Abstract
This review presents representative examples illustrating how the Lewis acidic character of the Zn(II) metal center in Zn(salen)-type complexes, as well as in complexes of other tetradentate ligands, and the nature of the medium govern their supramolecular aggregation, leading to the formation of a variety of supramolecular structures, either in solution or in the solid state. Stabilization of these Lewis acidic complexes is almost always reached through an axial coordination of a Lewis base, leading to a penta-coordinated square-pyramidal geometry around the metal center. The coverage is not exhaustive, mainly focused on their crystallographic structures, but also on their aggregation and sensing properties in solution, and on their self-assembled and responsive nanostructures, summarizing their salient aspects. The axial ligands can easily be displaced, either in solution or in the solid state, with suitable Lewis bases, thus being responsive supramolecular structures useful for sensing. This contribution represents the first attempt to relate some common features of the chemistry of different families of Zn(II) complexes of tetradentate ligands to their intrinsic Lewis acidic character.
1. Introduction
Supramolecular chemistry deals with weak interactions between molecular units, leading to self-assembled molecular architectures. It is an interdisciplinary field including basic concepts and applications, involving chemists, physicists, and biologists. In fact, supramolecular chemistry can not only be considered as a means to assemble molecules into supramolecular architectures, but also represents a powerful tool for new functional molecular materials for various applications, namely, as receptors of neutral and charged guests, for molecular recognition and sensing, drug delivery, molecular imaging, and mimicking biological systems, as efficient catalysts, or as molecular machines for optoelectronics [1,2,3,4].
Although supramolecular chemistry mainly includes noncovalent intermolecular interactions, in a more general view, it seems more appropriate to consider the properties of the molecular assemblies, rather than the type of intermolecular interactions involved [5]. Therefore, other examples that also involve covalent interactions can be included. In particular, coordination-driven self-assembly defines a new approach for the self-assembly process [6].
Zinc(II) complexes of salen-type Schiff-base ligands, i.e., of tetradentate (N2O2) ligands derived from the condensation of substituted salicylaldehydes with 1,2-diamines, are characterized by interesting aggregation and sensing properties, mostly related to the Lewis acidic character of the metal center [7,8,9,10,11]. Stabilization of these complexes mainly occurs by the axial interaction with a suitable donor, with formation of supramolecular architectures, governed by the solvent medium and/or the presence of proper Lewis bases.
This review contribution is focused on the ability of Zn(salen)-type complexes (Figure 1) to aggregate as a consequence of their Lewis acidic character (Section 2.1), leading to the formation of a variety of supramolecular structures. The coverage is not exhaustive, mainly focused on their crystallographic structures (Section 2.2.1, Section 2.2.2, Section 2.2.3, Section 2.2.4), but also on their aggregation (Section 2.2.5) and sensing (Section 2.2.6) properties in solution, and on their self-assembled and responsive nanostructures (Section 2.3), summarizing their salient aspects. Moreover, examples of other families of Zn(II) complexes of tetradentate ligands, whose properties can be view within this framework, are briefly commented (Section 3). This represents the first attempt described in the literature to relate some common features of the chemistry of different families of Zn(II) complexes of tetradentate ligands to their intrinsic Lewis acidic character.
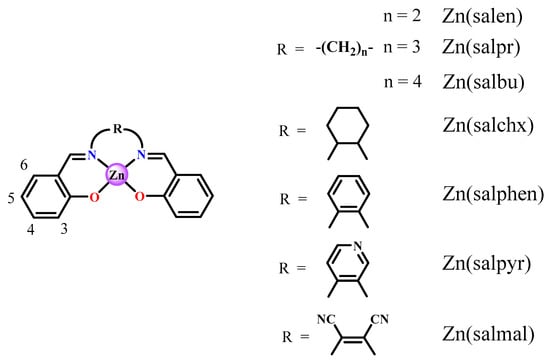
Figure 1.
Some basic structures of Zn(salen)-type complexes and abbreviations used.
2. Lewis Acidic Zn(II) Salen-Type Complexes
2.1. Origin of the Lewis Acidic Character
The Lewis acidic properties of Zn(salen)-type complexes can be attributed to the 3d10 electron configuration of the Zn(II) metal ion and the structure of the tetradentate Schiff-base ligand. The latter constrains the metal center in a pseudo-planar coordination. Without any further coordination, this would imply a coordinative unsaturation of the metal center. Therefore, stabilization of these complexes is often achieved through an axial coordination of a Lewis base, leading to a penta-coordinated square-pyramidal geometry. On the other hand, bidentate ligands almost always lead to a tetrahedral coordination of the metal center.
Although the stabilization by a penta-coordination was involved since the early studies by MacLachlan et al. [12] and Kleij [7,8] to explain the aggregation properties of these complexes, only recently has a systematic combined theoretical/experimental investigation focused on their Lewis acidic character been done [13]. In particular, a series of Zn(salen)-type complexes on changing the structure of the 1,2-diimine bridge has been investigated. Thus, complexes having conjugated diamine bridges possess a stronger Lewis acidic character than those having non-conjugated bridges, governed by the relative stability of the monomeric adducts with respect to that of the aggregates. These results have been further supported by more recent DFT calculations [14].
The penta-coordination in Zn(salen)-type complexes can be fulfilled in different ways, depending on the absence or presence of Lewis bases (Scheme 1). In the first case, stabilization is achieved by intermolecular Zn···O interactions, involving the phenolic oxygen atoms of the ligand framework. Reciprocal interactions lead to the formation of self-assembled dimers, I; otherwise, by non-mutual interactions, larger oligomeric aggregates, II, are formed. These structures have been found either in the solid state or in solution. Instead, in the presence of Lewis bases, monomeric adducts, III, or supramolecular structures, IV, are found, in relation to the mono- or polytopic nature of the Lewis base, respectively. Finally, appropriate donor substituents on the ligand framework can lead to a variety of supramolecular architectures, V. Exceptions to the penta-coordination mode are encountered in relation to the chemical structure/stereochemistry of the 1,2-diimine bridge. For example, in the case of non-conjugated bridges, the occurrence of structures, VI, with a tetrahedral coordination is found (Scheme 1). Representative examples of these structures are reported in the following paragraphs.
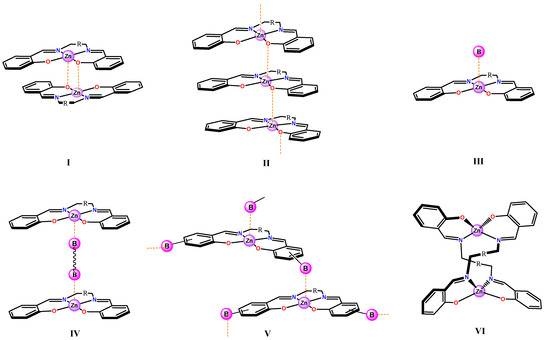
Scheme 1.
Possible structures of Zn(salen)-type complexes by axial coordination of a Lewis base (I–V), or by tetrahedral coordination (VI).
