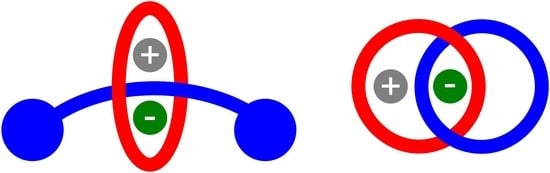
Heteroditopic Rotaxanes and Catenanes for Ion Pair Recognition
Abstract
:1. Introduction
2. Early Examples of Rotaxanes Incorporating Heteroditopic Macrocycles
3. Ion-Induced Motion in Rotaxanes and Catenanes Incorporating Heteroditopic Macrocycles
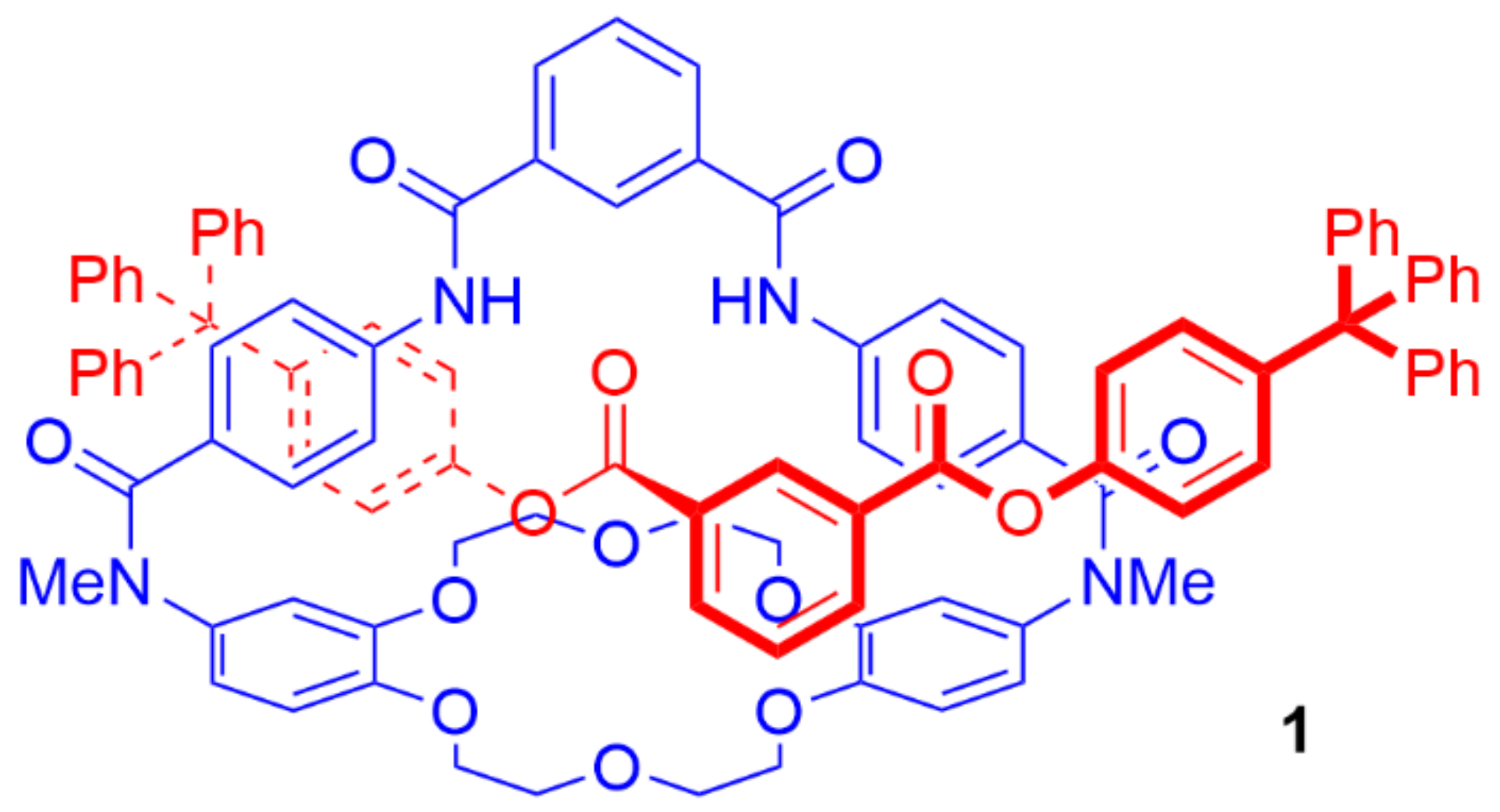
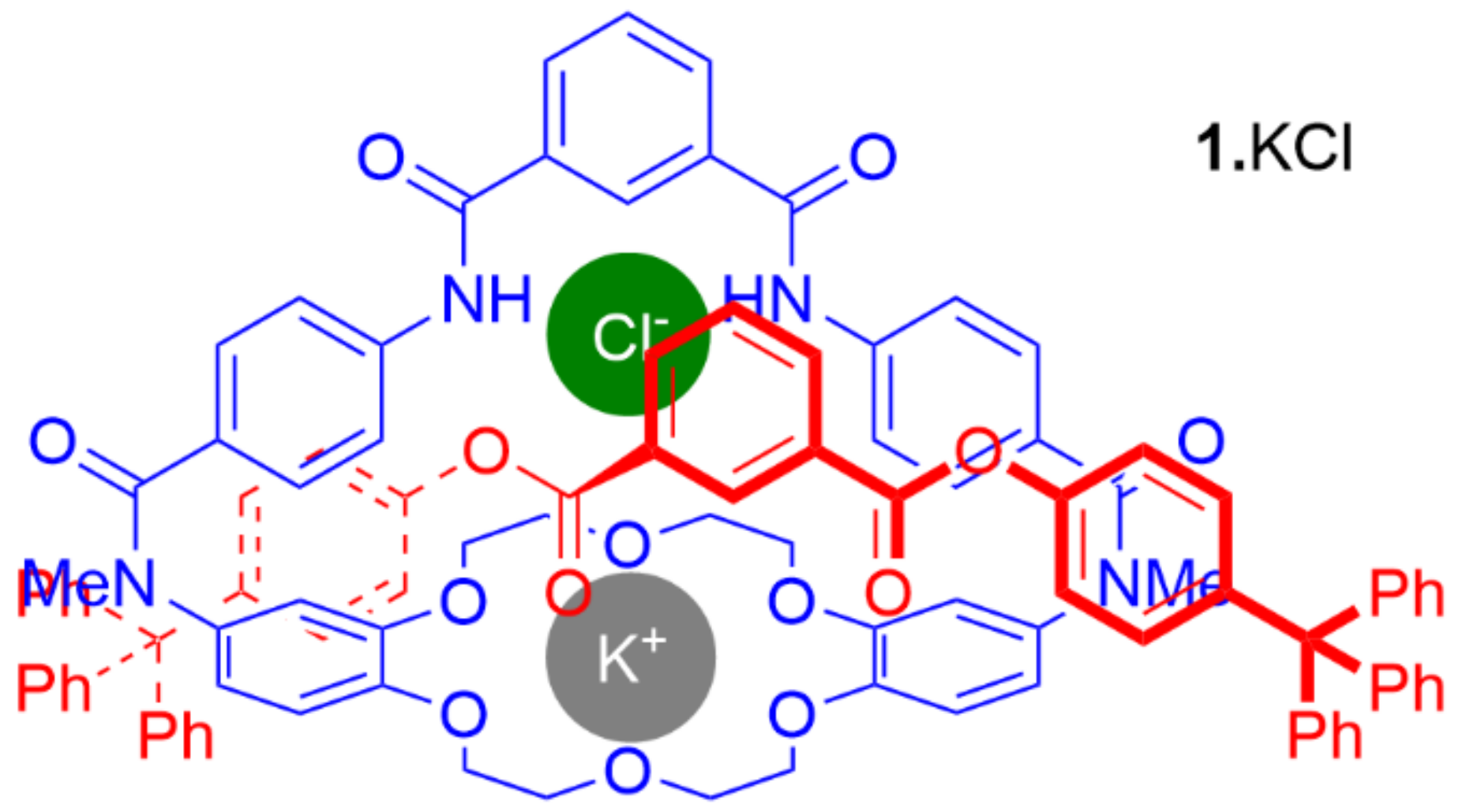
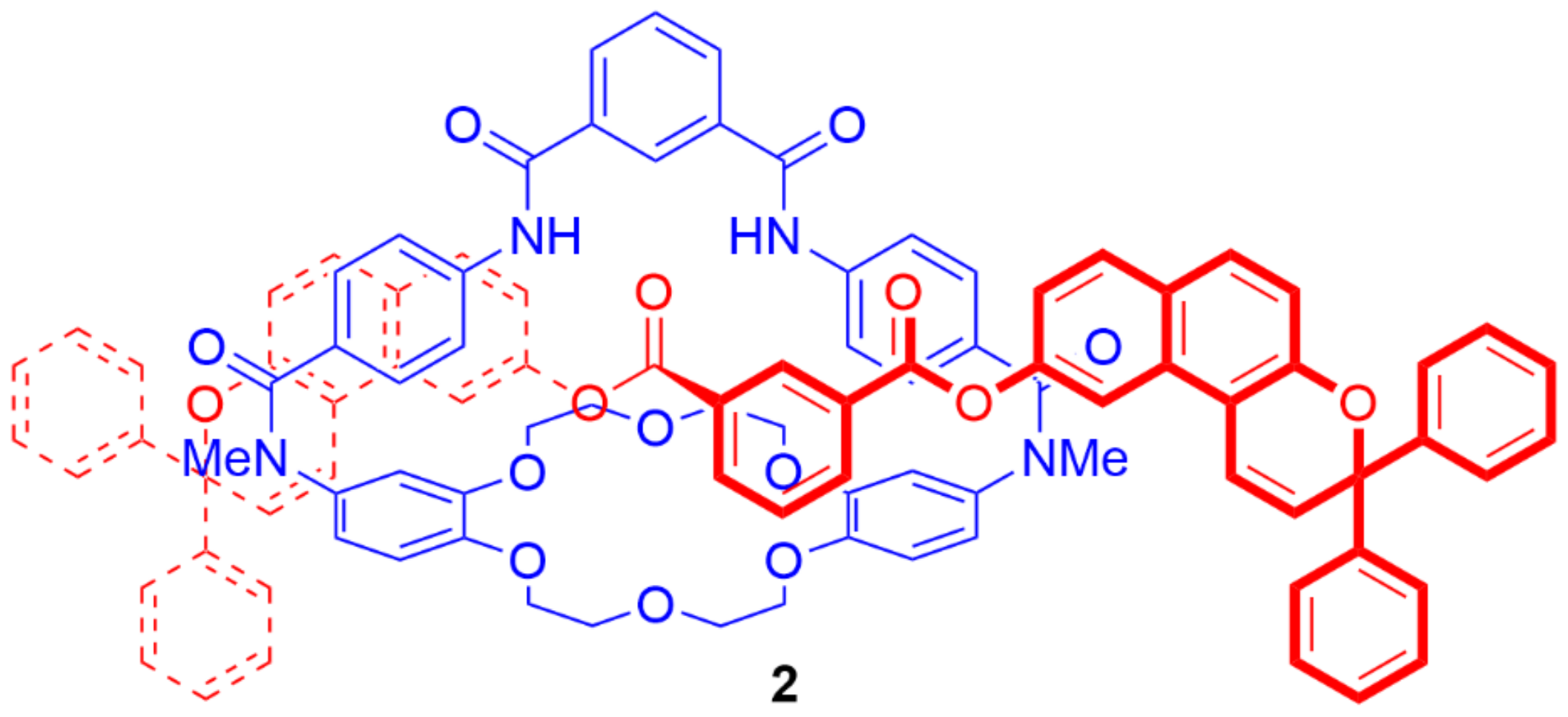
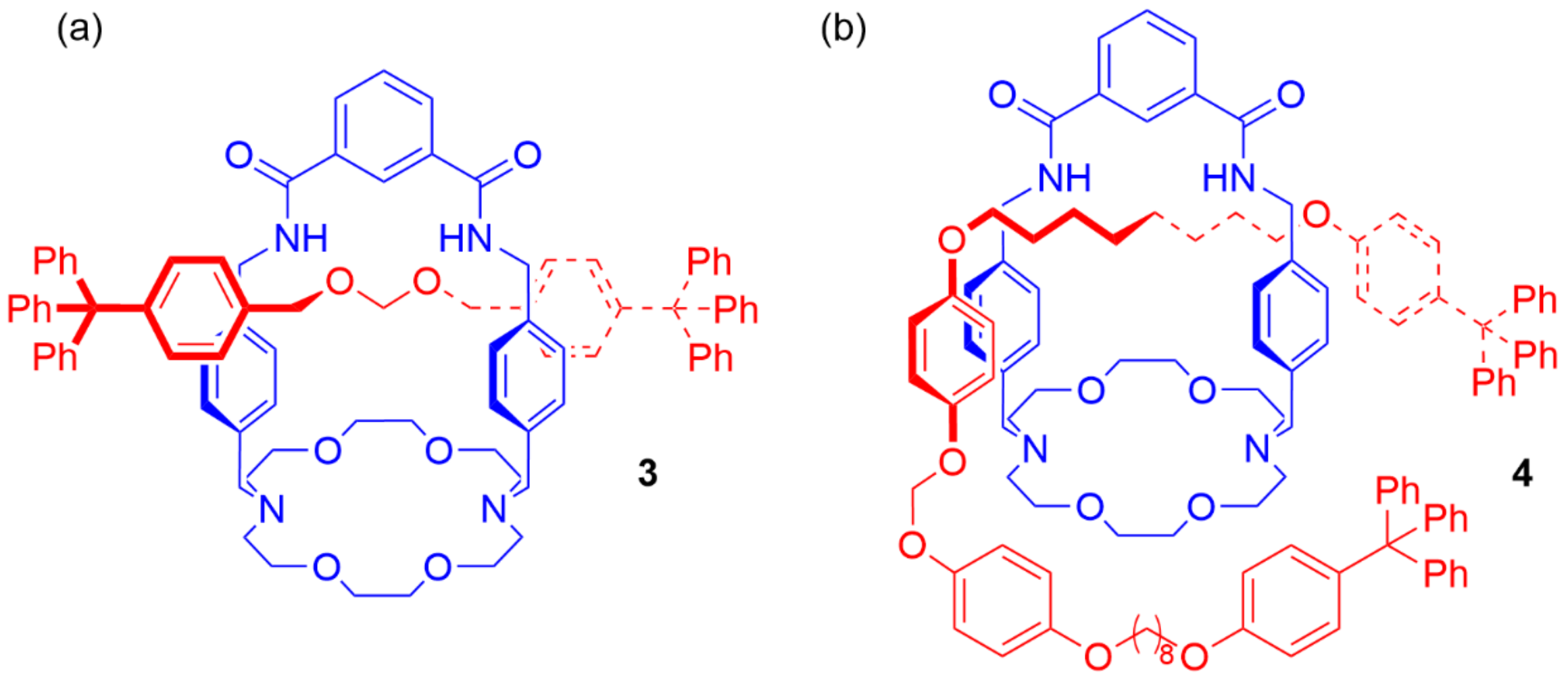
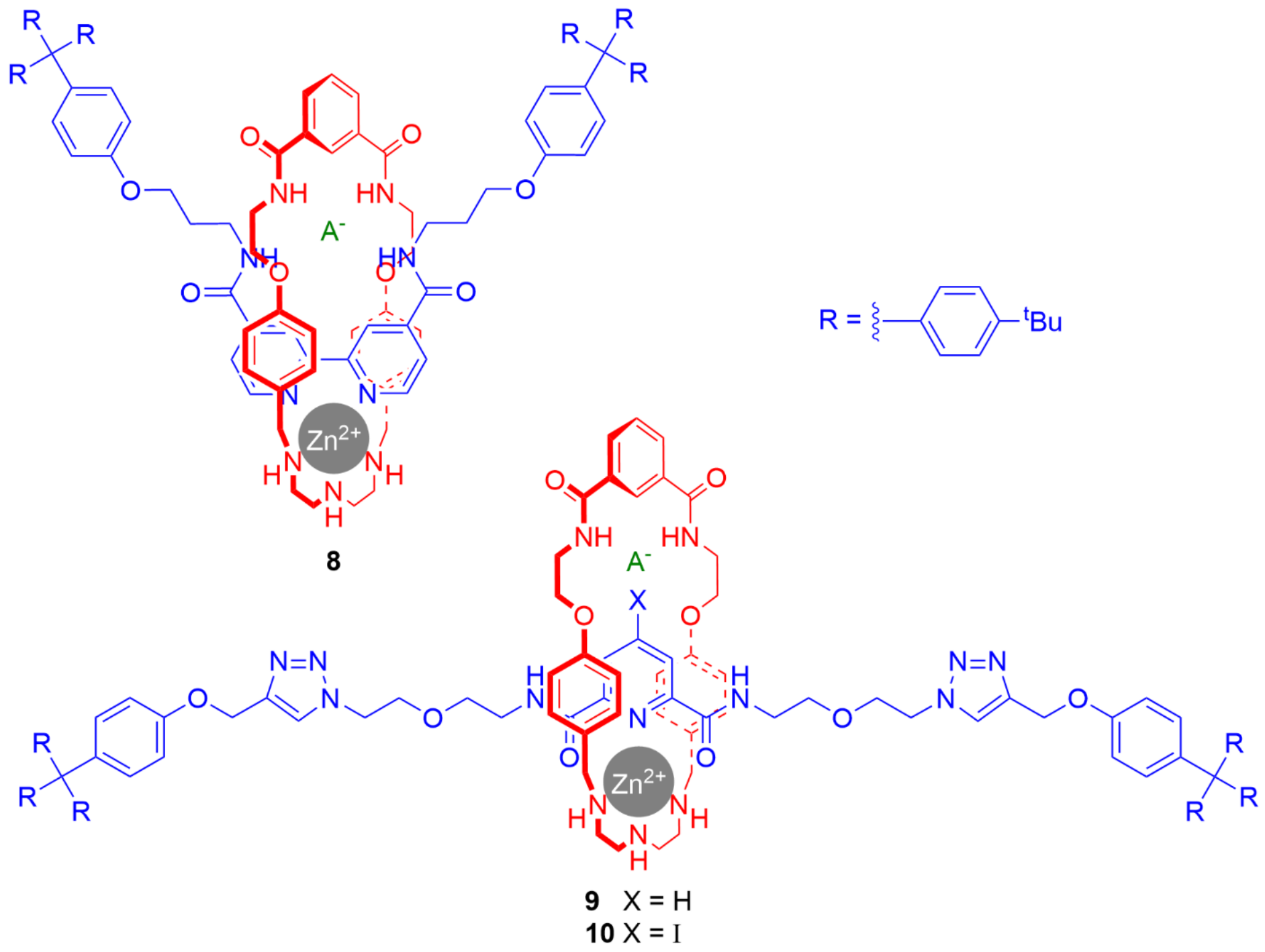
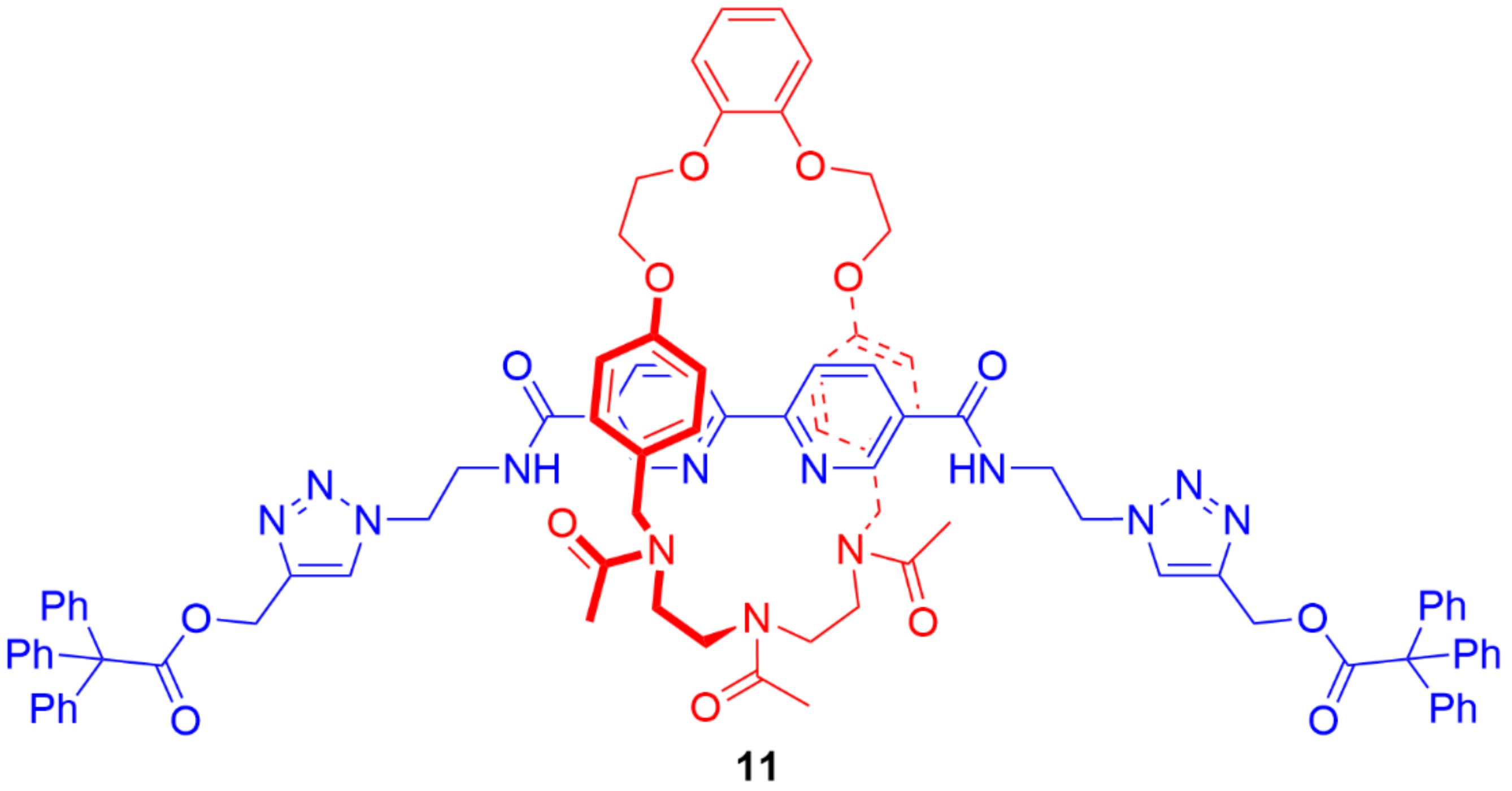
4. Further Examples of Heteroditopic Rotaxanes Binding Ion Pairs
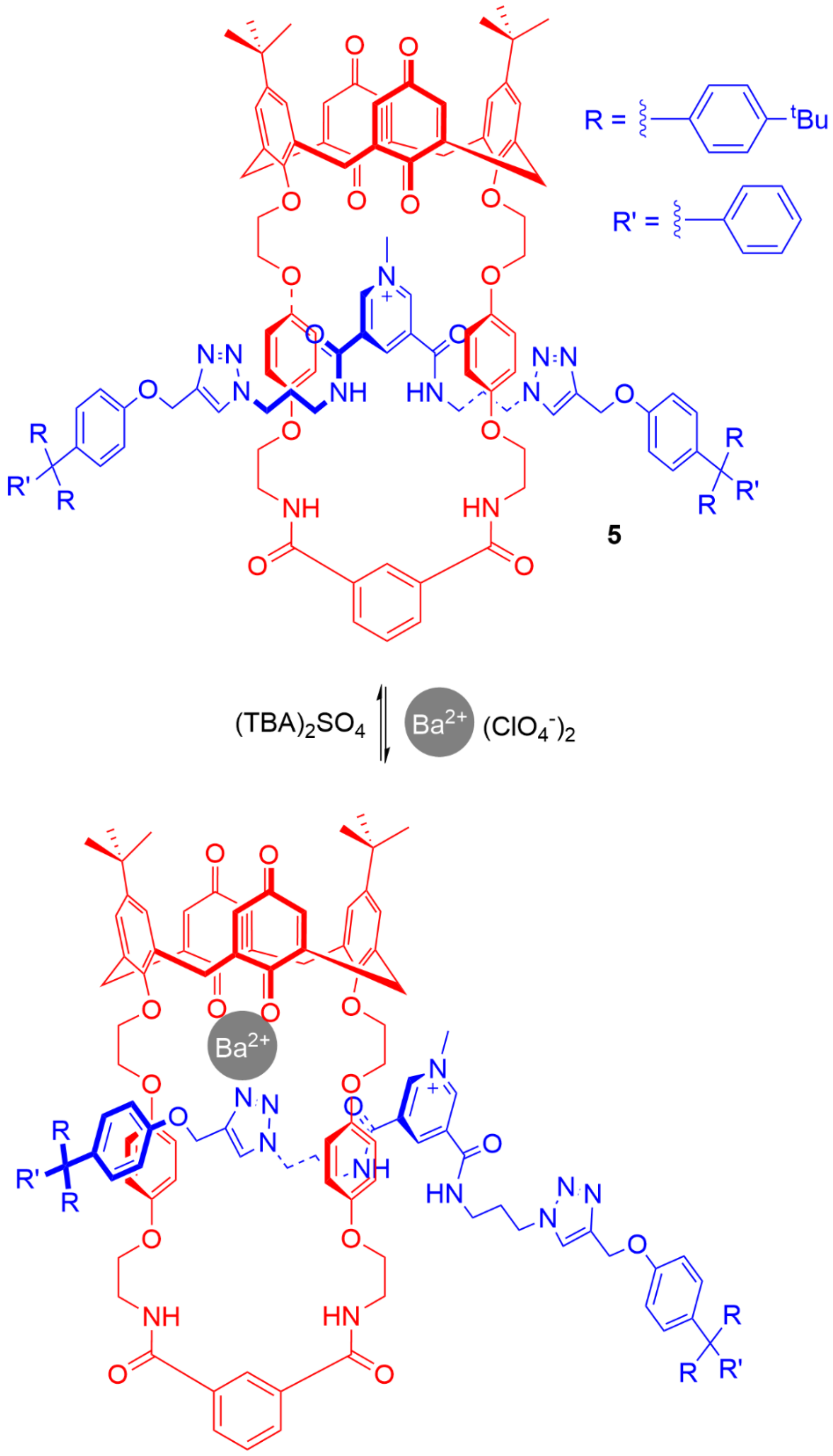
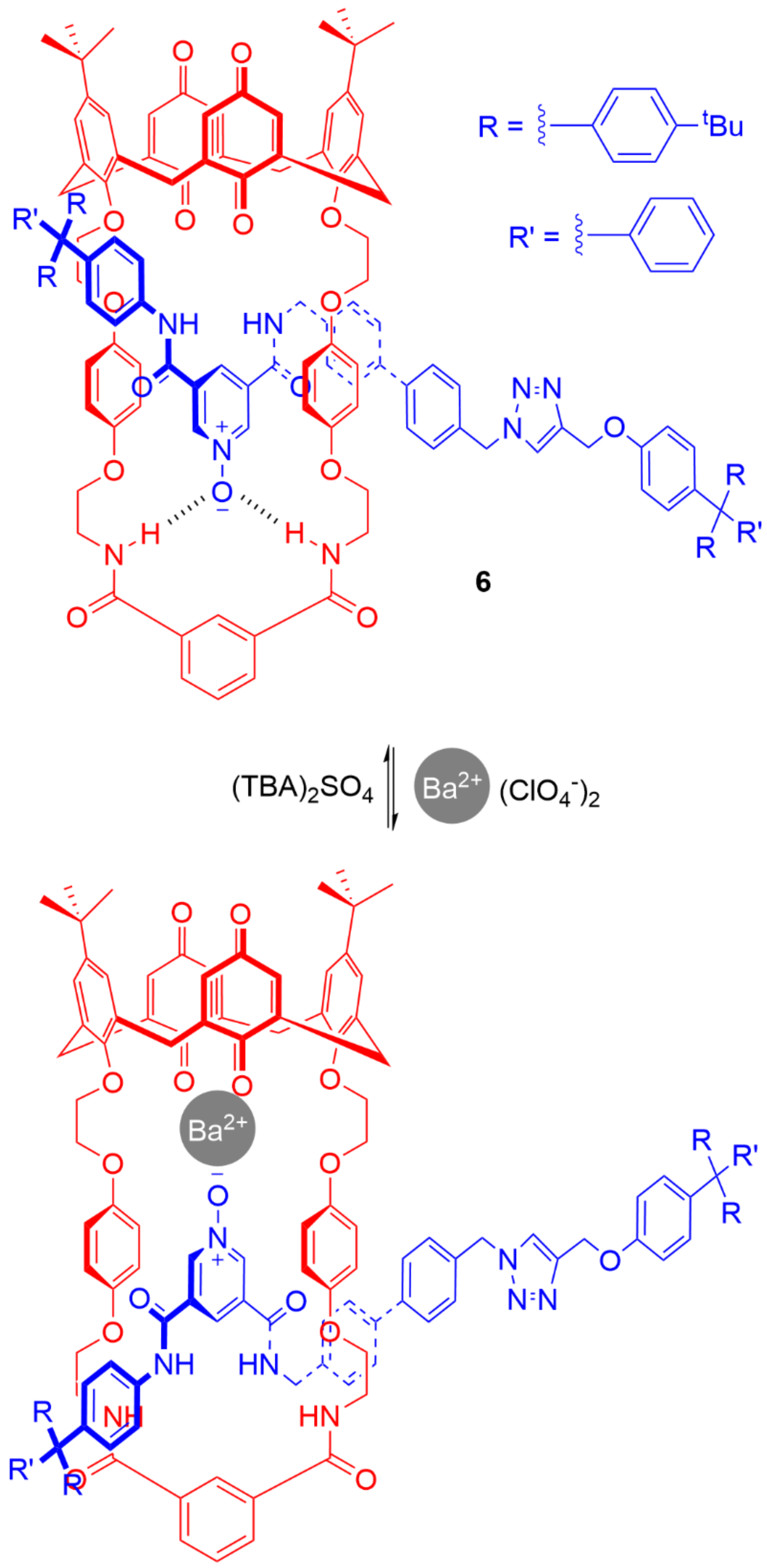
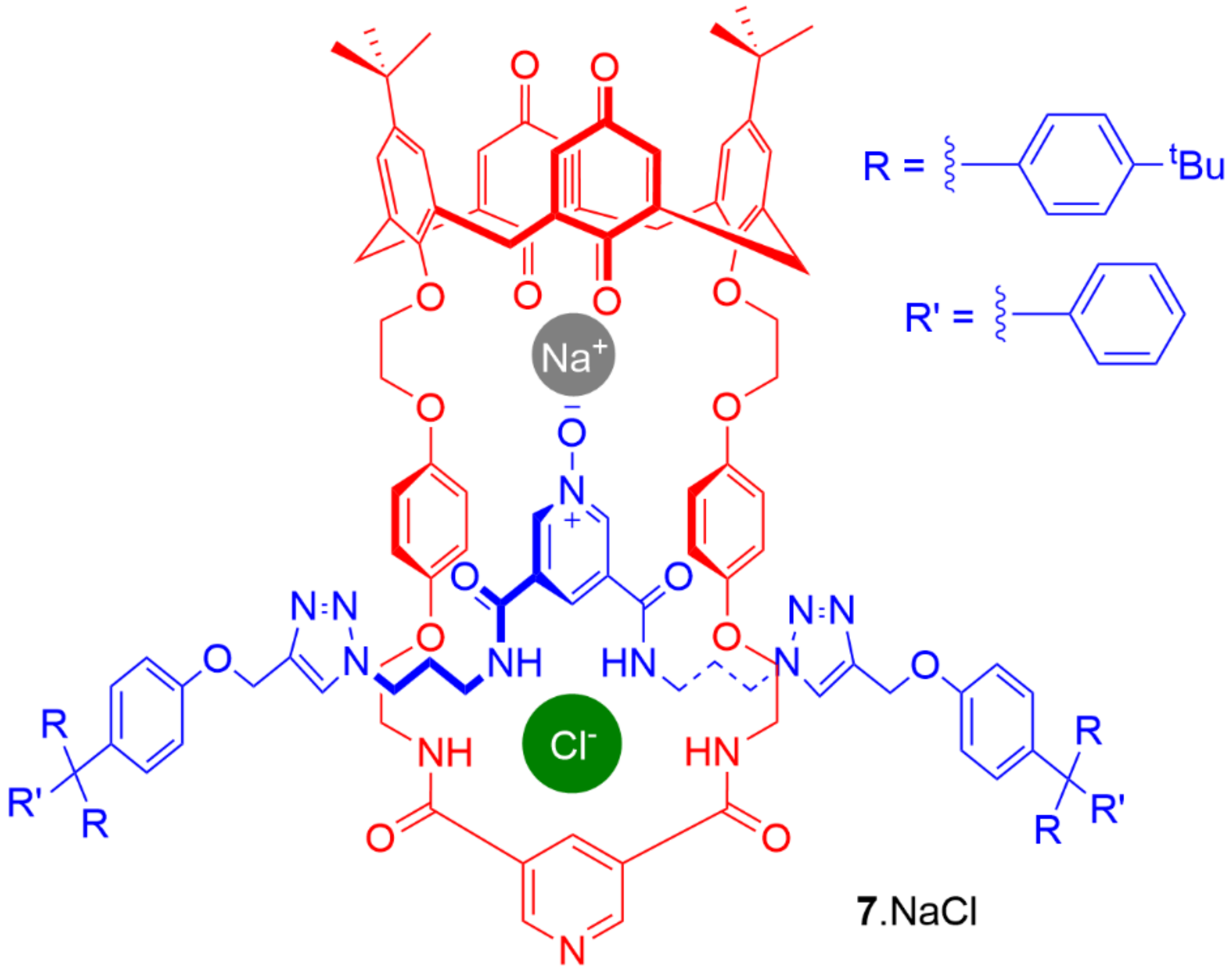
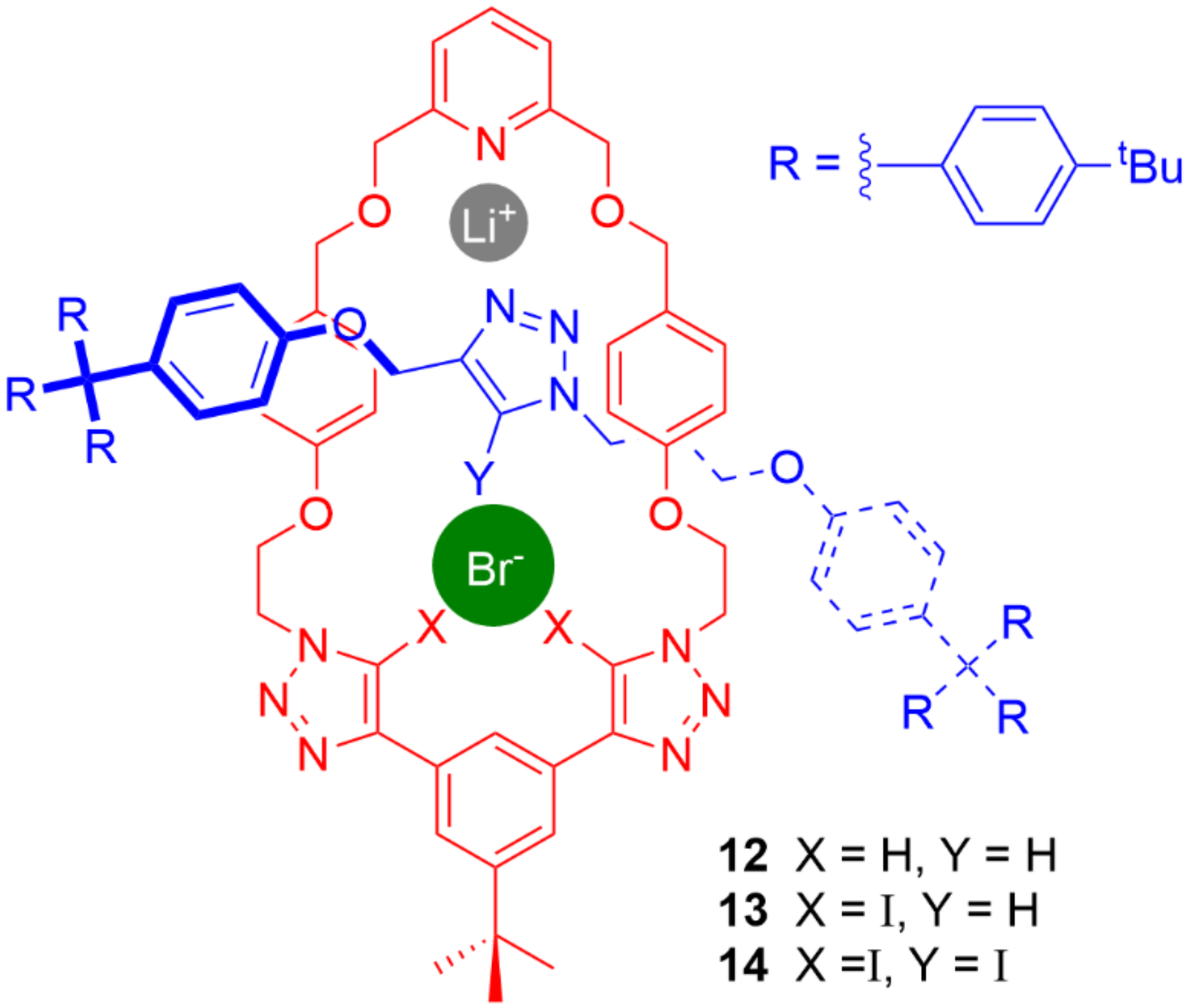
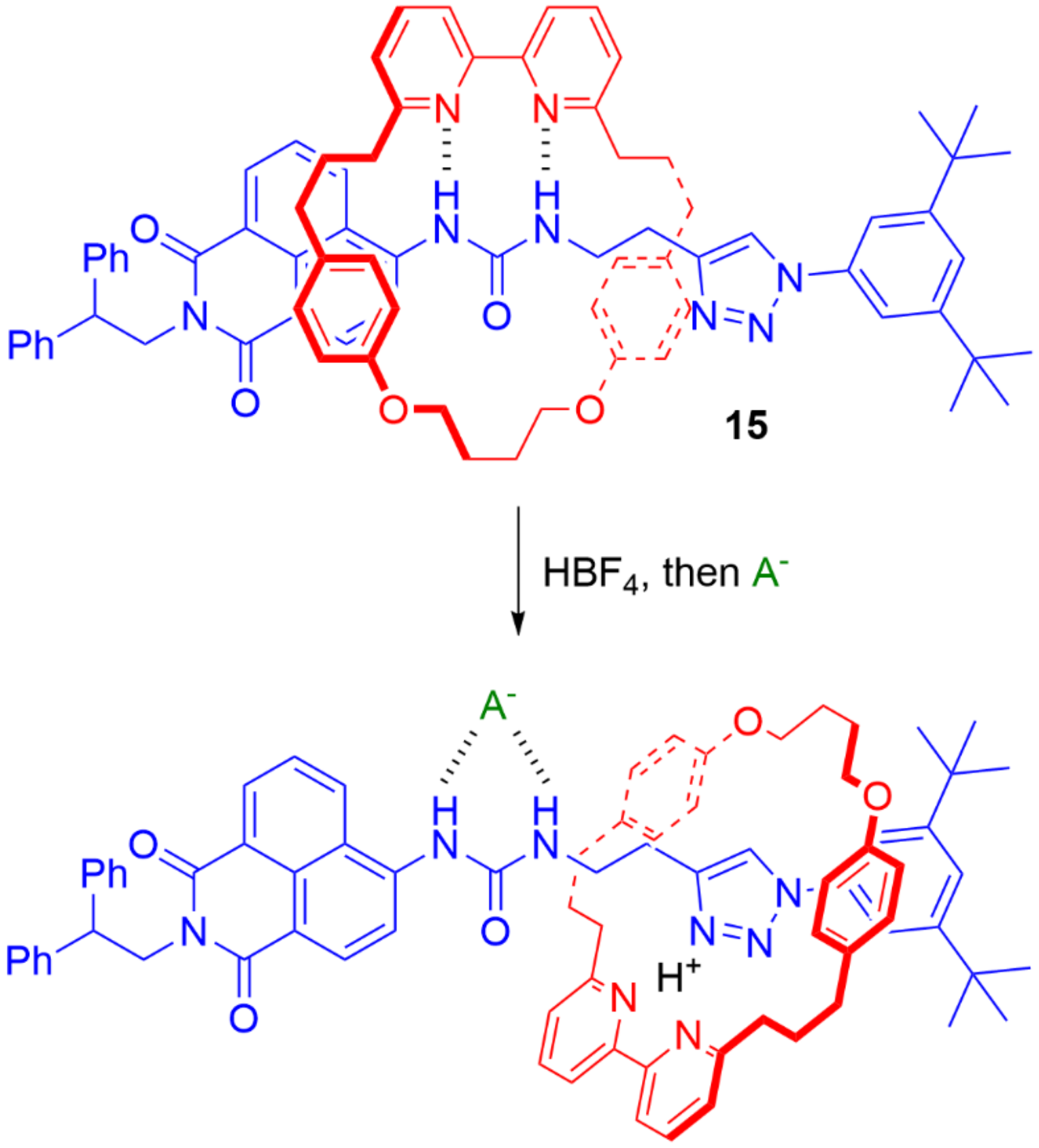
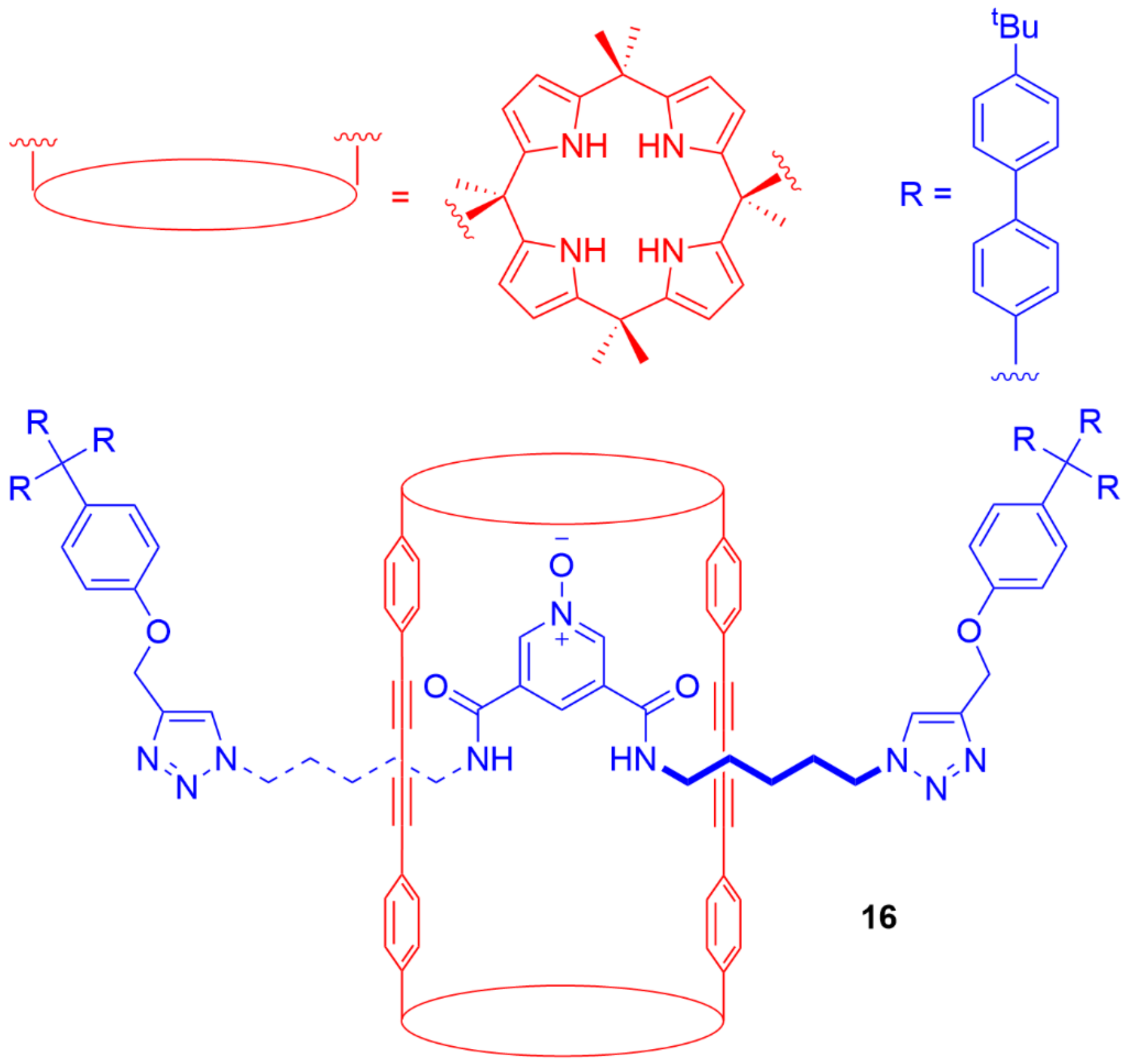
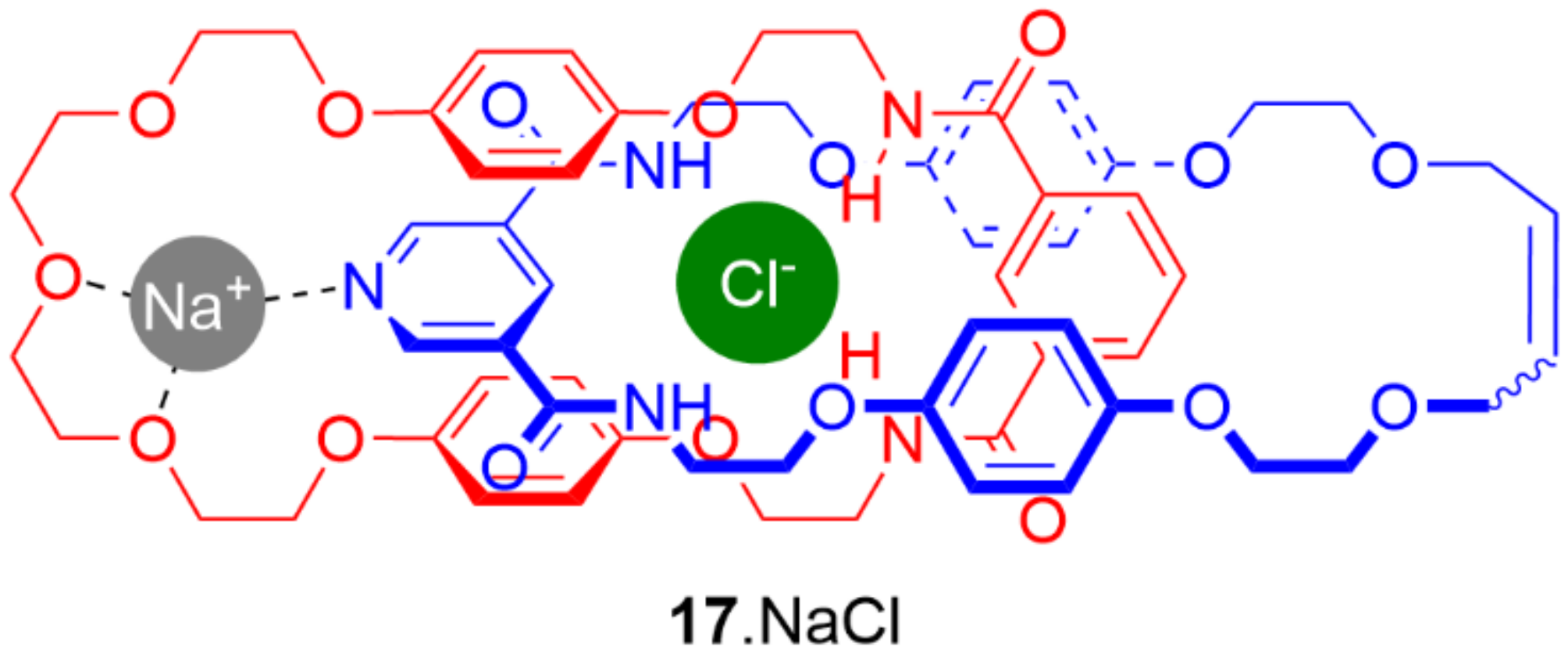
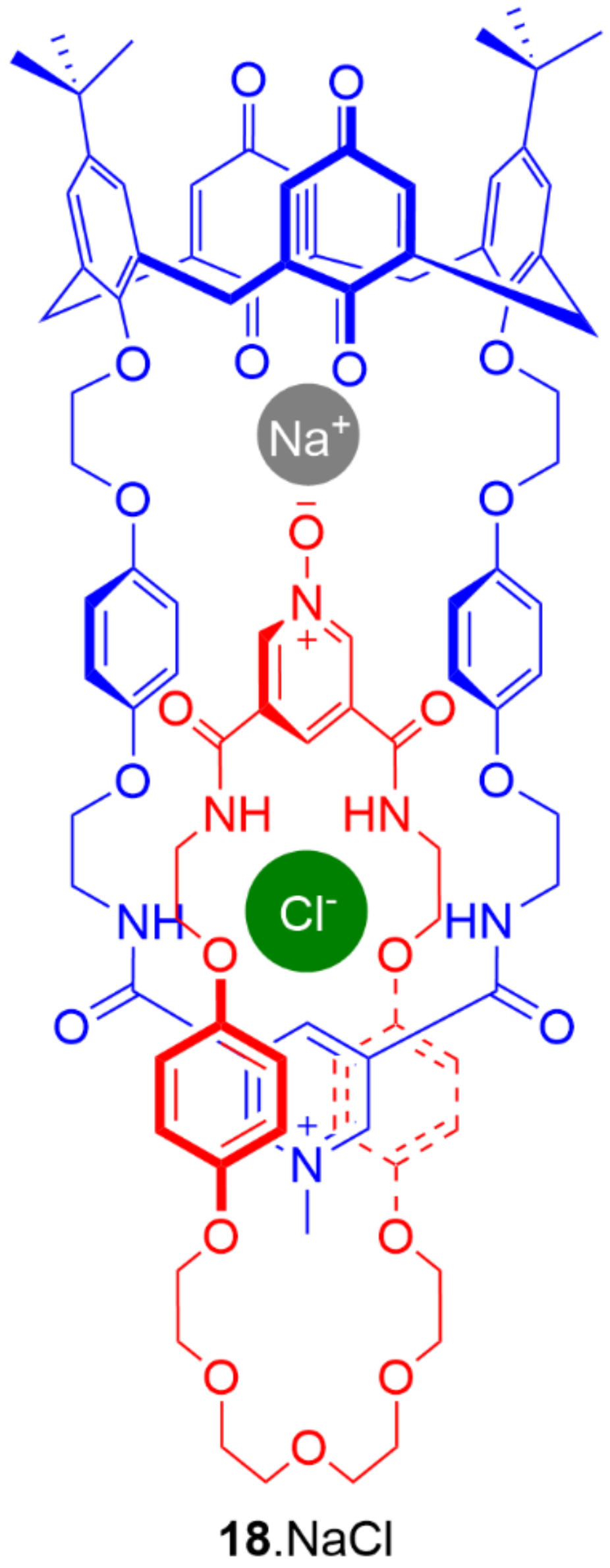
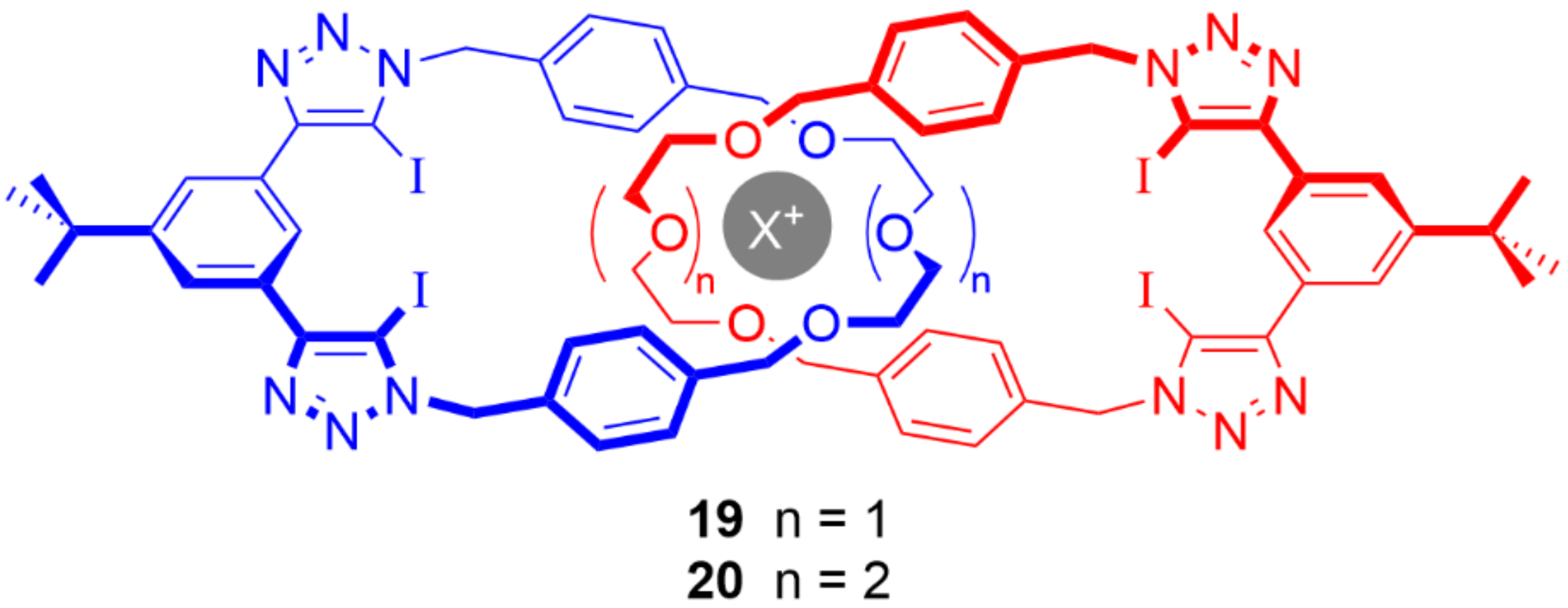
5. Heteroditopic Catenanes Binding Ion Pairs
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular chemistry—Scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar]
- Cram, D.J. The design of molecular hosts, guests, and their complexes (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1009–1020. [Google Scholar]
- Pedersen, C.J. The discovery of crown ethers (Noble Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar]
- Izatt, R.M.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.J. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar]
- Sessler, J.L.; Gale, P.A.; Cho, W.-S. Anion Receptor Chemistry; Royal Society of Chemistry: London, UK, 2006. [Google Scholar]
- Evans, N.H.; Beer, P.D. Advances in anion supramolecular chemistry: From recognition to chemical applications. Angew. Chem. Int. Ed. 2014, 53, 11716–11754. [Google Scholar]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar]
- Kim, S.K.; Sessler, J.L. Ion pair receptors. Chem. Soc. Rev. 2010, 39, 3784–3809. [Google Scholar]
- McConnell, A.J.; Beer, P.D. Heteroditopic receptors for ion-pair recognition. Angew. Chem. Int. Ed. 2012, 51, 5052–5061. [Google Scholar]
- He, Q.; Vargas-Zúñiga, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as ion pair receptors. Chem. Rev. 2019, 119, 9753–9835. [Google Scholar]
