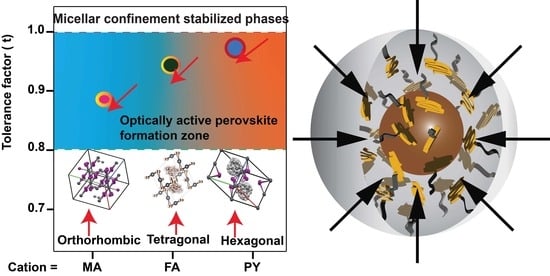
Unusual Phase Behaviour for Organo-Halide Perovskite Nanoparticles Synthesized via Reverse Micelle Templating
Abstract
:1. Introduction
2. Materials and Methods
3. Results
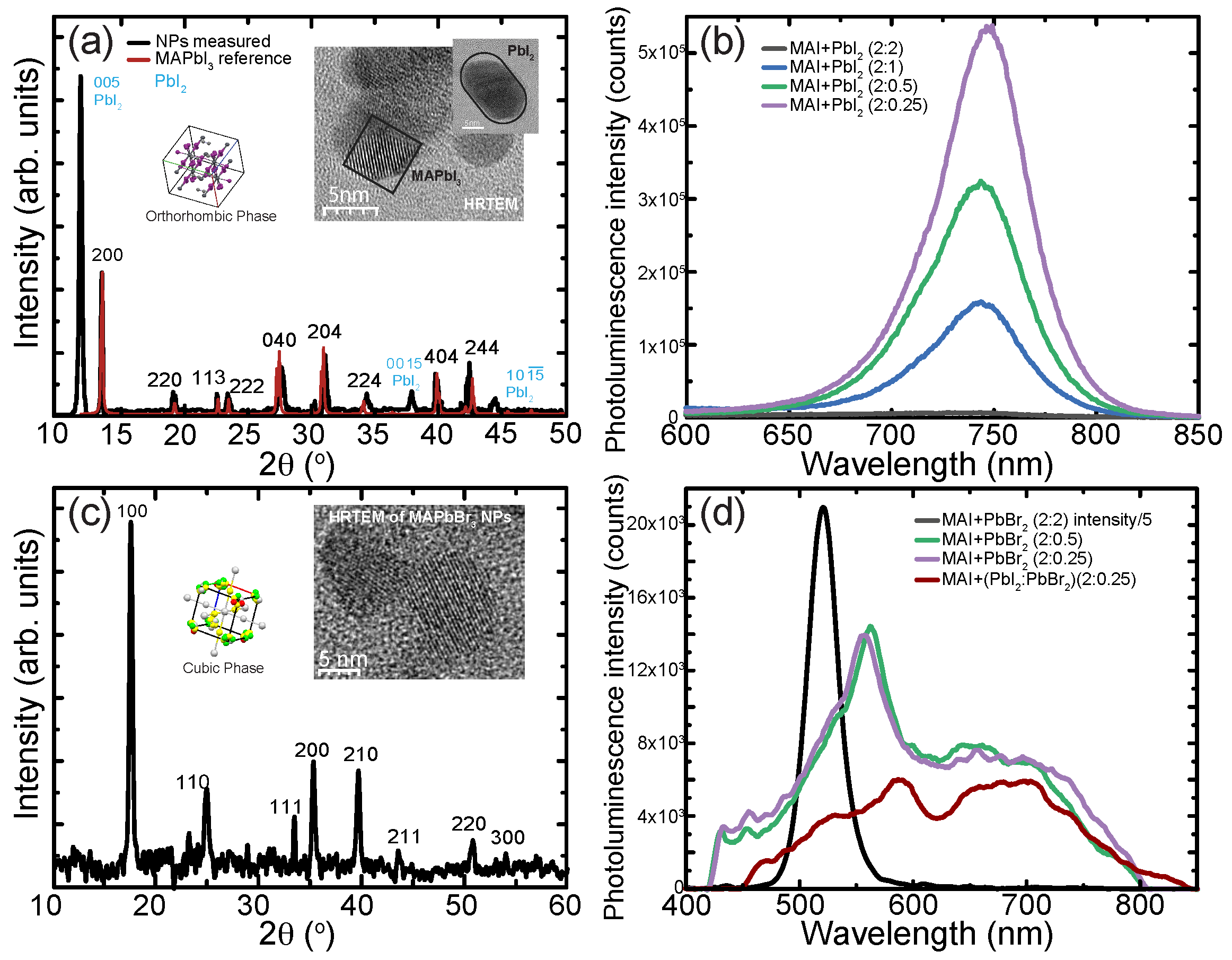
Methylammonium-Based Perovskite Nanoparticles
4. Formadinium-Based Perovskite Nanoparticles
5. Pyrrolidinium Based Perovskite Nanoparticles
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ling, Y.; Yuan, Z.; Tian, Y.; Wang, X.; Wang, J.C.; Xin, Y.; Hanson, K.; Ma, B.; Gao, H. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite Nanoplatelets. Adv. Mater. 2016, 28, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal Halide Perovskites for Light-Emitting Diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J. Present Status and Future Prospects of Perovskite Photovoltaics. Nat. Mater. 2018, 17, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and Challenges of Perovskite Solar Cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef]
- Moon, J.; Mehta, Y.; Gundogdu, K.; So, F.; Gu, Q. Metal-Halide Perovskite Lasers: Cavity Formation and Emission Characteristics. Adv. Mater. 2023, 2211284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shang, Q.; Su, R.; Do, T.T.H.; Xiong, Q. Halide Perovskite Semiconductor Lasers: Materials, Cavity Design, and Low Threshold. Nano Lett. 2021, 21, 1903–1914. [Google Scholar] [CrossRef]
- Eaton, S.W.; Lai, M.; Gibson, N.A.; Wong, A.B.; Dou, L.; Ma, J.; Wang, L.W.; Leone, S.R.; Yang, P. Lasing in Robust Cesium Lead Halide Perovskite Nanowires. Proc. Natl. Acad. Sci. USA 2016, 113, 1993–1998. [Google Scholar] [CrossRef]
- Sutherland, B.R.; Sargent, E.H. Perovskite Photonic Sources. Nat. Photonics 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Munir, M.; Tan, J.; Arbi, R.; Oliveira, P.; Leeb, E.; Salinas, Y.; Scharber, M.C.; Sariciftci, N.S.; Turak, A. Enhanced Stokes Shift and Phase Stability by Cosynthesizing Perovskite Nanoparticles (MAPbI3/MAPbBr3) in a Single Solution. Adv. Photonics Res. 2022, 3, 2100372. [Google Scholar] [CrossRef]
- Tan, J.; Munir, M.; Arbi, R.; Turak, A. Matching Emission and Absorption Profiles for Improved Performance in OPVs with Perovskite Nanoparticle Down-Converters. In Proceedings of the 2023 Photonics North PN, Montreal, QC, Canada, 12–15 June 2023; IEEE: Montreal QC, Canada, 2023; Volume PN58661, p. 10222968. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Du, Z.; Li, Z. Recent Advances in Metal Halide Perovskite Photocatalysts: Properties, Synthesis and Applications. J. Energy Chem. 2021, 54, 770–785. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Zhang, X.; Chen, B.; Suo, H.; Xing, Z.; Wang, Y.; Wei, H.L.; Chen, J.; Guo, Y.; et al. Emerging Halide Perovskite Ferroelectrics. Adv. Mater. 2023, 35, 2205410. [Google Scholar] [CrossRef] [PubMed]
- De Wolf, S.; Holovsky, J.; Moon, S.J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.J.; Yum, J.H.; Ballif, C. Organometallic Halide Perovskites: Sharp Optical Absorption Edge and Its Relation to Photovoltaic Performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Maes, J.; Balcaen, L.; Drijvers, E.; Zhao, Q.; De Roo, J.; Vantomme, A.; Vanhaecke, F.; Geiregat, P.; Hens, Z. Light Absorption Coefficient of CsPbBr3 Perovskite Nanocrystals. J. Phys. Chem. Lett. 2018, 9, 3093–3097. [Google Scholar] [CrossRef]
- Hintermayr, V.A.; Richter, A.F.; Ehrat, F.; Döblinger, M.; Vanderlinden, W.; Sichert, J.A.; Tong, Y.; Polavarapu, L.; Feldmann, J.; Urban, A.S. Tuning the Optical Properties of Perovskite Nanoplatelets through Composition and Thickness by Ligand-Assisted Exfoliation. Adv. Mater. 2016, 28, 9478–9485. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Shi, T.; Yan, Y. Unusual Defect Physics in CH3NH3PbI3 Perovskite Solar Cell Absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Turren-Cruz, S.H.; Hagfeldt, A.; Saliba, M. Methylammonium-Free, High-Performance, and Stable Perovskite Solar Cells on a Planar Architecture. Science 2018, 362, 449–453. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, S.; Xu, Z.; Qiao, B.; Song, P.; Gao, D.; Xu, X. Shape-Controlled Synthesis of All-Inorganic CsPbBr3 Perovskite Nanocrystals with Bright Blue Emission. ACS Appl. Mater. Interfaces 2016, 8, 28824–28830. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Filip, M.R.; Eperon, G.E.; Snaith, H.J.; Giustino, F. Steric Engineering of Metal-Halide Perovskites with Tunable Optical Band Gaps. Nat. Commun. 2014, 5, 5757. [Google Scholar] [CrossRef]