2.2. Molecular Aggregates
2.2.1. Structures of Mononuclear Complexes
The concepts outlined in the previous paragraph (see Section 2.1), i.e., the formation of dimeric aggregates, I, in the absence of Lewis bases, are reflected in almost all crystallographic structures of Zn(salen)-type complexes reported in the literature, whose crystallization was achieved in non-coordinating solvents, or in coordinating solvents having a weak Lewis basicity, unless a sterical hindrance by bulky substituents in the salicylidene rings occurs. On the other hand, the formation of monomeric adducts, III, often take place in the presence of suitable Lewis bases. Some aspects of the aggregation properties of these complexes, involving early studies, have previously been reviewed by Kleij [8].
The simpler case is represented by the unsubstituted Zn(salen) (1, Figure 2). This complex, crystallized from methanol, leads to the formation of a centrosymmetric dimeric structure, with a distorted penta-coordinated square-pyramidal geometry around the Zn(II) atoms, defined by the (N2O2) atoms of the tetradentate salen ligand as the basal plane and one bridging O atom of the adjacent ligand as the apical atom (Figure 2a) [15]. The same Zn(salen) complex when crystallized from pyridine shows a monomeric structure with a square-based pyramidal configuration (Figure 2b) [16], isostructural with that of the Zn(salen) monohydrate complex [17], the latter crystallized from aqueous methanol. An analogous behavior is observed on increasing the length of the non-conjugated bridge of the salen-type ligand. Thus, the unsubstituted Zn(salpr) complex, even if synthesized in pyridine, upon recrystallization from dichloromethane-diethylether gives a pseudo-centrosymmetric dimer, analogous to a Zn(salen) dimer, in which the Zn(II) is penta-coordinated in a distorted pyramidal structure [16].
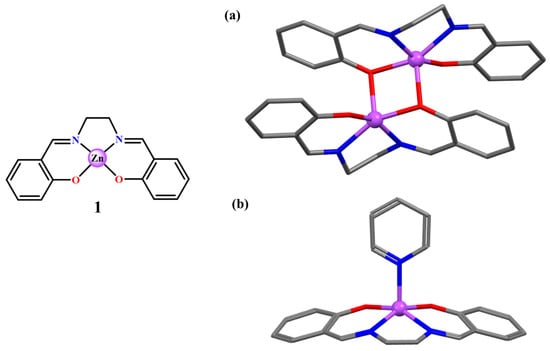
Figure 2.
Molecular drawing of the Zn(salen) complex 1, and crystal structures of the (1)2 dimer (a) and the 1·py adduct (b). Adapted from Refs. [15,16].
The further increase of the bridge length in Zn(salbu) complexes, due to the increased flexibility of the ligand, leads to more complex structures. For example, crystals obtained by slow evaporation of a saturated acetone:acetonitrile (1:1) solution of a Zn(salbu) complex, derived from the 2,5-dihydroxybenzaldehyde, reveal the formation of a dinuclear complex [18]. The latter results from the pairing of two mononuclear subunits through a double μ-phenoxo bridge, whose two units adopt two different geometries. The Zn(1) geometry can be considered as square-based pyramidal, whereas Zn(2) is better described as trigonal bipyramidal. In both cases, a penta-coordination around the Zn(II) atoms is satisfied. Instead, the Zn(salbu) complex in the presence of acetate ions leads to the formation of a trinuclear complex, μ-bridged by two acetate ions [16].
Substitution of a bulky t-Bu group at the 3,3′-positions of the salicylidene rings of the H2salen ligand, (H2-t-salen, Figure 3), prevents the formation of the dimeric aggregate. Instead, unexpectedly, in this case, a coordination mode VI is observed. In fact, even when crystallized from acetonitrile, the formation of a 2:2 t-salen–Zn(II) complex, Zn2(t-salen)2, is found, having a helical structure in which the Zn(II) atoms show a tetrahedral coordination (2, Figure 3) [19], similar to that observed in Zn(II) complexes of bidentate (NO) Schiff-base ligands [20,21,22,23]. Analogously, the H2-t-salen-like ligand, derived from the 2,2′-diaminodiphenyl ether, leads to a dinuclear complex with a helical structure [24]. Therefore, in these cases, although the aggregation mode, I, is inhibited by the presence of the bulky t-Bu groups, the “flexibility” of the salen ligand having non-conjugated bridges allows the formation of a stable structure in a tetrahedral coordination.
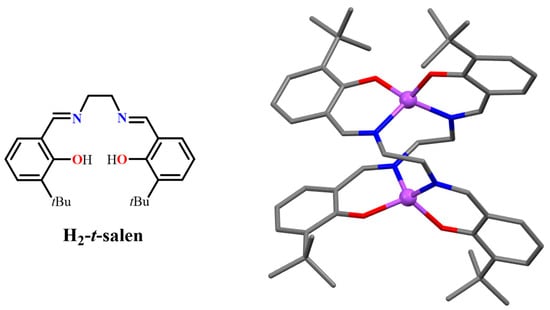
Figure 3.
Molecular drawing of the H2-t-salen ligand (left) and crystal structure of the Zn2(t-salen)2 complex 2 (right). Adapted from Ref. [19].
Crystallographic structures of various Zn(salphen) complexes were investigated (Figure 4). The Zn(salphen) derivative (3, Figure 4), having two bulky t-Bu groups at the 3,5-positions of the salicylidene rings, was first investigated by Atwood et al., to increase its solubility in common solvents and to gain access to a tetra-coordinated, ideally strong Lewis acidic complex [25]. However, the formation of the monomeric adducts, either with THF or pyridine, with the Zn(II) atom in a penta-coordinated square-pyramidal geometry, with the solvent occupying the apical position, were obtained. Moreover, any attempt to prepare the tetra-coordinated, desolvated derivative was unsuccessful. Analogous monomeric adducts of complex 3 with pyridine, ethanol, and THF were subsequently reported by other authors [26].
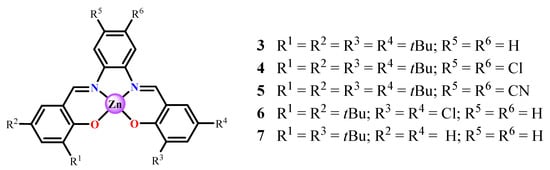
Figure 4.
Structure of investigated substituted Zn(salphen) complexes.
It is therefore expected that the presence of bulky t-Bu groups in the salicylidene rings in Zn(salphen) complexes hinders their dimerization process. In fact, complexes having two further substituents in the phenyl ring, in addition to the bulky t-Bu groups at the 3,5-positions of the salicylidene rings (4, 5, Figure 4), crystallize as monomeric adducts even in a weak Lewis base, such as methanol [27,28], as consequence of the their very encumbered structure. Conversely, the analogous nonsymmetrical complex (6, Figure 4) crystallizes as a dimer, even in acetonitrile (Figure 5) [27]. The axial oxygen coordination gives rise to a distorted square-pyramidal coordination around the Zn(II) atoms. The two salphen units have an anti-parallel orientation, and the t-Bu groups of the individual unit point away from the adjacent unit in order to minimize steric repulsions.
