Copper-Catalyzed Four-Component A3-Based Cascade Reaction: Facile Synthesis of 3-Oxetanone-Derived Spirocycles
Abstract
1. Introduction
2. Materials and Methods
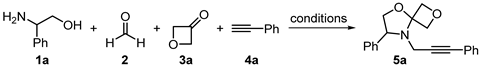
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liu, J.; Dransfield, P.J.; Zhu, L.; Wang, Z.; Du, X.; Jiao, X.; Su, Y.; Li, A.; Brown, S.P.; et al. Discovery and Optimization of Potent GPR40 Full Agonists Containing Tricyclic Spirocycles. ACS Med. Chem. Lett. 2013, 4, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.W.; Puentes, L.N.; Wilson, K.; Chia-Ju Hsieh, C.-J.; Weng, C.-C.; Makvandi, M.; Mach, R.H. Examination of Diazaspiro Cores as Piperazine Bioisosteres in the Olaparib Framework Shows Reduced DNA Damage and Cytotoxicity. J. Med. Chem. 2018, 61, 5367–5379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Song, X.; Pan, Y.; Li, M.; Chen, L.; Xiao, P.; Du, R.; Dong, Z.; Yang, G. Design, Synthesis, and Biological Evaluation of Novel Spirocyclic Compounds as Potential Anti-Glioblastoma Agents. Eur. J. Med. Chem. 2023, 258, 115595. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.A.; Croft, R.A.; Davis, O.A.; Doran, R.; Morgan, K.F. Oxetanes: Recent Advances in Synthesis, Reactivity, and Medicinal Chemistry. Chem. Rev. 2016, 116, 12150–12233. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.J.; Bull, J.A. Oxetanes in Drug Discovery Campaigns. J. Med. Chem. 2023, 66, 12697–12709. [Google Scholar] [CrossRef]
