, H.; Arumugam, N.; Almansour, A.I.; Elumalai, K.; Singh, A. AChE Inhibitor: A Regio- and Stereo-selective 1,3-Dipolar Cycloaddition for the Synthesis of Novel Substituted 5,6-Dimethoxy Spiro[5.3′]-oxindole-Spiro-[6.3″]-2,3-Dihydro-1H-Inden-1″-one-7-(substituted aryl)-Tetrahydro-1H-Pyrrolo[1,2-c][1,3]thiazole. Bioorg. Med. Chem. Lett. 2012, 22, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhou, Y.; Yu, Q.; Fang, Y.; Jiang, Y.; Zhao, Y.; Yuan, C.; Xie, W. Synthesis and Anticancer Activity of New Spirooxindoles Incorporating[1,2,4]Triazolo[3,4-b][1,3,4]Thiadiazine Moiety. J. Mol. Struct. 2021, 1227, 129406. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Sivakumar, P.M.; Doble, M.; Perumal, P.T. Synthesis, Antibacterial Activity Evaluation and QSAR Studies of Novel Dispiropyrrolidines. Eur. J. Med. Chem. 2010, 45, 3446–3452. [Google Scholar] [CrossRef]
- Quiroga, J.; Portillo, S.; Pérez, A.; Gálvez, J.; Abonia, R.; Insuasty, B. An Efficient Synthesis of Pyrazolo[3,4-b]Pyridine-4-Spiroindolinones by a Three-Component Reaction of 5-Aminopyrazoles, Isatin, and Cyclic β-Diketones. Tetrahedron Lett. 2011, 52, 2664–2666. [Google Scholar] [CrossRef]
- Ashraf, A.; Shafiq, Z.; Khan Jadoon, M.S.; Tahir, M.N.; Pelletier, J.; Sevigny, J.; Yaqub, M.; Iqbal, J. Synthesis, Characterization, and in Silico Studies of Novel Spirooxindole Derivatives as Ecto-5′-Nucleotidase Inhibitors. ACS Med. Chem. Lett. 2020, 11, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, M.; Azimi, S.C.; Khavasi, H.R.; Bazgir, A. A Novel Reaction of 6-Amino-Uracils and Isatins. Tetrahedron 2008, 64, 7307–7311. [Google Scholar] [CrossRef]
- Jadidi, K.; Ghahremanzadeh, R.; Mirzaei, P.; Bazgir, A. Three-Component Synthesis of Spiro[Indoline-3,5′-Pyrimido[4,5-b]Quinoline]-Triones in Water. J. Heterocycl. Chem. 2011, 48, 1014–1018. [Google Scholar] [CrossRef]
- Ghahremanzadeh, R.; Sayyafi, M.; Ahadi, S.; Bazgir, A. Novel One-Pot, Three-Component Synthesis of Spiro[Indoline-Pyrazolo[4′,3′:5,6]Pyrdo[2,3-d]Pyrimidine]Trione Library. J. Comb. Chem. 2009, 11, 393–396. [Google Scholar] [CrossRef]
- Elinson, M.N.; Merkulova, V.; Ilovaisky, A.; Nikishin, G.I. Cascade Assembling of Isatins and Barbituric Acids: Facile and Efficient Way to 2″H-Dispiro[Indole-3,5′-Furo[2,3-d]Pyrimidine-6′,5″-Pyrimidine]-2,2′,2″,4′,4″,6″-(1H,1′H,1″H,3′H,3″H)-Hexone Scaffold. J. Heterocycl. Chem. 2013, 50, 1236–1241. [Google Scholar] [CrossRef]
- Ponnala, S.; Kumar, R.; Maulik, P.R.; Sahu, D.P. One Pot Synthesis of Novel Dispiro[Oxindole-Thiazolidinedione/Thioxo-Thiazolidinone/Dihydro Pyrazolone]-Pyrrolidines via 1,3-Dipolar Cycloaddition Reaction of Azomethine Ylides. J. Heterocycl. Chem. 2006, 43, 1635–1640. [Google Scholar] [CrossRef]