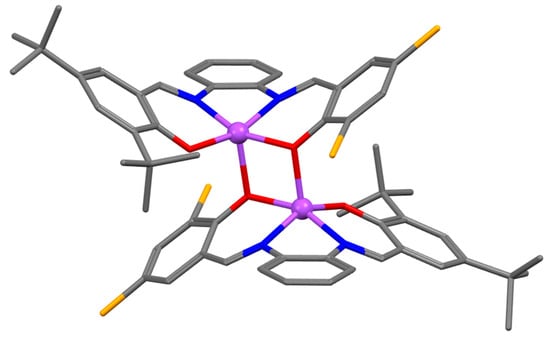
Figure 5.
Crystal structure of the (6)2 dimer. Adapted from Ref. [27].
The strong tendency towards dimerization was further observed in a series of Zn(salphen) complexes with various symmetrical and non-symmetrical substitution patterns in the 3,5-positions of the salicylidene rings [29]. The formation of dimers (in acetonitrile) was unexpectedly found even from the derivative with the t-Bu groups at the 3,3′-positions (7, Figure 4), in which the two monomeric units in the dimers are twisted about 90° to minimize the steric repulsions of bulky substituents. In each case, the formation of these assemblies shows the capability of these dimers to release the steric strain that accompanies the assembly process by choosing a sterically less-congested structure.
On the other hand, the formation of monomeric adducts always occurs in the presence of sufficiently strong Lewis bases [30]. In fact, penta-coordinated monomer adducts, from either Zn(salphen) or Zn(salchx) derivatives having 3,5-di-t-Bu groups or 5-(4-pyridyl) substituents in the salicylidene rings, were achieved by crystallizing these complexes from pyridine or piperidine [31]. The formation of monomeric adducts was also found for a derivative of Zn(salpyr) [32] or 2,3-diaminophenazine [33] complexes crystallized from pyridine or DMSO, respectively.
Regarding Zn(salmal) derivatives, the relevant X-ray structures reported in the literature are related to monomeric adducts either when crystallized from DMF [34], or from methanol [35], in the latter case for complexes having 3- or 4-hydroxy substituents in the salicylidene rings. This is in sharp contrast to the dimeric structures found for Zn(salen) [15] or Zn(salphen) complexes [27,29], likely as a consequence of their stronger Lewis acidic character [13].
The X-ray crystal structure of the 4-methoxy substituted complex derived from cis-1,2-diaminocyclohexane (8, Figure 6), crystallized from chloroform, indicates the existence of an asymmetric dimer with a very rigid backbone [36]. The structure is the consequence of the cis-cyclohexane ring because of its energetically favored chair conformation, thus preventing the formation of a symmetric arrangement. Conversely, the Zn(II) complex derivative from the (1S,2S)-(+)-trans-1,2-diaminocyclohexane and 3-tert-butyl-2-hydroxybenzaldehyde leads to a dinuclear double-stranded helicate structure, with a tetrahedral coordination around the Zn(II) atoms (9, Figure 7) [37], instead of a dimer with a penta-coordination around the Zn(II) atoms. The authors demonstrated that the formation of the helicate structure is not due to 3,3′-t-Bu substituents in the salicylidene rings, but due to the defined stereochemistry of the trans-1,2-diaminocyclohexane chelate bridge. Note that, as mentioned above, a tetrahedral coordination around the Zn(II) atom always occurs in complexes of bidentate (NO) Schiff-base ligands [20,21,22,23], independently of the presence of Lewis bases. In other terms, in the absence of sterical constraints, the formation of tetrahedral structures is favored.
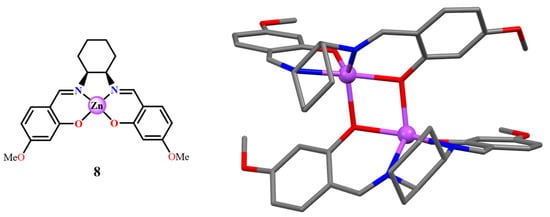
Figure 6.
Molecular drawing of the 4-OMe substituted Zn(salchx) complex 8 (left), and crystal structure of the (8)2 dimer (right). Adapted from Ref. [36].
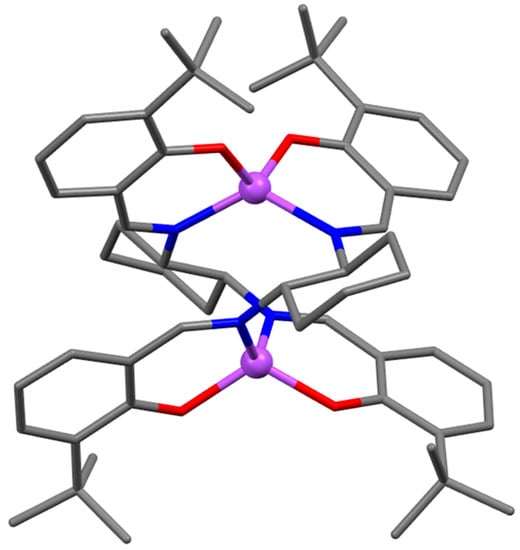
Figure 7.
Crystal structure of P-(S,S)-9. Adapted from Ref. [37].
2.2.2. Structures of Polynuclear and Macrocycle Complexes
The observed aggregation properties of mononuclear Zn(salen) complexes illustrated in the previous paragraph are also reflected in polynuclear complexes [38,39]. The formation of adducts of dinuclear complexes with nicotine [40], i.e., a pyridine-based alkaloid, or of adducts of macrocyclic complexes with pyridine [41] are observed using strong Lewis bases. However, starting from various bis-salphen scaffolds, e.g., the H4-bis(t-salphen) ligand (Figure 8), upon metalation is observed the formation of a trinuclear (10, Figure 8) or a hexanuclear structure. In these structures, each Zn(II) atom is resultantly still penta-coordinated by a bridging acetate and n-hexylamine, when crystals were achieved from acetonitrile in the presence of n-hexylamine, or by four hydroxo (OH) bridging ligands, by crystallization from dichoromethane, whose Zn(OH)2 units are presumably originated from the synthetic procedure [42].
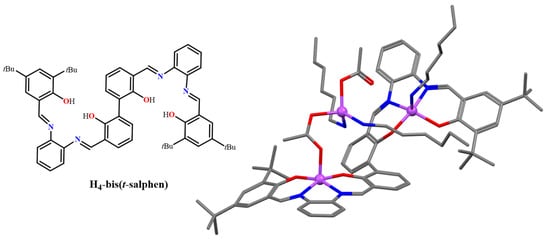
Figure 8.
Molecular drawing of the H4-bis(t-salphen) ligand (left), and crystal structure of the trinuclear complex 10 (right) crystallized in the presence of hexylamine. Adapted from Ref. [42].
A macrocyclic calix[4]arene comprising of two 2,6-bis-(iminomethyl) phenolate chelating ligands gives a Zn(II) complex which, in the absence of external coligands, aggregates in a dimeric structure via intermolecular Zn-Nimine bonds, to afford two penta-coordinated Zn(II) atoms with distorted trigonal-bipyramidal coordination environments [43].
Other polynuclear Zn(II) complexes are presented in the following paragraphs (Section 2.2.3 and Section 2.2.4), while heterometallic polynuclear complexes will not be described in this contribution.
