In Pursuit of Next Generation N-Heterocyclic Carbene-Stabilized Copper and Silver Precursors for Metalorganic Chemical Vapor Deposition and Atomic Layer Deposition Processes
Abstract
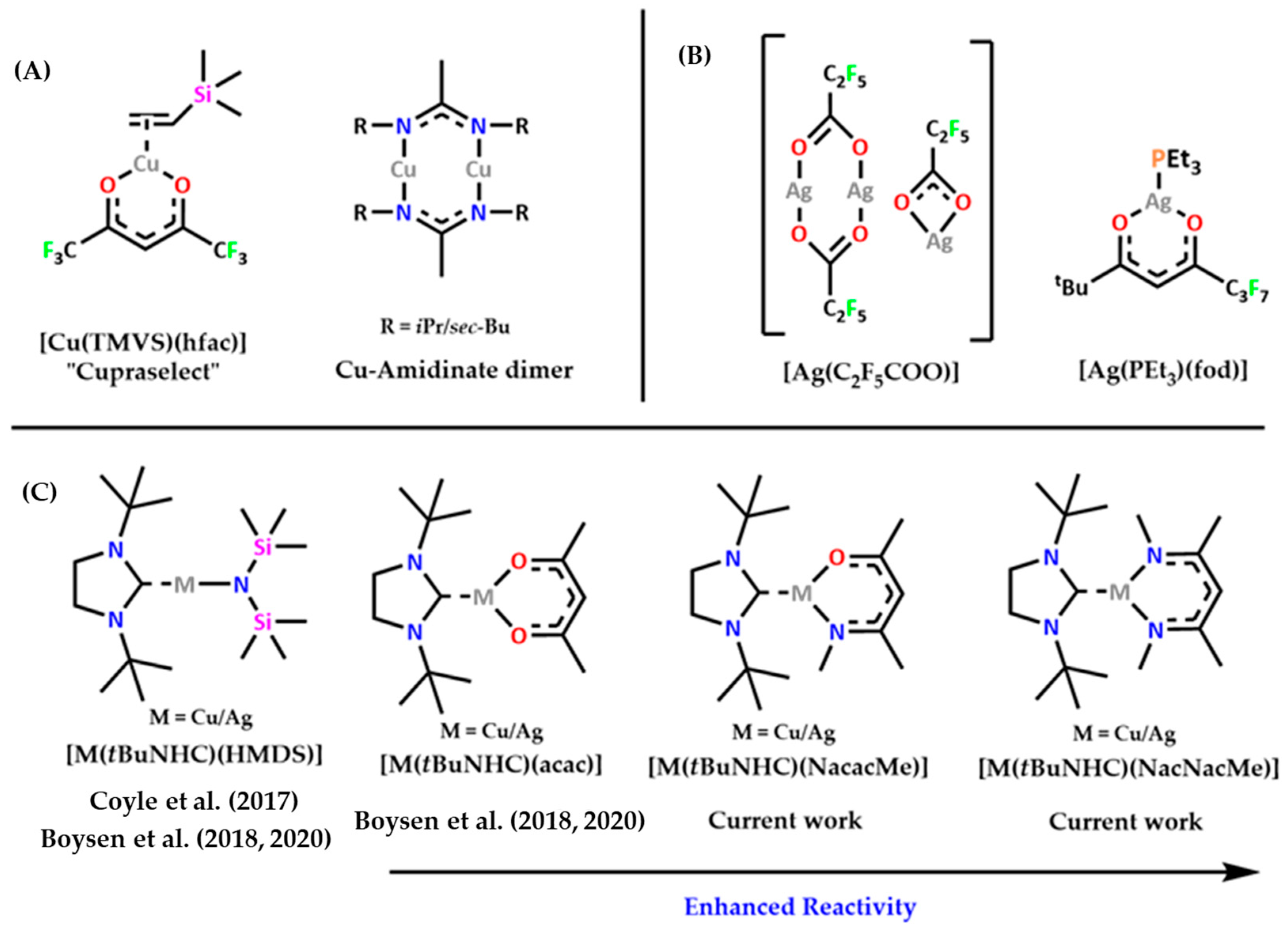
:1. Introduction
2. Materials and Methods
2.1. General Experimental
2.2. Preparation of Anionic Ligands
2.2.1. 4-(Methylamino)pent-3-en-2-one (NacacMe)
2.2.2. N-Methyl-4-(methylimino)pent-2-en-2-Amine (NacNacMe)
2.3. Preparation of Starting Material Complexes
2.3.1. [Cu(tBuNHC) (HMDS)]
2.3.2. [Ag(tBuNHC) (HMDS)]
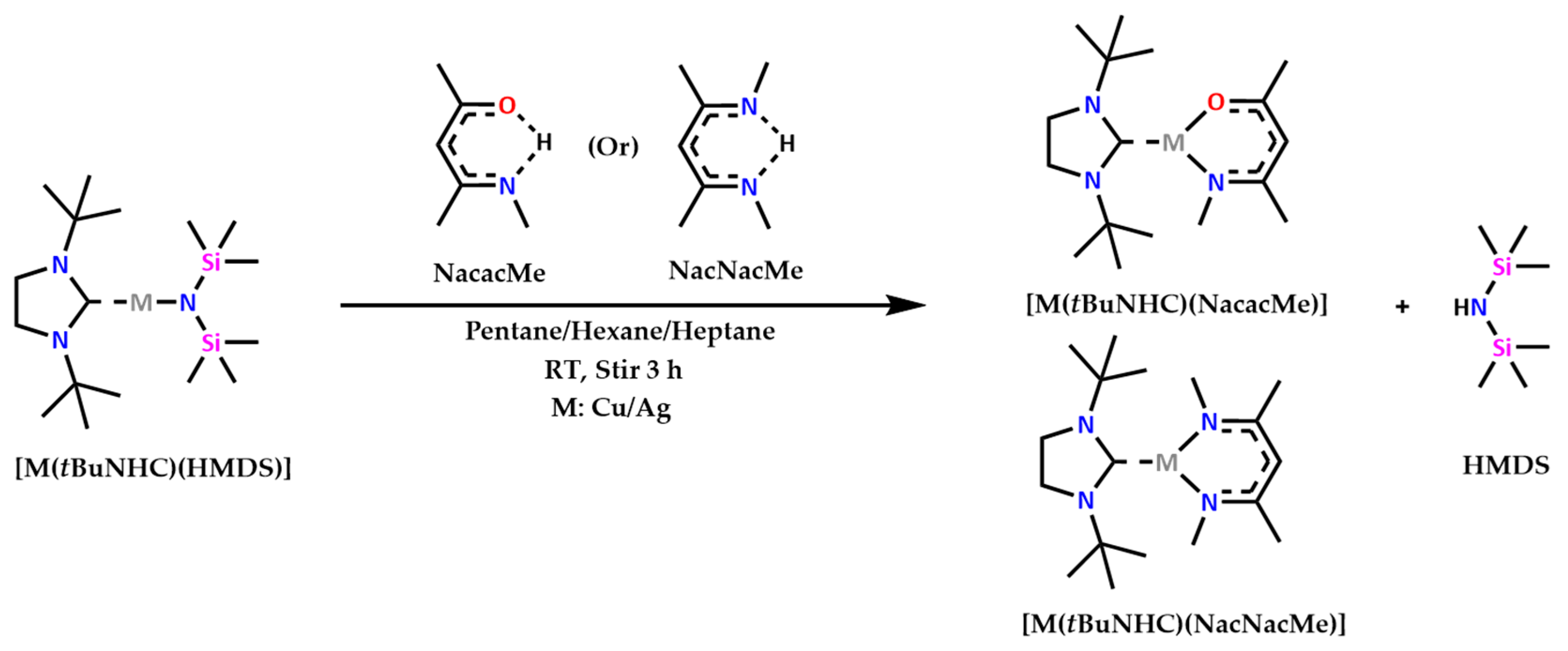
2.4. Preparation of Precursor Complexes
2.4.1. [Cu(tBuNHC) (NacacMe)] (1)
2.4.2. [Ag(tBuNHC) (NacacMe)] (2)