- Liu, H.; Zou, Y.; Hu, Y.; Shi, D.-Q. An Efficient One-Pot Synthesis of Dispiropyrrolidine Derivatives Through 1,3-Dipolar Cycloaddition Reactions Under Ultrasound Irradiation. J. Heterocycl. Chem. 2011, 48, 877–881. [Google Scholar] [CrossRef]
- Hu, Y.; Zou, Y.; Wu, H.; Shi, D. A Facile and Efficient Ultrasound-Assisted Synthesis of Novel Dispiroheterocycles through 1,3-Dipolar Cycloaddition Reactions. Ultrason. Sonochem. 2012, 19, 264–269. [Google Scholar] [CrossRef]
- Puerto Galvis, C.E.; Kouznetsov, V.V. Regio- and Stereoselective Synthesis of Spirooxindole 1′-Nitro Pyrrolizidines with Five Concurrent Stereocenters under Aqueous Media and Their Bioprospection Using the Zebrafish (Danio rerio) Embryo Model. Org. Biomol. Chem. 2013, 11, 7372–7386. [Google Scholar] [CrossRef]
- Al-Majid, A.M.; Ghawas, H.M.; Islam, M.S.; Soliman, S.M.; El-Senduny, F.F.; Badria, F.A.; Ali, M.; Shaik, M.R.; Ghabbour, H.A.; Barakat, A. Synthesis of Spiroindolone Analogue via Three Components Reaction of Olefin with Isatin and Sarcosine: Anti-Proliferative Activity and Computational Studies. J. Mol. Struct. 2020, 1204, 127500. [Google Scholar] [CrossRef]
- Ghosh, R.; Vitor, J.B.; Mendes, E.; Paulo, A.; Acharya, P.C. Stereoselective Synthesis of Spirooxindole Derivatives Using One-Pot Multicomponent Cycloaddition Reaction and Evaluation of Their Antiproliferative Efficacy. ACS Omega 2020, 5, 27332–27343. [Google Scholar] [CrossRef] [PubMed]
- Toumi, A.; Boudriga, S.; Hamden, K.; Sobeh, M.; Cheurfa, M.; Askri, M.; Knorr, M.; Strohmann, C.; Brieger, L. Synthesis, Antidiabetic Activity and Molecular Docking Study of Rhodanine-Substitued Spirooxindole Pyrrolidine Derivatives as Novel α-Amylase Inhibitors. Bioorg. Chem. 2021, 106, 104507. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, Y.; Insuasty, B.; Abonia, R.; Ortiz, A.; Romo, P.; Quiroga, J. Three-Component One-Pot Synthesis of New Spiro[Indoline-Pyrrolidine] Derivatives Mediated by 1,3-Dipolar Reaction and DFT Analysis. Monatsh. Chem. 2021, 152, 497–506. [Google Scholar] [CrossRef]
- Kukushkin, M.E.; Kondratyeva, A.A.; Zyk, N.V.; Majouga, A.G.; Beloglazkina, E.K. First Example of [3+2] Cycloaddition of Azomethine Ylides to 5-Methylidene-3-Phenylhydantoin. Russ. Chem. Bull. 2019, 68, 2088–2091. [Google Scholar] [CrossRef]
- Barashkin, A.A.; Polyakov, V.S.; Shikut, N.L.; Putilova, A.D.; Gorovoy, A.R.; Degtiarev, A.D.; Tafeenko, V.A.; Tarasevich, B.N.; Zyk, N.V.; Beloglazkina, E.K. Diastereoselective Cycloaddition of Isatin Azomethine Ylides to 5-Arylidene-2-Thiohydantoins Bearing 3-Positioned Chiral Substituent. Mendeleev Commun. 2022, 32, 221–223. [Google Scholar] [CrossRef]