2.2.3. Neutral and Charged Polytopic Lewis Bases Templating the Supramolecular Architecture
The synthesis of Zn(salen)-type complexes in the presence of polytopic Lewis bases leads to the formation of varied supramolecular structures IV [44,45]. Thus, using various Zn(salen)-type complexes, e.g., 4, or chiral Zn(salen) complexes, and ditopic species, such as 4,4ʹ-bipyridine (bipy) or 1,4-diazabicyclo[2.2.2]octane, the X-ray structure of 2:1 adducts was characterized [46,47]. Alternatively, starting from the dinuclear bis-Zn(salphen) complex (11, Figure 9), in the presence of various ditopic bipy ligands, the formation of 2:2 supramolecular box-type assemblies was found, either in solution and in the solid state, e.g., (11)2·(bipy)2 (Figure 9) [46].
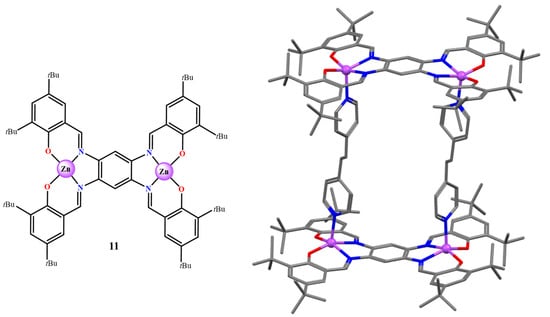
Figure 9.
Molecular drawing of complex 11 (left) and crystal structure of the assembly of (11)2·(bipy)2 in the solid state (right). Adapted from Ref. [46].
As discussed in Section 2.2.2, the presence of bulky t-Bu groups in the salicylidene rings in Zn(salphen) complexes inhibits the dimerization process. However, complex 3 in the presence of alcohols and acetone self-assemble through a unique combination of hydrogen bonds and weak oxygen···oxygen interactions, giving rise to discrete supramolecular dimers [48].
The formation of molecular assemblies also occurs in the presence of anions. Kleij et al. first demonstrated the anion-templated formation of supramolecular assemblies [49]. For example, using the Zn(salphen) derivative having a bulky t-Bu group at the 3,3′-positions of the salicylidene rings (7), the acetate anion leads to a 2:1 assembly, (7)2⋅NBu4OAc, in which both complexes are bridged by one acetate.
2.2.4. Self-Assembly of Zn(salen)-Type Complexes by Donor Substituents on the Ligand Framework
Another approach to supramolecular architectures for these Lewis acidic Zn(salen)-type complexes can be achieved by using mono- or polynuclear complexes having appropriate donor substituents on the ligand framework, e.g., pyridyl groups, or ammonium halides in the salicylidene rings, or substituents in the 1,2-diimine bridge, e.g., Zn(salpyr) complexes. In these cases, the formation of supramolecular structures, V, is observed.
For example, using the dinuclear complex 11 and the 3,5-di-t-Bu-substituted Zn(salpyr) complex, the formation of a (1:2) assembly was characterized [50], while the 3,3´-t-Bu-substituted Zn(salpyr) complex self-organizes into a stable tetrameric vase structure via co-operative intermolecular Zn−Npyr interactions [51]. Likewise, symmetrical Zn(salmal) complexes, having 4,4´-alkyl ammonium bromide as substituents in the salicylidene rings, lead to the formation of branched nanostructures [52].
The H4-bis(pyr-t-salphen) ligand (H4-12, Figure 10), having 5,5′-(4-pyridinyl) substituents in the salicylidene rings, coordinates two Zn(II) atoms (12, Figure 10), and one of the pyridyl groups on each side of the bis(salphen) further coordinates with a bis(salphen)-coordinated Zn(II) atom of another dinuclear complex to give a 2D sheet structure (Figure 11), having uncoordinated pyridyl groups exposed to 1D channels [53]. In turn, 2D sheets are stacked together to form a 3D-like structure containing 1D channels.
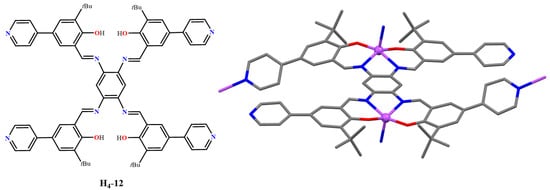
Figure 10.
Molecular drawing of the H4-12 ligand (left) and crystal structure of the dinuclear unit of the complex 12 (right). Adapted from Ref. [53].
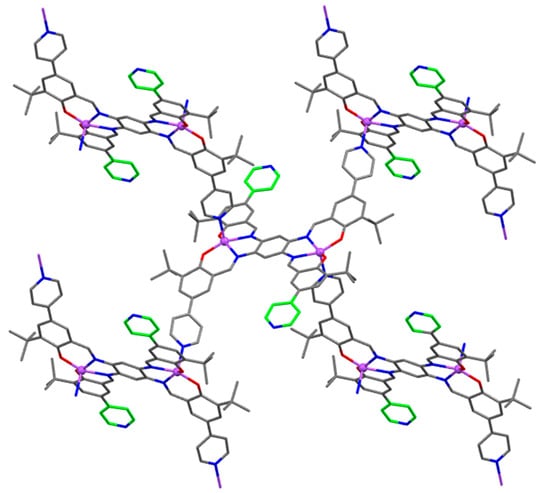
Figure 11.
2D structure of 12 showing uncoordinated pyridyl groups exposed to 1D channels. Openly accessible pyridyl groups are highlighted in green. Adapted from Ref. [53].
2.2.5. Studies in Solution
The aggregation characteristics of Zn(salen)-type complexes discussed in the previous paragraphs are often also encountered in solution [12,29,35,36,41,42,46,49,50,52]. In these cases, in addition to crystallographic investigations, parallel studies in solution have demonstrated the existence of analogous aggregate species.
Di Bella et al. reported systematic studies in solution on the aggregation/deaggregation properties of non-coordinating/coordinating solvents for a series of Zn(salen)-type complexes on changing the conjugated [54,55,56,57] or non-conjugated [36,58] structure of the 1,2-diamine bridge, including complexes derived from chiral 1,2-diamines [59,60,61]. The main features that emerge from these studies are: (i) the existence of aggregate species in solution of non-coordinating solvents; (ii) the formation of monomeric adducts in solution of coordinating solvents; and (iii) deaggregation upon the addition of an appropriate Lewis base in non-coordinating solvents. Moreover, deaggregation is accompanied by relevant changes in their spectroscopic properties that can appropriately be exploited for sensing Lewis bases (see, Section 2.2.6). Further details on the aggregation/deaggregation, 1H NMR, and optical spectroscopic properties of Zn(salen)-type complexes in solution have been described in recent accounts [10,11].
More recent studies involved the aggregation properties of dinuclear Zn(salmal)-type complexes, having a non-conjugated flexible spacer between each molecular unit (e.g., 13, Figure 12) [62]. It is worth noting that, in the solution of non-coordinating solvents, these complexes form very stable intramolecular aggregates. In comparison to intermolecular aggregates, these intramolecular aggregates have a larger thermodynamic stability, even larger than that of the aggregates of conjugated multinuclear complexes. Moreover, these dinuclear complexes preferentially deaggregate with ditopic Lewis bases forming stable 1:1 adducts, with higher binding constants in comparison to those related to monotopic species, thus behaving as molecular tweezers of ditopic guests having a strong Lewis basicity.