2.4.3. [Cu(tBuNHC) (NacNacMe)] (3)
2.4.4. [Ag(tBuNHC) (NacNacMe)] (4)
3. Results and Discussion
3.1. Synthesis of Metal Complexes
3.2. 1H-NMR Characterization
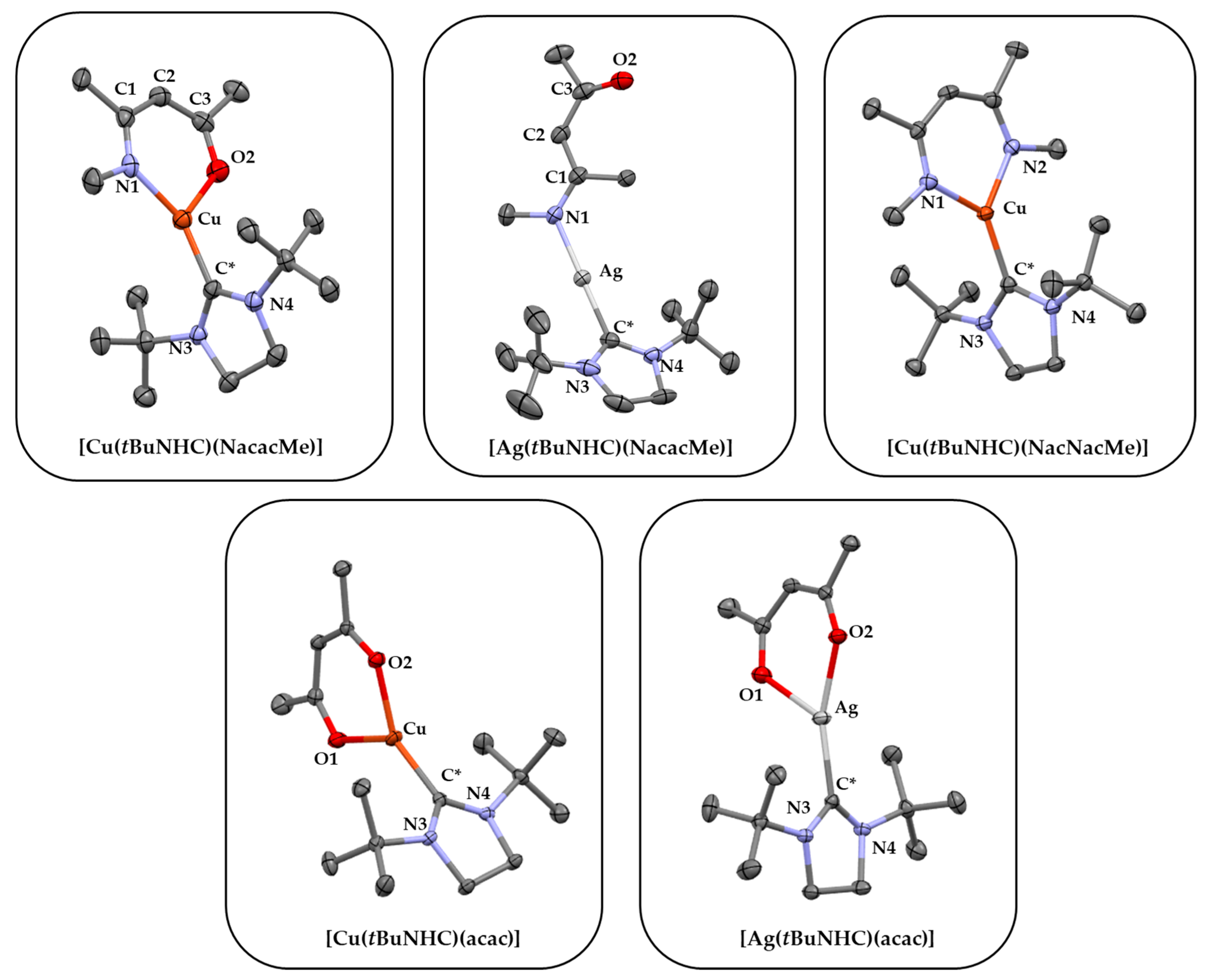
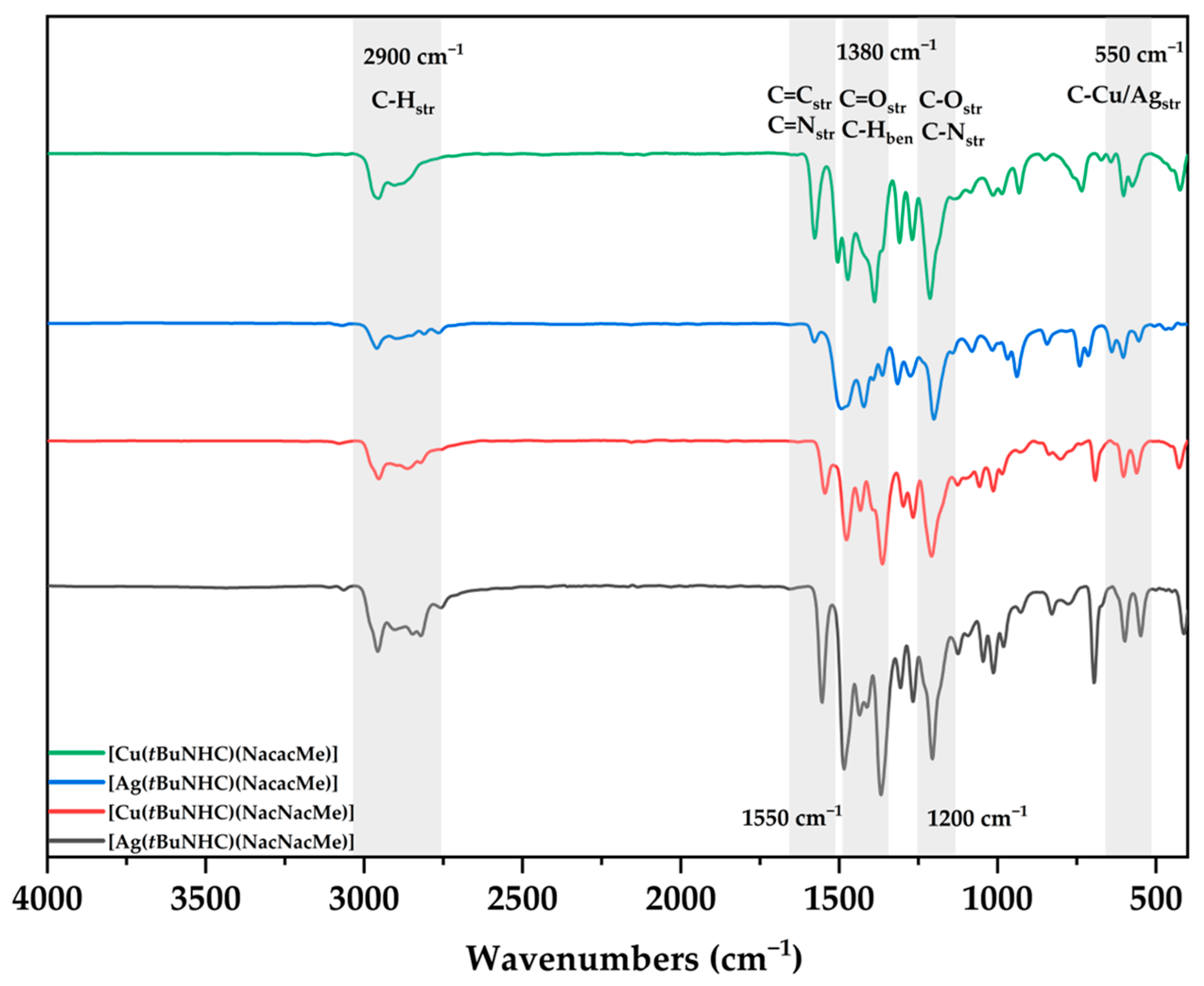
3.3. Single Crystal XRD and FT-IR Analysis
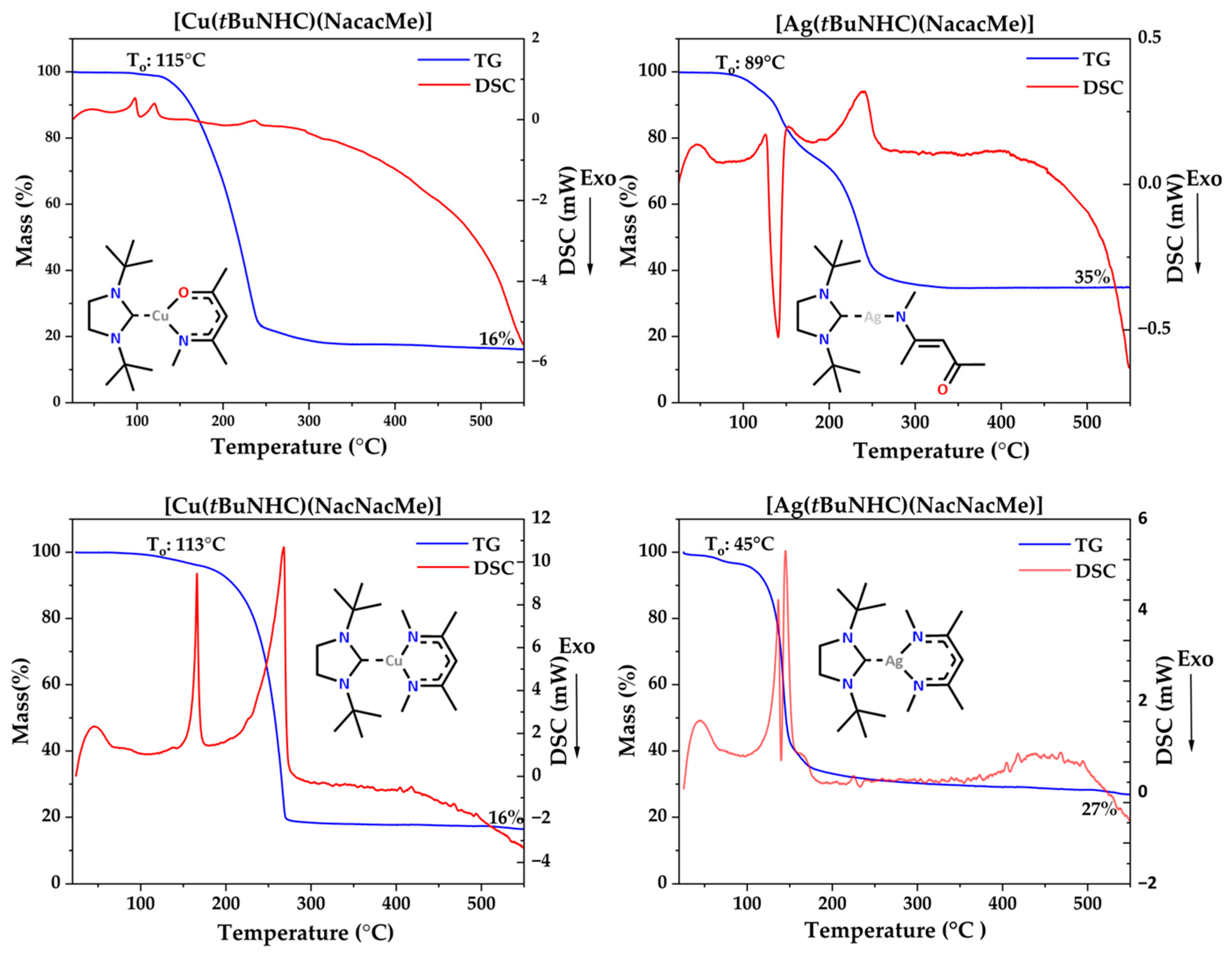
3.4. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matula, R.A. Electrical Resistivity of Copper, Gold, Palladium, and Silver. J. Phys. Chem. Ref. Data 1979, 8, 1147–1298. [Google Scholar] [CrossRef]
- Nath, P.; Chopra, K.L. Thermal Conductivity of Copper Films. Thin Solid Film. 1974, 20, 53–62. [Google Scholar] [CrossRef]