- Huang, G.; Hucek, D.; Cierpicki, T.; Grembecka, J. Applications of Oxetanes in Drug Discovery and Medicinal Chemistry. Eur. J. Med. Chem. 2023, 261, 115802. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Thornton, P.D.; Painter, T.O.; Jain, P.; Downard, J.; Douglas, J.T.; Santini, C. Synthesis of a Family of Spirocyclic Scaffolds: Building Blocks for the Exploration of Chemical Space. J. Org. Chem. 2013, 78, 6529–6539. [Google Scholar] [CrossRef] [PubMed]
- Carreira, E.M.; Fessard, T.C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Tian, R.; Duan, Z. Activation of CS2 with the 2H-Phosphindole Complex to Construct P,S-Polycycles. Org. Lett. 2022, 24, 6117–6121. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Z.; Wang, P.; Shi, Z.; Li, T.; Cui, X. Ruthenium(II)-Catalyzed Regioselective [3+2] Spiroannulation of 2H-Imidazoles with 2-Alkynoates. Org. Lett. 2020, 22, 6272–6276. [Google Scholar] [CrossRef]
- Marchese, A.D.; Durant, A.G.; Lautens, M. A Modular Approach for the Palladium-Catalyzed Synthesis of Bis-heterocyclic Spirocycles. Org. Lett. 2022, 24, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Larduinat, M.; Dokmak, E.; Verrier, C.; Sylvie, M.S.; Popowycz, F. From 5-HMF to Novel Cyclopentenone-Based Aza Spirocycles: An Intramolecular Aza-Piancatelli Reaction in Action. J. Org. Chem. 2024, 89, 9661–9665. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Proud, M.; Sridharan, V. Synthesis of Oxetane/Azetidine Containing Spirocycles via the 1,3-Dipolar Cycloaddition Reaction. Tetrahedron Lett. 2016, 57, 2811–2813. [Google Scholar] [CrossRef]