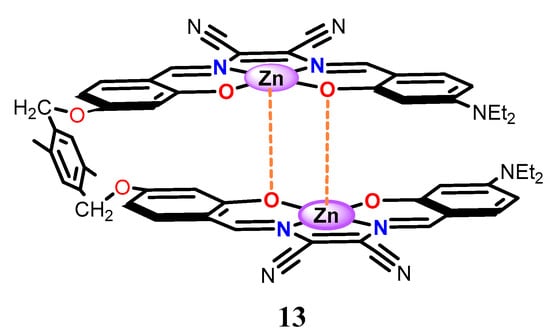
Figure 12.
Molecular structure of the intramolecular aggregate dinuclear complex 13.
2.2.6. Sensing Studies
As anticipated in the previous section, optical spectroscopic changes observed upon deaggregation of Zn(salen)-type complexes in non-coordinating solvents have been developed for sensing various Lewis bases. However, other sensing studies have been accomplished in coordinating solvents. In this regard, several review contributions have appeared in the recent literature [9,10,11,63,64].
Three main sensing mechanisms can be involved (Scheme 2). Briefly, beyond sensing studies by adduct formation upon deaggregation (mechanism A), including amines [65,66] and their derivatives [40,67,68], chiral species [69,70,71], and anions [72], other sensing studies involving anions have been performed in coordinating solvents, generally in solvents with a weak Lewis basicity, such as ethanol [73,74], or acetone [75]. In these cases, the sensing mechanism necessarily implies a displacement of the axial coordinated solvent with the analyte (mechanism B, Scheme 2). Other studies include aqueous solutions of Zn(salen)-type complexes for sensing α-aminoacids [76], anions [77], ATP and ADP [78], or monohydrogensulfide [79], but with different mechanisms, also involving secondary interactions. Moreover, given their very good fluorescent characteristics [80], Zn(salen)-type complexes have also been employed as optical probes for cell imaging [81,82,83,84,85].
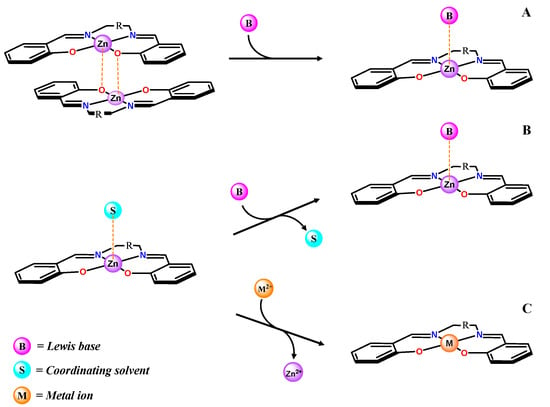
Scheme 2.
Main sensing mechanisms involved in Zn(salen)-type complexes.
Recent studies have involved the dinuclear complex 13 to explore its binding properties towards a series of ditopic diamines having a strong Lewis basicity, with different chain lengths and rigidities [86], and for the highly selective and sensitive colorimetric/fluorometric detection of relevant biogenic amines [87]. Using an analogous approach, a chiral spirobifluorene-based Zn(salen)-type complex has been shown to be a receptor for the highly enantioselective binding of chiral carboxylates [88].
The stability of the Zn(salen)-type Lewis base adducts in solution, mainly related to the Lewis basicity of the coordinated species, should also affect the ability of the Zn(II) ion to transmetalate with other metal ions (mechanism C, Scheme 2). From this perspective, in a recent study, it has been demonstrated that the 5,5′-t-Bu-substituted Zn(salmal) complex, 14, in acetonitrile solution gives an immediate and complete transmetalation with Cu2+, while DMF solutions of the complex are less prone to transmetalation, because of the greater stability of the related adducts [89]. The fast transmetallation of 14 in acetonitrile towards Cu2+ has been exploited for the selective and sensitive colorimetric and fluorometric detection of Cu2+ ions in aqueous solution [90].
2.3. Molecular Self-Assembled Nanostructures
As illustrated in Section 2.1, non-mutual intermolecular Zn···O interactions lead to the formation of larger aggregates II which, in turn, can self-assemble into nanostructures. These nanostructures, first investigated by MacLachlan et al. [91,92], have recently been reviewed in detail by one of us [11]. Therefore, they are briefly commented upon. These nanostructures include mainly Zn(salphen) complexes having alkoxy groups either in the bridge [91,92,93] or in the salicylidene rings [94,95,96], morphologically and structurally characterized by solids achieved by casting or solvent evaporation, from solutions of coordinating solvents.
Although intermolecular Zn···O interactions are the driving force for the formation of these nanostructures, the presence of the aromatic rings, by π–π stacking interactions, and alkyl side groups, by their interdigitation, contribute to their stabilization. The formation of nanostructures is shown in Figure 13 for Zn(salphen) complexes having 4,4′-alkoxy substituents in the salicylidene rings [95]. The induction of mesomorphism and photoluminescence via coordination with the Zn(II) ion has been reported for a series of Zn(salphen) and Zn(salen) complexes with longer 4,4′-n-alkoxysalicylidene substituents (n = 12, 14, and 16) [97,98,99,100]. The formation of these fluorescent columnar mesomorphic structures can be described with an analogous model.
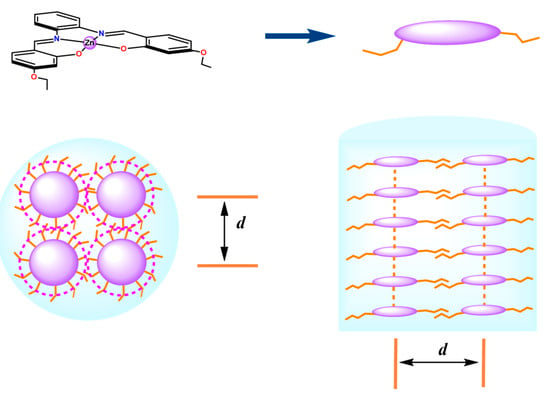
Figure 13.
Sketch of nanostructures by intermolecular Zn⋯O interactions for alkoxy-substituted Zn(salphen) complexes. Cross-sectional and axial representation of nanostructures in a columnar square (left) or a lamellar (right) structure. The distance, d, can be related to the spacing derived from the XRD patterns. Adapted from Ref. [95].
An analogous aggregation mode has also recently been utilized to explain the formation of even more interesting nanosized rings starting from a symmetrical dinuclear Zn(salphen) complex functionalized with phenyl groups [101].
Responsive Properties
Responsive nanomaterials are currently being extensively investigated for many applications. Those arising from Zn(salen)-type complexes, due to their interesting photophysical properties, represent emerging materials for sensing and optoelectronic applications [11,102].