- McConnell, A.J.; Docker, A.; Beer, P.D. From Heteroditopic to Multitopic Receptors for Ion-Pair Recognition: Advances in Receptor Design and Applications. ChemPlusChem 2020, 85, 1824–1841. [Google Scholar]
- Scheerder, J.; van Duynhoven, J.P.M.; Engbersen, J.F.J.; Reinhoudt, D.N. Solubilization of NaX salts in chloroform by bifunctional receptors. Angew. Chem. Int. Ed. Engl. 1996, 35, 1090–1093. [Google Scholar]
- Beer, P.D.; Hopkins, P.K.; McKinney, J.D. Cooperative halide, perrhenate anion-sodium cation binding and pertechnetate extraction and transport by a novel tripodal tris (amido benzo-15-crown-5) ligand. Chem. Commun. 1999, 13, 1253–1254. [Google Scholar]
- Galbraith, S.G.; Plieger, P.G.; Tasker, P.A. Cooperative sulfate binding by metal salt extractants containing 3-dialkylaminomethylsalicylaldimine units. Chem. Commun. 2002, 22, 2662–2663. [Google Scholar]
- Webber, P.R.A.; Beer, P.D. Ion-pair recognition by a ditopic calix[4] semitube receptor. Dalton Trans. 2003, 11, 2249–2252. [Google Scholar]
- Aydogan, A.; Coady, D.J.; Kim, S.K.; Akar, A.; Bielawski, C.W.; Marquez, M.; Sessler, J.L. Poly(methyl methacrylate)s with pendant calixpyrroles and crown ethers: Polymeric extractants for potassium halides. Angew. Chem. Int. Ed. 2008, 47, 9648–9652. [Google Scholar]
- Zakrzewski, M.; Kwietniewska, N.; Walczak, W.; Piątek, P. A non-multimacrocyclic heteroditopic receptor that cooperatively binds and effectively extracts KAcO salt. Chem. Commun. 2018, 54, 7018–7021. [Google Scholar]
- Jagleniec, D.; Dobrzycki, Ł.; Karbarz, M.; Romański, J. Ion-pair induced supramolecular assembly formation for selective extraction and sensing of potassium sulfate. Chem. Sci. 2019, 10, 9542–9547. [Google Scholar]
- Docker, A.; Stevens, J.G.; Beer, P.D. Halogen Bonding Heteroditopic Materials for Cooperative Sodium Iodide Binding and Extraction. Chem. Eur. J. 2021, 27, 14600–14604. [Google Scholar]
- Koulov, A.V.; Mahoney, J.M.; Smith, B.D. Facilitated transport of sodium or potassium chloride across vesicle membranes using a ditopic salt-binding macrobicycle. Org. Biomol. Chem. 2003, 1, 27–29. [Google Scholar]
- Lee, J.H.; Lee, J.H.; Choi, Y.R.; Kang, P.; Choi, M.-G.; Jeong, K.-S. Synthetic K+/Cl−-selective symporter across a phospholipid membrane. J. Org. Chem. 2014, 79, 6403–6409. [Google Scholar]
