Recognizing New Types of Stacking Interactions by Analyzing Data in the Cambridge Structural Database
Abstract
1. Introduction
2. Stacking Interactions of Chelate Rings
2.1. Chelate–Aryl Stacking Interactions
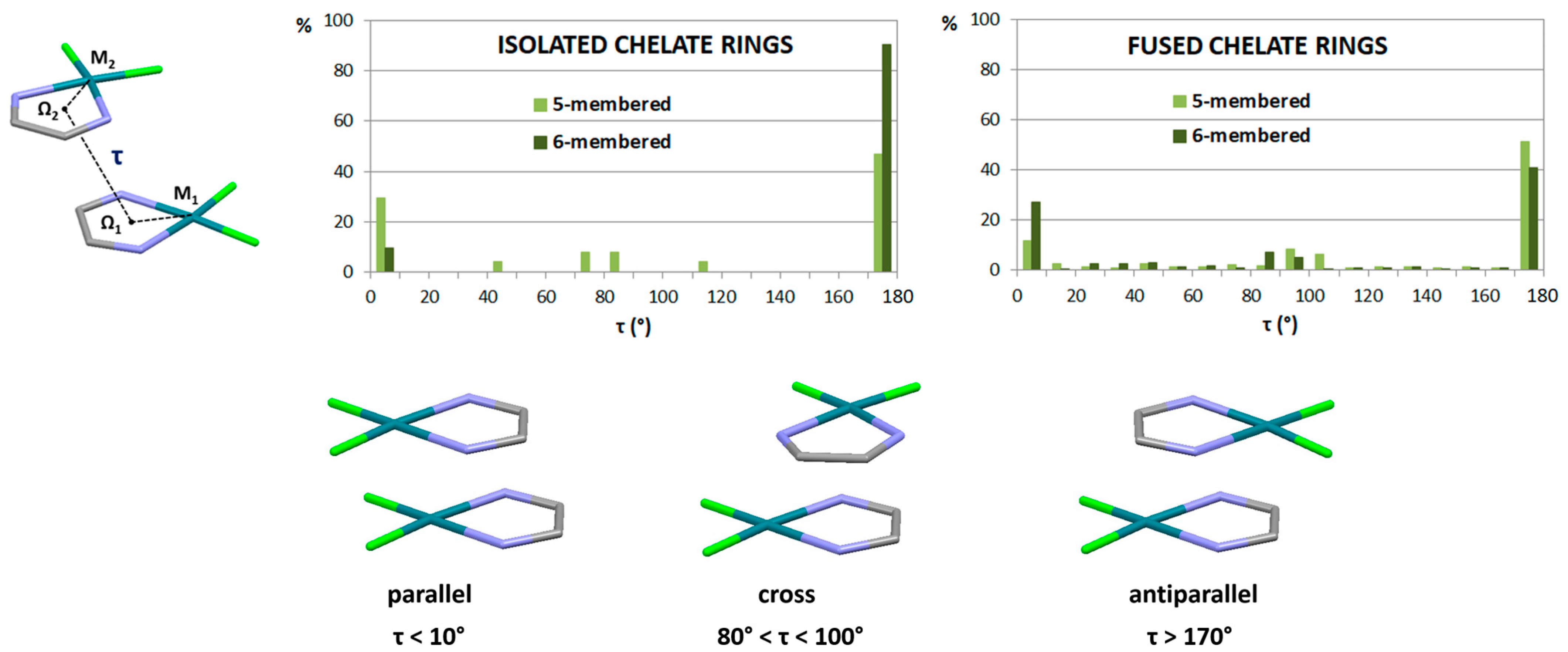
2.2. Chelate–Chelate Stacking Interactions
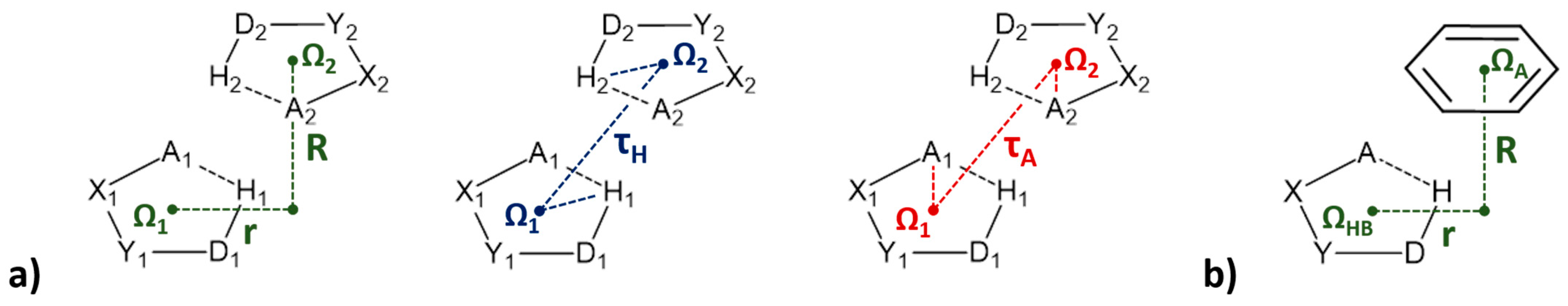
3. Stacking Interactions of Hydrogen-Bridged Rings
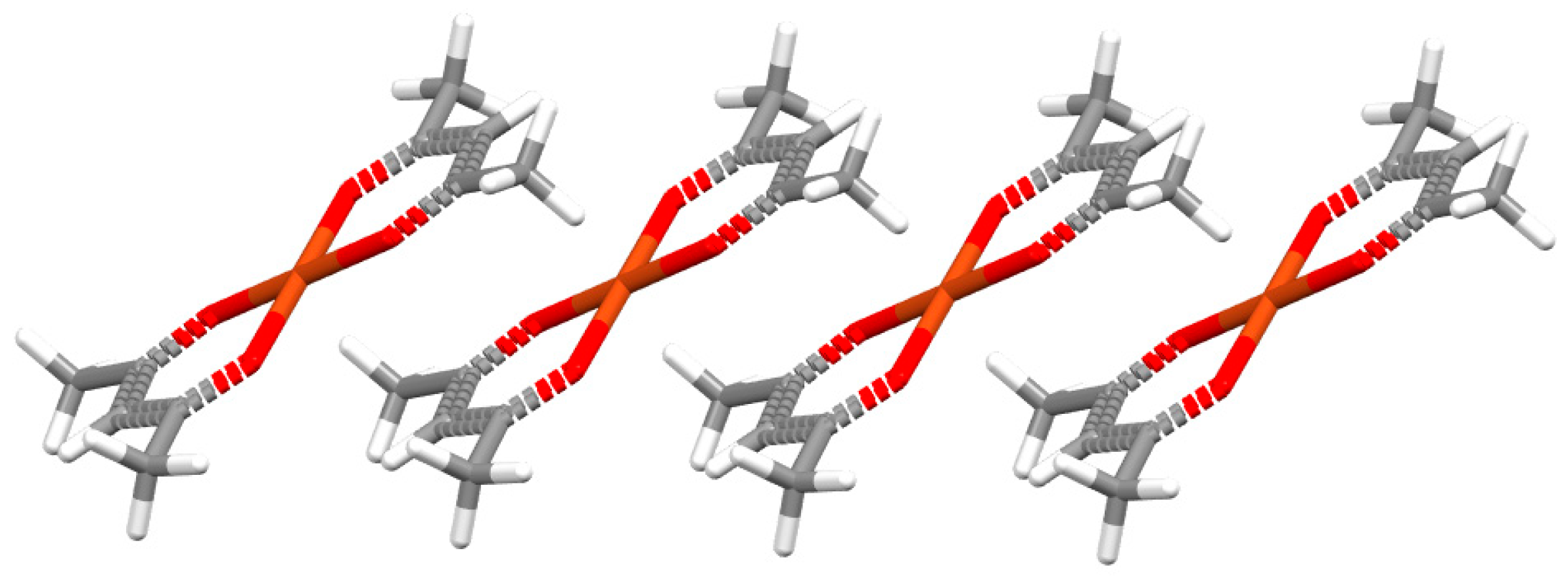
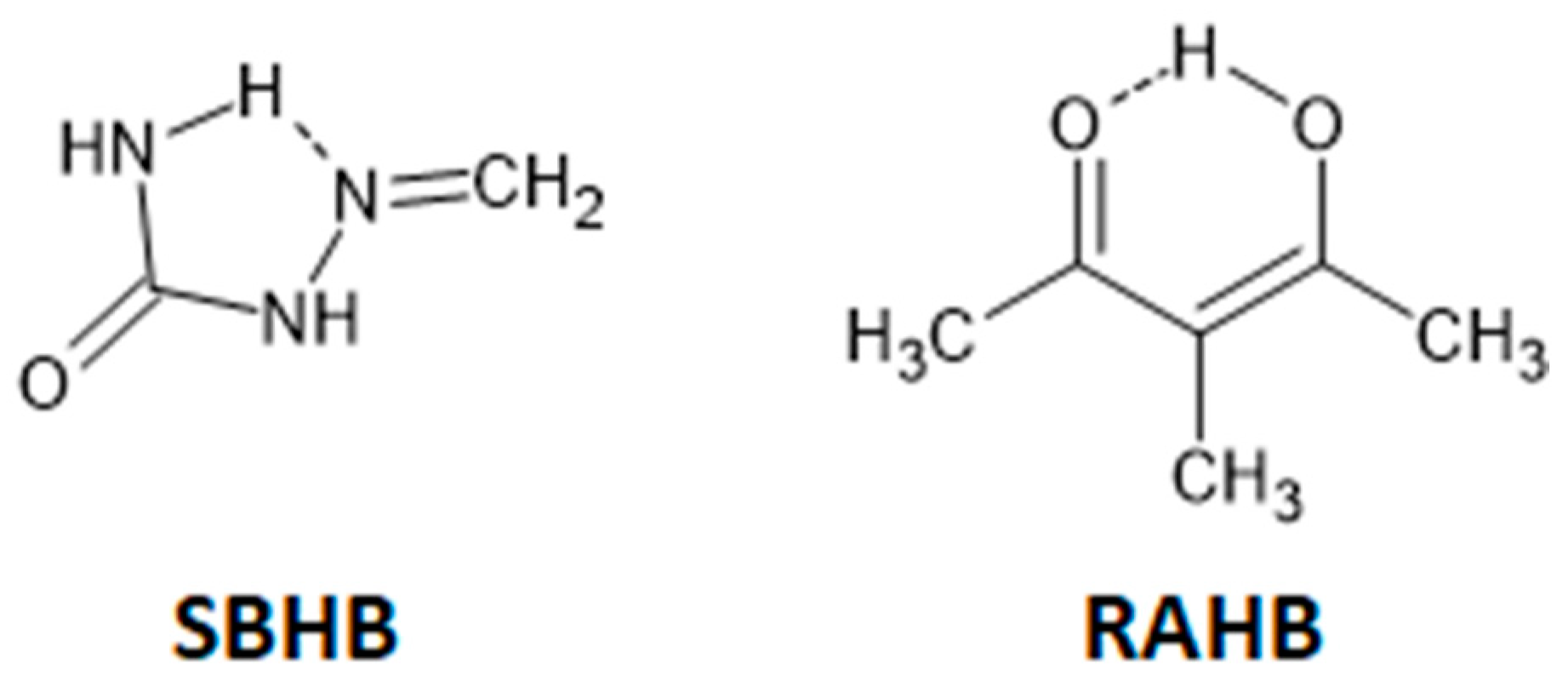
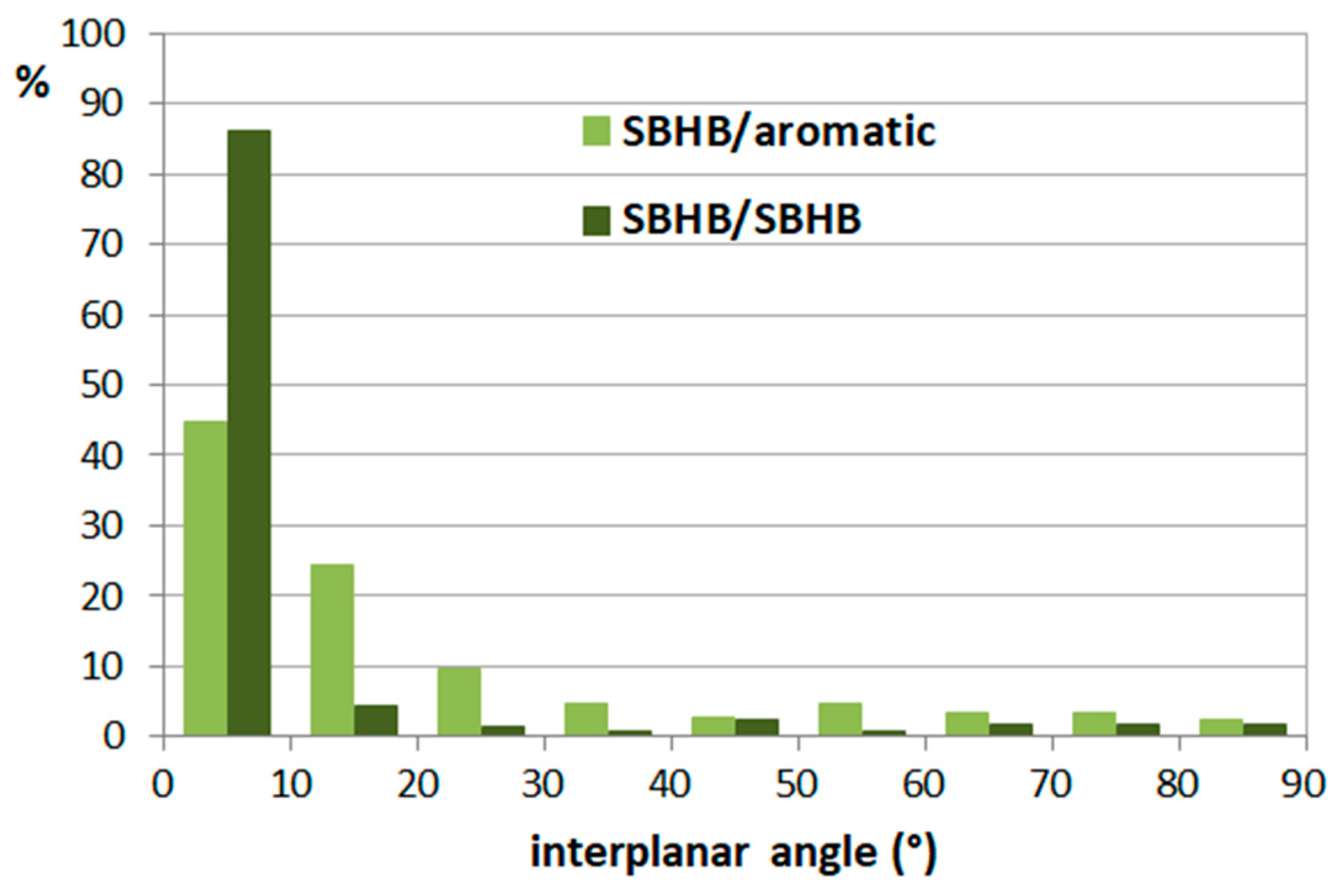
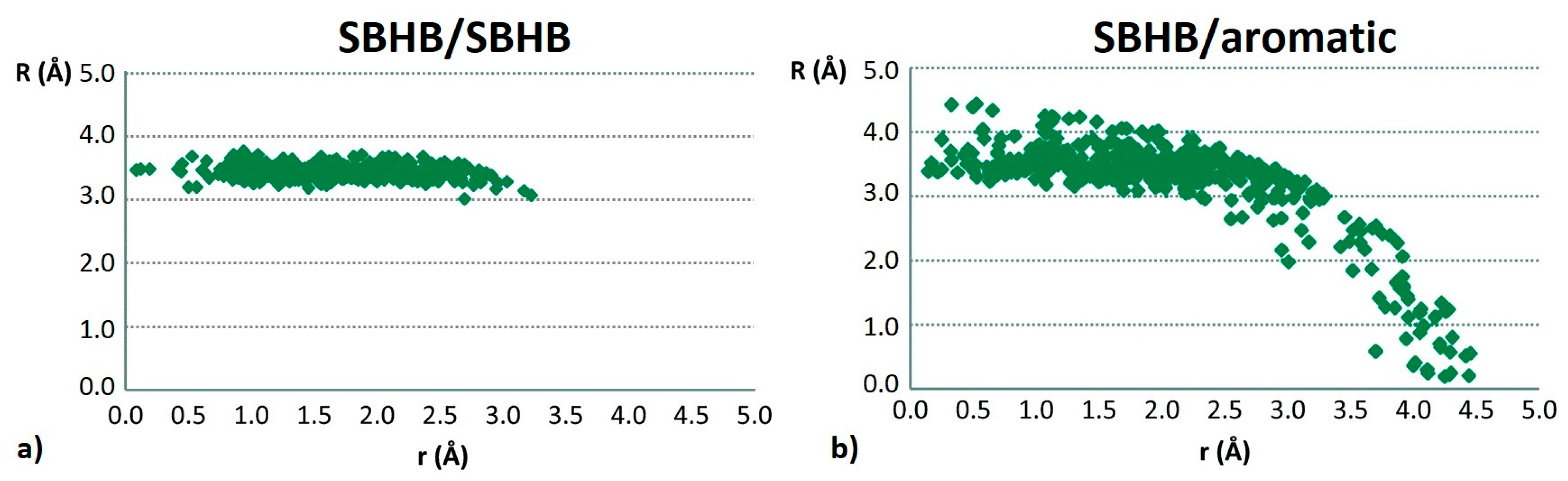
3.1. Stacking Interactions of Single-Bond Hydrogen-Bridged Rings
3.2. Stacking Interactions of Resonance-Assisted Hydrogen-Bridged Rings
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole Is Greater than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Malenov, D.P.; Janjić, G.V.; Medaković, V.B.; Hall, M.B.; Zarić, S.D. Noncovalent Bonding: Stacking Interactions of Chelate Rings of Transition Metal Complexes. Coord. Chem. Rev. 2017, 345, 318–341. [Google Scholar] [CrossRef]
- Ninković, D.B.; Janjić, G.V.; Veljković, D.Ž.; Sredojević, D.N.; Zarić, S.D. What Are the Preferred Horizontal Displacements in Parallel Aromatic-Aromatic Interactions? Significant Interactions at Large Displacements. ChemPhysChem 2011, 12, 3511–3514. [Google Scholar] [CrossRef]
- Janjić, G.V.; Veljković, D.Z.; Zarić, S.D.; Janjić, G.V.; Veljković, D.Ž.; Zarić, S.D. Water/Aromatic Parallel Alignment Interactions. Significant Interactions at Large Horizontal Displacements. Cryst. Growth Des. 2011, 11, 2680–2683. [Google Scholar] [CrossRef]
- Milovanović, M.R.; Stanković, I.M.; Živković, J.M.; Ninković, D.B.; Hall, M.B.; Zarić, S.D. Water: New Aspect of Hydrogen Bonding in the Solid State. IUCrJ 2022, 9, 639–647. [Google Scholar] [CrossRef]
- Blagojević, J.P.; Zarić, S.D. Stacking Interactions of Hydrogen-Bridged Rings—Stronger than the Stacking of Benzene Molecules. Chem. Commun. 2015, 51, 12989–12991. [Google Scholar] [CrossRef]
- Blagojević, J.P.; Veljković, D.Ž.; Zarić, S.D. Stacking Interactions between Hydrogen-Bridged and Aromatic Rings: Study of Crystal Structures and Quantum Chemical Calculations. CrystEngComm 2017, 19, 40–46. [Google Scholar] [CrossRef]
- Blagojević Filipović, J.P.; Hall, M.B.; Zarić, S.D. Stacking Interactions of Resonance-Assisted Hydrogen-Bridged Rings. A Systematic Study of Crystal Structures and Quantum-Chemical Calculations. Cryst. Growth Des. 2019, 19, 5619–5628. [Google Scholar] [CrossRef]
- Blagojević Filipović, J.P.; Hall, M.B.; Zarić, S.D. Stacking Interactions of Resonance-Assisted Hydrogen-Bridged Rings and C6-Aromatic Rings. Phys. Chem. Chem. Phys. 2020, 22, 13721–13728. [Google Scholar] [CrossRef]
- Bogdanović, G.A.; Biré, A.S.; Zarić, S.D. Evidence Based on Crystal Structures and Calculations of a C−H···π Interaction Between an Organic Moiety and a Chelate Ring in Transition Metal Complexes. Eur. J. Inorg. Chem. 2002, 2002, 1599–1602. [Google Scholar] [CrossRef]