The nanostructured self-assembled materials obtained from a Zn(salmal) complex having 4,4′-alkoxy substituents in the salicylidene rings exhibit marked vapochromic and chemiresistive characteristics when exposed to vapors of volatile Lewis bases [103,104]. The chemisorption of a volatile Lewis base, e.g., an aliphatic amine with the formation of a 1:1 adduct, is responsible for these properties. Moreover, the process is reversible after successive cycles of chemisorption and thermal desorption (Figure 14). Thus, the Lewis acidic character of these complexes is also reflected in their solid state properties. The same behavior was observed for the nanofibrillar molecular material composed of the Zn(salphen) complex derivative having 4,4′-decycloxy substituents as lateral groups [105]. Moreover, a Zn(salphen) derivative functionalized with L-valine residues, self-assembled into helical nanofibers, shows a gelator behavior in acetonitrile, responsive to the presence of anions [106].
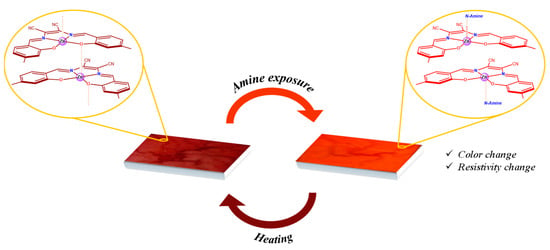
Figure 14.
Sketch of vapochromic and chemiresistive characteristics of the Zn(salmal) complex having 4,4′-alkoxy substituents in the salicylidene rings upon chemisorption/desorption of a volatile aliphatic amine.
In other recent investigations, using benzothiadiazole-bridging and phthalonitrile-bridging Zn(salphen) derivatives, a multi-responsive behavior of these nanostructured materials has been found, with mechanochromic characteristics, in addition to the vapochromic properties [107,108].
3. Zn(II) Complexes of Other Tetradentate Ligands
The aggregation properties of Zn(salen)-type complexes reviewed in the previous section have mainly been related to their Lewis acidic character, as a consequence of the structure of the tetradentate Schiff-base ligand which constrains the Zn(II) atom in a pseudo-planar coordination. Therefore, it is expected that Zn(II) complexes of other tetradentate ligands may also possess such a Lewis acidic character [11].
An interesting case is represented by Zn(salmal)-like complexes (15, Figure 15), from tetradentate Schiff base-like ligands which are derivatives of an alkyl acetoacetate with the 1,2-diaminomaleonitrile, recently developed by Weber et al. [109,110]. In fact, these complexes crystallize in a square-pyramidal coordination, having methanol, pyridine, or water axially penta-coordinated with the Zn(II) atom. Moreover, while these complexes are not emissive in chloroform, because of their aggregation, a green fluorescence can be switched on in the presence of strong Lewis bases. Encapsulation of a non-fluorescent Zn(salmal)-like complex into micelles derived from a family of non-fluorescent polystyrene-block-poly(4-vinylpyridine) diblock copolymers shows a turn-on emission leading to brightly emissive materials, whose quantum yields tend to increase with the amount of anchoring sites in the micelle cores [110].
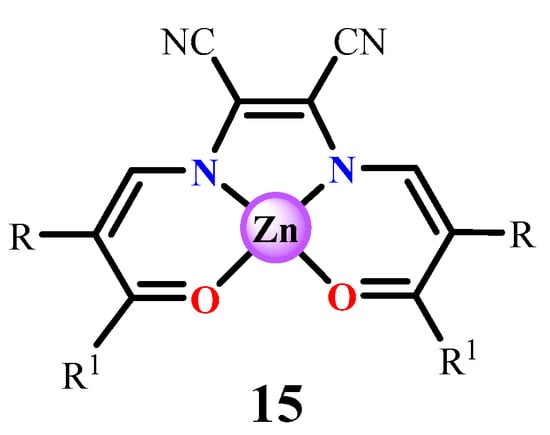
Figure 15.
Zn(salmal)-like complexes investigated by Weber et al. [109,110].
Some aspects of the chemistry of Zn(II) porphyrins can be related to the Lewis acidic character of the Zn(II) center. For example, in the presence of suitable Lewis bases, a series of Zn(porphyrinato) complexes crystallize with the base axially penta-coordinated with the Zn(II) center, leading to monomeric species [111]. Moreover, spectrophotometric titrations of Zn(DPP) (DPP2− = 2, 3, 5, 7, 8, 10, 12, 13, 15, 17, 18, 20-dodecaphenylporphyrinato anion) in chloroform solution, upon addition of various Lewis bases, indicate the axial coordination of the base, whose strength of binding is regulated not only by the Lewis basicity but also by the distortion of the porphyrinato ligand, which regulate the Lewis acidity of the metal center [111]. In general, the supramolecular chemistry of neutral Zn(II) porphyrins is largely related to axial coordination, leading to a variety of supramolecular architectures [112].
Stabilization by axial coordination of a Lewis base has been involved in Zn(II) cyclen complexes (cyclen = 1, 4, 7, 10-tetraazacyclododecane) as a consequence of their Lewis acidic character [113,114].
Other families of Zn(II) complexes of tetradentate (N2O2; N2S2; N4) ligands, whose properties can be viewed within this framework, could be identified and discussed. However, a comprehensive discussion would be beyond the scope of this contribution.
4. Conclusions
This contribution is mainly focused on the ability of Zn(salen)-type complexes to aggregate as a consequence of their Lewis acidic character, leading to the formation of a variety of responsive supramolecular structures, either in solution and in the solid state.
The Lewis acidic properties of these complexes can be ascribed to the structure of the tetradentate Schiff-base ligand, which constrains the metal center in a pseudo-planar coordination. Stabilization is almost always achieved through an axial coordination of a Lewis base, leading to a penta-coordinated square-pyramidal geometry around the metal center. The penta-coordination can be attained in different ways, depending on the absence or presence of Lewis bases. In the first case, stabilization is obtained by intermolecular Zn···O interactions, involving the phenolic oxygen atoms of the ligand framework. Mutual interactions lead to the formation of dimers; otherwise, larger oligomeric aggregates with the formation of self-assembled nanostructures are found. By contrast, in the presence of Lewis bases, in relation to their mono- or polytopic nature, monomeric adducts or supramolecular structures are formed. A further aggregation mode can be achieved by appropriate donor substituents on the ligand framework, leading to a variety of supramolecular architectures. Exceptions to the penta-coordination mode, with the occurrence of a tetrahedral coordination, are encountered in relation to the chemical structure/stereochemistry of the non-conjugated 1,2-diimine bridge.
The axial ligands in these supramolecular structures can easily be displaced, either in solution or in the solid state, with suitable Lewis bases. This process is accompanied by relevant changes to their optical properties. Therefore, they behave as responsive supramolecular structures useful for sensing.
Finally, examples of other families of Zn(II) complexes of tetradentate ligands, whose properties can be related to the Lewis acidic character of the Zn(II) center, are briefly commented upon. This represents the first attempt described in the literature to relate some common features of the chemistry of different families of Zn(II) complexes of tetradentate ligands to their intrinsic Lewis acidic character.
The knowledge of the Lewis acidic characteristics of these Zn(II) complexes is relevant not only for the design of new responsive supramolecular architectures, e.g., selective and sensitive chemosensors of specific analytes, for health, environmental, and biological applications, but also for the development of more efficient catalysts and new molecular devices.