- Roelens, S.; Vacca, A.; Francesconi, O.; Venturi, C. Ion-Pair Binding: Is Binding Both Binding Better? Chem. Eur. J. 2009, 15, 8296–8302. [Google Scholar]
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Mena-Hernando, S.; Pérez, E.M. Mechanically interlocked materials. Rotaxanes and catenanes beyond the small molecule. Chem. Soc. Rev. 2019, 48, 5016–5032. [Google Scholar]
- Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Development of Pseudorotaxanes and Rotaxanes: From synthesis to stimuli-responsive motions to applications. Chem. Rev. 2015, 115, 7398–7501. [Google Scholar]
- Gil-Ramírez, G.; Leigh, D.A.; Stephans, A.J. Catenanes: Fifty years of molecular links. Angew. Chem. Int. Ed. 2015, 54, 6110–6150. [Google Scholar]
- Langton, M.J.; Beer, P.D. Rotaxane and catenane host structures for sensing charged guest species. Acc. Chem. Res. 2014, 47, 1935–1949. [Google Scholar]
- Lewis, J.E.M.; Beer, P.D.; Loeb, S.J.; Goldup, S.M. Metal ions in the synthesis of interlocked molecules and materials. Chem. Soc. Rev. 2017, 46, 2577–2591. [Google Scholar]
- Bąk, K.M.; Porfyrakis, K.; Davis, J.J.; Beer, P.D. Exploiting the mechanical bond for molecular recognition and sensing of charged species. Mater. Chem. Front. 2020, 4, 1052–1073. [Google Scholar]
- Tay, H.M.; Beer, P.D. Optical sensing of anions by macrocyclic and interlocked hosts. Org. Biomol. Chem. 2021, 19, 4652–4677. [Google Scholar]
- Dietrich-Buchecker, C.; Sauvage, J.-P.; Kern, J.-M. Synthesis and electrochemical studies of catenates: Stabilization of low oxidation states by interlocked macrocyclic ligands. J. Am. Chem. Soc. 1989, 111, 7791–7800. [Google Scholar]
- Zhu, S.S.; Carroll, P.J.; Swager, T.M. Conducting polymetallorotaxanes: A supramolecular approach to transition metal ion sensors. J. Am. Chem. Soc. 1996, 118, 8713–8714. [Google Scholar]
- Zhu, S.S.; Swager, T.M. Conducting polymetallorotaxanes: Metal ion mediated enhancements in conductivity and charge localization. J. Am. Chem. Soc. 1997, 119, 12568–12577. [Google Scholar]
- Hiratani, K.; Kaneyana, M.; Nagawa, Y.; Koyama, E.; Kanesato, M. Synthesis of [1]rotaxane via covalent bond formation and its unique fluorescent response by energy transfer in the presence of lithium ion. J. Am. Chem. Soc. 2004, 126, 13568–13569. [Google Scholar]
- Nagawa, Y.; Suga, J.; Hiratani, K.; Koyama, E.; Kanesato, M. [3]Rotaxane synthesized via covalent bond formation can recognize cations forming a sandwich structure. Chem. Commun. 2005, 6, 749–751. [Google Scholar]