- Kieslich, G.; Sun, S.; Cheetham, A.K. Solid-State Principles Applied to Organic–Inorganic Perovskites: New Tricks for an Old Dog. Chem. Sci. 2014, 5, 4712–4715. [Google Scholar] [CrossRef]
- Fu, Y.; Hautzinger, M.P.; Luo, Z.; Wang, F.; Pan, D.; Aristov, M.M.; Guzei, I.A.; Pan, A.; Zhu, X.; Jin, S. Incorporating Large A Cations into Lead Iodide Perovskite Cages: Relaxed Goldschmidt Tolerance Factor and Impact on Exciton–Phonon Interaction. ACS Cent. Sci. 2019, 5, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.S.; Beswick, C.; Getachew, A.; Heilbrunner, H.; Liang, K.; Hanta, G.; Arbi, R.; Munir, M.; Dawood, H.; Isik Goktas, N.; et al. Reverse Micelle Templating Route to Ordered Monodispersed Spherical Organo-Lead Halide Perovskite Nanoparticles for Light Emission. ACS Appl. Nano Mater. 2019, 2, 4121–4132. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.; Wang, L.; Pang, S. Review of Stability Enhancement for Formamidinium-Based Perovskites. Sol. RRL 2019, 3, 1900215. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium Lead Trihalide: A Broadly Tunable Perovskite for Efficient Planar Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, X.; Bai, Y.; Wang, Q.; Zhao, J.; Huang, J. Surfactant-Controlled Ink Drying Enables High-Speed Deposition of Perovskite Films for Efficient Photovoltaic Modules. Nat. Energy 2018, 3, 560–566. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.H.; Ma, B. Low Dimensional Metal Halide Perovskites and Hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, C.; Tian, Y.; Siegrist, T.; Ma, B. Low-Dimensional Organometal Halide Perovskites. ACS Energy Lett. 2018, 3, 54–62. [Google Scholar] [CrossRef]
- Yang, B.; Bogachuk, D.; Suo, J.; Wagner, L.; Kim, H.; Lim, J.; Hinsch, A.; Boschloo, G.; Nazeeruddin, M.K.; Hagfeldt, A. Strain Effects on Halide Perovskite Solar Cells. Chem. Soc. Rev. 2022, 51, 7509–7530. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Biesold-McGee, G.V.; Liu, Y.; Kang, Z.; Lin, Z. Doping and Ion Substitution in Colloidal Metal Halide Perovskite Nanocrystals. Chem. Soc. Rev. 2020, 49, 4953–5007. [Google Scholar] [CrossRef]
- Liu, D.; Luo, D.; Iqbal, A.N.; Orr, K.W.P.; Doherty, T.A.S.; Lu, Z.H.; Stranks, S.D.; Zhang, W. Strain Analysis and Engineering in Halide Perovskite Photovoltaics. Nat. Mater. 2021, 20, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Sa, R.; Xu, G. Tuning Electronic and Optical Properties of CsPbI3 by Applying Strain: A First-Principles Theoretical Study. Chem. Phys. Lett. 2019, 732, 136642. [Google Scholar] [CrossRef]
- Edri, E.; Kirmayer, S.; Kulbak, M.; Hodes, G.; Cahen, D. Chloride Inclusion and Hole Transport Material Doping to Improve Methyl Ammonium Lead Bromide Perovskite-Based High Open-Circuit Voltage Solar Cells. J. Phys. Chem. Lett. 2014, 5, 429–433. [Google Scholar] [CrossRef]
- Ahlawat, P.; Hinderhofer, A.; Alharbi, E.A.; Lu, H.; Ummadisingu, A.; Niu, H.; Invernizzi, M.; Zakeeruddin, S.M.; Dar, M.I.; Schreiber, F.; et al. A Combined Molecular Dynamics and Experimental Study of Two-Step Process Enabling Low-Temperature Formation of Phase-Pure α-FAPbI3. Sci. Adv. 2021, 7, eabe3326. [Google Scholar] [CrossRef]
- Steele, J.A.; Yuan, H.; Tan, C.Y.X.; Keshavarz, M.; Steuwe, C.; Roeffaers, M.B.J.; Hofkens, J. Direct Laser Writing of δ- to α-Phase Transformation in Formamidinium Lead Iodide. ACS Nano 2017, 11, 8072–8083. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Mhaisalkar, S.G.; Mathews, N.; Boix, P.P. Perovskite Nanoparticles: Synthesis, Properties, and Novel Applications in Photovoltaics and LEDs. Small Methods 2019, 3, 1800231. [Google Scholar] [CrossRef]
- Prochazkova, A.J.; Mayr, F.; Gugujonovic, K.; Hailegnaw, B.; Krajcovic, J.; Salinas, Y.; Brüggemann, O.; Sariciftci, N.S.; Scharber, M.C. Anti-Stokes Photoluminescence Study on a Methylammonium Lead Bromide Nanoparticle Film. Nanoscale 2020, 12, 16556–16561. [Google Scholar] [CrossRef]