- Ho, C.Y.; Ackerman, M.W.; Wu, K.Y.; Oh, S.G.; Havill, T.N. Thermal Conductivity of Ten Selected Binary Alloy Systems. J. Phys. Chem. Ref. Data 1978, 7, 959–1178. [Google Scholar] [CrossRef]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, X.; Deng, Y.; Li, T.; Shao, Y.; Gruverman, A.; Shield, J.; Huang, J. Is Cu a Stable Electrode Material in Hybrid Perovskite Solar Cells for a 30-Year Lifetime? Energy Environ. Sci. 2016, 9, 3650–3656. [Google Scholar] [CrossRef]
- Bellchambers, P.; Walker, M.; Huband, S.; Dirvanauskas, A.; Hatton, R.A. Enhanced Oxidation Stability of Transparent Copper Films Using a Hybrid Organic-Inorganic Nucleation Layer. ChemNanoMat 2019, 5, 619–624. [Google Scholar] [CrossRef]
- Li, B.; Sullivan, T.D.; Lee, T.C.; Badami, D. Reliability Challenges for Copper Interconnects. Microelectron. Reliab. 2004, 44, 365–380. [Google Scholar] [CrossRef]
- Andricacos, P.C.; Uzoh, C.; Dukovic, J.O.; Horkans, J.; Deligianni, H. Damascene Copper Electroplating for Chip Interconnections. IBM J. Res. Dev. 1998, 42, 567–574. [Google Scholar] [CrossRef]
- Buchanan, K. The Evolution of Interconnect Technology for Silicon Integrated Circuitry. In Proceedings of the GaAs Mantech Conference, San Diego, CA, USA, 19 April 2002; Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=1098682b0717c1267a22eb343405ee87b21f26f1 (accessed on 15 September 2023).