- Zhou, W.; Li, J.; He, X.; Li, C.; Lv, J.; Li, Y.; Wang, S.; Liu, H.; Zhu, D. A molecular shuttle for driving a multilevel fluorescence switch. Chem. Eur. J. 2008, 14, 754–763. [Google Scholar]
- Baggi, G.; Loeb, S.J. Rotationally Active Ligands: Dialing-Up the Co-conformations of a [2]Rotaxane for Metal Ion Binding. Angew. Chem. Int. Ed. 2016, 55, 12533–12537. [Google Scholar]
- Baggi, G.; Loeb, S.J. Rotationally Active Ligands: Dialing-Up Multiple Interlocked Co-Conformations for Silver (I) Coordination. Chem. Eur. J. 2017, 23, 14163–14166. [Google Scholar]
- Denis, M.; Pancholi, J.; Jobe, K.; Watkinson, M.; Goldup, S.M. Chelating rotaxane ligands as fluorescent sensors for metal ions. Angew. Chem. Int. Ed. 2018, 57, 5310–5314. [Google Scholar]
- Nandi, M.; Bej, S.; Ghosh, T.K.; Ghosh, P. A multifunctional catenated host for the efficient binding of Eu3+ and Gd3+. Chem. Commun. 2019, 55, 3085–3088. [Google Scholar]
- Chan, S.-M.; Tang, F.-K.; Kwan, C.-S.; Lam, C.-Y.; Hau, S.C.K.; Leung, K.C.-F. Water-compatible fluorescent [2]rotaxanes for Au3+ detection and bioimaging. Mat. Chem. Front. 2019, 3, 2388–2396. [Google Scholar]
- Colley, N.D.; Nosiglia, M.A.; Li, L.; Amir, F.; Chang, C.; Greene, A.F.; Fisher, J.M.; Li, R.; Li, X.; Barnes, J.C. One-Pot Synthesis of a Linear [4]Catenate Using Orthogonal Metal Templation and Ring-Closing Metathesis. Inorg. Chem. 2020, 59, 10450–10460. [Google Scholar]
- Wisner, J.A.; Beer, P.D.; Drew, M.G.B.; Sambrook, M.R. Anion-templated rotaxane formation. J. Am. Chem. Soc. 2002, 124, 12469–12476. [Google Scholar]
- Sambrook, M.R.; Beer, P.D.; Wisner, J.A.; Paul, R.L.; Cowley, A.R. Anion-templated assembly of a [2]catenane. J. Am. Chem. Soc. 2004, 126, 15364–15365. [Google Scholar]
- Bayly, S.R.; Gray, T.M.; Chmiełweski, M.J.; Davis, J.J.; Beer, P.D. Anion templated surface assembly of a redox-active sensory rotaxane. Chem. Commun. 2007, 22, 2234–2236. [Google Scholar]
- Kilah, N.L.; Wise, M.D.; Serpell, C.J.; Thompson, A.L.; White, N.G.; Christensen, K.E.; Beer, P.D. Enhancement of anion recognition exhibited by a halogen-bonding rotaxane host system. J. Am. Chem. Soc. 2010, 132, 11893–11895. [Google Scholar]