- Grigg, R.; Thianpatanagul, S. Decarboxylative Transamination. Mechanism and Applications to the Synthesis of Heterocyclic Compounds. J. Chem. Soc. Chem. Commun. 1984, 3, 180–181. [Google Scholar] [CrossRef]
- Grigg, R.; Aly, M.F.; Sridharan, V.; Thianpatanagul, S. Decarboxylative Transamination. A New Route to Spirocyclic and Bridgehead-nitrogen Compounds. Relevance to α-Amino Acid Decarboxylases. J. Chem. Soc. Chem. Commun. 1984, 3, 182–183. [Google Scholar] [CrossRef]
- Quiroga, J.; Romo, P.; Cobo, J.; Glidewell, C. Synthesis of Spiro[Indoline-3,3′-Pyrrolizines] by 1,3-Dipolar Reactions between Isatins, L-Proline and Electron-Deficient Alkenes. Acta Crystallogr. 2017, C73, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Romo, P.E.; Quiroga, J.; Cobo, J.; Glidewell, C. Synthesis and Spectroscopic and Structural Characterization of Spiro[Indoline-3,3′-Indolizine]s Formed by 1,3-Dipolar Cycloadditions between Isatins, Pipecolic Acid and an Electron-Deficient Alkene. Acta Crystallogr. 2021, C77, 496–504. [Google Scholar] [CrossRef] [PubMed]
- The Clinical and Laboratory Standards Institute (CLSI) M100. Performance Standards for Antimicrobial Susceptibility Testing, 24th ed.; The Clinical and Laboratory Standards Institute (CLSI): Pittsburgh, PA, USA, 2024; ISBN 9781684401048. [Google Scholar]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics 2021, 10, 1545. [Google Scholar] [CrossRef]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Conceição, K.; Konno, K.; Richardson, M.; Antoniazzi, M.M.; Jared, C.; Daffre, S.; Camargo, A.C.M.; Pimenta, D.C. Isolation and Biochemical Characterization of Peptides Presenting Antimicrobial Activity from the Skin of Phyllomedusa hypochondrialis. Peptides 2006, 27, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Mejia-Gutierrez, M.; Vásquez-Paz, B.D.; Fierro, L.; Maza, J.R. In Silico Repositioning of Dopamine Modulators with Possible Application to Schizophrenia: Pharmacophore Mapping, Molecular Docking and Molecular Dynamics Analysis. ACS Omega 2021, 6, 14748–14764. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swiss ADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
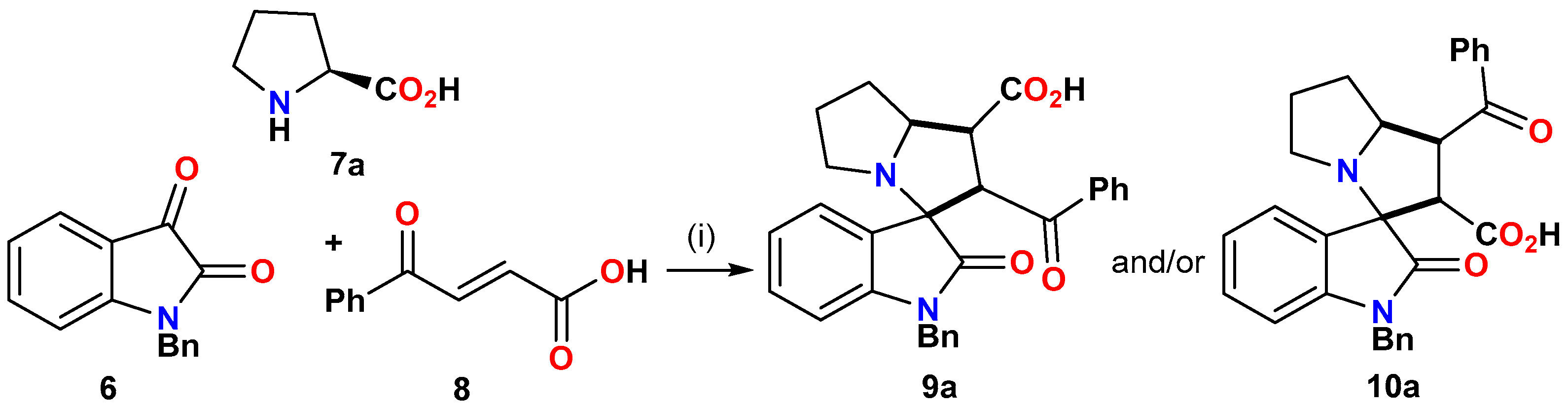






| Entry | Condition (i) | Time (h) | Yield of 9a/10a (%) |
|---|---|---|---|
| 1 | EtOH, reflux | 2 | 26 |
| 2 | ACN, reflux | 2 | 56 |
| 3 | H2O, reflux | 3 | 59 |
| 4 | MeOH, reflux | 2 | 61 |
| 5 | THF, reflux | 4 | 45 |
 | |||