- Bhaumik, S.; Veldhuis, S.A.; Ng, Y.F.; Li, M.; Muduli, S.K.; Sum, T.C.; Damodaran, B.; Mhaisalkar, S.; Mathews, N. Highly Stable, Luminescent Core–Shell Type Methylammonium–Octylammonium Lead Bromide Layered Perovskite Nanoparticles. Chem. Commun. 2016, 52, 7118–7121. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, D.; Xu, Y.; Wang, A.; Deng, Z. Ligand-Mediated Synthesis of Shape-Controlled Cesium Lead Halide Perovskite Nanocrystals via Reprecipitation Process at Room Temperature. ACS Nano 2016, 10, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Demchyshyn, S.; Roemer, J.M.; Groiß, H.; Heilbrunner, H.; Ulbricht, C.; Apaydin, D.; Böhm, A.; Rütt, U.; Bertram, F.; Hesser, G.; et al. Confining Metal-Halide Perovskites in Nanoporous Thin Films. Sci. Adv. 2017, 3, e1700738. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ladi, N.H.; Shai, X.; Li, H.; Shen, Y.; Wang, M. Will Organic–Inorganic Hybrid Halide Lead Perovskites Be Eliminated from Optoelectronic Applications? Nanoscale Adv. 2019, 1, 1276–1289. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Zhong, Y.; Schötz, K.; Tripathi, B.; Köhler, A.; Huettner, S.; Panzer, F. Investigating Two-Step MAPbI 3 Thin Film Formation during Spin Coating by Simultaneous in Situ Absorption and Photoluminescence Spectroscopy. J. Mater. Chem. A 2020, 8, 5086–5094. [Google Scholar] [CrossRef]
- Esaka, H.; Kurz, W. Columnar Dendrite Growth: Experiments on Tip Growth. J. Cryst. Growth 1985, 72, 578–584. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Lin, X.; Wu, T.; Han, Q.; Zhang, Y.; Han, L. Effects of A Site Doping on the Crystallization of Perovskite Films. J. Mater. Chem. A 2021, 9, 1372–1394. [Google Scholar] [CrossRef]
- Hoke, E.T.; Slotcavage, D.J.; Dohner, E.R.; Bowring, A.R.; Karunadasa, H.I.; McGehee, M.D. Reversible Photo-Induced Trap Formation in Mixed-Halide Hybrid Perovskites for Photovoltaics. Chem. Sci. 2015, 6, 613–617. [Google Scholar] [CrossRef]
- Min, H.; Kim, M.; Lee, S.U.; Kim, H.; Kim, G.; Choi, K.; Lee, J.H.; Seok, S.I. Efficient, Stable Solar Cells by Using Inherent Bandgap of α-Phase Formamidinium Lead Iodide. Science 2019, 366, 749–753. [Google Scholar] [CrossRef]
- Turak, A. Reverse Micelles as a Universal Route to Solution Processed Nanoparticles for Optical, Optoelectronic and Photonic Applications: A Story of Salt Complexation, Micellar Stability, and Nanoparticle Spatial Distribution. Vid. Proc. Adv. Mater. 2021, 2, 2103166. [Google Scholar]
- Turak, A. Perovskite Nanoparticles on Demand: Highly Stable Nanoparticles Using Reverse Micelle Templating. In Proceedings of the 2023 Photonics North PN, Montreal, QC, Canada, 12–15 June 2023; IEEE: Montreal QC, Canada, 2023; Volume PN58661, p. 10222995. [Google Scholar] [CrossRef]
- Hui, L.S.; Munir, M.; Vuong, A.; Hilke, M.; Wong, V.; Fanchini, G.; Scharber, M.C.; Sariciftci, N.S.; Turak, A. Universal Transfer Printing of Micelle-Templated Nanoparticles Using Plasma-Functionalized Graphene. ACS Appl. Mater. Interfaces 2020, 12, 46530–46538. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Arbi, R.; Scharber, M.C.; Salinas, Y.; Sariciftci, N.S.; Turak, A. Anionic Exchange Route to Synthesize Highly Uniform, Stable and Luminescent MAPBr Nanoparticles. OSA Tech. Dig. 2020, 2020, PvTu3G.5. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials: Tools and Challenges. In Nanomaterials for Food Applications; López Rubio, A., Fabra Rovira, M.J., Martínez Sanz, M., Gómez-Mascaraque, L.G., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar] [CrossRef]
- Stavis, S.M.; Fagan, J.A.; Stopa, M.; Liddle, J.A. Nanoparticle Manufacturing–Heterogeneity through Processes to Products. ACS Appl. Nano Mater. 2018, 1, 4358–4385. [Google Scholar] [CrossRef]
- Moerman, D.; Eperon, G.E.; Precht, J.T.; Ginger, D.S. Correlating Photoluminescence Heterogeneity with Local Electronic Properties in Methylammonium Lead Tribromide Perovskite Thin Films. Chem. Mater. 2017, 29, 5484–5492. [Google Scholar] [CrossRef]
- He, R.; Wang, W.; Yi, Z.; Lang, F.; Chen, C.; Luo, J.; Zhu, J.; Thiesbrummel, J.; Shah, S.; Wei, K.; et al. Improving Interface Quality for 1-Cm2 All-Perovskite Tandem Solar Cells. Nature 2023, 618, 80–86. [Google Scholar] [CrossRef]