- Yakovchuk, P.; Protozanova, E.; Frank-Kamenetskii, M.D. Base-Stacking and Base-Pairing Contributions into Thermal Stability of the DNA Double Helix. Nucleic Acids Res. 2006, 34, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Sarma, S.P. Engineering Aromatic–Aromatic Interactions To Nucleate Folding in Intrinsically Disordered Regions of Proteins. Biochemistry 2017, 56, 4346–4359. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-R.; Wang, Y.; Cui, P.-F.; Xing, L.; Lee, J.; Kim, D.; Jiang, H.-L.; Oh, Y.-K. Applications of π-π Stacking Interactions in the Design of Drug-Delivery Systems. J. Control Release 2019, 294, 311–326. [Google Scholar] [CrossRef]
- Ahmed, E.; Karothu, D.P.; Naumov, P. Crystal Adaptronics: Mechanically Reconfigurable Elastic and Superelastic Molecular Crystals. Angew. Chem. Int. Ed. 2018, 57, 8837–8846. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Liu, J. π–π Stacking Interaction: A Nondestructive and Facile Means in Material Engineering for Bioapplications. Cryst. Growth Des. 2018, 18, 2765–2783. [Google Scholar] [CrossRef]
- Bludský, O.; Rubeš, M.; Soldán, P.; Nachtigall, P. Investigation of the Benzene-Dimer Potential Energy Surface: DFT/CCSD(T) Correction Scheme. J. Chem. Phys. 2008, 128, 114102. [Google Scholar] [CrossRef]
- Lee, E.C.; Kim, D.; Jurečka, P.; Tarakeshwar, P.; Hobza, P.; Kim, K.S. Understanding of Assembly Phenomena by Aromatic−Aromatic Interactions: Benzene Dimer and the Substituted Systems. J. Phys. Chem. A 2007, 111, 3446–3457. [Google Scholar] [CrossRef]
- Bettinger, H.F.; Kar, T.; Sánchez-García, E.; Sánchez-García, E. Borazine and Benzene Homo- And Heterodimers. J. Phys. Chem. A 2009, 113, 3353–3359. [Google Scholar] [CrossRef]
- Pitoňák, M.; Neogrády, P.; Řezáč, J.; Jurečka, P.; Urban, M.; Hobza, P. Benzene Dimer: High-Level Wave Function and Density Functional Theory Calculations. J. Chem. Theory Comput. 2008, 4, 1829–1834. [Google Scholar] [CrossRef]
- Grimme, S. Do Special Noncovalent π-π Stacking Interactions Really Exist? Angew. Chem. Int. Ed. 2008, 47, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.W.G.; Wheeler, S.E. Taking the Aromaticity out of Aromatic Interactions. Angew. Chem. Int. Ed. 2011, 50, 7847–7849. [Google Scholar] [CrossRef] [PubMed]
- Tomic, Z.D.; Leovac, V.M.; Pokorni, S.V.; Zobel, D.; Zaric, S.D. Crystal Structure of Bis[Acetone-1-Naphthoylhydrazinato(-1)]Copper(II) and Investigations of Intermolecular Interactions. Eur. J. Inorg. Chem. 2003, 2003, 1222–1226. [Google Scholar] [CrossRef]
- Castiñeiras, A.; Sicilia-Zafra, A.G.; González-Pérez, J.M.; Choquesillo-Lazarte, D.; Niclós-Gutiérrez, J. Intramolecular “Aryl−Metal Chelate Ring” π,π-Interactions as Structural Evidence for Metalloaromaticity in (Aromatic α,α′-Diimine)−Copper(II) Chelates: Molecular and Crystal Structure of Aqua(1,10-Phenanthroline)(2-Benzylmalonato)Copper(II) Three-Hydrate. Inorg. Chem. 2002, 41, 6956–6958. [Google Scholar] [CrossRef] [PubMed]