|---|---|---|---|
| Compound | R1 | R2 | Yield of 9 (%) |
| 9a | Bn | H | 61 |
| 9b | CH3 | H | 44 |
| 9c | H | H | 72 |
| 9d * | n-Hexyl | H | 60 |
| 9e | CH3 | 5-Cl | 58 |
| 9f | Bn | 5-Cl | 41 |
| 9g | H | 5,7-di-Cl | 58 |
| 9h | H | 5-CH3 | 49 |
| 9i | H | 5-NO2 | 40 |
| 9j | Bn | 5,7-di-Cl | 86 |
| 9k | Bn | 5-F | 80 |
| 9l | H | 5-F | 71 |
| 9m | n-Hexyl | 5-Cl | 78 |
 | |||
|---|---|---|---|
| Compound | R1 | R2 | Yield of 11 (%) |
| 11a * | H | 5-CH3 | 48 |
| 11b * | H | 5-F | 68 |
| 11c * | CH3 | H | 49 |
| 11d | H | 5-Cl | 68 |
| 11e | Bn | H | 45 |
| 11f * | n-Hexyl | H | 48 |
| 11g * | CH3 | 5-Cl | 69 |
| 11h | H | H | 53 |
| 11i | Bn | 5-Cl | 67 |
| 11j | n-Hexyl | 5-Cl | 65 |
| Compound | R1 | R2 | Result |
|---|---|---|---|
| 9a | Bn | H | Not measured |
| 9b | CH3 | H | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, N. gonorrhoeae |
| 9c | H | H | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, N. gonorrhoeae |
| 9d | Hexyl | H | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, Active against N. gonorrhoeae MIC. 62.5 µg/mL |
| 9e | CH3 | 5-Cl | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, N. gonorrhoeae |
| 9f | Bn | 5-Cl | Not active against P. aeruginosa, K. pneumoniae, E. coli Active against S. aureus ATCC 25923 MIC: 8 µg/mL S. aureus ATCC 43300 MIC: 8 µg/mL N. gonorrhoeae MIC: 250 µg/mL |
| 9g | H | 5,7-di-Cl | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, N. gonorrhoeae |
| 9h | H | 5-CH3 | |
| 9i | H | 5-NO2 | Not measured |
| 9j | Bn | 5,7-di-Cl | Not active against P. aeruginosa, K. pneumoniae, E. coli Active against S. aureus ATCC 25923 MIC: >500 µg/mL S. aureus ATCC 43300 MIC > 500 µg/mL N. gonorrhoeae MIC. 125 µg/mL |
| 9k | Bn | 5-F | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, N. gonorrhoeae |
| 9l | H | 5-F | |
| 9m | Hexyl | 5-Cl | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, Active against N. gonorrhoeae MIC. 62.5 µg/mL |
| Product | R1 | R2 | Result |
|---|---|---|---|
| 11a | H | 5-CH3 | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, N. gonorrhoeae |
| 11b | H | 5-F | |
| 11c | CH3 | H | |
| 11d | H | 5-Cl | |
| 11e | Bn | H | |
| 11f | Hexyl | H | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus Active against N. gonorrhoeae MIC: 1mg/mL |
| 11g | CH3 | 5-Cl | Not active against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, N. gonorrhoeae |
| 11h | H | H | |
| 11i | Bn | 5-Cl | Not active against P. aeruginosa, K. pneumoniae, E. coli. Active against S. aureus ATCC 25923 MIC: 4 µg/mL S. aureus ATCC 43300 MIC: 16.12 µg/mL N. gonorrhoeae MIC: 500 µg/mL |