- Zilberberg, K.; Riedl, T. Metal-Nanostructures—A Modern and Powerful Platform to Create Transparent Electrodes for Thin-Film Photovoltaics. J. Mater. Chem. A 2016, 4, 14481–14508. [Google Scholar] [CrossRef]
- Hu, T.; Becker, T.; Pourdavoud, N.; Zhao, J.; Brinkmann, K.O.; Heiderhoff, R.; Gahlmann, T.; Huang, Z.; Olthof, S.; Meerholz, K.; et al. Indium-Free Perovskite Solar Cells Enabled by Impermeable Tin-Oxide Electron Extraction Layers. Adv. Mater. 2017, 29, 1606656. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Lu, X.; Ma, Z.; Xie, C.; Zheng, Z. Chemical Formation of Soft Metal Electrodes for Flexible and Wearable Electronics. Chem. Soc. Rev. 2018, 47, 4611–4641. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ambwani, P.; Manno, M.; Lindquist, N.C.; Nagpal, P.; Oh, S.-H.; Leighton, C.; Norris, D.J. Single-Crystalline Silver Films for Plasmonics. Adv. Mater. 2012, 24, 3988–3992. [Google Scholar] [CrossRef] [PubMed]
- Santbergen, R.; Temple, T.L.; Liang, R.; Smets, A.H.M.; van Swaaij, R.A.C.M.M.; Zeman, M. Application of Plasmonic Silver Island Films in Thin-Film Silicon Solar Cells. J. Opt. 2012, 14, 024010. [Google Scholar] [CrossRef]
- Mansuripur, M.; Zakharian, A.R.; Lesuffleur, A.; Oh, S.-H.; Jones, R.J.; Lindquist, N.C.; Im, H.; Kobyakov, A.; Moloney, J.V. Plasmonic Nano-Structures for Optical Data Storage. Opt. Express 2009, 17, 14001–14014. [Google Scholar] [CrossRef]
- Lee, K.J.; Wei, R.; Wang, Y.; Zhang, J.; Kong, W.; Chamoli, S.K.; Huang, T.; Yu, W.; ElKabbash, M.; Guo, C. Gigantic Suppression of Recombination Rate in 3D Lead-Halide Perovskites for Enhanced Photodetector Performance. Nat. Photonics 2023, 17, 236–243. [Google Scholar] [CrossRef]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface Plasmon Subwavelength Optics. Nature 2003, 424, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Baburin, A.S.; Merzlikin, A.M.; Baryshev, A.V.; Ryzhikov, I.A.; Panfilov, Y.V.; Rodionov, I.A. Silver-Based Plasmonics: Golden Material Platform and Application Challenges [Invited]. Opt. Mater. Express 2019, 9, 611–642. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Pierson, H.O. 15—CVD in Optoelectronic and Ferroelectric Applications. In Handbook of Chemical Vapor Deposition (CVD), 2nd ed.; Pierson, H.O., Ed.; William Andrew Publishing: Norwich, NY, USA, 1999; pp. 384–402. ISBN 978-0-8155-1432-9. [Google Scholar]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Schalk, N.; Tkadletz, M.; Mitterer, C. Hard Coatings for Cutting Applications: Physical vs. Chemical Vapor Deposition and Future Challenges for the Coatings Community. Surf. Coat. Technol. 2022, 429, 127949. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition (ALD): From Precursors to Thin Film Structures. Thin Solid Film. 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Dias, H.V.R. Polyfluorinated ligand-supported organometallic complexes of copper, silver, and gold. Pure Appl. Chem. 2010, 82, 649–656. [Google Scholar] [CrossRef]
- Hagen, D.J.; Pemble, M.E.; Karppinen, M. Atomic Layer Deposition of Metals: Precursors and Film Growth. Appl. Phys. Rev. 2019, 6, 041309. [Google Scholar] [CrossRef]
- Cohen, S.L.; Liehr, M.; Kasi, S. Mechanisms of Copper Chemical Vapor Deposition. Appl. Phys. Lett. 1992, 60, 50–52. [Google Scholar] [CrossRef]
- Li, Z.; Rahtu, A.; Gordon, R.G. Atomic Layer Deposition of Ultrathin Copper Metal Films from a Liquid Copper(I) Amidinate Precursor. J. Electrochem. Soc. 2006, 153, C787. [Google Scholar] [CrossRef]