- Milčić, M.K.; Ostojić, B.D.; Zarić, S.D. Are Chelate Rings Aromatic? Calculations of Magnetic Properties of Acetylacetonato and o -Benzoquinonediimine Chelate Rings. Inorg. Chem. 2007, 46, 7109–7114. [Google Scholar] [CrossRef]
- Tomić, Z.D.; Novaković, S.B.; Zarić, S.D. Intermolecular Interactions between Chelate Rings and Phenyl Rings in Square-Planar Copper(II) Complexes. Eur. J. Inorg. Chem. 2004, 2215–2218. [Google Scholar] [CrossRef]
- Tomić, Z.D.; Sredojević, D.; Zarić, S.D. Stacking Interactions between Chelate and Phenyl Rings in Square-Planar Transition Metal Complexes. Cryst. Growth Des. 2006, 6, 29–31. [Google Scholar] [CrossRef]
- Sredojević, D.N.; Tomić, Z.D.; Zarić, S.D. Influence of Metal and Ligand Types on Stacking Interactions of Phenyl Rings with Square-Planar Transition Metal Complexes. Cent. Eur. J. Chem. 2007, 5, 20–31. [Google Scholar] [CrossRef]
- Yang, X.-J.; Drepper, F.; Wu, B.; Sun, W.-H.; Haehnel, W.; Janiak, C. From Model Compounds to Protein Binding: Syntheses, Characterizations and Fluorescence Studies of [Ru II (Bipy)(Terpy)L] 2+ Complexes (Bipy = 2,2′-Bipyridine; Terpy = 2,2′:6′,2″-Terpyridine; L = Imidazole, Pyrazole and Derivatives, Cytochrome C). Dalt. Trans. 2005, 256–267. [Google Scholar] [CrossRef]
- Mosae Selvakumar, P.; Suresh, E.; Subramanian, P.S. Single Stranded Helical Supramolecular Architecture with a Left Handed Helical Water Chain in Ternary Copper(II) Tryptophan/Diamine Complexes. Polyhedron 2009, 28, 245–252. [Google Scholar] [CrossRef]
- Allali, M.; Jaud, J.; Habbadi, N.; Dartiguenave, M.; Beauchamp, A.L.; Benoist, E. Structural Evidence for an Unusual Conformation and Weak Interligand Interactions in Two Copper Chelates with (o-Nitrophenyl)-Ethylenediaminediacetic Acid. Eur. J. Inorg. Chem. 2009, 2009, 1488–1494. [Google Scholar] [CrossRef]
- Philip, V.; Suni, V.; Prathapachandra Kurup, M.R.; Nethaji, M. Structural and Spectral Studies of Nickel(II) Complexes of Di-2-Pyridyl Ketone N4,N4-(Butane-1,4-Diyl) Thiosemicarbazone. Polyhedron 2004, 23, 1225–1233. [Google Scholar] [CrossRef]
- Sredojević, D.N.; Vojislavljević, D.Z.; Tomić, Z.D.; Zarić, S.D. Parallel Stacking Interactions in Square-Planar Transition-Metal Complexes Containing Fused Chelate and C6-Aromatic Rings. Acta Crystallogr. B 2012, 68, 261–265. [Google Scholar] [CrossRef]
- Malenov, D.P.; Ninković, D.B.; Sredojević, D.N.; Zarić, S.D. Stacking of Benzene with Metal Chelates: Calculated CCSD(T)/CBS Interaction Energies and Potential-Energy Curves. ChemPhysChem 2014, 15, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Malenov, D.P.; Ninković, D.B.; Zarić, S.D. Stacking of Metal Chelates with Benzene: Can Dispersion-Corrected DFT Be Used to Calculate Organic-Inorganic Stacking? ChemPhysChem 2015, 16, 761–768. [Google Scholar] [CrossRef]