- Tan, S.; Huang, T.; Yavuz, I.; Wang, R.; Yoon, T.W.; Xu, M.; Xing, Q.; Park, K.; Lee, D.K.; Chen, C.H.; et al. Stability-Limiting Heterointerfaces of Perovskite Photovoltaics. Nature 2022, 605, 268–273. [Google Scholar] [CrossRef]
- Mitzi, D.B. Synthesis, Structure, and Properties of Organic-Inorganic Perovskites and Related Materials. In Progress in Inorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1999; pp. 1–121. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible- Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Im, J.H.; Lee, C.R.; Lee, J.W.; Park, S.W.; Park, N.G. 6.5% Efficient Perovskite Quantum-Dot-Sensitized Solar Cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Quarti, C.; Mosconi, E.; Ball, J.M.; D’Innocenzo, V.; Tao, C.; Pathak, S.; Snaith, H.J.; Petrozza, A.; De Angelis, F. Structural and Optical Properties of Methylammonium Lead Iodide across the Tetragonal to Cubic Phase Transition: Implications for Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 155–163. [Google Scholar] [CrossRef]
- Ball, J.M.; Stranks, S.D.; Hörantner, M.T.; Hüttner, S.; Zhang, W.; Crossland, E.J.W.; Ramirez, I.; Riede, M.; Johnston, M.B.; Friend, R.H.; et al. Optical Properties and Limiting Photocurrent of Thin-Film Perovskite Solar Cells. Energy Environ. Sci. 2015, 8, 602–609. [Google Scholar] [CrossRef]
- Ziffer, M.E.; Mohammed, J.C.; Ginger, D.S. Electroabsorption Spectroscopy Measurements of the Exciton Binding Energy, Electron–Hole Reduced Effective Mass, and Band Gap in the Perovskite CH3NH3PbI3. ACS Photonics 2016, 3, 1060–1068. [Google Scholar] [CrossRef]
- Maiti, A.; Pal, A.J. Carrier Recombination in CH3NH3PbI3: Why Is It a Slow Process? Rep. Prog. Phys. 2022, 85, 024501. [Google Scholar] [CrossRef]
- Chen, Z.; Turedi, B.; Alsalloum, A.Y.; Yang, C.; Zheng, X.; Gereige, I.; AlSaggaf, A.; Mohammed, O.F.; Bakr, O.M. Single-Crystal MAPbI3 Perovskite Solar Cells Exceeding 21% Power Conversion Efficiency. ACS Energy Lett. 2019, 4, 1258–1259. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Chen, M.; Zong, Y.; Huang, J.; Pang, S.; Padture, N.P. Doping and Alloying for Improved Perovskite Solar Cells. J. Mater. Chem. A 2016, 4, 17623–17635. [Google Scholar] [CrossRef]
- Tenuta, E.; Zheng, C.; Rubel, O. Thermodynamic Origin of Instability in Hybrid Halide Perovskites. Sci. Rep. 2016, 6, 37654. [Google Scholar] [CrossRef]
- Vybornyi, O.; Yakunin, S.; Kovalenko, M.V. Polar-Solvent-Free Colloidal Synthesis of Highly Luminescent Alkylammonium Lead Halide Perovskite Nanocrystals. Nanoscale 2016, 8, 6278–6283. [Google Scholar] [CrossRef]
- Aharon, S.; Etgar, L. Two Dimensional Organometal Halide Perovskite Nanorods with Tunable Optical Properties. Nano Lett. 2016, 16, 3230–3235. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.Y. Lead Halide Perovskite Nanowire Lasers with Low Lasing Thresholds and High Quality Factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Wang, L.; Williams, N.E.; Malachosky, E.W.; Otto, J.P.; Hayes, D.; Wood, R.E.; Guyot-Sionnest, P.; Engel, G.S. Scalable Ligand-Mediated Transport Synthesis of Organic–Inorganic Hybrid Perovskite Nanocrystals with Resolved Electronic Structure and Ultrafast Dynamics. ACS Nano 2017, 11, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Levchuk, I.; Herre, P.; Brandl, M.; Osvet, A.; Hock, R.; Peukert, W.; Schweizer, P.; Spiecker, E.; Batentschuk, M.; Brabec, C.J. Ligand-Assisted Thickness Tailoring of Highly Luminescent Colloidal CH3NH3PbX3 (X = Br and I) Perovskite Nanoplatelets. Chem. Commun. 2017, 53, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Milot, R.L.; Eperon, G.E.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Temperature-Dependent Charge-Carrier Dynamics in CH3NH3PbI3 Perovskite Thin Films. Adv. Funct. Mater. 2015, 25, 6218–6227. [Google Scholar] [CrossRef]
- Deretzis, I.; Magna, A.L. Exploring the Orthorhombic–Tetragonal Phase Transition in CH3NH3PbI3: The Role of Atom Kinetics. Nanoscale 2017, 9, 5896–5903. [Google Scholar] [CrossRef] [PubMed]
