Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film
Abstract
1. Introduction
2. Materials and Methods
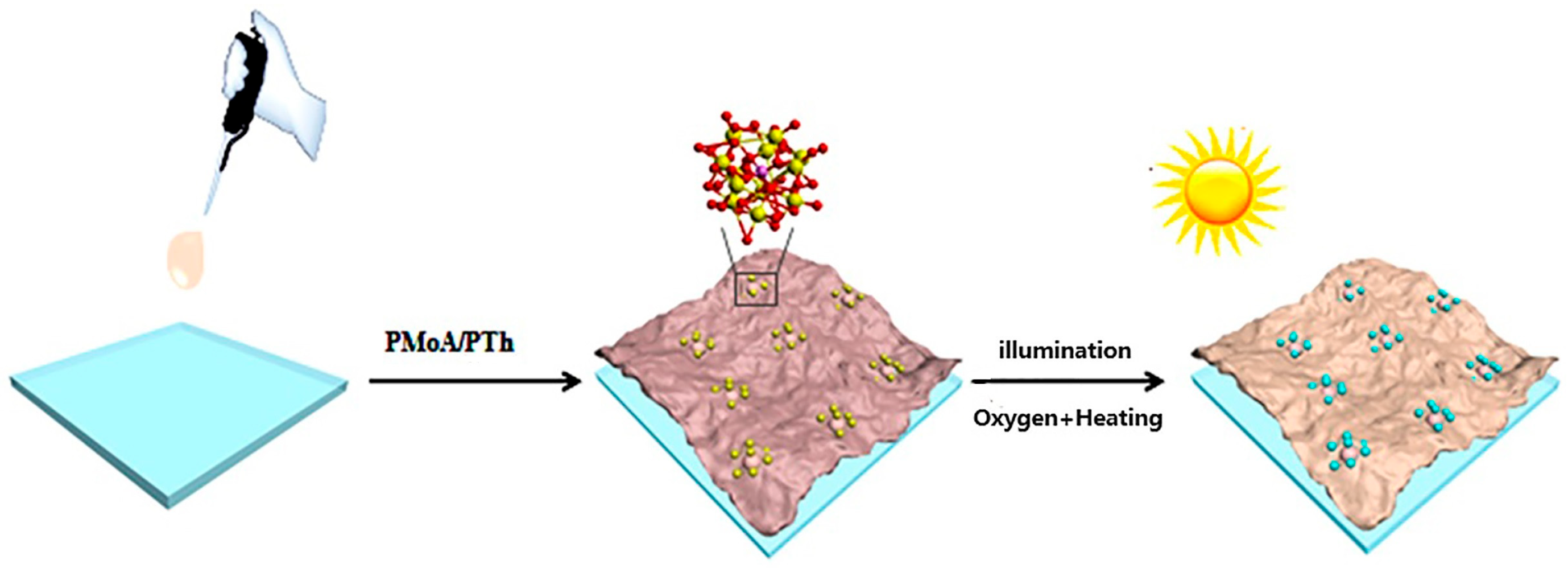
2.1. Preparation
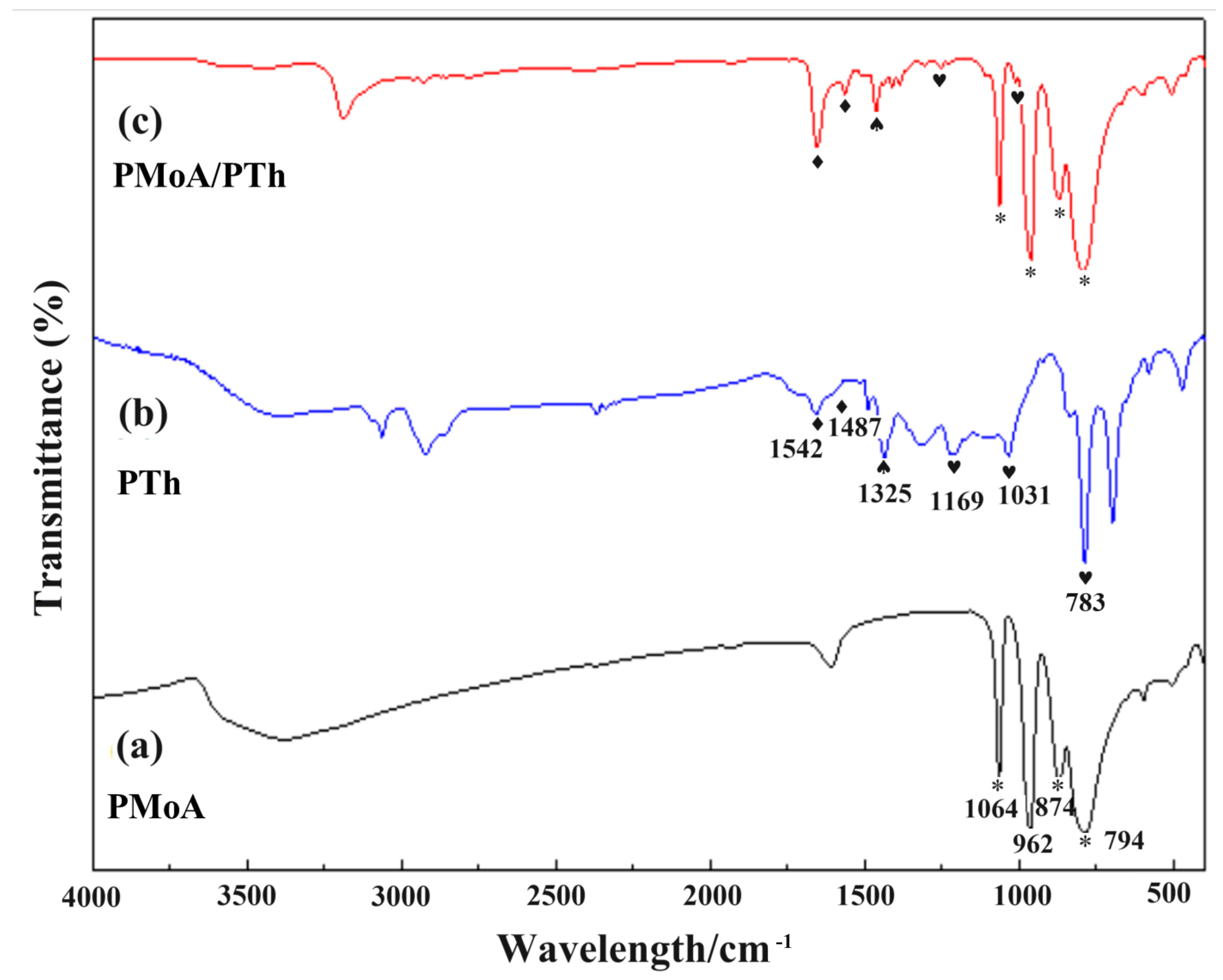
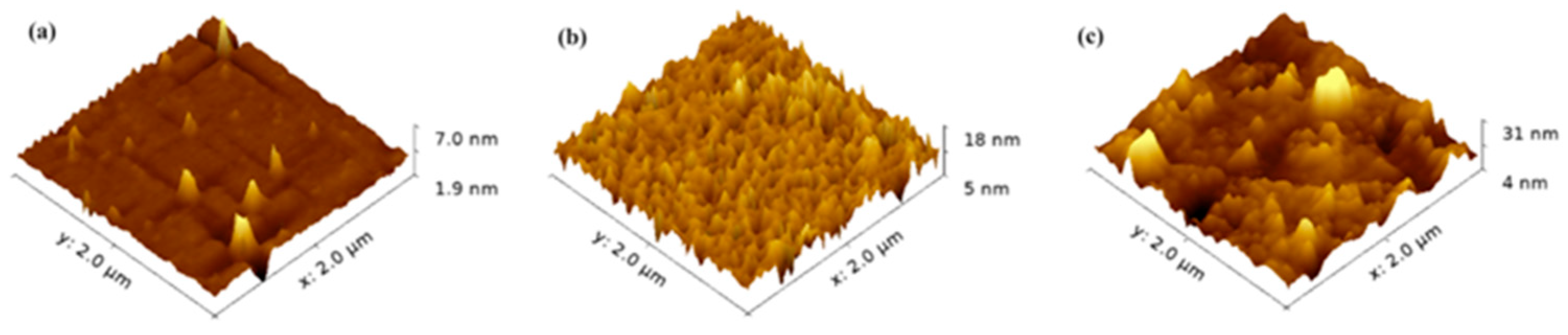
2.2. Characterization
2.3. Experiments
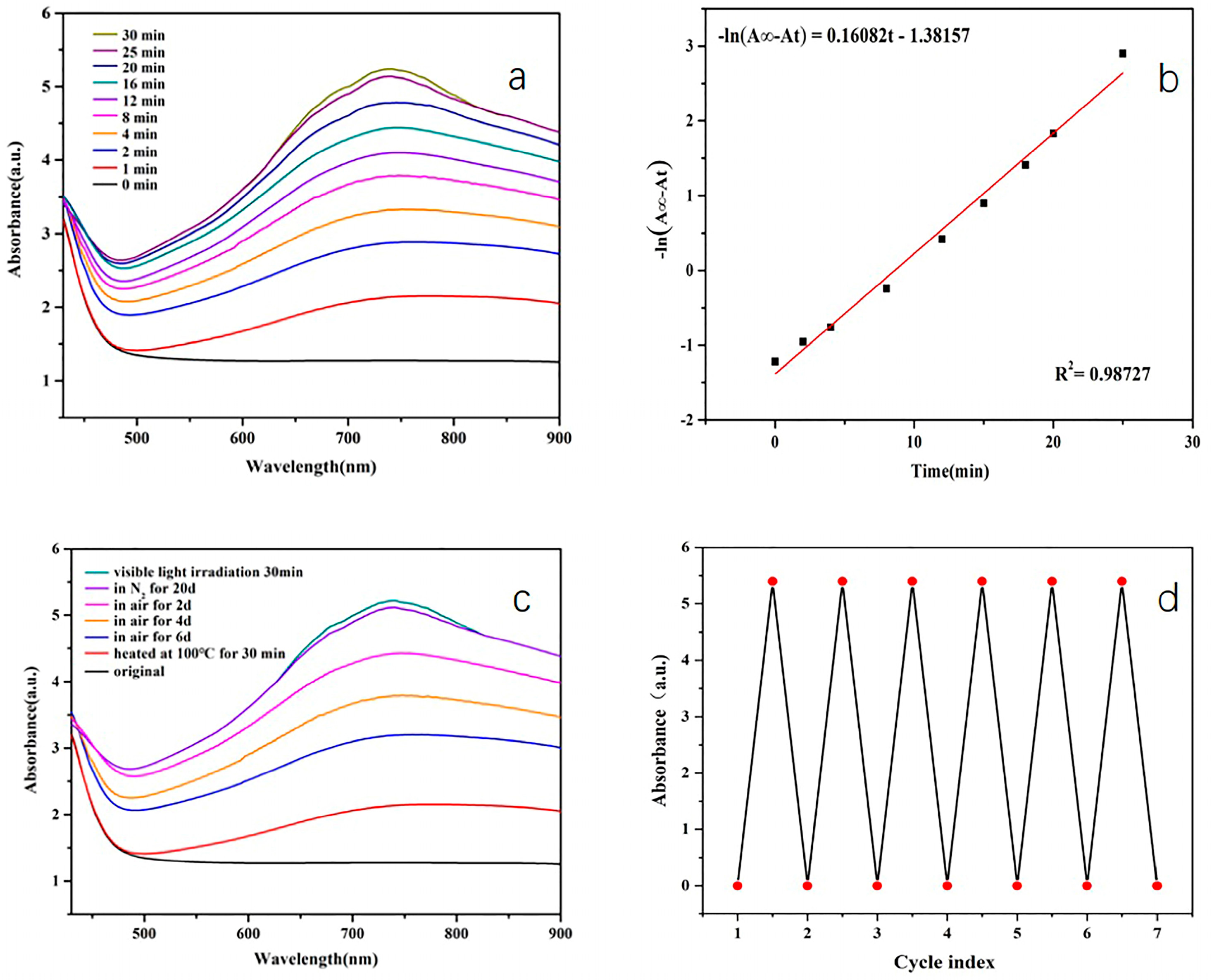
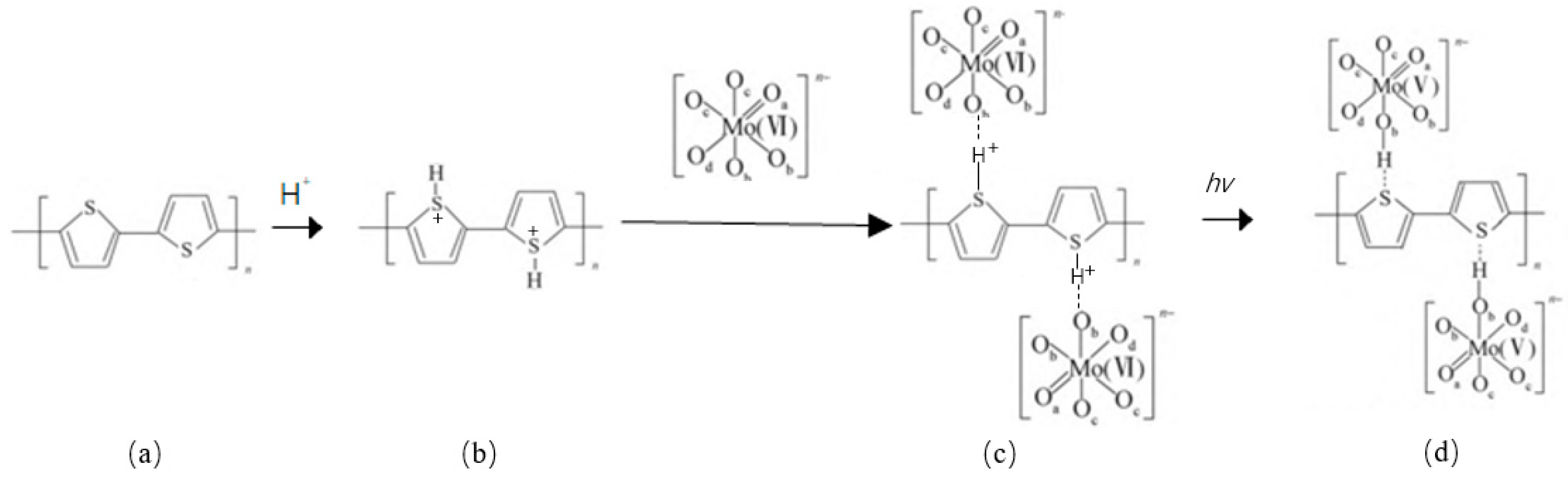
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, B.; Chen, H.Y.; Liang, H.; Fu, L.; Liu, X.; Qiu, X.; Liu, S.; Song, R.; Tang, Z. Reversible photoswitchable fluorescence in thin films of inorganic nanoparticle and polyoxometalate assemblies. J. Am. Chem. Soc. 2010, 132, 2886–2888. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.X.; Bi, L.H.; Fu, Y.; Wang, N.; Liu, S.; Tang, Z. Multistate electrically controlled photoluminescence switching. Chem. Sci. 2013, 4, 4371–4377. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Ma, P.; Singh, V.; Zhang, C.; Niu, J.; Wang, J. Photochromic behavior of a new polyoxomolybdate/alkylamine composite in solid state. J. Mater. Sci. 2018, 53, 3078–3086. [Google Scholar] [CrossRef]