- Malenov, D.P.; Hall, M.B.; Zarić, S.D. Influence of Metal Ion on Chelate-Aryl Stacking Interactions. Int. J. Quantum Chem. 2018, 118, e25629. [Google Scholar] [CrossRef]
- Malenov, D.P.; Veljković, D.Ž.; Hall, M.B.; Brothers, E.N.; Zarić, S.D. Influence of Chelate Ring Type on Chelate–Chelate and Chelate–Aryl Stacking: The Case of Nickel Bis(Dithiolene). Phys. Chem. Chem. Phys. 2019, 21, 1198–1206. [Google Scholar] [CrossRef]
- Malenov, D.P.; Zarić, S.D. Strong Stacking Interactions of Metal–Chelate Rings Are Caused by Substantial Electrostatic Component. Dalton Trans. 2019, 48, 6328–6332. [Google Scholar] [CrossRef]
- Sredojević, D.N.; Ninković, D.B.; Janjić, G.V.; Zhou, J.; Hall, M.B.; Zarić, S.D. Stacking Interactions of Ni(Acac) Chelates with Benzene: Calculated Interaction Energies. ChemPhysChem 2013, 14, 1797–1800. [Google Scholar] [CrossRef]
- MacKie, I.D.; DiLabio, G.A. Approximations to Complete Basis Set-Extrapolated, Highly Correlated Non-Covalent Interaction Energies. J. Chem. Phys. 2011, 135, 134318. [Google Scholar] [CrossRef]
- Jeziorski, B.; Moszynski, R.; Szalewicz, K. Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van Der Waals Complexes. Chem. Rev. 1994, 94, 1887–1930. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Density Fitting and Cholesky Decomposition Approximations in Symmetry-Adapted Perturbation Theory: Implementation and Application to Probe the Nature of π-π Interactions in Linear Acenes. J. Chem. Phys. 2010, 132, 184111. [Google Scholar] [CrossRef]
- Gonthier, J.F.; Sherrill, C.D. Density-Fitted Open-Shell Symmetry-Adapted Perturbation Theory and Application to π -Stacking in Benzene Dimer Cation and Ionized DNA Base Pair Steps. J. Chem. Phys. 2016, 145, 134106. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, E.G.; Sherrill, C.D. Effects of Heteroatoms on Aromatic π-π Interactions: Benzene-Pyridine and Pyridine Dimer. J. Phys. Chem. A 2009, 113, 878–886. [Google Scholar] [CrossRef]
- Ninković, D.B.; Blagojević Filipović, J.P.; Hall, M.B.; Brothers, E.N.; Zarić, S.D. What Is Special about Aromatic-Aromatic Interactions? Significant Attraction at Large Horizontal Displacement. ACS Cent. Sci. 2020, 6, 420–425. [Google Scholar] [CrossRef]
- Janiak, C. A Critical Account on π-π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc. Dalton Trans. 2000, 95, 3885–3896. [Google Scholar] [CrossRef]
- Sredojević, D.N.; Tomić, Z.D.; Zarić, S.D. Evidence of Chelate-Chelate Stacking Interactions in Crystal Structures of Transition-Metal Complexes. Cryst. Growth Des. 2010, 10, 3901–3908. [Google Scholar] [CrossRef]
- Brock, A.J.; Whittaker, J.J.; Powell, J.A.; Pfrunder, M.C.; Grosjean, A.; Parsons, S.; McMurtrie, J.C.; Clegg, J.K. Elastically Flexible Crystals Have Disparate Mechanisms of Molecular Movement Induced by Strain and Heat. Angew. Chem. Int. Ed. 2018, 57, 11325–11328. [Google Scholar] [CrossRef]