- Brivio, F.; Frost, J.M.; Skelton, J.M.; Jackson, A.J.; Weber, O.J.; Weller, M.T.; Goñi, A.R.; Leguy, A.M.A.; Barnes, P.R.F.; Walsh, A. Lattice Dynamics and Vibrational Spectra of the Orthorhombic, Tetragonal, and Cubic Phases of Methylammonium Lead Iodide. Phys. Rev. B 2015, 92, 144308. [Google Scholar] [CrossRef]
- Reinoso, M.Á.; Otálora, C.A.; Gordillo, G. Improvement Properties of Hybrid Halide Perovskite Thin Films Prepared by Sequential Evaporation for Planar Solar Cells. Materials 2019, 12, 1394. [Google Scholar] [CrossRef]
- Munir, M.; Arbi, R.; Tan, J.; Turak, A. Core-Shell Perovskite-TiO2 Nanoparticles for High Stability. In Proceedings of the 2023 Photonics North PN, Montreal, QC, Canada, 12–15 June 2023; IEEE: Montreal QC, Canada, 2023; Volume PN58661, p. 10223000. [Google Scholar] [CrossRef]
- Cui, L.; Oliveira, P.Q.; Sask, K.N.; Turak, A.Z. PeakForce Quantitative Nanomechanical Information for Reverse Micelle Synthesis. In Proceedings of the 2023 Photonics North PN, Montreal, QC, Canada, 12–15 June 2023; IEEE: Montreal QC, Canada, 2023; Volume PN58661, p. 10222973. [Google Scholar] [CrossRef]
- Arbi, R.; Ibrahim, A.; Goldring-Vandergeest, L.; Liang, K.; Hanta, G.; Hui, L.S.; Turak, A. Role of Hydration and Micellar Shielding in Tuning the Structure of Single Crystalline Iron Oxide Nanoparticles for Designer Applications. Nano Sel. 2021, 2, 2419–2431. [Google Scholar] [CrossRef]
- Aloisi, G.G.; Beggiato, G.; Mazzucato, U. Charge Transfer Complexes between Halogens and Pyridines. Part 4—Effect of the Acid Strength of the Acceptors. Trans. Faraday Soc. 1970, 66, 3075–3080. [Google Scholar] [CrossRef]
- Shin, W.J.; Kim, J.Y.; Cho, G.; Lee, J.S. Highly Selective Incorporation of SiO2 Nanoparticles in PS-b-P2VP Block Copolymers by Quaternization. J. Mater. Chem. 2009, 19, 7322. [Google Scholar] [CrossRef]
- Zhang, Z.; Rahman, M.M.; Abetz, C.; Bajer, B.; Wang, J.; Abetz, V. Quaternization of a Polystyrene-block-poly(4-Vinylpyridine) Isoporous Membrane: An Approach to Tune the Pore Size and the Charge Density. Macromol. Rapid Commun. 2019, 40, 1800729. [Google Scholar] [CrossRef]
- Bumstead, M.; Liang, K.; Hanta, G.; Hui, L.S.; Turak, A. disLocate: Tools to Rapidly Quantify Local Intermolecular Structure to Assess Two-Dimensional Order in Self-Assembled Systems. Sci. Rep. 2018, 8, 1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; Li, L.C.; Shellaiah, M.; Wen Sun, K. Structural and Photophysical Properties of Methylammonium Lead Tribromide (MAPbBr3) Single Crystals. Sci. Rep. 2017, 7, 13643. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.; Lin, Y.; Beavers, C.M.; Voss, J.; Mao, W.L.; Karunadasa, H.I. High-Pressure Single-Crystal Structures of 3D Lead-Halide Hybrid Perovskites and Pressure Effects on Their Electronic and Optical Properties. ACS Cent. Sci. 2016, 2, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.G.; Gordillo, G.; Plazas, M.C.; Landínez Téllez, D.A.; Roa-Rojas, J. Optical Features of PbBr2 Semiconductor Thin Films for Radiation Attenuation Application. J. Mater. Sci. Mater. Electron. 2021, 32, 16937–16944. [Google Scholar] [CrossRef]
- Lehmann, F.; Franz, A.; Többens, D.M.; Levcenco, S.; Unold, T.; Taubert, A.; Schorr, S. The Phase Diagram of a Mixed Halide (Br, I) Hybrid Perovskite Obtained by Synchrotron X-ray Diffraction. RSC Adv. 2019, 9, 11151–11159. [Google Scholar] [CrossRef]
- Merlevede, C.F.; Maes, G. Vibrational Spectra of Charge Transfer Complexes of Pyridines and Halogens:: Part 1. A Laser Raman Study of the Iodine Stretching Vibration in Complexes Pyridine .Iodine. Comparison with Pyridine .Bromine. Adv. Mol. Relax. Interact. Process. 1980, 16, 111–130. [Google Scholar] [CrossRef]
- Brunetti, B.; Cavallo, C.; Ciccioli, A.; Gigli, G.; Latini, A. On the Thermal and Thermodynamic (In)Stability of Methylammonium Lead Halide Perovskites. Sci. Rep. 2016, 6, 31896. [Google Scholar] [CrossRef]
- Nagabhushana, G.P.; Shivaramaiah, R.; Navrotsky, A. Direct Calorimetric Verification of Thermodynamic Instability of Lead Halide Hybrid Perovskites. Proc. Natl. Acad. Sci. USA 2016, 113, 7717–7721. [Google Scholar] [CrossRef]
- Ivanov, I.L.; Steparuk, A.S.; Bolyachkina, M.S.; Tsvetkov, D.S.; Safronov, A.P.; Zuev, A.Y. Thermodynamics of Formation of Hybrid Perovskite-Type Methylammonium Lead Halides. J. Chem. Thermodyn. 2018, 116, 253–258. [Google Scholar] [CrossRef]