- Hancock, L.M.; Gilday, L.C.; Carvalho, S.; Costa, P.J.; Felix, V.; Serpell, C.J.; Kilah, N.L.; Beer, P.D. Rotaxanes capable of recognising chloride in aqueous media. Chem. Eur. J. 2010, 16, 13082–13094. [Google Scholar]
- Evans, N.H.; Serpell, C.J.; Beer, P.D. A redox-active [3]rotaxane capable of binding and electrochemically sensing chloride and sulfate anions. Chem. Commun. 2011, 47, 8775–8777. [Google Scholar]
- Hancock, L.M.; Marchi, E.; Ceroni, P.; Beer, P.D. Anion sensing in aqueous media by photo-active transition-metal bipyridyl rotaxanes. Chem. Eur. J. 2012, 18, 11277–11283. [Google Scholar]
- Langton, M.J.; Duckworth, L.C.; Beer, P.D. Nitrate anion templated assembly of a [2]rotaxane for selective nitrate recognition in aqueous solvent mixtures. Chem. Commun. 2013, 49, 8608–8610. [Google Scholar]
- Lehr, J.; Lang, T.; Blackburn, O.A.; Barendt, T.A.; Faulkner, S.; Davis, J.J.; Beer, P.D. Anion sensing by solution-and surface-assembled osmium(II)bipyridyl rotaxanes. Chem. Eur. J. 2013, 19, 15898–15906. [Google Scholar]
- Langton, M.J.; Robinson, S.W.; Marques, I.; Felix, V.; Beer, P.D. Halogen bonding in water results in enhanced anion recognition in acyclic and rotaxane hosts. Nature Chem. 2014, 6, 1039–1043. [Google Scholar]
- Mullaney, B.R.; Thompson, A.; Beer, P.D. An all-halogen bonding rotaxane for selective sensing of halides in aqueous media. Angew. Chem. Int. Ed. 2014, 53, 11458–11462. [Google Scholar]
- Langton, M.J.; Xiong, Y.; Beer, P.D. Active-Metal Template Synthesis of a Halogen-Bonding Rotaxane for Anion Recognition. Chem. Eur. J. 2015, 21, 18910–18914. [Google Scholar]
- Barendt, T.A.; Docker, A.; Marques, I.; Felix, V.; Beer, P.D. Selective Nitrate Recognition by a Halogen-Bonding Four-Station [3]Rotaxane Molecular Shuttle. Angew. Chem. Int. Ed. 2016, 55, 11069–11076. [Google Scholar]
- Lim, J.Y.C.; Marques, I.; Thompson, A.L.; Christensen, K.E.; Felix, V.; Beer, P.D. Chalcogen bonding macrocycles and [2]rotaxanes for anion recognition. J. Am. Chem. Soc. 2017, 139, 3122–3133. [Google Scholar]
- Lim, J.Y.C.; Marques, I.; Felix, V.; Beer, P.D. Enantioselective anion recognition by chiral halogen-bonding [2]rotaxanes. J. Am. Chem. Soc. 2017, 139, 12228–12239. [Google Scholar]
- Serpell, C.J.; Park, A.Y.; Robinson, C.V.; Beer, P.D. Imidazolium-based catenane host for bromide recognition in aqueous media. Chem. Commun. 2021, 57, 101–104. [Google Scholar]