- Cook, J.; Zusi, F.C.; McDonald, I.M.; King, D.; Hill, M.D.; Iwuagwu, C.; Mate, R.A.; Fang, H.Q.; Zhao, R.L.; Wang, B.; et al. Design and Synthesis of a New Series of 4-Heteroarylamino-1′-Azaspiro[Oxazole-5,3′-Bicyclo[2.2.2]Octanes as α7 Nicotinic Receptor Agonists. 1. Development of Pharmacophore and Early Structure–Activity Relationship. J. Med. Chem. 2016, 59, 11171–11181. [Google Scholar]
- Wuitschik, G.; Carreira, E.M.; Wagner, B.; Fischer, H.; Parrilla, I.; Schuler, F.; Mark, R.E.; Müller, K. Oxetanes in Drug Discovery: Structural and Synthetic Insights. J. Med. Chem. 2010, 53, 3227–3246. [Google Scholar] [CrossRef]
- Vasylyev, M.; Alper, H. Diastereoselective Synthesis of Hexahydropyrrolo[2,1-b]Oxazoles by a Rhodium-Catalyzed Hydroformylation/Silica-Promoted Deformylation Sequence. Angew. Chem. Int. Ed. 2009, 48, 1287–1290. [Google Scholar] [CrossRef]
- Waller, R.W.; Diorazio, L.J.; Taylor, B.A.; Motherwell, W.B.; Sheppard, T.D. Isocyanide Based Multicomponent Reactions of Oxazolidines and Related Systems. Tetrahedron 2010, 66, 6496–6507. [Google Scholar] [CrossRef]
- Vasylyev, M.; Alper, H. Synthesis of Morpholin-2-ones by Chemoselective Intramolecular Rhodium-Catalyzed Reductive Ring Expansion of Oxazolidines. Org. Lett. 2008, 10, 1357–1359. [Google Scholar] [CrossRef]