- Han, Q.; Bae, S.H.; Sun, P.; Hsieh, Y.T.; Yang, Y.M.; Rim, Y.S.; Zhao, H.; Chen, Q.; Shi, W.; Li, G.; et al. Single Crystal Formamidinium Lead Iodide (FAPbI3): Insight into the Structural, Optical, and Electrical Properties. Adv. Mater. 2016, 28, 2253–2258. [Google Scholar] [CrossRef]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-Organic-Cation Perovskite Photovoltaics for Enhanced Solar-Light Harvesting. Angew. Chem. Int. Ed. 2014, 53, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Seol, D.J.; Cho, A.N.; Park, N.G. High-Efficiency Perovskite Solar Cells Based on the Black Polymorph of HC(NH2)2PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.M.; Fu, K.; Fang, Y.; Chen, S.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G.; Boix, P.P.; Baikie, T. Formamidinium-Containing Metal-Halide: An Alternative Material for Near-IR Absorption Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 16458–16462. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-Performance Photovoltaic Perovskite Layers Fabricated through Intramolecular Exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Hu, H.; Zhang, J.; Lv, S.; Yu, Y.; Wei, F.; Qin, T.; Xu, H.; Liu, Z.; Cui, G. NH2CH=NH2PbI3: An Alternative Organolead Iodide Perovskite Sensitizer for Mesoscopic Solar Cells. Chem. Mater. 2014, 26, 1485–1491. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled Growth of Perovskite Layers with Volatile Alkylammonium Chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, B.; Wang, C.; Shi, P.; Zhang, X.; Liu, C.; Yang, Y.; Gao, X.; Wang, R.; Tao, L.; et al. Highly Efficient and Stable FAPbI3 Perovskite Solar Cells and Modules Based on Exposure of the (011) Facet. Nano-Micro Lett. 2023, 15, 138. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, C.; Jha, S.K.; Li, Z.; Zhu, K.; Priya, S. Improved Phase Stability of Formamidinium Lead Triiodide Perovskite by Strain Relaxation. ACS Energy Lett. 2016, 1, 1014–1020. [Google Scholar] [CrossRef]
- Li, G.; Su, Z.; Canil, L.; Hughes, D.; Aldamasy, M.H.; Dagar, J.; Trofimov, S.; Wang, L.; Zuo, W.; Jerónimo-Rendon, J.J.; et al. Highly Efficient P-i-n Perovskite Solar Cells That Endure Temperature Variations. Science 2023, 379, 399–403. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A Mixed-Cation Lead Mixed-Halide Perovskite Absorber for Tandem Solar Cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Quarti, C.; Grancini, G.; Mosconi, E.; Bruno, P.; Ball, J.M.; Lee, M.M.; Snaith, H.J.; Petrozza, A.; Angelis, F.D. The Raman Spectrum of the CH3NH3PbI3 Hybrid Perovskite: Interplay of Theory and Experiment. J. Phys. Chem. Lett. 2014, 5, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sun, Y.; Zhu, W.; Jiang, J.; Zhao, X.; Lin, D.; Xu, W.; Duan, X.; Francisco, J.S.; Zeng, X.C. Two-Dimensional Monolayer Salt Nanostructures Can Spontaneously Aggregate Rather than Dissolve in Dilute Aqueous Solutions. Nat. Commun. 2021, 12, 5602. [Google Scholar] [CrossRef]
- Kusumawati, Y.; Ivansyah, A.L.; Ali, B.T.I.; Kurnia, K.A.; Hutama, A.S.; Fansuri, H. Photophysical Properties of Ammonium, Pyrrolidinium, Piperidinium, Imidazolium, and Pyridinium as a Guide to Prepare Ionic-Organic Hybrid Materials. Heliyon 2022, 8, e09121. [Google Scholar] [CrossRef]
- Jin, J.C.; Shen, N.N.; Wang, Z.P.; Peng, Y.C.; Huang, X.Y. Photoluminescent Ionic Metal Halides Based on S2 Typed Ions and Aprotic Ionic Liquid Cations. Coord. Chem. Rev. 2021, 448, 214185. [Google Scholar] [CrossRef]
- Selivanov, N.I.; Rozhkova, Y.A.; Kevorkyants, R.; Emeline, A.V.; Bahnemann, D.W. The Effect of Organic Cations on the Electronic, Optical and Luminescence Properties of 1D Piperidinium, Pyridinium, and 3-Hydroxypyridinium Lead Trihalides. Dalton Trans. 2020, 49, 4390–4403. [Google Scholar] [CrossRef]
- Xu, A.F.; Liu, N.; Xie, F.; Song, T.; Ma, Y.; Zhang, P.; Bai, Y.; Li, Y.; Chen, Q.; Xu, G. Promoting Thermodynamic and Kinetic Stabilities of FA-based Perovskite by an in Situ Bilayer Structure. Nano Lett. 2020, 20, 3864–3871. [Google Scholar] [CrossRef]