- Chae, M.K.; Suk, J.; Jeong, K.S. A catenated anion receptor based on indolocarbazole. Tetrahedron Lett. 2010, 51, 4240–4242. [Google Scholar]
- Zhao, Y.; Li, Y.; Li, Y.; Zheng, H. Construction of an interpenetrated structure of macrocycles. Chem. Commun. 2010, 46, 5698–5700. [Google Scholar]
- Gassensmith, J.J.; Matthys, S.; Lee, J.-J.; Wojcik, A.; Kamat, P.V.; Smith, B.D. Squaraine rotaxane as a reversible optical chloride sensor. Chem. Eur. J. 2010, 16, 2916–2921. [Google Scholar]
- Collins, C.G.; Peck, E.M.; Kramer, P.J.; Smith, B.D. Squaraine rotaxane shuttle as a ratiometric deep-red optical chloride sensor. Chem. Sci. 2013, 4, 2557–2563. [Google Scholar]
- Ayme, J.F.; Beves, J.E.; Campbell, C.J.; Gil-Ramierz, G.; Leigh, D.A.; Stephens, A.J. Strong and selective anion binding within the central cavity of molecular knots and links. J. Am. Chem. Soc. 2015, 137, 9812–9815. [Google Scholar]
- August, D.P.; Borsley, S.; Cockroft, S.L.; della Sala, F.; Leigh, D.A.; Webb, S.J. Transmembrane ion channels formed by a Star of David [2]catenane and a molecular pentafoil knot. J. Am. Chem. Soc. 2020, 142, 18859–18865. [Google Scholar]
- Crowley, J.D.; Goldup, S.M.; Lee, A.-L.; Leigh, D.A.; McBurney, R.T. Active metal template synthesis of rotaxanes, catenanes and molecular shuttles. Chem. Soc. Rev. 2009, 38, 1530–1541. [Google Scholar]
- Denis, M.; Goldup, S.M. The active template approach to interlocked molecules. Nat. Rev. Chem. 2017, 1, 0061. [Google Scholar]
- Shukla, R.; Deetz, M.J.; Smith, B.D. [2]Rotaxane with a cation-binding wheel. Chem. Commun. 2000, 23, 2397–2398. [Google Scholar]
- Reuter, C.; Wienand, W.; Hübner, G.M.; Seel, C.; Vögtle, F. High-Yield Synthesis of Ester, Carbonate, and Acetal Rotaxanes by Anion Template Assistance and their Hydrolytic Dethreading. Chem. Eur. J. 1999, 5, 2692–2697. [Google Scholar]
- Deetz, M.J.; Shukla, R.; Smith, B.D. Recognition-directed assembly of salt-binding [2]rotaxanes. Tetrahedron 2002, 58, 799–805. [Google Scholar]
- Fradera, X.; Marquez, M.; Smith, B.D.; Orozco, M.; Luque, F.J. Molecular dynamics study of [2]rotaxanes: Influence of solvation and cation on co-conformation. J. Org. Chem. 2003, 68, 4663–4673. [Google Scholar]
- Shilova, E.A.; Perevalov, V.P.; Suslov, V.V.; Moustrou, C. Synthesis of new [2]rotaxane including a macrocyclic receptor and a photochromic unit. Tetrahedron Lett. 2008, 49, 3454–3457. [Google Scholar]