- Ruider, S.A.; Müller, S.; Carreira, E.M. Ring Expansion of 3-Oxetanone-Derived Spirocycles: Facile Synthesis of Saturated Nitrogen Heterocycles. Angew. Chem. Int. Ed. 2013, 52, 11908–11911. [Google Scholar] [CrossRef]
- Brady, P.B.; Carreira, E.M. Addition of Trifluoroborates to Oxetanyl N,O-Acetals: Entry into Spiro and Fused Saturated Heterocycles. Org. Lett. 2015, 17, 3350–3353. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Tesch, M.; Wang, L.; Daniliuc, C.G.; Studer, A. Synthesis of a Bulky Nitroxide and Its Application in the Nitroxidemediated Radical Polymerization. Tetrahedron 2016, 72, 7665–7671. [Google Scholar] [CrossRef]
- Adarsh, D.R.; Ravi Teja, T.; Sridhar, B.; Subba Reddy, B.V. Highly Diastereoselective Total Synthesis of Vibegron, a Drug for Overactive Bladder Disease. Tetrahedron Lett. 2025, 155, 155429. [Google Scholar] [CrossRef]
- Tanaka, K.; Matsumoto, R.; Pradipta, A.R.; Kitagawa, Y.; Okumura, M.; Manabe, Y.; Fukase, K. Facile Preparation of 1,5-Diazacyclooctanes from Unsaturated Imines: Effects of the Hydroxyl Groups on [4+4] Dimerization. Synlett 2014, 25, 1026–1030. [Google Scholar] [CrossRef]
- Jha, R.R.; Danodia, A.K.; Kumar, S.; Verma, A.K. Au(III)-Catalyzed Regio- and Stereoselective Tandem Synthesis of Oxazolo Fused Naphthyridines and Isoquinolines from O-Alkynylaldehydes. Tetrahedron Lett. 2014, 55, 610–615. [Google Scholar] [CrossRef]