| 11j | Hexyl | 5-Cl | Not active against P. aeruginosa, K. pneumoniae, E. coli. Active against S. aureus ATCC 25923 MIC: >500 µg/mL S. aureus ATCC 43300 MIC > 500 µg/mL N. gonorrhoeae MIC 250 µg/mL |
| Compound | 9d | 9f | 9j | 9m | 11i | 11j |
|---|---|---|---|---|---|---|
| Hepatotoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Consensus scoring (CS) | 0.77 | 0.70 | 0.70 | 0.72 | 0.70 | 0.71 |
| Neurotoxicity | Active | Active | Active | Active | Active | Active |
| Consensus scoring (CS) | 0.62 | 0.73 | 0.73 | 0.75 | 0.77 | 0.77 |
| Cardiotoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Consensus scoring (CS) | 0.80 | 0.79 | 0.79 | 0.80 | 0.80 | 0.81 |
| Nephrotoxicity | Active | Active | Active | Active | Active | Active |
| Consensus scoring (CS) | 0.60 | 0.63 | 0.63 | 0.60 | 0.63 | 0.59 |
| Respiratory toxicity | Active | Active | Active | Active | Active | Active |
| Consensus scoring (CS) | 0.75 | 0.72 | 0.72 | 0.77 | 0.78 | 0.79 |
| Immunotoxicity | Active | Inactive | Inactive | Active | Inactive | Active |
| Consensus scoring (CS) | 0.71 | 0.76 | 0.65 | 0.88 | 0.85 | 0.81 |
| Carcinogenicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Consensus scoring (CS) | 0.56 | 0.61 | 0.61 | 0.59 | 0.62 | 0.60 |
| Mutagenicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Consensus scoring (CS) | 0.67 | 0.68 | 0.68 | 0.63 | 0.68 | 0.63 |
| Cytotoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Consensus scoring (CS) | 0.62 | 0.58 | 0.58 | 0.58 | 0.61 | 0.59 |
| Clinical toxicity | Active | Active | Active | Active | Active | Active |
| Consensus scoring (CS) | 0.69 | 0.74 | 0.74 | 0.67 | 0.78 | 0.70 |
| Blood brain barrier toxicity | Inactive | Active | Active | Inactive | Active | Active |
| Consensus scoring (CS) | 0.57 | 0.54 | 0.54 | 0.56 | 0.53 | 0.57 |
| GABA receptor event | Active | Inactive | Inactive | Active | Inactive | Active |
| Consensus scoring (CS) | 0.57 | 0.61 | 0.61 | 0.53 | 0.59 | 0.52 |
| Cytochrome inhibition (P450 2C9) | Inactive | Active | Active | Active | Active | Active |
| Consensus scoring (CS) | 0.50 | 0.57 | 0.57 | 0.60 | 0.55 | 0.57 |
| LD50(mg/Kg) | 208 | 769 | 769 | 500 | 729 | 325 |
| Toxicity class | 3 | 4 | 4 | 4 | 4 | 4 |
| Protein Target | Compound | Docking Score (Kcal/mol) a |
|---|---|---|
| 6TYM | 11e | −10.4 |
| 6TYM | 11i | −10.6 |
| 6TYM | Native ligand b (08A) | −10.7 |
| 4JYG | 9b | −10.5 |
| 4JYG | Native ligand (1NY) | −9.7 |
| 5KIR | 9c | −10.6 |
| 5KIR | 9l | −10.2 |
| 5KIR | Native ligand (RCX) | −6.4 |
| 5UZK | 9g | −10 |
| 5UZK | Native ligand (504) | −9.8 |
| 7Q6S | 9b | −10 |
| 7Q6S | 11e | −10.1 |