- Mahoney, J.M.; Shukla, R.; Marshall, R.A.; Beatty, A.M.; Zajicek, J.; Smith, B.D. Templated conversion of a crown ether-containing macrobicycle into [2]rotaxanes. J. Org. Chem. 2002, 67, 1436–1440. [Google Scholar]
- Leontiev, A.V.; Jemmett, C.A.; Beer, P.D. Anion Recognition and Cation-Induced Molecular Motion in a Heteroditopic [2]Rotaxane. Chem. Eur. J. 2011, 17, 816–825. [Google Scholar]
- Leontiev, A.V.; Serpell, C.J.; White, N.G.; Beer, P.D. Cation-induced molecular motion of spring-like [2]catenanes. Chem. Sci. 2011, 2, 922–927. [Google Scholar]
- Hancock, L.M.; Beer, P.D. Sodium and barium cation-templated synthesis and cation-induced molecular pirouetting of a pyridine-N-oxide containing [2]rotaxane. Chem. Commun. 2011, 47, 6012–6014. [Google Scholar]
- Knighton, R.C.; Beer, P.D. Axle component separated ion-pair recognition by a neutral heteroditopic [2]-rotaxane. Chem. Commun. 2014, 50, 1540–1542. [Google Scholar]
- Brown, A.; Mennie, K.M.; Mason, O.; White, N.G.; Beer, P.D. Copper (II)-directed synthesis of neutral heteroditopic [2]-rotaxane ion-pair host systems incorporating hydrogen and halogen bonding anion binding cavities. Dalton Trans. 2017, 46, 13376–13385. [Google Scholar]
- Santra, S.; Ghosh, P. Rotamer-Induced Dynamic Nature of a [2]Rotaxane and Control of the Dynamics by External Stimuli. Eur. J. Org. Chem. 2017, 82, 1583–1593. [Google Scholar]
- Santra, S.; Ghosh, P. Fluorophoric [2]rotaxanes: Post-synthetic functionalization, conformational fluxionality and metal ion chelation. N. J. Chem. 2020, 44, 5947–5964. [Google Scholar]
- Bej, S.; Nandi, M.; Ghosh, P. A Cd (II) and Zn (II) selective naphthyl based [2]rotaxane acts as an exclusive Zn (II) sensor upon further functionalization with pyrene. Dalton Trans. 2021, 50, 294–303. [Google Scholar]
- Nandi, M.; Bej, S.; Ghosh, P. NDI-integrated rotaxane/catenane and their interactions with anions. Dalton Trans. 2022, 51, 13507–13514. [Google Scholar]
- Munasinghe, V.K.; Pancholi, J.; Manawadu, D.; Zhang, Z.; Beer, P.D. Mechanical Bond Enhanced Lithium Halide Ion-Pair Binding by Halogen Bonding Heteroditopic Rotaxanes. Chem. Eur. J. 2022, 28, e202201209. [Google Scholar]