- Li, Z.; Barry, S.T.; Gordon, R.G. Synthesis and Characterization of Copper(I) Amidinates as Precursors for Atomic Layer Deposition (ALD) of Copper Metal. Inorg. Chem. 2005, 44, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.P.; Dey, G.; Sirianni, E.R.; Kemell, M.L.; Yap, G.P.A.; Ritala, M.; Leskelä, M.; Elliott, S.D.; Barry, S.T. Deposition of Copper by Plasma-Enhanced Atomic Layer Deposition Using a Novel N-Heterocyclic Carbene Precursor. Chem. Mater. 2013, 25, 1132–1138. [Google Scholar] [CrossRef]
- Boysen, N.; Misimi, B.; Muriqi, A.; Wree, J.-L.; Hasselmann, T.; Rogalla, D.; Haeger, T.; Theirich, D.; Nolan, M.; Riedl, T.; et al. A Carbene Stabilized Precursor for the Spatial Atomic Layer Deposition of Copper Thin Films. Chem. Commun. 2020, 56, 13752–13755. [Google Scholar] [CrossRef]
- Szłyk, E.; Piszczek, P.; Grodzicki, A.; Chaberski, M.; Goliński, A.; Szatkowski, J.; Błaszczyk, T. CVD of AgI Complexes with Tertiary Phosphines and Perfluorinated Carboxylates—A New Class of Silver Precursors. Chem. Vap. Depos. 2001, 7, 111–116. [Google Scholar] [CrossRef]
- Shapiro, M.J.; Lackey, W.J.; Hanigofsky, J.A.; Hill, D.N.; Carter, W.B.; Barefield, E.K. Chemical Vapor Deposition of Silver Films for Superconducting Wire Applications. J. Alloys Compd. 1992, 187, 331–349. [Google Scholar] [CrossRef]
- Boysen, N.; Hasselmann, T.; Karle, S.; Rogalla, D.; Theirich, D.; Winter, M.; Riedl, T.; Devi, A. An N-Heterocyclic Carbene Based Silver Precursor for Plasma-Enhanced Spatial Atomic Layer Deposition of Silver Thin Films at Atmospheric Pressure. Angew. Chem. Int. Ed. 2018, 57, 16224–16227. [Google Scholar] [CrossRef] [PubMed]
- Hasselmann, T.; Misimi, B.; Boysen, N.; Zanders, D.; Wree, J.-L.; Rogalla, D.; Haeger, T.; Zimmermann, F.; Brinkmann, K.O.; Schädler, S.; et al. Silver Thin-Film Electrodes Grown by Low-Temperature Plasma-Enhanced Spatial Atomic Layer Deposition at Atmospheric Pressure. Adv. Mater. Technol. 2023, 8, 2200796. [Google Scholar] [CrossRef]
- Boysen, N.; Philip, A.; Rogalla, D.; Karppinen, M.; Devi, A. Role of Anionic Backbone in NHC-Stabilized Coinage Metal Complexes: New Precursors for Atomic Layer Deposition. Chem. Eur. J. 2022, 28, e202103798. [Google Scholar] [CrossRef] [PubMed]
- Devi, A. ‘Old Chemistries’ for New Applications: Perspectives for Development of Precursors for MOCVD and ALD Applications. Coord. Chem. Rev. 2013, 257, 3332–3384. [Google Scholar] [CrossRef]
- Huster, N.; Ghiyasi, R.; Zanders, D.; Rogalla, D.; Karppinen, M.; Devi, A. SnO Deposition via Water Based ALD Employing Tin(II) Formamidinate: Precursor Characterization and Process Development. Dalton Trans. 2022, 51, 14970–14979. [Google Scholar] [CrossRef]
- Zanders, D.; Obenlüneschloß, J.; Wree, J.-L.; Jagosz, J.; Kaur, P.; Boysen, N.; Rogalla, D.; Kostka, A.; Bock, C.; Öhl, D.; et al. Unveiling Ruthenium(II) Diazadienyls for Gas Phase Deposition Processes: Low Resistivity Ru Thin Films and Their Performance in the Acidic Oxygen Evolution Reaction. Adv. Mater. Interfaces 2022, 9, 2201709. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Simoni, D.; Manfredini, S. An Improved Preparation of Enaminones from 1,3-Diketones and Ammonium Acetate or Amine Acetates. Synthesis 1983, 1983, 902–903. [Google Scholar] [CrossRef]
- Taftaf, M.; Zuideveld, M.A.; Batinas-Geurts, A.A.; Sainani, J.B.; Vimalkumar, M.P.; Zakharov, V.A.; Bukatov, G.D.; Sergeev, S.A.; Ghalit, N. Catalyst Composition for Polymerization of Olefins. U.S. Patent 10059656B2, 19 December 2014. [Google Scholar]
- Stalzer, M.M.; Lohr, T.L.; Marks, T.J. Synthesis, Characterization, and Thermal Properties of N-alkyl β-Diketiminate Manganese Complexes. Inorg. Chem. 2018, 57, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- McGeachin, S.G. Synthesis and Properties of Some β-Diketimines Derived from Acetylacetone, and Their Metal Complexes. Can. J. Chem. 1968, 46, 1903–1912. [Google Scholar] [CrossRef]