- Graca, V.C.; Sousa, C.M.; Coelho, P. Towards grey coloring photochromic materials using vinylidene-naphthofurans. Dye. Pigment. 2020, 176. [Google Scholar] [CrossRef]
- Bao, H.F.; Wang, X.Y.; Yang, G.Q.; Li, H.; Zhang, F.; Feng, W. UV-light and visible-light photochromism of inorganic–organic multilayer films based on polyoxometalate and poly(acrylamide). Colloid Polym. Sci. 2014, 292, 2883–2889. [Google Scholar] [CrossRef]
- Jing, X.F.; Zou, D.L.; Meng, Q.Q.; Zhang, W.; Zhang, F.; Feng, W.; Han, X. Fabrication and visible-light photochromism of novel hybrid inorganic–organic film based on polyoxometalates and ethyl cellulose. Inorg. Chem. Commun. 2014, 46, 149–154. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Q.; Tian, H. Photochromic materials: More than meets the eye. Adv. Mater. 2013, 25, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Toshihiro, Y. Photo- and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–326. [Google Scholar]
- Chen, J.; MeiAi, L.; Feng, W.; Xiong, D.Q.; Liu, Y.; Cai, W.M. Preparation and photochromism of nanocomposite thin film based on polyoxometalate and polyethyleneglycol. Mater. Lett. 2007, 61, 5247–5249. [Google Scholar] [CrossRef]
- Zeng, O.R.; Guo, S.Y.; Sun, Y.B.; Li, Z.; Feng, W. Protonation Induced Enhanced Optical-Light Photochromic Properties of an lnorganic-Organic Phosphomolybdic Acid/Polyaniline Hybrid Thin Film. Nanomaterials 2020, 10, 1839. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Sun, Y.; Liu, J.L.; Wang, X.; Liu, S.L.; Feng, W. Enhanced photochromism of heteropolyacid/polyvinylpyrolidone composite film by TiO2 doping. J. Appl. Polym. Sci. 2015, 132, 41583. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Lu, Y.; Xuan, L.; Xia, S.; Feng, W.; Han, X. Preparation and visible-light photochromism of phosphomolybdic acid/polyvinylpyrrolidone hybrid film. Chem. Res. Chin. Univ. 2014, 30, 703–708. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Dong, Z.; Feng, W. Phosphomolybdic acid-modified highly organized TiO2 nanotube arrays with rapid photochromic performance. J. Mater. Sci. Technol. 2019, 35, 1951–1958. [Google Scholar] [CrossRef]