- Joksimović, N.; Janković, N.; Petronijević, J.; Baskić, D.; Popovic, S.; Todorović, D.; Zarić, M.; Klisurić, O.; Vraneš, M.; Tot, A.; et al. Synthesis, Anticancer Evaluation and Synergistic Effects with Cis Platin of Novel Palladium Complexes: DNA, BSA Interactions and Molecular Docking Study. Med. Chem. 2020, 16, 78–92. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Castiñeiras, A.; Garczarek, P.; Bauzá, A.; Rheingold, A.L.; Kinzhybalo, V.; Frontera, A. Synthesis, X-Ray Characterization, DFT Calculations and Hirshfeld Surface Analysis of Thiosemicarbazone Complexes of Mn+ Ions (n = 2, 3; M = Ni, Cd, Mn, Co and Cu). CrystEngComm 2016, 18, 1009–1023. [Google Scholar] [CrossRef]
- Holland, L.; Shen, W.Z.; Von Grebe, P.; Sanz Miguel, P.J.; Pichierri, F.; Springer, A.; Schalley, C.A.; Lippert, B. A Neutral Pt 3 Stack Unsupported by Any Bridging Ligand. Dalton Trans. 2011, 40, 5159–5161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laurila, E.; Oresmaa, L.; Niskanen, M.; Hirva, P.; Haukka, M. Metal−Metal Interactions in Stacked Mononuclear and Dinuclear Rhodium 2,2′-Biimidazole Carbonyl Complexes. Cryst. Growth Des. 2010, 10, 3775–3786. [Google Scholar] [CrossRef]
- Hosseini-Monfared, H.; Pousaneh, E.; Sadighian, S.; Ng, S.W.; Tiekink, E.R.T.T. Syntheses, Structures, and Catalytic Activity of Copper(II)-Aroylhydrazone Complexes. Z. Anorg. Allg. Chem. 2013, 639, 435–442. [Google Scholar] [CrossRef]
- Malenov, D.P.; Zarić, S.D. Chelated Metal Ions Modulate the Strength and Geometry of Stacking Interactions: Energies and Potential Energy Surfaces for Chelate–Chelate Stacking. Phys. Chem. Chem. Phys. 2018, 20, 14053–14060. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Bellucci, F.; Ferretti, V.; Bertolasi, V. Evidence for Resonance-Assisted Hydrogen Bonding from Crystal-Structure Correlations on the Enol Form of the .Beta.-Diketone Fragment. J. Am. Chem. Soc. 1989, 111, 1023–1028. [Google Scholar] [CrossRef]
- Gilli, P.; Bertolasi, V.; Ferretti, V.; Gilli, G. Evidence for Intramolecular N-H···O Resonance-Assisted Hydrogen Bonding in β-Enaminones and Related Heterodienes. A Combined Crystal-Structural, IR and NMR Spectroscopic, and Quantum-Mechanical Investigation. J. Am. Chem. Soc. 2000, 122, 10405–10417. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Pombeiro, A.J.L. Resonance-Assisted Hydrogen Bonding as a Driving Force in Synthesis and a Synthon in the Design of Materials. Chem. Eur. J. 2016, 22, 16356–16398. [Google Scholar] [CrossRef]
- Shapenova, D.S.; Shiryaev, A.A.; Bolte, M.; Kukułka, M.; Szczepanik, D.W.; Hooper, J.; Babashkina, M.G.; Mahmoudi, G.; Mitoraj, M.P.; Safin, D.A. Resonance Assisted Hydrogen Bonding Phenomenon Unveiled through Both Experiments and Theory: A New Family of Ethyl N-Salicylideneglycinate Dyes. Chem. Eur. J. 2020, 26, 12987–12995. [Google Scholar] [CrossRef]