- Coyle, J.P.; Sirianni, E.R.; Korobkov, I.; Yap, G.P.A.; Dey, G.; Barry, S.T. Study of Monomeric Copper Complexes Supported by N-Heterocyclic and Acyclic Diamino Carbenes. Organometallics 2017, 36, 2800–2810. [Google Scholar] [CrossRef]
- Dilworth, J.R.; Hussain, W.; Hutson, A.J.; Jones, C.J.; Mcquillan, F.S.; Mayer, J.M.; Arterburn, J.B. Tetrahalo Oxorhenate Anions. In Inorganic Syntheses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 257–262. ISBN 978-0-470-13262-3. [Google Scholar]
- Weber, M.; Boysen, N.; Graniel, O.; Sekkat, A.; Dussarrat, C.; Wiff, P.; Devi, A.; Muñoz-Rojas, D. Assessing the Environmental Impact of Atomic Layer Deposition (ALD) Processes and Pathways to Lower It. ACS Mater. Au 2023, 3, 274–298. [Google Scholar] [CrossRef]
- Hitchcock, P.B.; Lappert, M.F.; Pierssens, L.J.-M. Synthesis and X-ray Molecular Structures of the Silver(I) Amides [{Ag[µ-N(SiMe3)2]}4] and [{Ag[µ-NCMe2(CH2)3CMe2]}4]. Chem. Commun. 1996, 10, 1189–1190. [Google Scholar] [CrossRef]
- Teyssot, M.-L.; Nauton, L.; Canet, J.-L.; Cisnetti, F.; Chevry, A.; Gautier, A. Aromatic Nitrogen Donors for Efficient Copper(I)–NHC CuAAC under Reductant-Free Conditions. Eur. J. Org. Chem. 2010, 2010, 3507–03515. [Google Scholar] [CrossRef]
- Pellei, M.; Gandin, V.; Marzano, C.; Marinelli, M.; Del Bello, F.; Santini, C. The First Water-Soluble Copper(I) Complexes Bearing Sulfonated Imidazole- and Benzimidazole-Derived N-Heterocyclic Carbenes: Synthesis and Anticancer Studies. Appl. Organomet. Chem. 2018, 32, e4185. [Google Scholar] [CrossRef]
- Boysen, N.; Devi, A. Liquid Injection Field Desorption/Ionization as a Powerful Tool to Characterize Volatile, Labile, and Reactive Metal–Organic Complexes. Eur. J. Mass Spectrom. 2023, 29, 12–20. [Google Scholar] [CrossRef]
- Price, D. Vapor Pressure Determination by Thermogravimetry. Thermochim. Acta 2001, 367, 253–262. [Google Scholar] [CrossRef]
- Smura, C.F.; Parker, D.R.; Zbiri, M.; Johnson, M.R.; Gál, Z.A.; Clarke, S.J. High-Spin Cobalt(II) Ions in Square Planar Coordination: Structures and Magnetism of the Oxysulfides Sr2CoO2Cu2S2 and Ba2CoO2Cu2S2 and Their Solid Solution. J. Am. Chem. Soc. 2011, 133, 2691–2705. [Google Scholar] [CrossRef]
- Owen, E.A.; Williams, G.I. A Low-Temperature X-Ray Camera. J. Sci. Instrum. 1954, 31, 49. [Google Scholar] [CrossRef]
- Moon, D.-Y.; Kim, W.-S.; Kim, T.-S.; Kang, B.-W.; Park, J.-W.; Yeom, S.J.; Kim, J.H. Atomic Layer Deposition of Copper Seed Layers from a (Hfac)Cu(VTMOS) Precursor. JKPS 2009, 54, 1330–1333. [Google Scholar] [CrossRef]
- Minjauw, M.M.; Solano, E.; Sree, S.P.; Asapu, R.; Van Daele, M.; Ramachandran, R.K.; Heremans, G.; Verbruggen, S.W.; Lenaerts, S.; Martens, J.A.; et al. Plasma-Enhanced Atomic Layer Deposition of Silver Using Ag(Fod) (PEt3) and NH3-Plasma. Chem. Mater. 2017, 29, 7114–7121. [Google Scholar] [CrossRef]
| [Cu(tBuNHC) (NacacMe)] | [Ag(tBuNHC) (NacacMe)] | [Cu(tBuNHC) (NacNacMe)] | |
|---|---|---|---|
| CCDC code | 2279240 | 2279241 | 2279242 |
| Empirical formula | C17H32CuN3O | C17H32AgN3O | C18H35CuN4 |
| Formula weight | 357.99 | 402.32 | 371.04 |
| Temperature/K | 109.99 (14) | 100.2 (4) | 109.9 (6) |
| Crystal system | monoclinic | monoclinic | orthorhombic |
| Space group | P21/n | I2/a | Pbca |
| a/Å | 14.4287 (4) | 23.29733 (10) | 11.16780 (10) |
| b/Å | 14.2916 (2) | 16.04937 (8) | 11.70100 (10) |
| c/Å | 19.2653 (5) | 41.1905 (2) | 29.6636 (2) |
| α/° | 90 | 90 | 90 |
| β/° | 109.120 (3) | 97.4656 (4) | 90 |
| γ/° | 90 | 90 | 90 |
| Volume/Å3 | 3753.53 (16) | 15,270.88 (12) | 3876.27 (5) |
| Z | 8 | 32 | 8 |
| ρcalcg/cm3 | 1.267 | 1.400 | 1.272 |
| μ/mm−1 | 1.671 | 8.502 | 1.610 |