- Yue, T.; Han, B.; Wang, X.; Bai, L.; Feng, W. Instantaneous visible-light photochromic performance of composite powders based on PMoA and ZnO nanotubes. Chem. Lett. 2019, 48, 851–854. [Google Scholar] [CrossRef]
- Liu Qi Hu Ch Wang, X. Hydrothermal synthesis of oxygen-deficiency tungsten oxide quantum dots with excellent photochromic reversibility. Appl. Surf. Sci. 2019, 480, 404–409. [Google Scholar]
- Li, D.; Wei, J.; Dong, S.; Li, H.; Xia, Y.; Jiao, X.; Wang, T.; Chen, D. Novel PVP/HTA hybrids for multifunctional rewritable paper. ACS Appl. Mater. Interfaces 2018, 10, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Qaid, S.M.; Alharbi, F.H.; Bedja, I.; Nazeeruddin, M.K.; Aldwayyan, A.S. Reducing Amplified Spontaneous Emission Threshold in CsPbBr3 Quantum Dot Films by Controlling TiO2 Compact Layer. Nanomaterials 2020, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Li, J.; Deng, Q.; Gao, Y. Preparation, characterization, photochromic properties, and mechanism of PMoA/ZnO/PVP composite film. Molecules 2023, 28, 7605. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, A.K.; Devi, M.; Gonsalves, K.E.; Pradeep, C.P. Engineering Multifunctionality in Hybrid Polyoxometalates: Aromatic Sulfonium Octamolybdates as Excellent Photochromic Materials and Self-Separating Catalysts for Epoxidation. Inorg. Chem. 2017, 56, 10325–10336. [Google Scholar] [CrossRef] [PubMed]
| Photochromic Material | The Thickness of Samples | The Maximum Absorbance | Reference |
|---|---|---|---|
| PMoA/PANI Hybridizing Thin Film | 1.8 μm | 3.46 | [10] |
| ZnO/PMoA | - | 0.21 | [14] |
| WO3-x QDs | - | 2.75 | [15] |
| PVP/HTA hybrids | - | 0.78 | [16] |
| CsPbBr3 Quantum Dot Films | - | 0.78 | [17] |
| PMoA/ZnO/PVP composite film | - | 0.32 | [18] |
| Aromatic Sulfonium Octamolybdates | solid-state | 3.2 | [19] |
| PMoA/PTh composite film | 2.0 μm | 5.27 | This work |
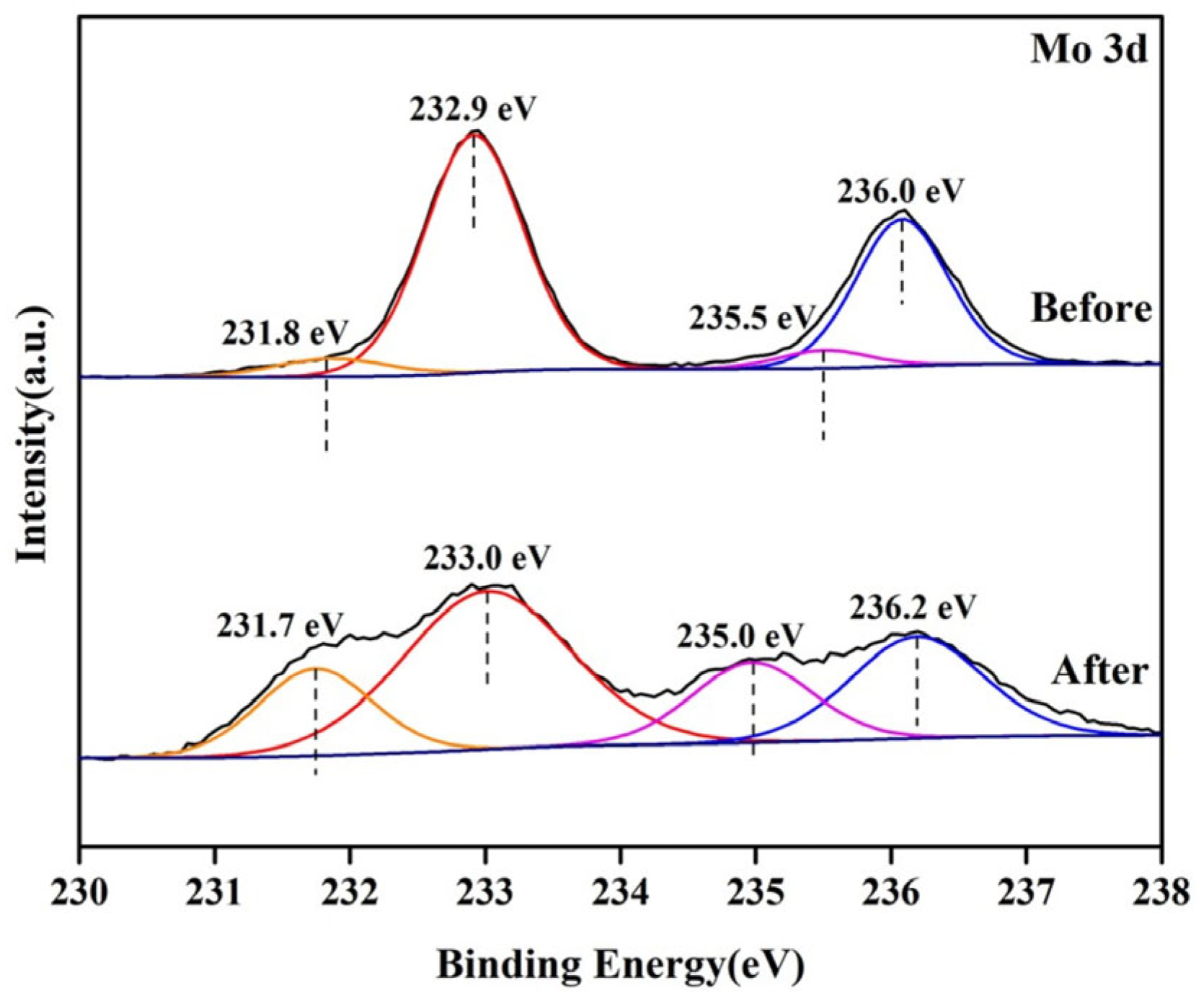
| Sample | Mo5+ | Mo6+ | Mo5+/Mo Ratios | ||
|---|---|---|---|---|---|
| 3d3/2 | 3d5/2 | 3d3/2 | 3d5/2 | ||
| Before | 231.8 | 235.5 | 232.9 | 236.0 | 0.09 |
| After | 231.7 | 235.0 | 233.0 | 236.2 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zhao, H.; Feng, W.; Zhao, H. Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film. Chemistry 2024, 6, 469-475. https://doi.org/10.3390/chemistry6030026
Zhao W, Zhao H, Feng W, Zhao H. Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film. Chemistry. 2024; 6(3):469-475. https://doi.org/10.3390/chemistry6030026
Chicago/Turabian StyleZhao, Wanqing, Hongmei Zhao, Wei Feng, and Honggang Zhao. 2024. "Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film" Chemistry 6, no. 3: 469-475. https://doi.org/10.3390/chemistry6030026
APA StyleZhao, W., Zhao, H., Feng, W., & Zhao, H. (2024). Visible-Light Photochromic Properties of an Inorganic-Organic Phosphomolybdic Acid/Polythiophene Hybrid Thin Film. Chemistry, 6(3), 469-475. https://doi.org/10.3390/chemistry6030026