- Sanz, P.; Mó, O.; Yáñez, M.; Elguero, J. Resonance-Assisted Hydrogen Bonds: A Critical Examination. Structure and Stability of the Enols of β-Diketones and β-Enaminones. J. Phys. Chem. A 2007, 111, 3585–3591. [Google Scholar] [CrossRef]
- Jabłoński, M.; Kaczmarek, A.; Sadlej, A.J. Estimates of the Energy of Intramolecular Hydrogen Bonds. J. Phys. Chem. A 2006, 110, 10890–10898. [Google Scholar] [CrossRef]
- Mason, P.E.; Dempsey, C.E.; Neilson, G.W.; Kline, S.R.; Brady, J.W. Preferential Interactions of Guanidinum Ions with Aromatic Groups over Aliphatic Groups. J. Am. Chem. Soc. 2009, 131, 16689–16696. [Google Scholar] [CrossRef] [PubMed]
- Negi, I.; Jangra, R.; Gharu, A.; Trant, J.F.; Sharma, P. Guanidinium–Amino Acid Hydrogen-Bonding Interactions in Protein Crystal Structures: Implications for Guanidinium-Induced Protein Denaturation. Phys. Chem. Chem. Phys. 2022, 25, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.S.; Sashikanth, S.; Reddy, P.P.; Cherukupally, P. An Efficient Synthesis of Vinylogous Carbamates from Alkyl Azides. Tetrahedron Lett. 2007, 48, 8709–8711. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Interplay between Resonance-Assisted Hydrogen Bonding and Coordination in Sulfo-Functionalized Arylhydrazones of Active Methylene Compounds. Chempluschem 2014, 79, 1523–1531. [Google Scholar] [CrossRef]
- Saccone, M.; Pfletscher, M.; Kather, S.; Wölper, C.; Daniliuc, C.; Mezger, M.; Giese, M. Improving the Mesomorphic Behaviour of Supramolecular Liquid Crystals by Resonance-Assisted Hydrogen Bonding. J. Mater. Chem. C 2019, 7, 8643–8648. [Google Scholar] [CrossRef]
- Vetrichelvan, M.; Valiyaveettil, S. Intramolecular Hydrogen-Bond-Assisted Planarization of Asymmetrically Functionalized Alternating Phenylene-Pyridinylene Copolymers. Chem. Eur. J. 2005, 11, 5889–5898. [Google Scholar] [CrossRef]
- Blagojević, J.; Janjić, G.; Zarić, S. Very Strong Parallel Interactions Between Two Saturated Acyclic Groups Closed with Intramolecular Hydrogen Bonds Forming Hydrogen-Bridged Rings. Crystals 2016, 6, 34. [Google Scholar] [CrossRef]
- Filipović, J.P.B.; Zarić, S.D. Significant Stacking Interactions of Resonance-Assisted Hydrogen-Bridged (RAHB) Rings at Large Horizontal Displacements. Cryst. Growth Des. 2021, 21, 4947–4958. [Google Scholar] [CrossRef]
- Lobana, T.S.; Sharma, R.; Castineiras, A.; Hundal, G.; Butcher, R.J. The Influence of Substituents (R) at N1 Atom of Thiophene-2-Carbaldehyde Thiosemicarbazones {(C4H3S)HC2N3–N(H)–C1(S)N1HR} on Bonding, Nuclearity and H-Bonded Networks of Copper(I) Complexes. Inorg. Chim. Acta 2009, 362, 3547–3554. [Google Scholar] [CrossRef]
- Saravanan, S.; Muthusubramanian, S. {3-[4-(N,N-Dimethylamino)Phenyl]-1-(4-Methylphenyl)-3-(Phenylsulfanyl)Propylidene}semicarbazide. Acta Crystallogr. E 2004, 60, o1895–o1897. [Google Scholar] [CrossRef]