| F(000) | 1536.0 | 6720.0 | 1600.0 |
| Crystal size/mm3 | 0.279 × 0.197 × 0.165 | 0.183 × 0.141 × 0.087 | 0.269 × 0.177 × 0.087 |
| Radiation | Cu Kα (λ = 1.54184) | Cu Kα (λ = 1.54184) | Cu Kα (λ = 1.54184) |
| 2Θ range for data collection/° | 6.708 to 146.116 | 5.916 to 155.772 | 5.958 to 153.434 |
| Index ranges | −17 ≤ h ≤ 16, −17 ≤ k ≤ 11, −20 ≤ l ≤ 23 | −29 ≤ h ≤ 29, −19 ≤ k ≤ 20, −52 ≤ l ≤ 49 | −14 ≤ h ≤ 13, −14 ≤ k ≤ 14, −28 ≤ l ≤ 36 |
| Reflections collected | 14,117 | 97,805 | 34,158 |
| Independent reflections | 7242 [Rint = 0.0447, Rsigma = 0.0588] | 16,028 [Rint = 0.0336, Rsigma = 0.0190] | 3937 [Rint = 0.0405, Rsigma = 0.0202] |
| Data/restraints/parameters | 7242/0/415 | 16028/116/861 | 3937/0/218 |
| Goodness-of-fit on F2 | 1.069 | 1.067 | 1.045 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0539, wR2 = 0.1440 | R1 = 0.0304, wR2 = 0.0805 | R1 = 0.0329, wR2 = 0.0945 |
| Final R indexes [all data] | R1 = 0.0708, wR2 = 0.1517 | R1 = 0.0328, wR2 = 0.0822 | R1 = 0.0356, wR2 = 0.0970 |
| Largest diff. peak/hole/e Å−3 | 0.44/−0.68 | 0.70/−0.83 | 0.47/−0.40 |
| Complex Name | Solvent System | Product Appearance | Yield (%) |
|---|---|---|---|
| [Cu(tBuNHC) (NacacMe)] | Hexane: THF (7:1) | Light yellow crystals | 76 |
| [Ag(tBuNHC) (NacacMe)] | Hexane: THF (7:2) | Colorless cubicle | 66 |
| [Cu(tBuNHC) (NacNacMe)] | Hexane: THF (7:2) | Colorless cubicle crystals | 57 |
| [Ag(tBuNHC) (NacNacMe)] | Hexane: THF (7:3) | Grey cubicle crystals | 45 |
| Parameters | [Cu(tBuNHC) (NacacMe)] | [Ag(tBuNHC) (NacacMe)] | [Cu(tBuNHC) (NacNacMe)] | [Cu(tBuNHC) (acac)] | [Ag(tBuNHC) (acac)] |
|---|---|---|---|---|---|
| C*–M | 1.899 Å | 2.096 Å | 1.900 Å | 1.888 Å | 2.091 Å |
| (O1/N1)-M | 1.943 Å | 2.093 Å | 1.966 Å | 2.051 Å | 2.341 Å |
| (O2/N2)-M | 2.031 Å | - | 1.966 Å | 1.969 Å | 2.180 Å |
| (N1/O1)-M-(N2/O2) | 94.76° | - | 97.25° | 92.09° | 82.44° |
| N3-C*-N4 | 108.38° | 109.33° | 107.4° | 108.10° | 108.92° |
| Structure | Monoclinic | Monoclinic | Orthorhombic | Monoclinic | Monoclinic |
| Complex Name | 1% Mass Loss (°C) | Melting Point (°C) | Decomposition (°C) | Residual Mass (%) | 1 Torr Temperature (°C) |
|---|---|---|---|---|---|
| [Cu(tBuNHC) (NacacMe)] | 115 | 99 | - | 16 | 185.02 |
| [Ag(tBuNHC) (NacacMe)] | 89 | - | 140 | 35 | - |
| [Cu(tBuNHC) (NacNacMe)] | 113 | 165 | - | 16 | 186 |
| [Ag(tBuNHC) (NacNacMe)] | 45 | 136 | 139 | 27 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvakumar, I.; Boysen, N.; Bürger, M.; Devi, A. In Pursuit of Next Generation N-Heterocyclic Carbene-Stabilized Copper and Silver Precursors for Metalorganic Chemical Vapor Deposition and Atomic Layer Deposition Processes. Chemistry 2023, 5, 2038-2055. https://doi.org/10.3390/chemistry5030138
Selvakumar I, Boysen N, Bürger M, Devi A. In Pursuit of Next Generation N-Heterocyclic Carbene-Stabilized Copper and Silver Precursors for Metalorganic Chemical Vapor Deposition and Atomic Layer Deposition Processes. Chemistry. 2023; 5(3):2038-2055. https://doi.org/10.3390/chemistry5030138
Chicago/Turabian StyleSelvakumar, Ilamparithy, Nils Boysen, Marco Bürger, and Anjana Devi. 2023. "In Pursuit of Next Generation N-Heterocyclic Carbene-Stabilized Copper and Silver Precursors for Metalorganic Chemical Vapor Deposition and Atomic Layer Deposition Processes" Chemistry 5, no. 3: 2038-2055. https://doi.org/10.3390/chemistry5030138
APA StyleSelvakumar, I., Boysen, N., Bürger, M., & Devi, A. (2023). In Pursuit of Next Generation N-Heterocyclic Carbene-Stabilized Copper and Silver Precursors for Metalorganic Chemical Vapor Deposition and Atomic Layer Deposition Processes. Chemistry, 5(3), 2038-2055. https://doi.org/10.3390/chemistry5030138