- Denis, M.; Qin, L.; Turner, P.; Joliffe, K.A.; Goldup, S.M. A Fluorescent Ditopic Rotaxane Ion-Pair Host. Angew. Chem. Int. Ed. 2018, 57, 5315–5319. [Google Scholar]
- Romero, J.R.; Aragay, G.; Ballester, P. Ion-pair recognition by a neutral [2]rotaxane based on a bis-calix[4]pyrrole cyclic component. Chem. Sci. 2017, 8, 491–498. [Google Scholar]
- Molina-Muriel, R.; Romero, J.R.; Li, Y.; Aragay, G.; Ballester, P. The effect of solvent on the binding of anions and ion-pairs with a neutral [2]rotaxane. Org. Biomol. Chem. 2021, 19, 9986–9995. [Google Scholar]
- Evans, N.H.; Serpell, C.J.; Beer, P.D. A [2] catenane displaying pirouetting motion triggered by debenzylation and locked by chloride anion recognition. Chem. Eur. J. 2011, 17, 7734–7738. [Google Scholar]
- Knighton, R.C.; Beer, P.D. Sodium cation-templated synthesis of an ion-pair binding heteroditopic [2]catenane. Org. Chem. Front. 2021, 8, 2468–2472. [Google Scholar]
- Tay, H.M.; Tse, Y.C.; Docker, A.; Gateley, C.; Thompson, A.L.; Kuhn, H.; Zhang, Z.; Beer, P.D. Halogen-Bonding Heteroditopic [2]Catenanes for Recognition of Alkali Metal/Halide Ion Pairs. Angew. Chem. Int. Ed. 2022, e202214785. [Google Scholar] [CrossRef]
- Tung, S.-T.; Lai, C.-C.; Liu, Y.-H.; Peng, S.-M.; Chiu, S.H. Synthesis of a [2]catenane from the sodium ion templated orthogonal arrangement of two diethylene glycol chains. Angew. Chem. Int. Ed. 2013, 52, 13269–13272. [Google Scholar]
- Inthasot, A.; Tung, S.-T.; Chiu, S.H. Using alkali metal ions to template the synthesis of interlocked molecules. Acc. Chem. Res. 2018, 51, 1324–1337. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholson, S.J.; Barlow, S.R.; Evans, N.H. Heteroditopic Rotaxanes and Catenanes for Ion Pair Recognition. Chemistry 2023, 5, 106-118. https://doi.org/10.3390/chemistry5010009
Nicholson SJ, Barlow SR, Evans NH. Heteroditopic Rotaxanes and Catenanes for Ion Pair Recognition. Chemistry. 2023; 5(1):106-118. https://doi.org/10.3390/chemistry5010009
Chicago/Turabian StyleNicholson, Steven J., Sean R. Barlow, and Nicholas H. Evans. 2023. "Heteroditopic Rotaxanes and Catenanes for Ion Pair Recognition" Chemistry 5, no. 1: 106-118. https://doi.org/10.3390/chemistry5010009
APA StyleNicholson, S. J., Barlow, S. R., & Evans, N. H. (2023). Heteroditopic Rotaxanes and Catenanes for Ion Pair Recognition. Chemistry, 5(1), 106-118. https://doi.org/10.3390/chemistry5010009