Author Contributions
Both authors contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the University of Catania, PIACERI 2020/2022, Linee di Intervento 2 e 3.
Data Availability Statement
The data used to support the findings of this research are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; Wiley: Chichester, UK, 2009; ISBN 978-0-470-51233-3. [Google Scholar]
- Ariga, K.; Kunitake, T. Supramolecular Chemistry—Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-3-540-01298-6. [Google Scholar]
- Beer, P.D.; Barendt, T.A.; Lim, J.Y.C. Supramolecular Chemistry: Fundamentals and Applications, 2nd ed.; Oxford University Press: Oxford, UK, 2022; ISBN 978-0-19-883284-3. [Google Scholar]
- Kolesnichenko, I.V.; Anslyn, E.V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 2385–2390. [Google Scholar] [CrossRef]
- Ragazzon, G.; Baroncini, M.; Ceroni, P.; Credi, A.; Venturi, M. Electrochemically Controlled Supramolecular Switches and Machines. In Comprehensive Supramolecular Chemistry II, 2nd ed.; Atwood, J.L., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 343–368. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Kleij, A.W. New Templating Strategies with Salen Scaffolds (Salen=N,N’-Bis(salicylidene)ethylenediamine Dianion). Chem. Eur. J. 2008, 14, 10520–10529. [Google Scholar] [CrossRef]
- Kleij, A.W. Zinc-centred salen complexes: Versatile and accessible supramolecular building motifs. Dalton Trans. 2009, 4635–4639. [Google Scholar] [CrossRef] [PubMed]
- Leoni, L.; Dalla Cort, A. The Supramolecular Attitude of Metal–Salophen and Metal–Salen Complexes. Inorganics 2018, 6, 42. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. On the Aggregation and Sensing Properties of Zinc(II) Schiff–Base Complexes of Salen–Type Ligands. Molecules 2019, 24, 2514. [Google Scholar] [CrossRef]
- Di Bella, S. Lewis acidic zinc(II) salen-type Schiff-base complexes: Sensing properties and responsive nanostructures. Dalton Trans. 2021, 50, 6050–6063. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.T.L.; MacLachlan, M.J. Supramolecular Assembly and Coordination-Assisted Deaggregation of Multimetallic Macrocycles. Angew. Chem. Int. Ed. 2005, 44, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Oliveri, I.P.; Consiglio, G.; Failla, S.; Di Bella, S. On the Lewis acidic character of bis(salicylaldiminato)zinc(II) Schiff-base complexes: A computational and experimental investigation on a series of compounds varying the bridging diimine. Dalton Trans. 2017, 46, 4571–4581. [Google Scholar] [CrossRef]
- Lamine, W.; Boughdiri, S.; Christ, L.; Morell, C.; Chermette, H. Coordination Chemistry of Zn2+ With Sal(ph)en Ligands: Tetrahedral Coordination or Penta-Coordination? A DFT Analysis. J. Comput. Chem. 2019, 40, 717–725. [Google Scholar] [CrossRef]
- Odoko, M.; Tsuchida, N.; Okabe, N. Bis{µ-2,2′-[ethane-1,3-diylbis(nitrilomethylidyne)]diphenolato}dizinc(II). Acta Cryst. 2006, E62, m708–m709. [Google Scholar] [CrossRef]
- Reglinski, J.; Morris, S.; Stevenson, D.A. Supporting conformational change at metal centres. Part 2: Four and five coordinate geometry. Polyhedron 2002, 21, 2175–2182. [Google Scholar] [CrossRef]
- Hall, D.; Moore, F.H. The Crystal Structure of NN’-Disalicylidene-ethylenediaminezinc(II) Mono hydrate. J. Chem. Soc. A 1966, 1822–1824. [Google Scholar] [CrossRef]
- Matalobos, J.S.; García-Deibe, A.M.; Fondo, M.; Navarro, D.; Bermejo, M.R. A di-μ-phenoxo bridged zinc dimer with unfamiliar spatial arrangement. Inorg. Chem. Comm. 2004, 7, 311–314. [Google Scholar] [CrossRef]
- Mizukami, S.; Houjou, H.; Nagawa, Y.; Kanesato, M. First helical zinc(II) complex with a salen ligand. Chem. Commun. 2003, 1148–1149. [Google Scholar] [CrossRef]
- Enamullah, M.; Vasylyeva, V.; Janiak, C. Chirality and diastereoselection of Δ/Λ-configured tetrahedral zinc(II) complexes with enantiopure or racemic Schiff base ligands. Inorg. Chim. Acta 2013, 408, 109–119. [Google Scholar] [CrossRef]
- Chamayou, A.-C.; Lüdeke, S.; Brecht, V.; Freedman, T.B.; Nafie, L.A.; Janiak, C. Chirality and Diastereoselection of Δ/Λ-Configured Tetrahedral Zinc Complexes through Enantiopure Schiff Base Complexes: Combined Vibrational Circular Dichroism, Density Functional Theory, 1H NMR, and X-ray Structural Studies. Inorg. Chem. 2011, 50, 11363–11374. [Google Scholar] [CrossRef]
- Onodera, T.; Akitsu, T. Tuning of the optical properties of chiral Schiff base Zn(II) complexes by substituents. Polyhedron 2013, 59, 107–114. [Google Scholar] [CrossRef]
- Evans, C.; Luneau, D. New Schiff base zinc(II) complexes exhibiting second harmonic generation. Dalton Trans. 2002, 83–86. [Google Scholar] [CrossRef]
- Malik, P.; Jain, I. Synthesis and characterization of a double helical dinuclear Zn–salen complex and its application in the detection of nitroaromatics. New J. Chem. 2022, 46, 15296–15300. [Google Scholar] [CrossRef]
- Singer, A.L.; Atwood, D.A. Five-coordinate Salen(tBu) complexes of zinc. Inorg. Chim. Acta 1998, 277, 157–162. [Google Scholar] [CrossRef]
- Germain, M.E.; Vargo, T.R.; Khalifah, P.G.; Knapp, M.J. Fluorescent Detection of Nitroaromatics and 2,3-Dimethyl- 2,3-dinitrobutane (DMNB) by a Zinc Complex: (salophen)Zn. Inorg. Chem. 2007, 46, 4422–4429. [Google Scholar] [CrossRef]
- Kleij, A.W.; Kuil, M.; Lutz, M.; Tooke, D.M.; Spek, A.L.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Supramolecular zinc(II)salphen motifs: Reversible dimerization and templated dimeric structures. Inorg. Chim. Acta 2006, 359, 1807–1814. [Google Scholar] [CrossRef]
- Song, J.-B.; Wang, P.; Yan, L.; Hao, L.; Khan, M.A.; Liu, G.-L.; Li, H. Crystal structures, red-shifted luminescence and iodide-anion recognition properties of four novel D–A type Zn(ii) complexes. Dalton Trans. 2020, 49, 4358–4368. [Google Scholar] [CrossRef]