- Rao, M.K.; Selvakumar, M.; Mahesha, M.G.; Paramasivam, S.; Dileep K, R.; Prabhu, N.S.; Veerappan, G.; Senthilkumar, S.; Kamath, S.D. Pyrrolidinium Induced Templated Growth of 1D-3D Halide Perovskite Heterostructure for Solar Cell Applications. Mater. Chem. Phys. 2023, 303, 127668. [Google Scholar] [CrossRef]
- Yang, M.J.; Tang, S.Y.; Weng, Y.R.; Zhou, F.; Shi, Y.; Bai, Y.J.; Ai, Y. H/F Substitution on the Spacer Cations Leads to 1D-to-2D Increment of the Pyrrolidinium-Containing Lead Iodide Hybrid Perovskites. Inorg. Chem. 2022, 61, 5836–5843. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.D.; Yang, Y.; Hoang, M.T.; Wang, T.; Tiong, V.T.; Wilson, G.J.; Wang, H. 1D Pyrrolidinium Lead Iodide for Efficient and Stable Perovskite Solar Cells. Energy Technol. 2020, 8, 1900918. [Google Scholar] [CrossRef]
- Xu, A.F.; Wang, R.T.; Yang, L.W.; Liu, N.; Chen, Q.; LaPierre, R.; Goktas, N.I.; Xu, G. Pyrrolidinium Containing Perovskites with Thermal Stability and Water Resistance for Photovoltaics. J. Mater. Chem. C 2019, 7, 11104–11108. [Google Scholar] [CrossRef]
- Xu, A.F.; Wang, R.T.; Yang, L.W.; Jarvis, V.; Britten, J.F.; Xu, G. Pyrrolidinium Lead Iodide from Crystallography: A New Perovskite with Low Bandgap and Good Water Resistance. Chem. Commun. 2019, 55, 3251–3253. [Google Scholar] [CrossRef]
- Ou, Q.; Bao, X.; Zhang, Y.; Shao, H.; Xing, G.; Li, X.; Shao, L.; Bao, Q. Band Structure Engineering in Metal Halide Perovskite Nanostructures for Optoelectronic Applications. Nano Mater. Sci. 2019, 1, 268–287. [Google Scholar] [CrossRef]
- Varin, R.A.; Bystrzycki, J.; Calka, A. Effect of Annealing on the Microstructure, Ordering and Microhardness of Ball Milled Cubic (L12) Titanium Trialuminide Intermetallic Powder. Intermetallics 1999, 7, 785–796. [Google Scholar] [CrossRef]
- Dongol, M.; El-Denglawey, A.; Abd El Sadek, M.S.; Yahia, I.S. Thermal Annealing Effect on the Structural and the Optical Properties of Nano CdTe Films. Optik 2015, 126, 1352–1357. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I.; et al. Dismantling the “Red Wall” of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium–Cesium Lead Iodide Nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef]
- Rambukwella, M.; Sakthivel, N.A.; Delcamp, J.H.; Sementa, L.; Fortunelli, A.; Dass, A. Ligand Structure Determines Nanoparticles’ Atomic Structure, Metal-Ligand Interface and Properties. Front. Chem. 2018, 6, 330. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Sneed, B.T.; Young, A.P.; Tsung, C.K. Building up Strain in Colloidal Metal Nanoparticle Catalysts. Nanoscale 2015, 7, 12248–12265. [Google Scholar] [CrossRef] [PubMed]
- Gheshlaghi, N.; Faraji, M.; Sedaghat Pisheh, H. Interfacial Strain and Shell Thickness Effect on Core Squeeze/Stretch in Core/Shell Quantum Dots. SN Appl. Sci. 2020, 2, 745. [Google Scholar] [CrossRef]
- Young, T.J.; Monclus, M.A.; Burnett, T.L.; Broughton, W.R.; Ogin, S.L.; Smith, P.A. The Use of the PeakForce TM Quantitative Nanomechanical Mapping AFM-based Method for High-Resolution Young’s Modulus Measurement of Polymers. Meas. Sci. Technol. 2011, 22, 125703. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Sokolov, I. Quantitative Mapping of the Elastic Modulus of Soft Materials with HarmoniX and PeakForce QNM AFM Modes. Langmuir 2012, 28, 16060–16071. [Google Scholar] [CrossRef] [PubMed]
- Hanta, G. Nanomechanical Dependence of Micelles on Salt Loading Ratios: A Story of Salt Complexation, Micellar Stability, and Nanoparticle Spatial Distribution. Master’s Thesis, McMaster University, Hamilton, ON, Canada, 2019. [Google Scholar]
- Liu, H.; Liang, X.; Nakajima, K. Nanoscale Strain–Stress Mapping for a Thermoplastic Elastomer Revealed Using a Combination of in Situ Atomic Force Microscopy Nanomechanics and Delaunay Triangulation. J. Polym. Sci. 2022, 60, 3134–3140. [Google Scholar] [CrossRef]
- Taubert, A. Heavy Elements in Ionic Liquids. In Ionic Liquids; Kirchner, B., Ed.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 127–159. [Google Scholar] [CrossRef]





| Observed Reflections | Expected Reflections MAPbI3 (252413-ICSD) | Expected Reflections PbI2 (23762-ICSD) | |||
|---|---|---|---|---|---|
| 2 | h k l | 2 | h k l | 2 | h k l |
| 12.60 | 0 0 5 (PbI2) | 12.70 | 0 0 5 | ||