- Feng, H.; Wang, F.; Cao, L.; Van der Eycken, E.V.; Yin, X. Switchable Mono- and Dipropargylation of Amino Alcohols: A Unique Property of the Iodide Anion in Controlling Ring-Opening Alkynylation. Eur. J. Org. Chem. 2021, 2021, 3676–3680. [Google Scholar] [CrossRef]
- Xu, X.; Feng, H.; Zhang, X.; Song, L.; Van Meervelt, L.; Van der Eycken, J.; Harvey, J.N.; Van der Eycken, E.V. Pd-Catalyzed Ring Restructuring of Oxazolidines with Alkenes Leading to Fused Polycyclic Indolizines. Org. Lett. 2022, 24, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Xu, X.; Zhong, L.; Cao, L.; Pan, Y.; Huang, J.; Feng, H. Metal- and Oxidant-Free Skeletal Reorganizing of Oxazolidines to Access N-Vinylpyrroles. Sustain. Chem. Pharm. 2024, 42, 101768. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Li, X.; Wang, L.; Feng, H. Chemo- and Diastereoselective Synthesis of N-Propargyl Oxazolidines through a Copper-Catalyzed Domino A3 Reaction. J. Org. Chem. 2019, 84, 5046–5055. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Shi, R.; Huang, L.; Huang, J.; Feng, H. Synthesis of Oxazolines through a Pd-Catalyzed Alkyne-Enabled C–N Activation of Oxazolidines. Tetrahedron Lett. 2024, 134, 154882. [Google Scholar] [CrossRef]