| 7Q6S | 11a | −10.1 |
| 7Q6S | 9h | −10 |
| 7Q6S | Native ligand (91M) | −12.6 |
| Compound | GI Absorption | MW | #H-Bond Acceptors | #H-Bond Donors | Log P | CYP2D6 Inhibitor | CYP3A4 Inhibitor |
|---|---|---|---|---|---|---|---|
| 9a | High | 466.53 | 5 | 1 | 2.74 | Yes | Yes |
| 9b | High | 390.43 | 5 | 1 | 1.47 | No | No |
| 9c | High | 376.41 | 5 | 2 | 1.37 | No | No |
| 9d | High | 460.56 | 5 | 1 | 3.28 | No | No |
| 9e | High | 424.88 | 5 | 1 | 2.10 | Yes | Yes |
| 9f | High | 500.97 | 5 | 1 | 3.24 | Yes | Yes |
| 9g | High | 445.3 | 5 | 2 | 2.48 | No | No |
| 9h | High | 390.43 | 5 | 2 | 1.69 | Yes | Yes |
| 9i | High | 421.4 | 7 | 2 | 0.63 | Yes | Yes |
| 9j | High | 535.42 | 5 | 1 | 3.72 | No | No |
| 9k | High | 484.52 | 6 | 1 | 3.01 | Yes | Yes |
| 9l | High | 394.4 | 6 | 2 | 1.68 | No | No |
| 9m | High | 495.01 | 5 | 1 | 3.76 | Yes | No |
| 11a | High | 404.46 | 5 | 2 | 1.98 | Yes | Yes |
| 11b | High | 408.42 | 6 | 2 | 1.98 | Yes | Yes |
| 11c | High | 404.46 | 5 | 1 | 1.82 | Yes | Yes |
| 11d | High | 424.88 | 5 | 2 | 2.16 | Yes | Yes |
| 11e | High | 480.55 | 5 | 1 | 2.91 | Yes | Yes |
| 11f | High | 474.59 | 5 | 1 | 3.55 | Yes | No |
| 11g | High | 438.9 | 5 | 1 | 2.41 | Yes | Yes |
| 11h | High | 390.43 | 5 | 2 | 1.62 | Yes | Yes |
| 11i | High | 515 | 5 | 1 | 3.51 | Yes | Yes |
| 11j | High | 509.04 | 5 | 1 | 4.02 | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romo, P.; Crespo, M.d.P.; Barreto, M.; Burbano, M.E.; Mejia-Gutierrez, M.; Quiroga, J.; Abonia, R. Pyrrolizine- and Indolizine-Derived Spirooxindoles: Synthesis, Antibacterial Activity and Inverse Docking Analysis. Chemistry 2025, 7, 18. https://doi.org/10.3390/chemistry7010018
Romo P, Crespo MdP, Barreto M, Burbano ME, Mejia-Gutierrez M, Quiroga J, Abonia R. Pyrrolizine- and Indolizine-Derived Spirooxindoles: Synthesis, Antibacterial Activity and Inverse Docking Analysis. Chemistry. 2025; 7(1):18. https://doi.org/10.3390/chemistry7010018
Chicago/Turabian StyleRomo, Pablo, María del Pilar Crespo, Mauricio Barreto, María Elena Burbano, Melissa Mejia-Gutierrez, Jairo Quiroga, and Rodrigo Abonia. 2025. "Pyrrolizine- and Indolizine-Derived Spirooxindoles: Synthesis, Antibacterial Activity and Inverse Docking Analysis" Chemistry 7, no. 1: 18. https://doi.org/10.3390/chemistry7010018
APA StyleRomo, P., Crespo, M. d. P., Barreto, M., Burbano, M. E., Mejia-Gutierrez, M., Quiroga, J., & Abonia, R. (2025). Pyrrolizine- and Indolizine-Derived Spirooxindoles: Synthesis, Antibacterial Activity and Inverse Docking Analysis. Chemistry, 7(1), 18. https://doi.org/10.3390/chemistry7010018