- Carballo, R.; Pino-Cuevas, A.; Vázquez-López, E.M. Crystal Structure of 1-(4-Formylbenzylidene)Thiosemicarbazone. Acta Crystallogr. E 2014, 70, o970. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wang, Y.; Ji, B.-M. Crystal Structure of Pyridine-2-Carbaldehyde Thiosemicarbazonium Chloride Hydrate, [C7H9N4S]Cl · H2O. Z. Kristallogr. NCS 2011, 226, 463–464. [Google Scholar] [CrossRef][Green Version]
- Hohenstein, E.G.; Sherrill, C.D. Density Fitting of Intramonomer Correlation Effects in Symmetry-Adapted Perturbation Theory. J. Chem. Phys. 2010, 133, 014101. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.; Hazell, R.G.; Larsen, C.; Nielsen, P.H.; Lindberg, A.A.; Jansen, G.; Lamm, B.; Samuelsson, B. The Crystal Structure of Thiosemicarbazide. Acta Chem. Scand. 1969, 23, 1359–1366. [Google Scholar] [CrossRef]
- Xu, L.-X.; Bai, X.-G.; Wang, J.-X.; Wang, Y.-C. (Z)-Methyl 3-(2,4-Dichlorophenyl)-3-Hydroxyacrylate. Acta Crystallogr. E 2012, 68, o4. [Google Scholar] [CrossRef]
- Ostrowska, K.; Musielak, B.; Szneler, E.; Dudek, Ł.; Gryl, M.; Stadnicka, K. Chelate Ring Size Effect as a Factor of Selective Fluorescent Recognition of Zn 2+ Ions by Pyrrolo[2,3-b]Quinoxaline with a Substituted 2-Pyridyl Group Receptor. Inorg. Chem. 2015, 54, 8423–8435. [Google Scholar] [CrossRef]
- Alpaslan, G.; Özdamar, Ö.; Odabaşoğlu, M.; Ocak Ískeleli, N.; Erdönmez, A. (Z)-Ethyl 2-[2-(4-Acetylphenyl)Hydrazono]-4-Chloro-3-Oxobutanoate. Acta Crystallogr. E 2007, 63, o2746. [Google Scholar] [CrossRef]
- Rojas, R.S.; Cabrera, A.R.; Peoples, B.C.; Spannhoff, K.; Valderrama, M.; Fröhlich, R.; Kehr, G.; Erker, G. Synthesis of [(π-Cyano-P-Nacnac)Cp] and [(π-Cyano-Nacnac)Cp]-Zirconium Complexes, and Their Remote Activation for Ethylene Polymerization. Dalton Trans. 2012, 41, 1243–1251. [Google Scholar] [CrossRef]
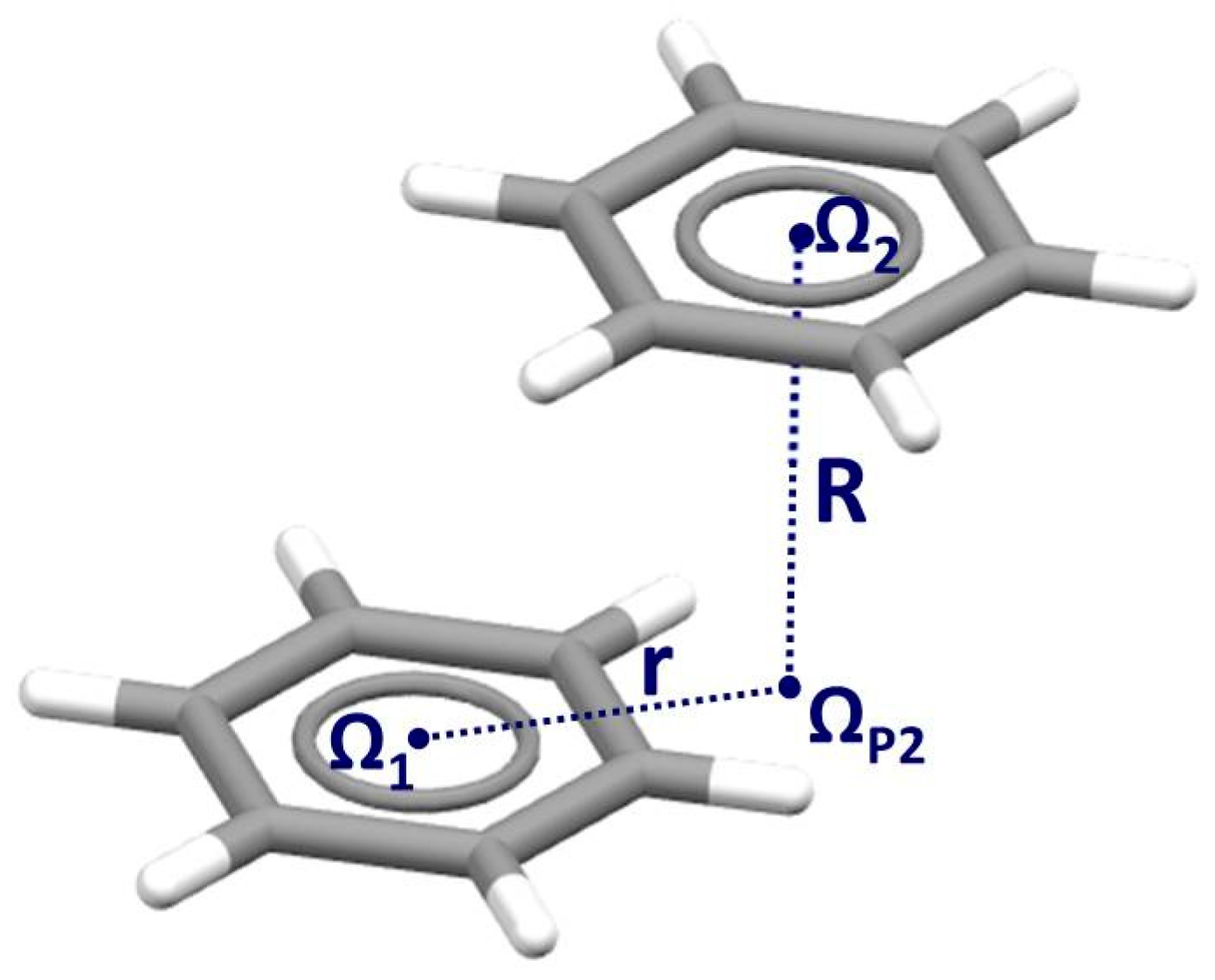
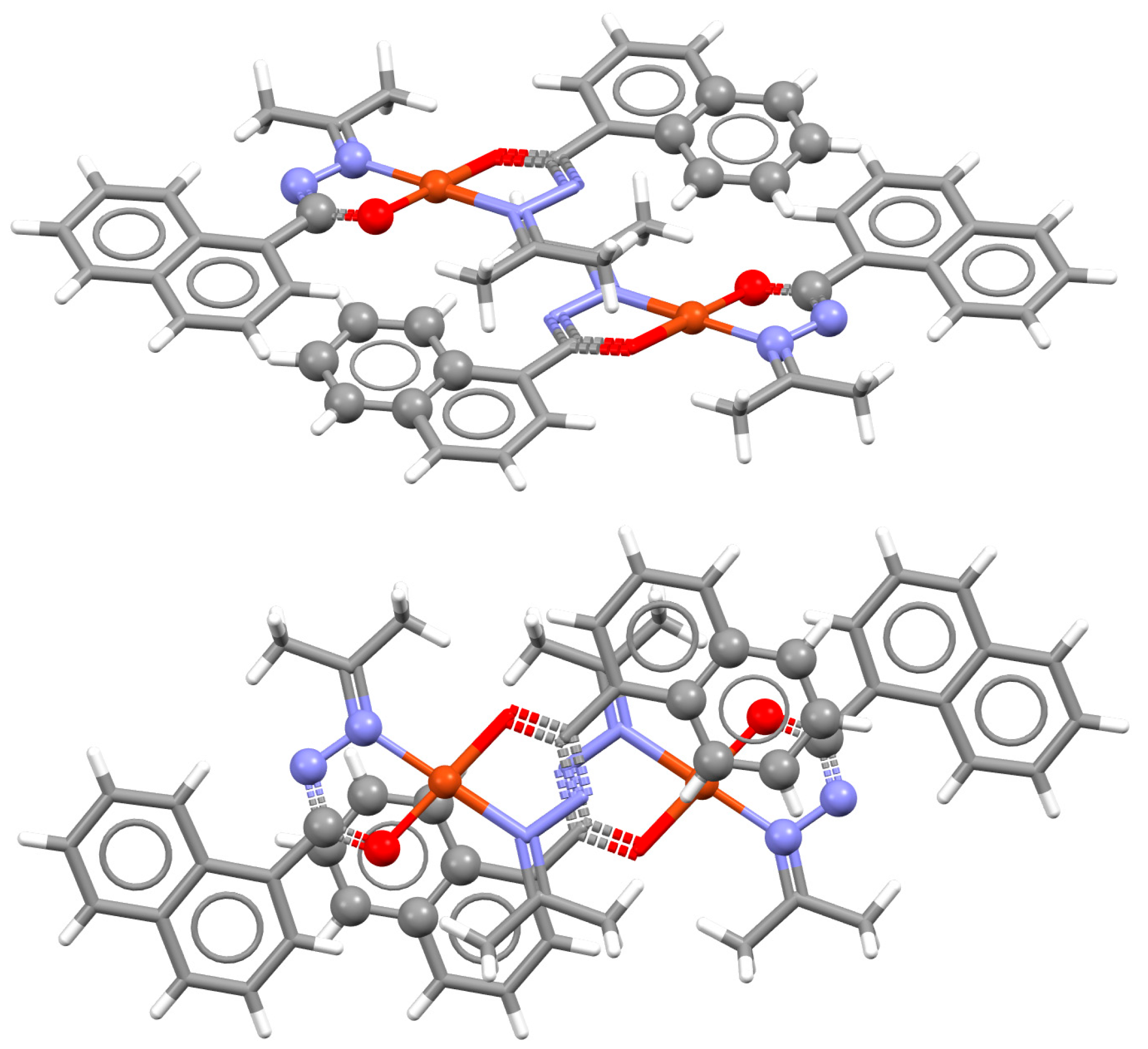
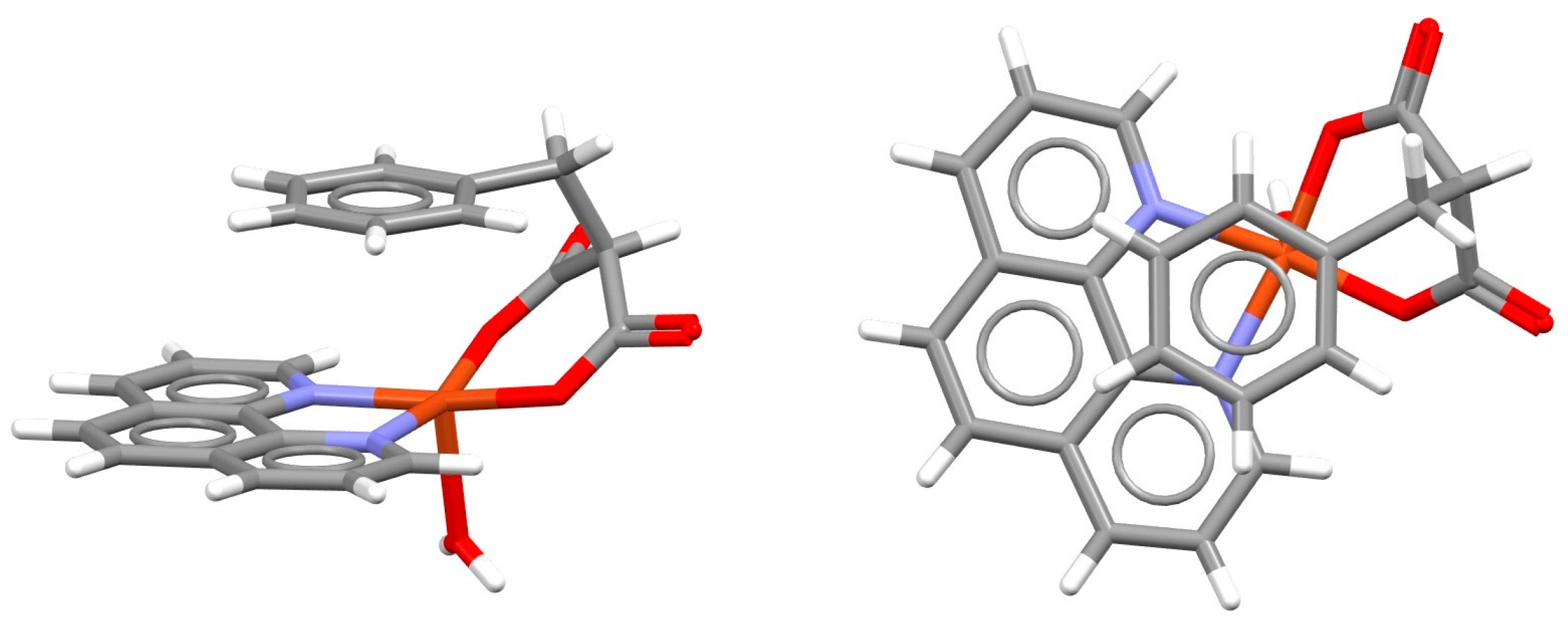
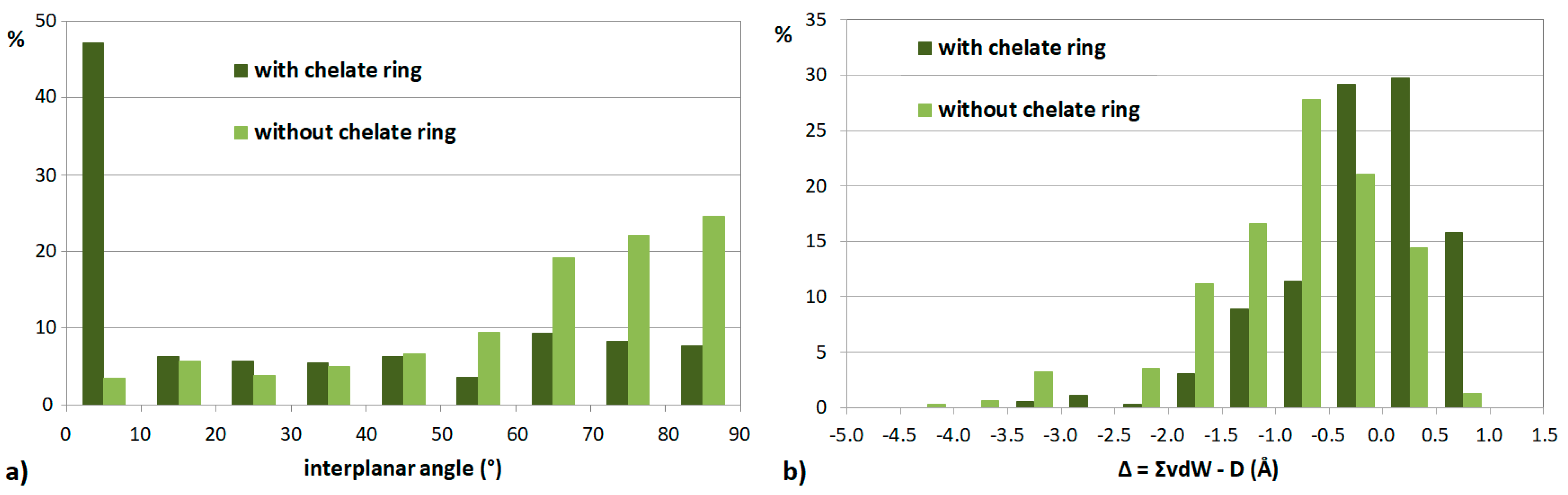
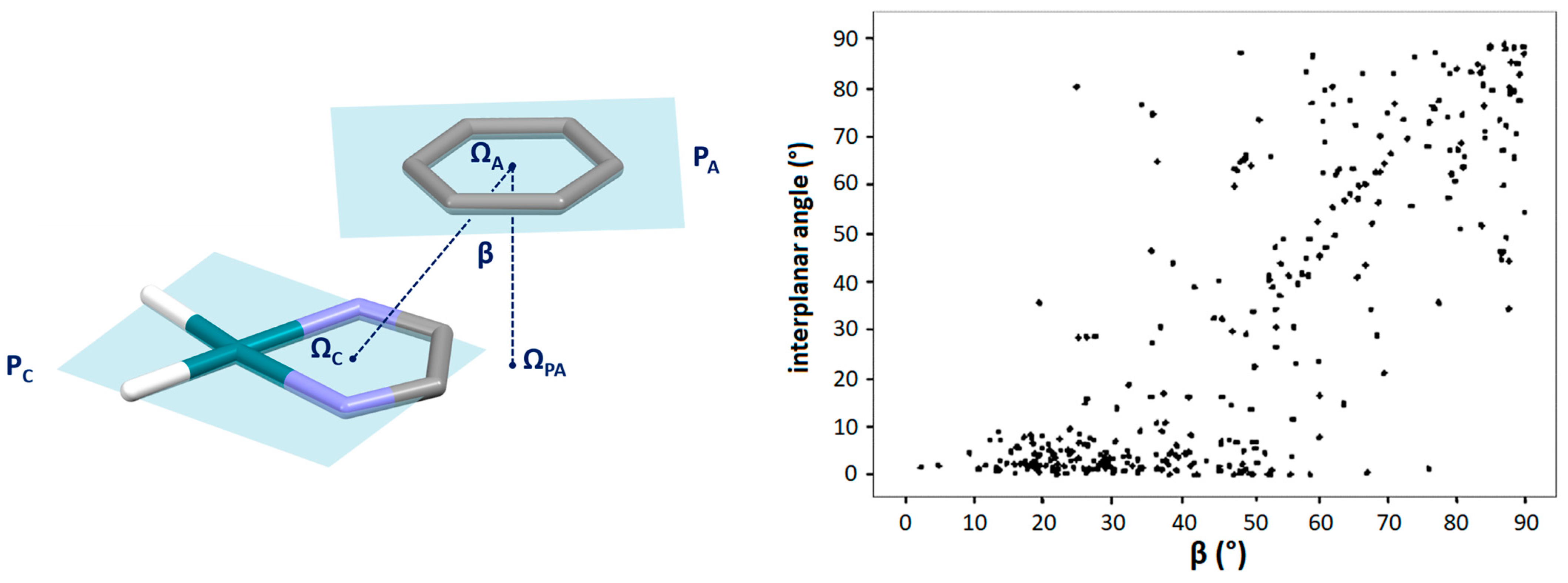
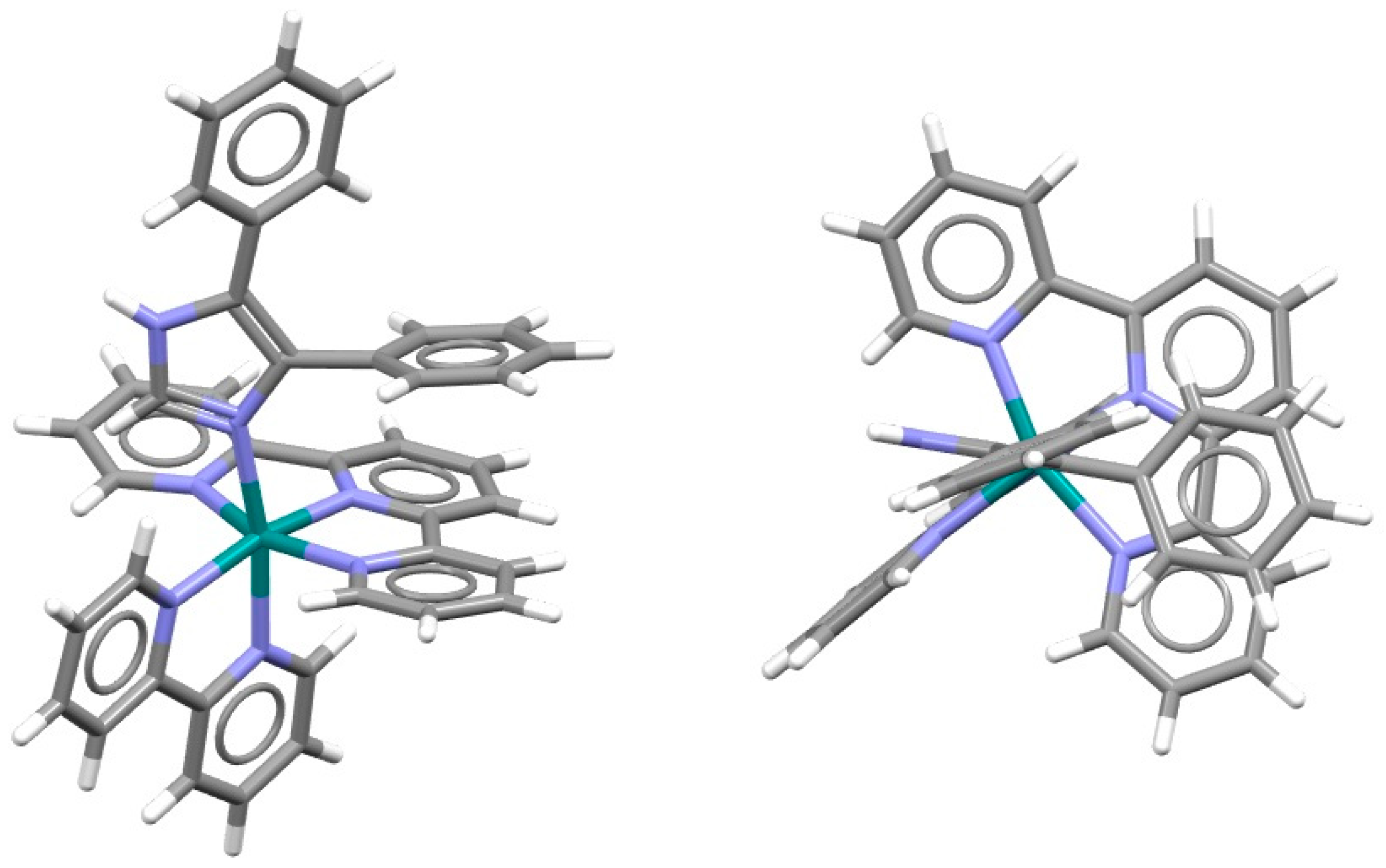

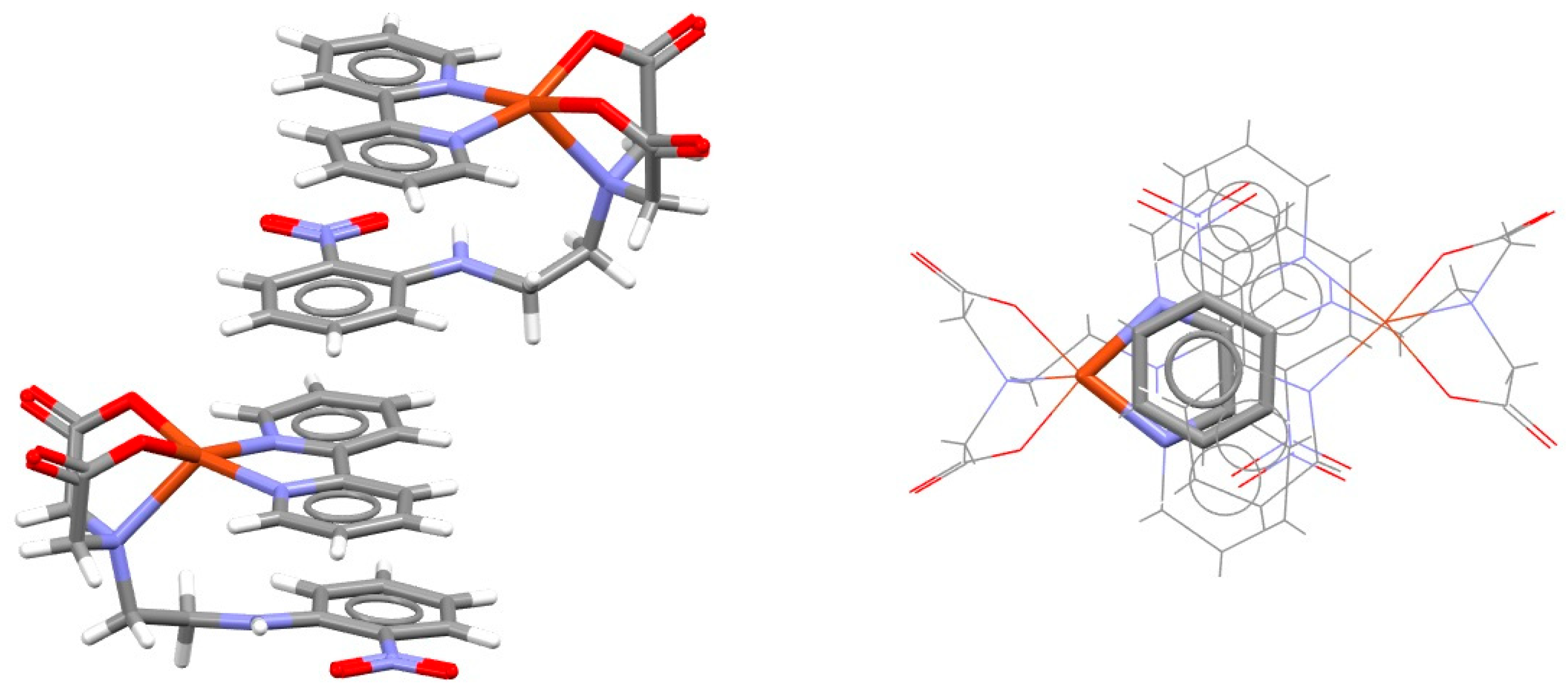

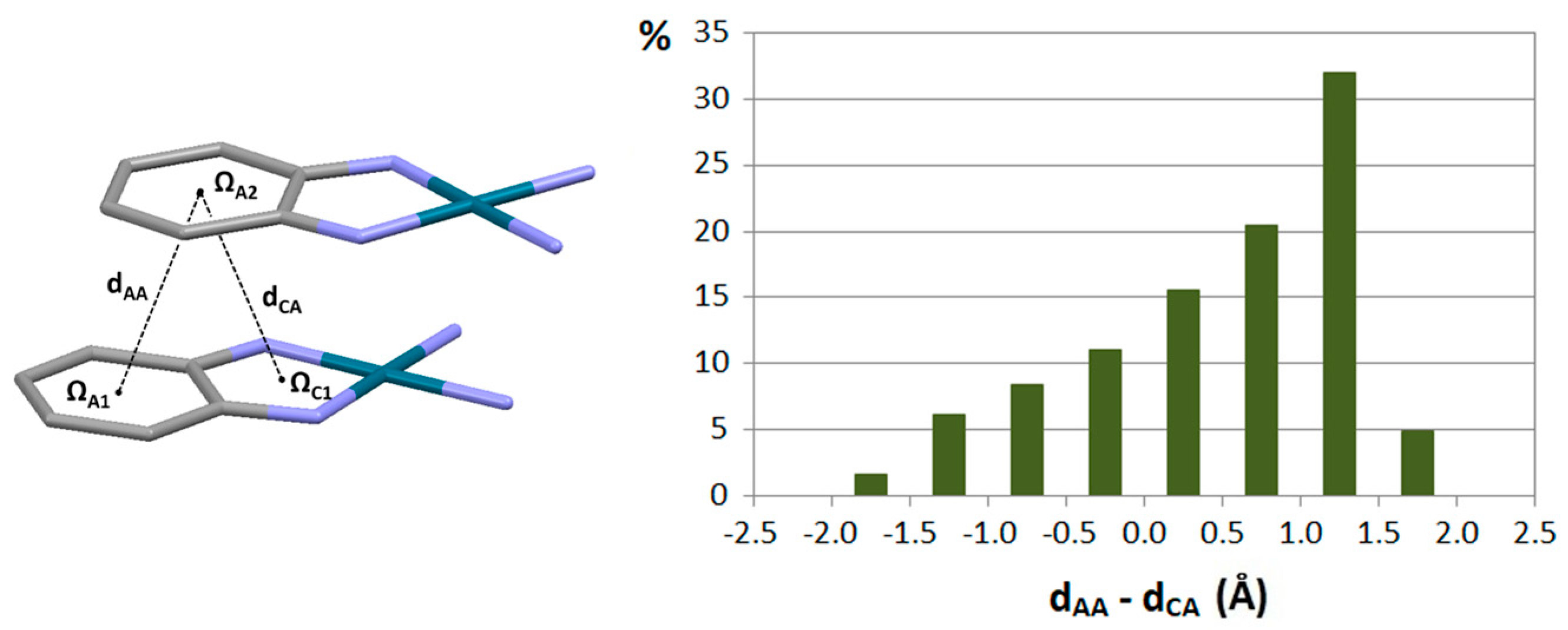
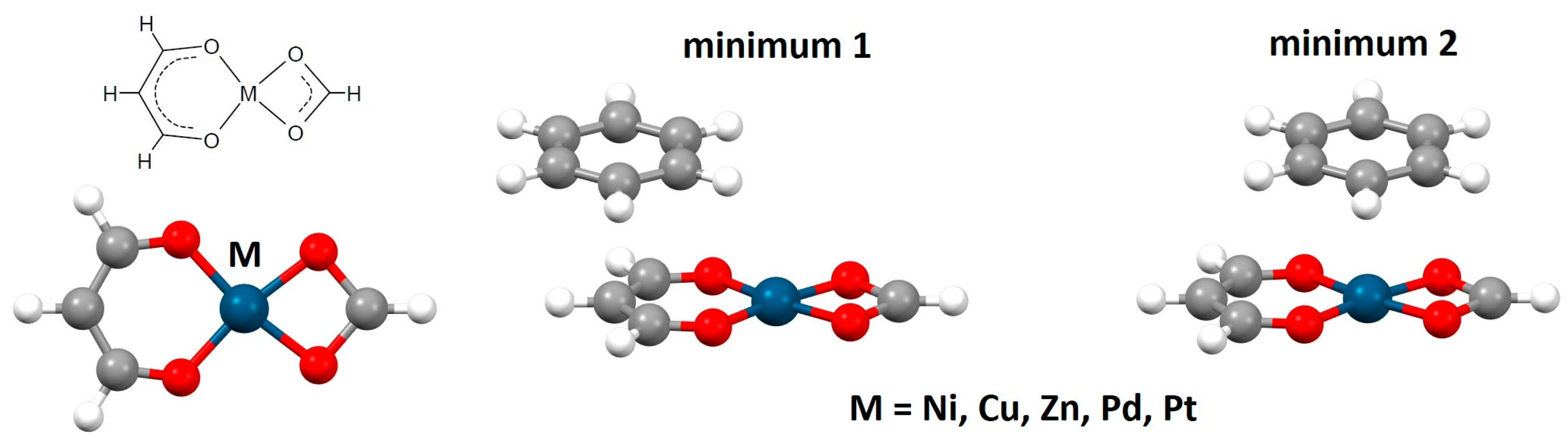
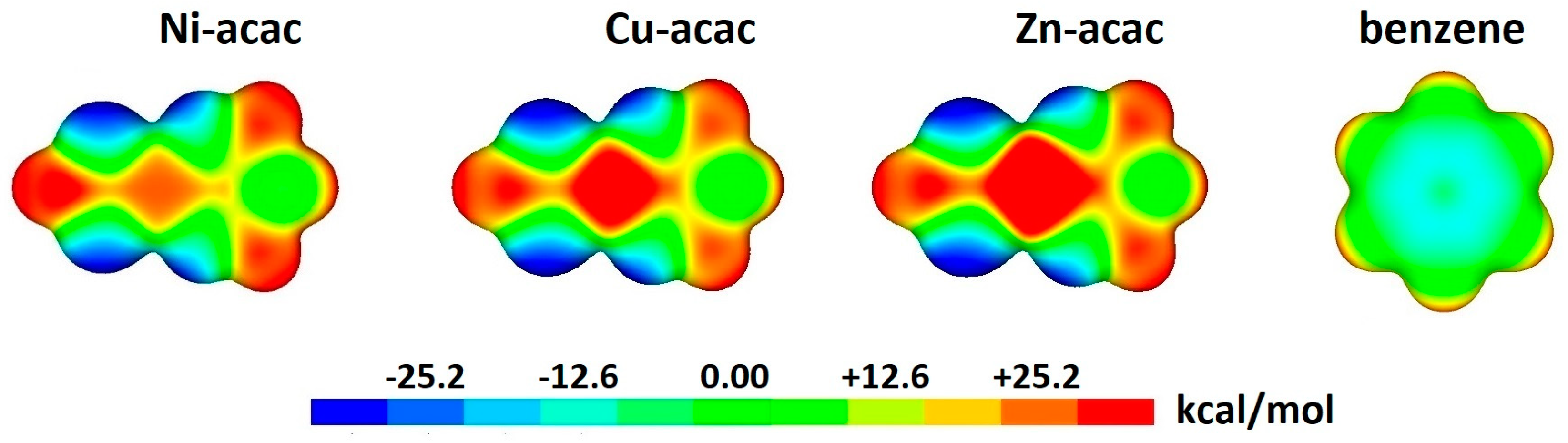

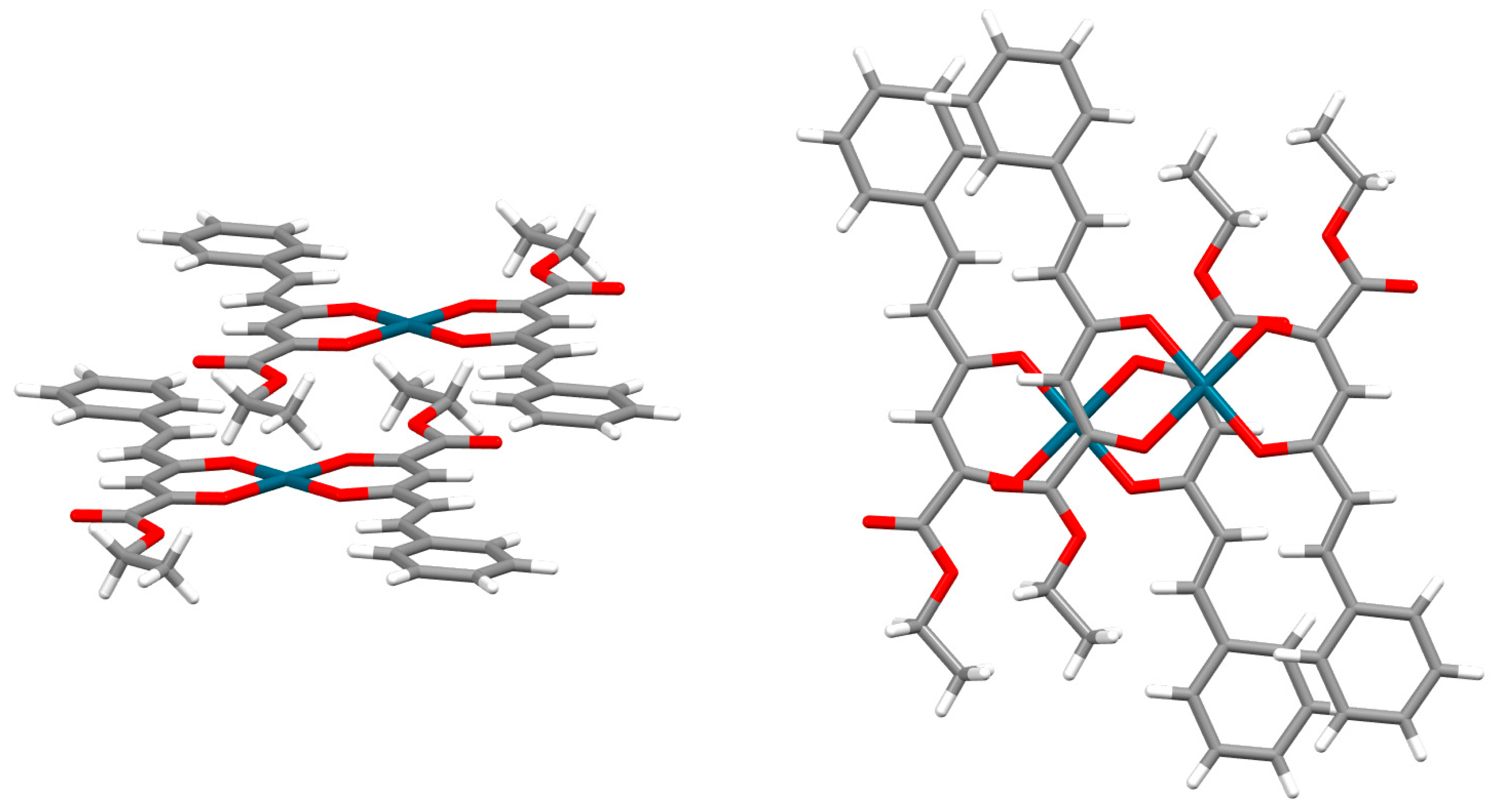

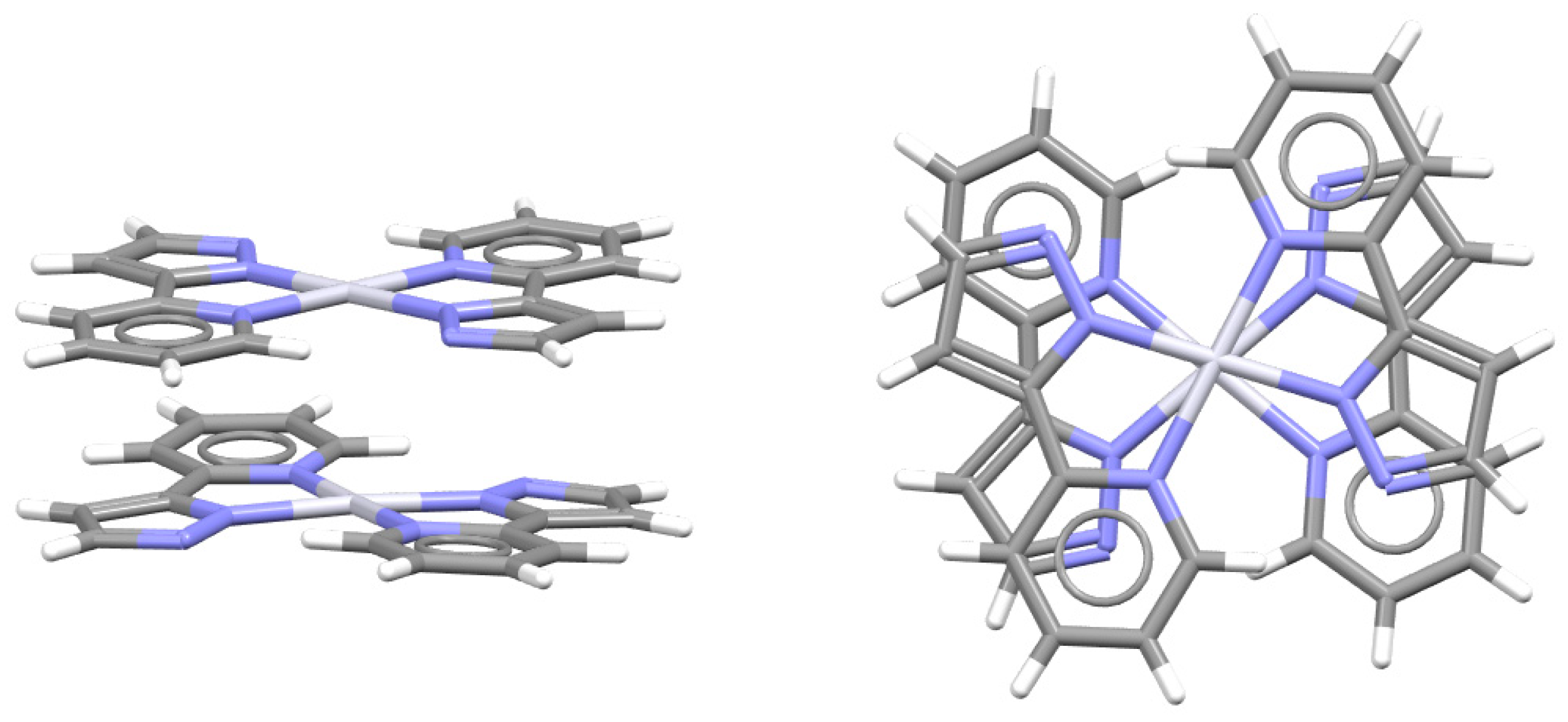
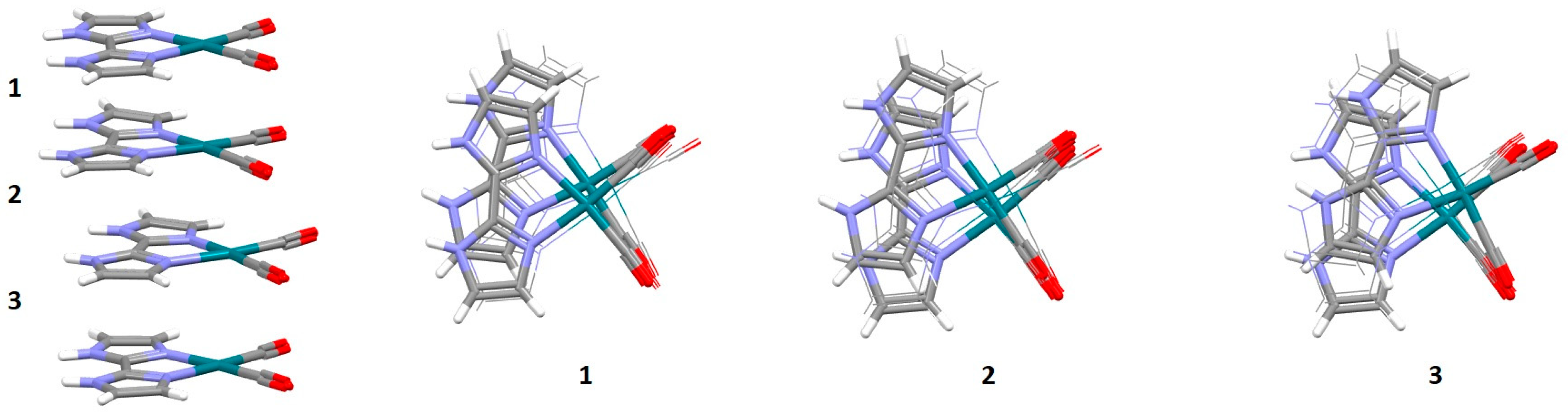
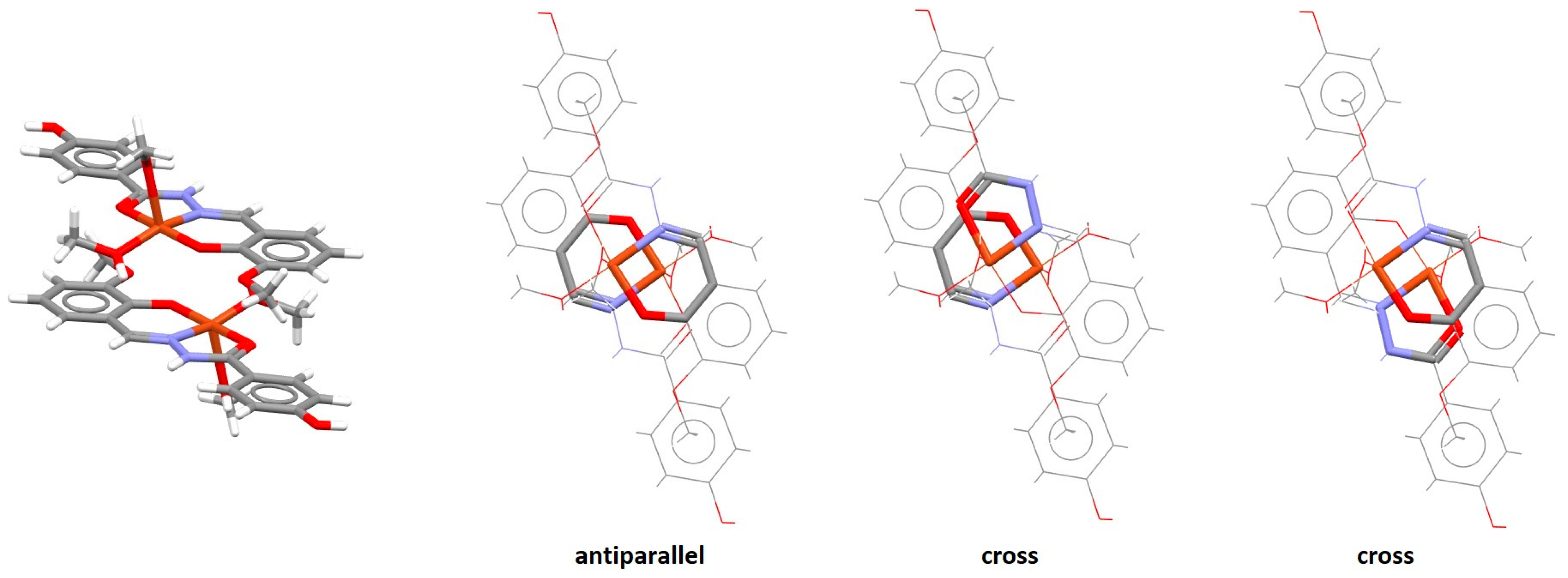

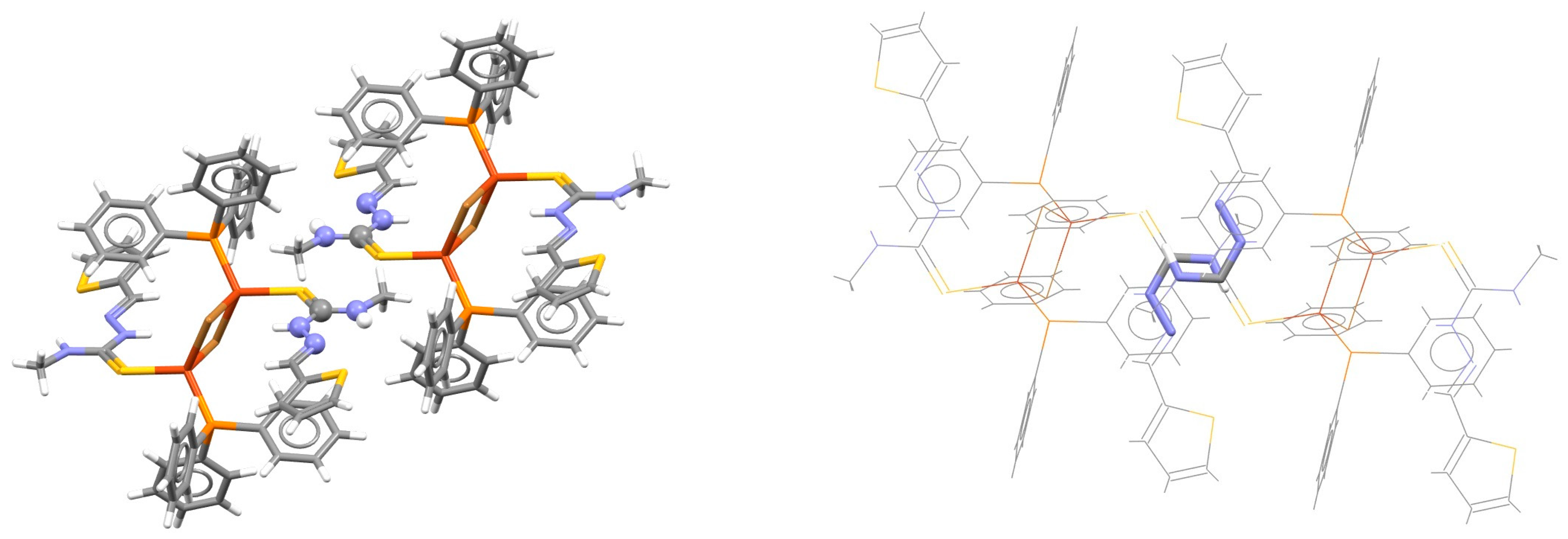
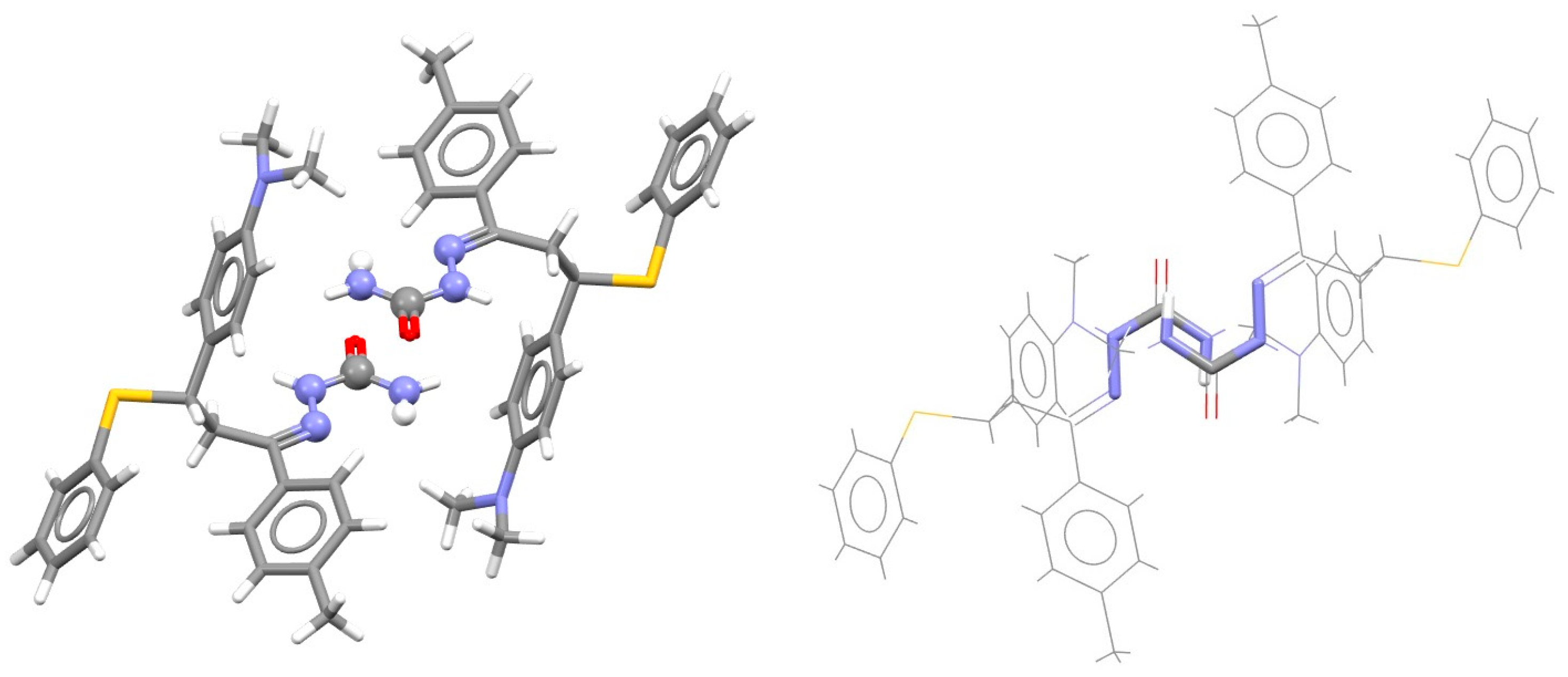
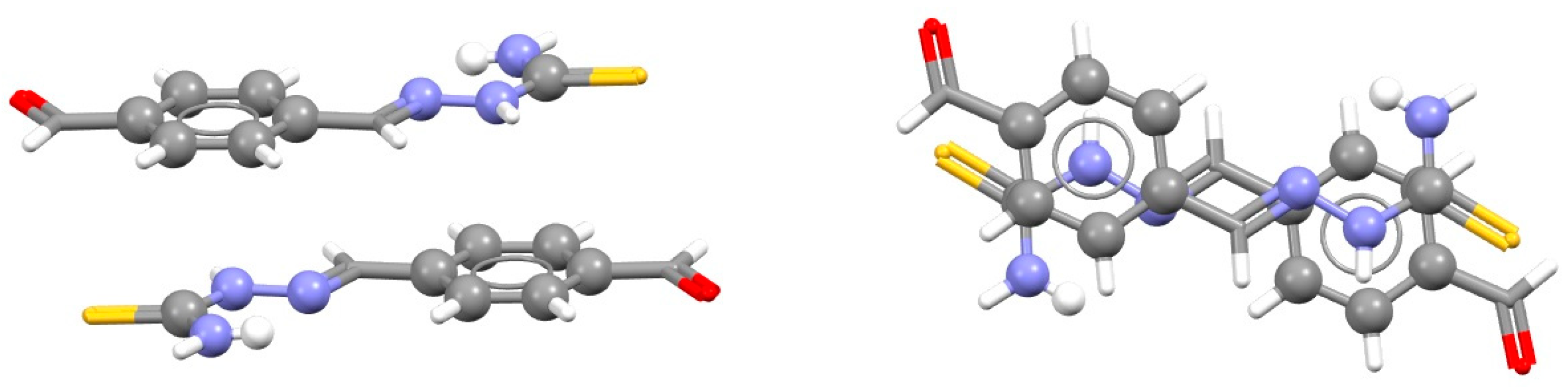
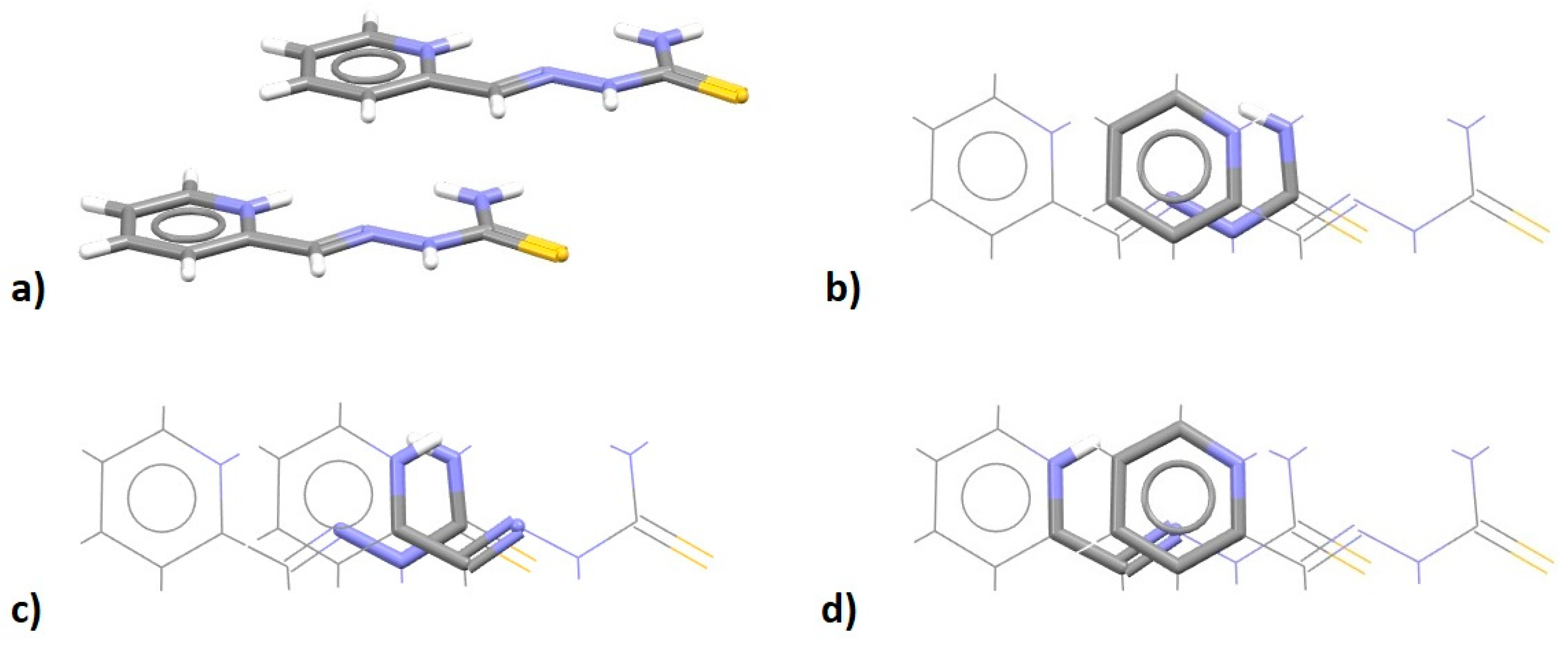

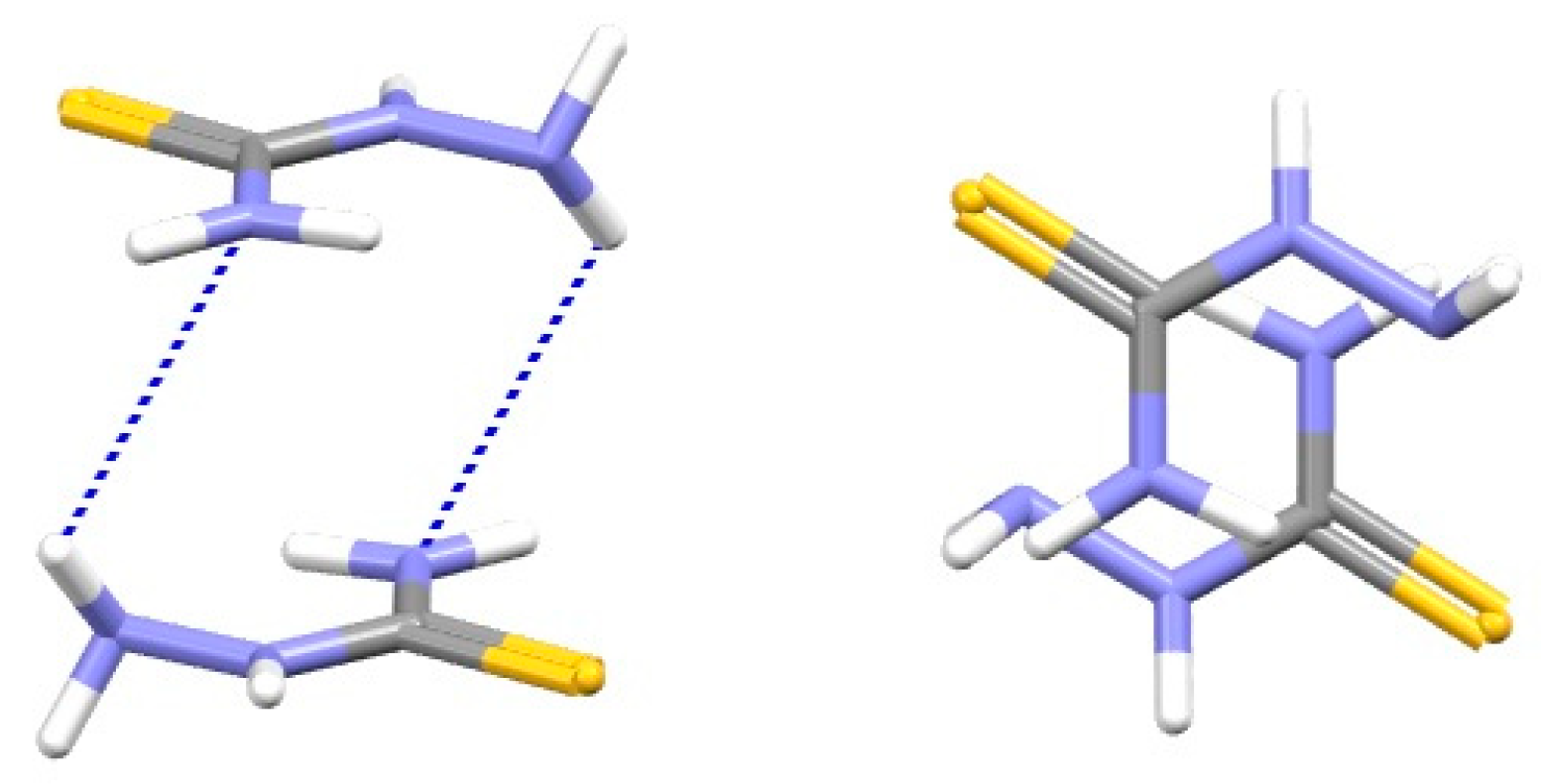


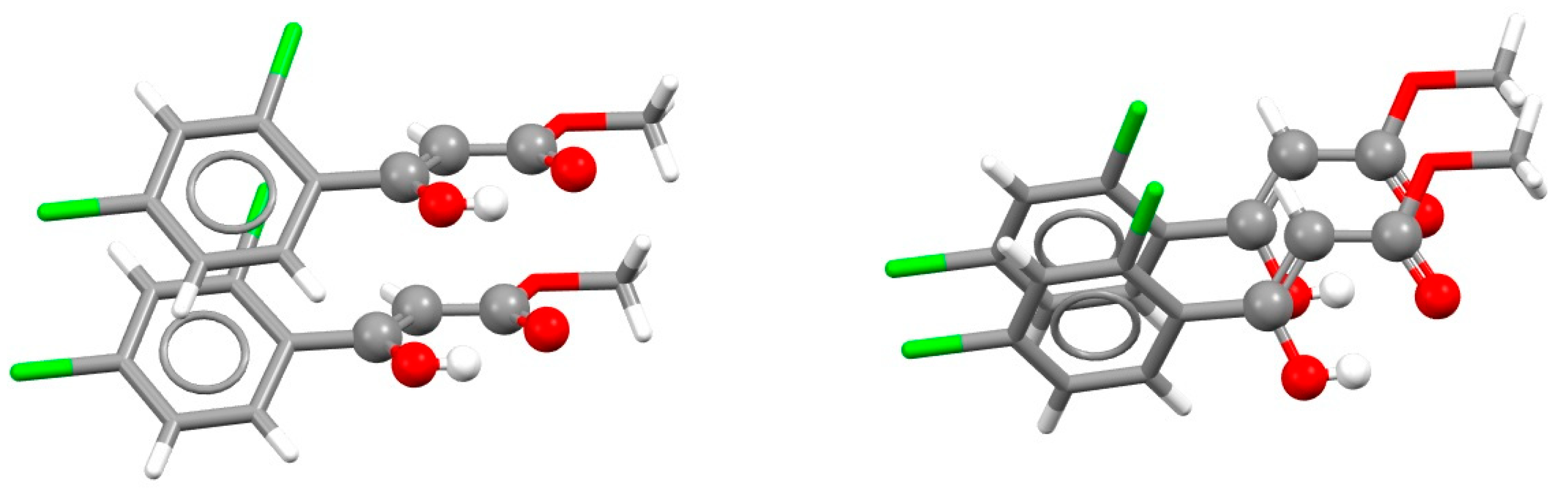
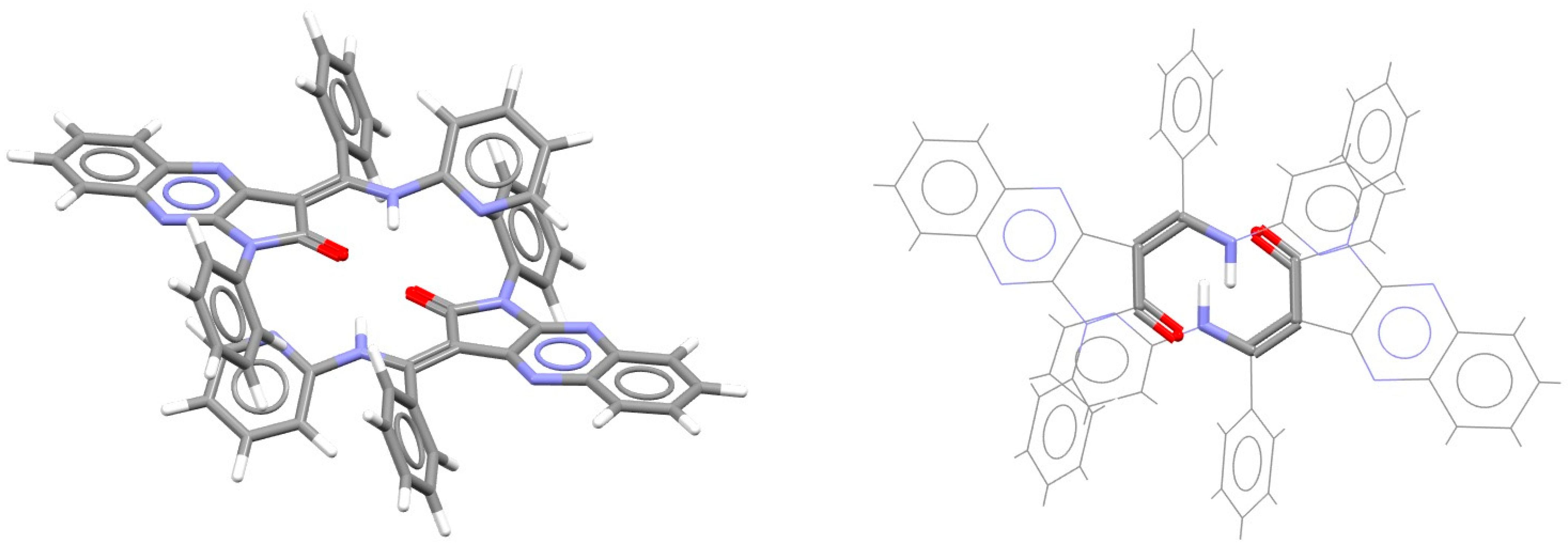
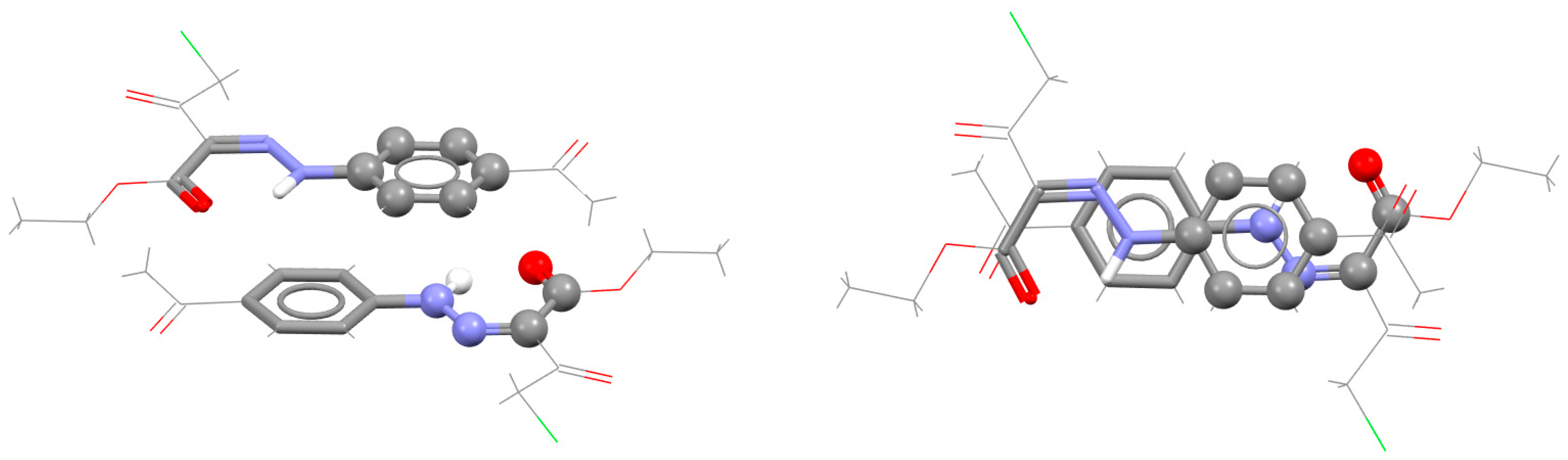


| Metal | Minimum 1 | Minimum 2 | ||||
|---|---|---|---|---|---|---|
| r | R | ΔE | r | R | ΔE | |
| Ni [38] | 1.3 | 3.40 | −4.82 | 1.4 | 3.37 | −5.52 |
| Cu [38] | 1.2 | 3.40 | −4.92 | 1.3 | 3.31 | −6.43 |
| Zn [38] | 1.0 | 3.43 | −4.93 | 1.3 | 3.27 | −7.56 |
| Pd [38] | 1.2 | 3.40 | −5.15 | 1.2 | 3.50 | −5.73 |
| Pt [36] | 1.2 | 3.40 | −5.36 | 1.2 | 3.50 | −5.27 |