- Calleja, J.; Pla, D.; Gorman, T.W.; Domingo, V.; Haffemayer, B.; Gaunt, M.J. A Steric Tethering Approach Enables Palladium-Catalysed C–H Activation of Primary Aamino Alcohols. Nat. Chem. 2015, 7, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, G.; Antonio, G.D.; Fiocchi, R.; Giuli, S.; Marcantoni, E.; Marcolini, M. Synthesis of an (R)-Garner-type Aldehyde from L-Serine: Useful Building Block for a (+)-Furanomycin Derivative. Synthesis 2009, 6, 0951–0956. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Basu, A.J.; Hsu, C.-S.; Monika, Y. Asymmetric 1,2-Carbamoyl Rearrangement of Lithiated Chiral Oxazolidine Carbamates and Diastereoselective Synthesis of α-Hydroxy Amides. Chem. Eur. J. 2022, 28, e202200941. [Google Scholar] [CrossRef]
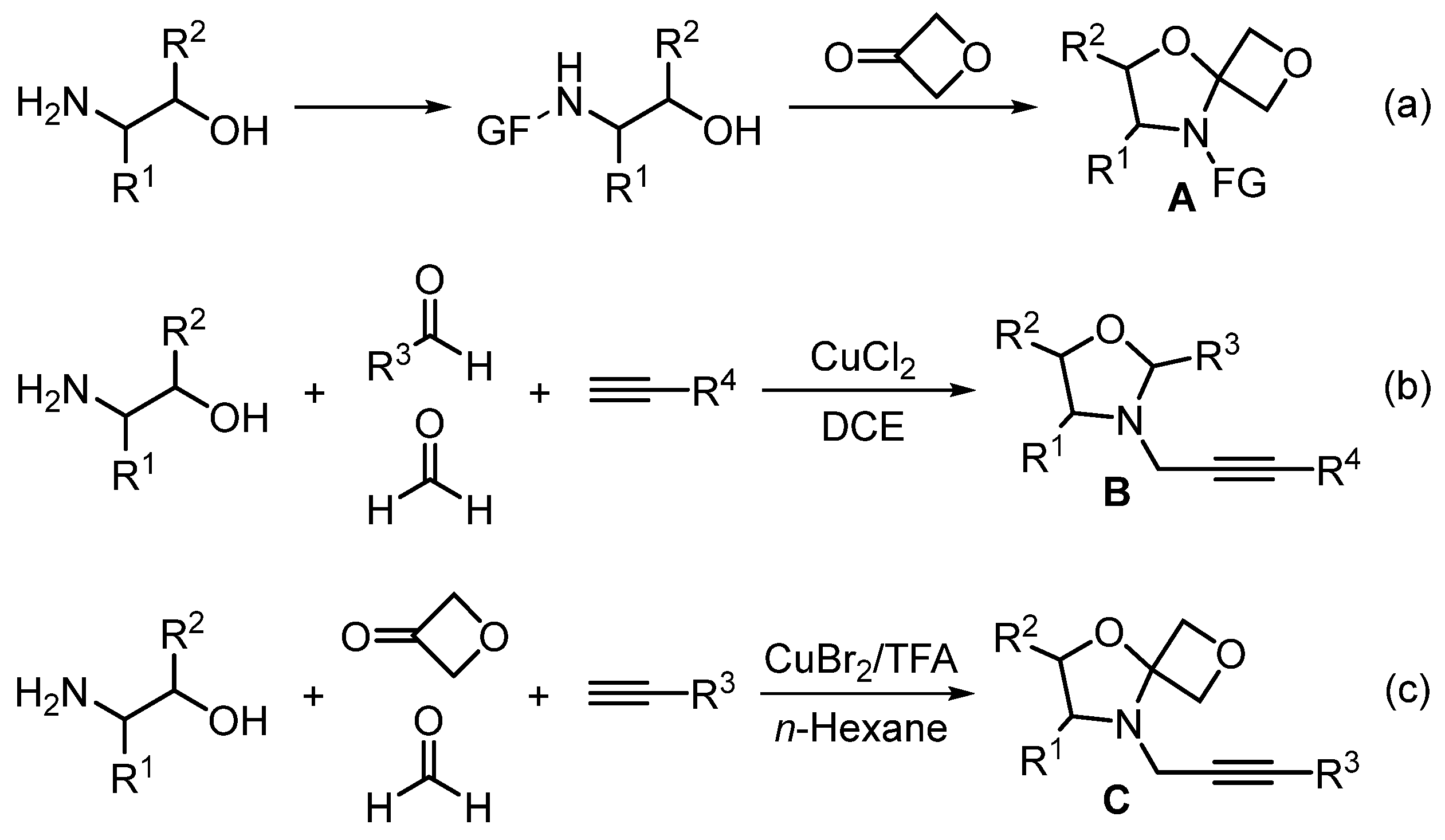
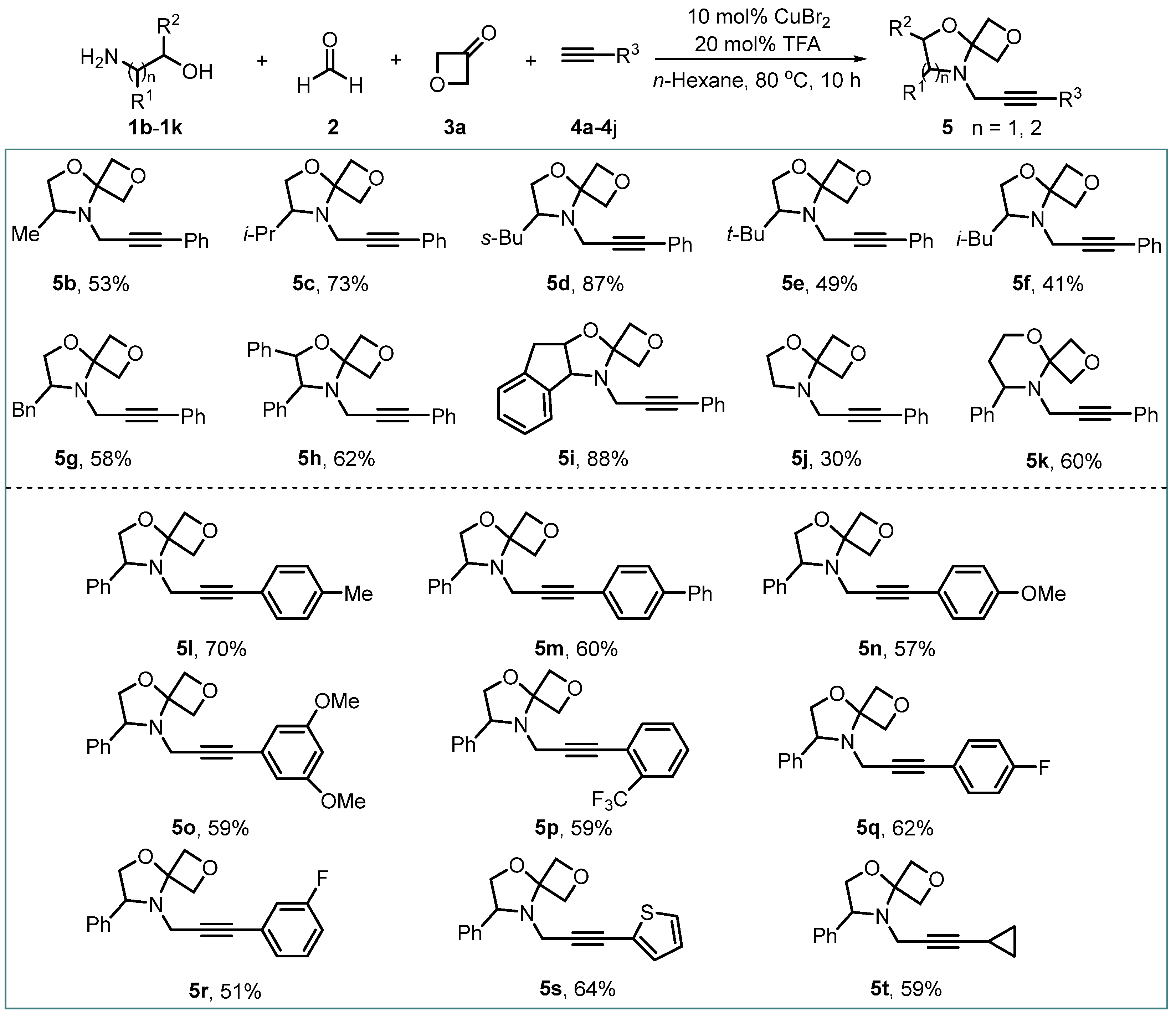
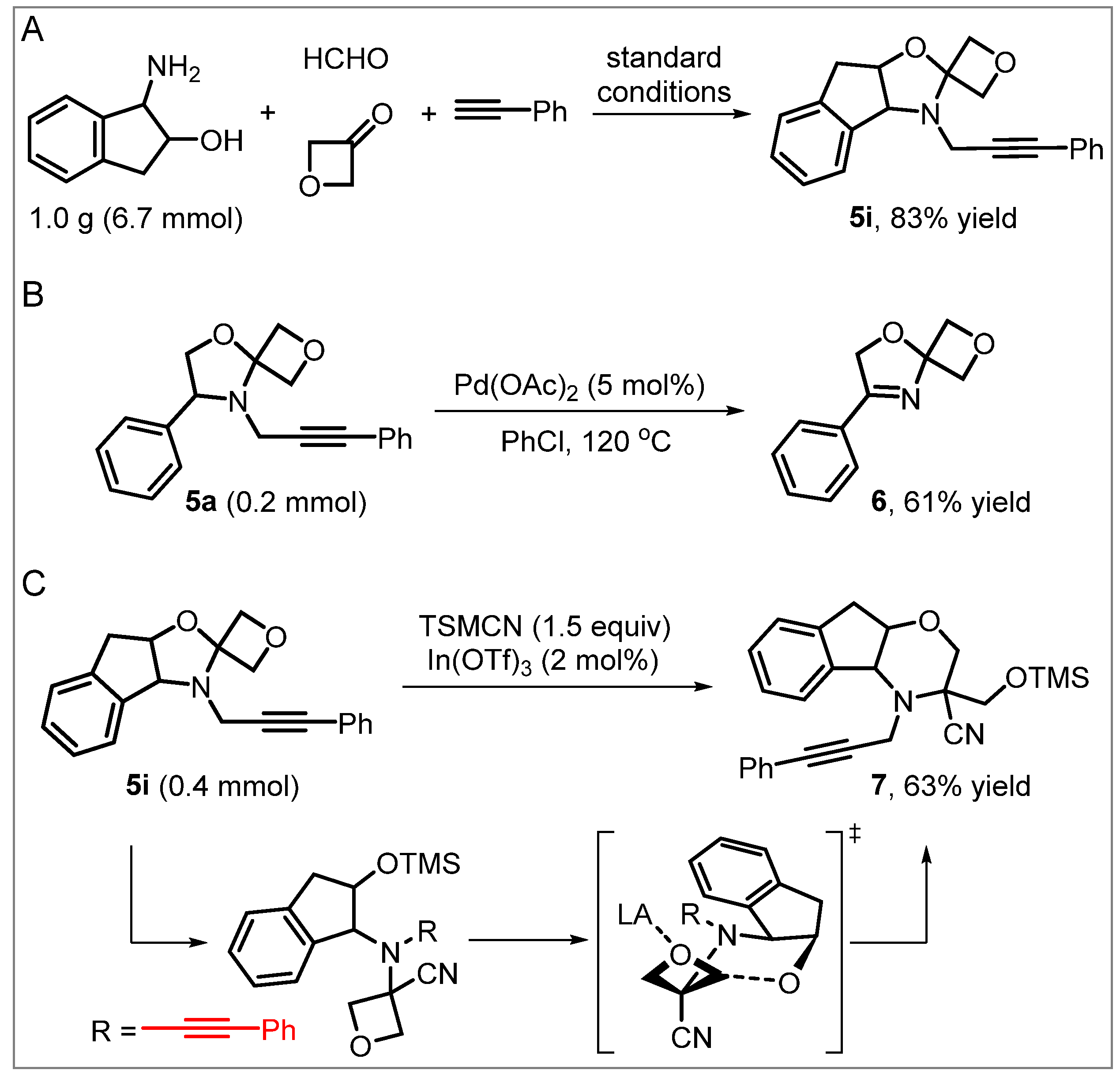
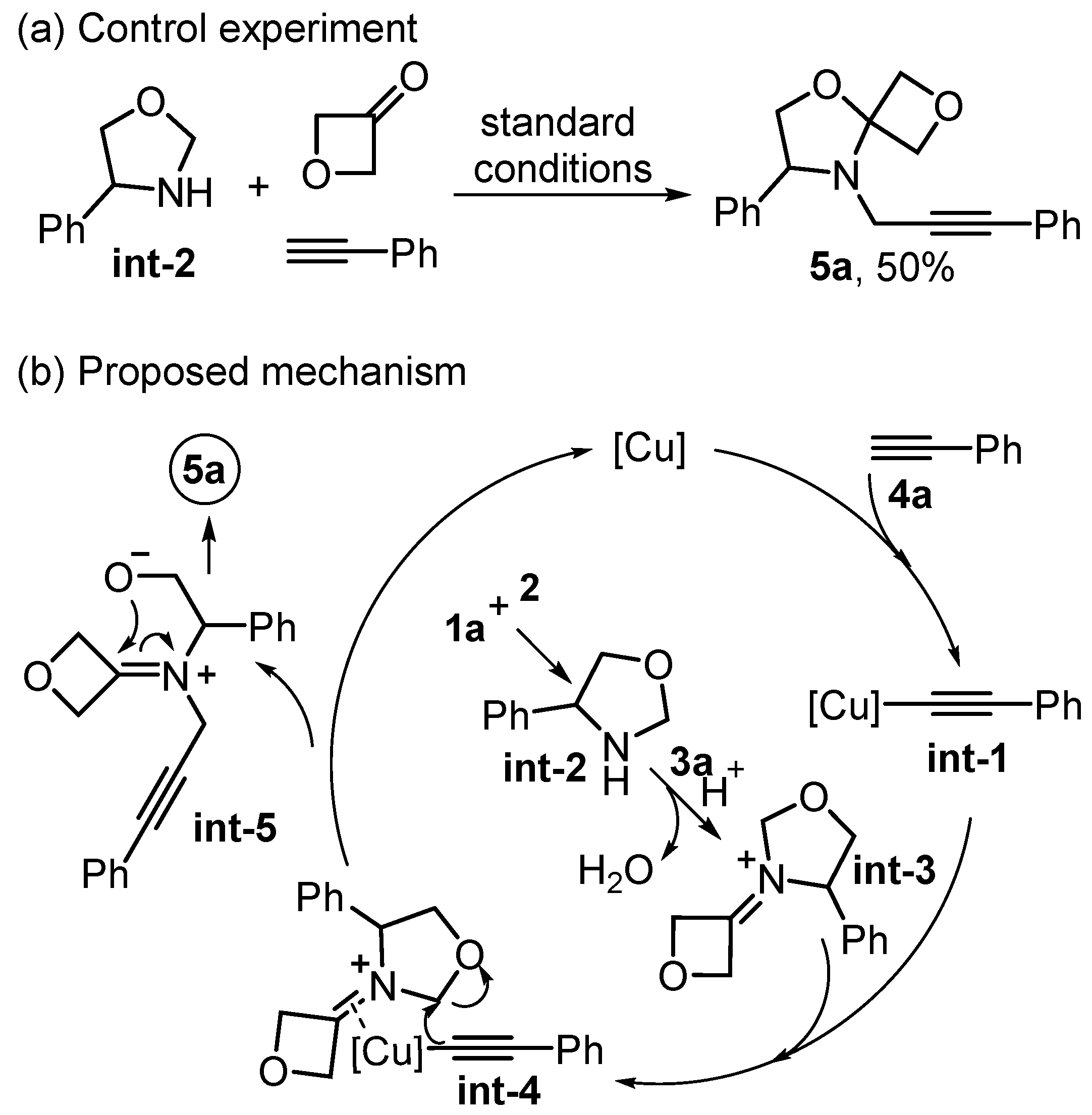
| Entry | Catalyst (mol%) | Solvent | Temperature (oC) | Time (h) | Yield (%) 2 |
|---|---|---|---|---|---|
| 1 | CuCl2 (20) | DCE | 80 | 10 | 34 |
| 2 | CuBr2 (20) | DCE | 80 | 10 | 41 |
| 3 | CuBr (20) | DCE | 80 | 10 | 28 |
| 4 | CuBr2 (30) | DCE | 80 | 10 | 40 |
| 5 | CuBr2 (10) | DCE | 80 | 10 | 54 |
| 6 | CuBr2 (10) | CHCl3 | 60 | 10 | 48 |
| 7 | CuBr2 (10) | 1,4-dioxane | 80 | 10 | 51 |
| 8 | CuBr2 (10) | toluene | 80 | 10 | 30 |
| 9 | CuBr2 (10) | CH3CN | 80 | 10 | trace |
| 10 | CuBr2 (10) | THF | 80 | 10 | 31 |
| 11 | CuBr2 (10) | petroleum ether | 80 | 10 | 57 |
| 12 | CuBr2 (10) | n-heptane | 80 | 10 | 55 |
| 13 | CuBr2 (10) | n-hexane | 80 | 10 | 59 |
| 14 | CuBr2 (10)/TFA (20) | n-hexane | 80 | 10 | 76 |