| 14.20 | 2 0 0 | 14.20 | 2 0 0 | ||
| 20.20 | 2 2 0 | 20.00 | 2 2 0 | ||
| 22.20 | 1 1 3 | 23.50 | 1 1 3 | 22.50 | 1 0 0 |
| 24.50 | 2 2 2 | 24.50 | 2 2 2 | 24.80 | 1 0 4 |
| 25.90 | 1 0 5 (PbI2) | 25.90 | 1 0 5 | ||
| 28.30 | 0 4 0 | 28.30 | 0 4 0 | ||
| 31.02 | 3 1 3 | ||||
| 31.70 | 2 0 4 | 31.70 | 2 0 4 | ||
| 34.30 | 1 0 10 | ||||
| 35.00 | 2 2 4 | 35.05 | 2 2 4 | ||
| 37.30 | 1 5 1 | ||||
| 38.70 | 0 0 15 (PbI2) | 38.70 | 0 0 15 | ||
| 39.60 | 2 1 | ||||
| 40.60 | 4 0 4 | ||||
| 42.70 | 5 3 1 | ||||
| 43.20 | 2 4 4 | 43.20 | 2 4 4 | ||
| 45.30 | 0 2 6 | 45.50 | 0 2 6 | ||
| 47.60 | 3 5 3 |
| Observed Reflections | Expected Reflections MAPbBr3 [88] | Expected Reflections PbBr2 [90] | |||
|---|---|---|---|---|---|
| 2 | h k l | 2 | h k l | 2 | h k l |
| 16.71 | 1 0 1 | ||||
| 17.40 | 1 0 0 | 17.44 | 1 0 0 | ||
| 21.62 | 0 0 2 | ||||
| 23.98 | 0 1 1 | ||||
| 24.70 | 1 1 0 | 24.69 | 1 1 0 | ||
| 25.38 | 1 2 0 | ||||
| 27.72 | 2 0 0 | ||||
| 31.40 | 1 1 1 | 30.34 | 1 1 1 | ||
| 33.54 | 2 1 0 | ||||
| 35.30 | 2 0 0 | 35.14 | 2 0 0 | ||
| 39.65 | 2 1 0 | 39.45 | 2 1 0 | ||
| 43.62 | 2 1 1 | 43.44 | 2 1 1 | ||
| 44.21 | 0 3 1 | ||||
| 46.82 | 3 1 0 | ||||
| 50.80 | 2 2 0 | 50.61 | 2 2 0 | ||
| 52.43 | 0 4 0 | ||||
| 53.27 | 3 7 0 | ||||
| 54.10 | 3 0 0 | 53.90 | 3 0 0 | ||
| 57.60 | 3 1 1/2 3 1 |
| Observed Reflections PyI-PbI Precursor -Based nps | Observed Reflections PyI-PbBr Precursor -Based nps | Calculated Reflections PyPbI3 | Calculated Reflections PyPbBr3 | Expected Reflections PbI2 (23762-ICSD) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | h k l | 2 | h k l | 2 | h k l | 2 | h k l | 2 | h k l |
| 9.85 | 1 0 0 (PyPbBr3) | 9.51 | 1 0 0 | ||||||
| 11.18 | 1 0 0 | 11.57 | 1 0 0 (PyPbI3) | 11.05 | 1 0 0 | ||||
| 12.79 | 0 0 5 (PbI2) | 12.85 | 0 0 5 (PbI2) | 12.7 | 0 0 5 | ||||
| 15.77 | 1 0 1 | 15.43 | 1 0 1 (PyPbI3) | 15.49 | 1 0 1 | ||||
| 19.29 | 2 0 | 19.55 | 2 0 (PyPbI3) 200 (PyPbBr3) unreacted precursors | 19.12 | 2 0 | 19.06 | 2 0 0 | ||
| 21.67 | 0 0 2 (PyPbBr3) | 21.34 | 2 0 | ||||||
| 22.14 | 0 0 2 | 21.95 | 0 0 2 | 21.92 | 0 0 2 | ||||
| 22.47 | 1 0 0 | ||||||||
| 24.10 | 1 0 2 (PyPbBr3) | 23.94 | 1 0 2 | ||||||
| 24.92 | 2 0 1 | 24.92 | 2 0 1 (PyPbI3) | 24.67 | 2 0 1 | 24.81 | 1 0 4 | ||
| 26.05 | 1 0 5 (PbI2) | 26.30 | 1 0 5 (PbI2) | 25.98 | 1 0 5 | ||||
| 28.76 | 3 0 0 | ||||||||
| 29.47 | 2 2 | 29.67 | 2 0 2 (PyPbBr3) | 29.21 | 2 2 | 29.21 | 2 0 2 | ||
| 30.50 | 3 0 (PyPbBr3) | 30.35 | 3 0 | ||||||
| 30.78 | 2 2 (PyPbBr3) | 30.78 | 2 2 | ||||||
| 31.63 | 3 1 | 31.50 | 3 1 (PyPbI3) | 31.33 | 3 1 | ||||
| 32.35 | 3 1 (PyPbBr3) | 32.35 | 3 1 | ||||||
| 33.70 | 3 0 0 (PyPbI3) | 33.25 | 3 0 0 | ||||||
| 34.74 | 1 0 10 (PbI2) | 34.24 | 1 0 10 | ||||||
| 35.33 | 1 0 3 | 35.20 | 34.98 | 1 0 3 | 34.56 | 1 0 3 | |||
| 37.32 | 3 2 | 36.88 | 3 2 | 36.46 | 3 0 2 | ||||
| 37.13 | 37.76 | 3 2 | |||||||
| 38.52 | 2 0 3 | ||||||||
| 39.2 | 4 0 | 38.7 | 4 0 | 38.7 | 0 0 15 | ||||
| 39.92 | 4 0 | 39.6 | 2 1 | ||||||
| 40.69 | 4 0 | 40.10 | 40.3 | 4 0 | 40.31 | 4 0 1 | |||
| 42.53 | 4 1 | 42.14 | 1 0 (PbI2) | 41.9 | 4 1 | 41.51 | 4 1 | 42.9 | 1 0 |
| 43.46 | 4 0 | ||||||||
| 44.71 | 0 0 4 | ||||||||
| 44.85 | 4 2 | 44.90 | 4 0 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, M.; Salib, A.; Hui, L.S.; Turak, A. Unusual Phase Behaviour for Organo-Halide Perovskite Nanoparticles Synthesized via Reverse Micelle Templating. Chemistry 2023, 5, 2490-2512. https://doi.org/10.3390/chemistry5040163
Munir M, Salib A, Hui LS, Turak A. Unusual Phase Behaviour for Organo-Halide Perovskite Nanoparticles Synthesized via Reverse Micelle Templating. Chemistry. 2023; 5(4):2490-2512. https://doi.org/10.3390/chemistry5040163
Chicago/Turabian StyleMunir, Muhammad, Arsani Salib, Lok Shu Hui, and Ayse Turak. 2023. "Unusual Phase Behaviour for Organo-Halide Perovskite Nanoparticles Synthesized via Reverse Micelle Templating" Chemistry 5, no. 4: 2490-2512. https://doi.org/10.3390/chemistry5040163
APA StyleMunir, M., Salib, A., Hui, L. S., & Turak, A. (2023). Unusual Phase Behaviour for Organo-Halide Perovskite Nanoparticles Synthesized via Reverse Micelle Templating. Chemistry, 5(4), 2490-2512. https://doi.org/10.3390/chemistry5040163