| System | r | R | ΔE | ELST | EXCH | IND | DISP | NET DISP |
|---|---|---|---|---|---|---|---|---|
| Ni(acac)-benzene | 1.4 | 3.37 | −5.97 | −4.07 | +7.91 | −0.80 | −9.00 | −1.09 |
| Cu(acac)-benzene | 1.3 | 3.31 | −6.80 | −5.38 | +9.53 | −1.16 | −9.78 | −0.25 |
| Zn(acac)-benzene | 1.3 | 3.27 | −7.59 | −6.38 | +10.52 | −1.61 | −10.12 | +0.40 |
| benzene–benzene | 1.5 | 3.50 | −2.83 | −1.50 | +6.58 | −0.70 | −7.21 | −0.62 |
| Metal | Antiparallel | Parallel | Cross | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | R | ΔE | r | R | ΔE | r | R | ΔE | |
| Ni [38] | 0.5 | 3.13 | −9.47 | 1.8 | 3.20 | −4.80 | 1.8 | 3.25 | −4.98 |
| Cu [38] | 0.4 | 3.01 | −11.70 | ||||||
| Zn [38] | 0.4 | 2.88 | −14.58 | ||||||
| Pd [54] | 2.7 | 3.30 | −9.30 | 1.8 | 3.20 | −5.97 | 1.8 | 3.30 | −5.56 |
| Pt [54] | 2.7 | 3.30 | −9.73 | 1.5 | 3.30 | −6.28 | 1.8 | 3.40 | −6.13 |
| Metal | r | R | ΔE | ELST | EXCH | IND | DISP | NET DISP |
|---|---|---|---|---|---|---|---|---|
| Ni | 0.5 | 3.13 | −10.11 | −11.99 | +15.60 | −1.82 | −11.89 | +3.71 |
| Cu | 0.4 | 3.01 | −12.54 | −16.67 | +21.42 | −3.47 | −13.82 | +7.60 |
| Zn | 0.4 | 2.88 | −15.39 | −23.21 | +30.67 | −6.67 | −16.18 | +14.49 |
| System | r | R | ΔE | SAPT | ELST | EXCH | IND | DISP |
|---|---|---|---|---|---|---|---|---|
| SBHB/SBHB [7] | 1.0 | 3.0 | −4.89 | |||||
| SBHB/benzene [8] | 1.5 | 3.2 | −4.38 | −4.44 | −4.29 | +9.49 | −1.04 | −8.59 |
| benzene/benzene [45] | 1.5 | 3.50 | −2.79 | −2.83 | −1.50 | +6.58 | −0.70 | −7.21 |
| Type of Stacking | RAHB Composition a | r | R | ΔE | SAPT | ELST | EXCH | IND | DISP |
|---|---|---|---|---|---|---|---|---|---|
| RAHB/RAHB [66] | HOCCCO (A) | 0.5 | 3.30 | −4.26 | −4.32 | −3.55 | +5.92 | −0.60 | −6.10 |
| HNCCCO (B) | 1.8 | 3.10 | −4.74 | −4.81 | −4.81 | +7.86 | −1.12 | −6.74 | |
| HNNCCO (C) | 1.8 | 3.35 | −2.23 | −2.09 | −0.89 | +3.76 | −0.33 | −4.64 | |
| RAHB/benzene [67] | HOCCCO (A) | 1.4 | 3.4 | −3.54 | |||||
| HNCCCO (B) | 1.8 | 3.3 | −3.47 | ||||||
| HNNCCO (C) | 1.5 | 3.4 | −3.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malenov, D.P.; Zarić, S.D. Recognizing New Types of Stacking Interactions by Analyzing Data in the Cambridge Structural Database. Chemistry 2023, 5, 2513-2541. https://doi.org/10.3390/chemistry5040164
Malenov DP, Zarić SD. Recognizing New Types of Stacking Interactions by Analyzing Data in the Cambridge Structural Database. Chemistry. 2023; 5(4):2513-2541. https://doi.org/10.3390/chemistry5040164
Chicago/Turabian StyleMalenov, Dušan P., and Snežana D. Zarić. 2023. "Recognizing New Types of Stacking Interactions by Analyzing Data in the Cambridge Structural Database" Chemistry 5, no. 4: 2513-2541. https://doi.org/10.3390/chemistry5040164
APA StyleMalenov, D. P., & Zarić, S. D. (2023). Recognizing New Types of Stacking Interactions by Analyzing Data in the Cambridge Structural Database. Chemistry, 5(4), 2513-2541. https://doi.org/10.3390/chemistry5040164