| 15 | CuBr2 (10)/AcOH (20) | n-hexane | 80 | 10 | 70 |
| 16 | CuBr2 (10)/conc.HCl (20) | n-hexane | 80 | 10 | 63 |
| 17 | CuBr2 (10)/TFA (10) | n-hexane | 80 | 10 | 73 |
| 18 | CuBr2 (10)/TFA (30) | n-hexane | 80 | 10 | 69 |
| 19 | CuBr2 (10)/TFA (20) | n-hexane | 70 | 10 | 70 |
| 20 | CuBr2 (10)/TFA (20) | n-hexane | 90 | 10 | 71 |
| 21 | CuBr2 (10)/TFA (20) | n-hexane | 80 | 8 | 66 |
| 22 | CuBr2 (10)/TFA (20) | n-hexane | 80 | 12 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Huang, L.; Gu, A.; Feng, H. Copper-Catalyzed Four-Component A3-Based Cascade Reaction: Facile Synthesis of 3-Oxetanone-Derived Spirocycles. Chemistry 2025, 7, 19. https://doi.org/10.3390/chemistry7010019
Zhang R, Huang L, Gu A, Feng H. Copper-Catalyzed Four-Component A3-Based Cascade Reaction: Facile Synthesis of 3-Oxetanone-Derived Spirocycles. Chemistry. 2025; 7(1):19. https://doi.org/10.3390/chemistry7010019
Chicago/Turabian StyleZhang, Rongkang, Liliang Huang, Aiguo Gu, and Huangdi Feng. 2025. "Copper-Catalyzed Four-Component A3-Based Cascade Reaction: Facile Synthesis of 3-Oxetanone-Derived Spirocycles" Chemistry 7, no. 1: 19. https://doi.org/10.3390/chemistry7010019
APA StyleZhang, R., Huang, L., Gu, A., & Feng, H. (2025). Copper-Catalyzed Four-Component A3-Based Cascade Reaction: Facile Synthesis of 3-Oxetanone-Derived Spirocycles. Chemistry, 7(1), 19. https://doi.org/10.3390/chemistry7010019