- Martínez Belmonte, M.; Wezenberg, S.J.; Haak, R.M.; Anselmo, D.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Self-Assembly of Zn(Salphen) Complexes: Steric Regulation, Stability Studies and Crystallographic Analysis Revealing an Unexpected Dimeric 3,3′-t-Bu-Substituted Zn(Salphen) Complex. Dalton Trans. 2010, 39, 4541–4550. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Maccarrone, G.; Di Bella, S. A Lewis Basicity Scale in Dichloromethane for Amines and Common Nonprotogenic Solvents Using a Zinc(II) Schiff-Base Complex as Reference Lewis Acid. J. Org. Chem. 2011, 76, 8879–8884. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.A.; Zhou, H.; Stern, C.L.; Nguyen, S.T. A General High-Yield Route to Bis(salicylaldimine) Zinc(II) Complexes: Application to the Synthesis of Pyridine-Modified Salen-Type Zinc(II) Complexes. Inorg. Chem. 2001, 40, 3222–3227. [Google Scholar] [CrossRef]
- Ouari, K.; Ourari, A.; Weiss, J. Synthesis and Characterization of a Novel Unsymmetrical Tetradentate Schiff Base Complex of Zinc(II) Derived from N,N′-bis(5-Bromosalicylidene) 2,3-Diaminopyridine (H2L): Crystal Structure of [Zn(II)L] Pyridine. J. Chem. Crystallogr. 2010, 40, 831–836. [Google Scholar] [CrossRef]
- Salassa, G.; Ryan, J.W.; Escudero-Adána, E.C.; Kleij, A.W. Spectroscopic properties of Zn(salphenazine) complexes and their application in small molecule organic solar cells. Dalton Trans. 2014, 43, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, V.; Oberhauser, W.; Pucci, A. Synthesis of new red photoluminescent Zn(II)-salicylaldiminato complex. Inorg. Chem. Commun. 2010, 13, 686–688. [Google Scholar] [CrossRef]
- Meng, Q.; Zhou, P.; Song, F.; Wang, Y.; Liu, G.; Li, H. Controlled Fluorescent Properties of Zn(II) Salen-Type Complex Based on Ligand Design. CrystEngComm 2013, 15, 2786–2790. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Punzo, F.; Thompson, A.L.; Di Bella, S.; Failla, S. Structure and Aggregation Properties of a Schiff-Base Zinc(II) Complex Derived from cis-1,2-Diaminocyclohexane. Dalton Trans. 2015, 44, 13040–13048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Q.; Proni, G. One-Pot Diastereoselective Assembly of Helicates Based on a Chiral Salen Scaffold. Inorg. Chem. Commun. 2014, 40, 47–50. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Salassa, G.; Kleij, A.W. Recent advances with π-conjugated salen systems. Chem. Soc. Rev. 2012, 41, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.M.; Storr, T. The chemistry and applications of multimetallic salen complexes. Dalton Trans. 2014, 43, 9380–9391. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Supramolecular Adsorption of Alkaloids by Metallosalphen Complexes. Inorg. Chem. 2008, 47, 4256–4263. [Google Scholar] [CrossRef]
- Salassa, G.; Castilla, A.M.; Kleij, A.J. Cooperative self-assembly of a macrocyclic Schiff base complex. Dalton Trans. 2011, 40, 5236–5243. [Google Scholar] [CrossRef]
- Escárcega-Bobadilla, M.V.; Anselmo, D.; Wezenberg, S.J.; Escudero-Adán, E.C.; Martínez Belmonte, M.; Martina, E.; Kleij, A.W. Metal-directed assembly of chiral bis-Zn(II) Schiff base structures. Dalton Trans. 2012, 41, 9766–9772. [Google Scholar] [CrossRef]
- Ullmann, S.; Börner, M.; Kahnt, A.; Abel, B.; Kersting, B. Green-Emissive Zn2+ Complex Supported by a Macrocyclic Schiff-Base/Calix[4]arene-Ligand: Crystallographic and Spectroscopic Characterization. Eur. J. Inorg. Chem. 2021, 2021, 3691–3698. [Google Scholar] [CrossRef]
- Crane, A.K.; MacLachlan, M.J. Portraits of Porosity: Porous Structures Based on Metal Salen Complexes. Eur. J. Inorg. Chem. 2012, 2012, 17–30. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Kleij, A.W. Material Applications for Salen Frameworks. Angew. Chem. Int. Ed. 2008, 47, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Kleij, A.W.; Kuil, M.; Tooke, D.M.; Lutz, M.; Spek, A.L.; Reek, J.N.H. ZnII-Salphen Complexes as Versatile Building Blocks for the Construction of Supramolecular Box Assemblies. Chem. Eur. J. 2005, 11, 4743–4750. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Martínez Belmonte, M.; Escudero-Adán, E.C.; Kleij, A.W. Exploring the Building-Block Potential of Readily Accessible Chiral [Zn(salen)] Complexes. Eur. J. Inorg. Chem. 2014, 2014, 4632–4641. [Google Scholar] [CrossRef]
- Groizard, T.; Kahlal, S.; Dorcet, V.; Roisnel, T.; Bruneau, C.; Halet, J.-F.; Gramage-Doria, R. Nonconventional Supramolecular Self-Assemblies of Zinc(II)-Salphen Building Blocks. Eur. J. Inorg. Chem. 2016, 2016, 5143–5151. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Anion-Templated Formation of Supramolecular Multinuclear Assemblies. Chem. Eur. J. 2009, 15, 5695–5700. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Versatile Approach toward the Self-Assembly of Heteromultimetallic Salen Structures. Inorg. Chem. 2008, 47, 2925–2927. [Google Scholar] [CrossRef]
- Kleij, A.W.; Kuil, M.; Tooke, D.M.; Spek, A.L.; Reek, J.N.H. Metal-Directed Self-Assembly of a ZnII-salpyr Complex into a Supramolecular Vase Structure. Inorg. Chem. 2007, 46, 5829–5831. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Failla, S.; Malandrino, G.; Di Bella, S. New Molecular Architectures by Aggregation of Tailored Zinc(II) Schiff-Base Complexes. New J. Chem. 2011, 35, 2826–2831. [Google Scholar] [CrossRef]
- Kim, W.-S.; Lee, K.Y.; Ryu, E.-H.; Gu, J.-M.; Kim, Y.; Lee, S.J.; Huh, S. Catalytic Transesterifications by a Zn–BisSalen MOF Containing Open Pyridyl Groups Inside 1D Channels. Eur. J. Inorg. Chem. 2013, 2013, 4228–4233. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Controlling the Molecular Aggregation. An Amphiphilic Schiff-Base Zinc(II) Complex as Supramolecular Fluorescent Probe. Dalton Trans. 2009, 10426–10428. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Supramolecular Aggregation/Deaggregation in Amphiphilic Dipolar Schiff-Base Zinc(II) Complexes. Inorg. Chem. 2010, 49, 5134–5142. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Di Bella, S. Aggregation Properties of Bis(Salicylaldiminato)Zinc(II) Schiff-Base Complexes and their Lewis Acidic Character. Dalton Trans. 2012, 41, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Failla, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Di Bella, S. Synthesis, Characterization, Optical Absorption/Fluorescence Spectroscopy, and Second-Order Nonlinear Optical Properties of Aggregate Molecular Architectures of Unsymmetrical Schiff-Base Zinc(II) Complexes. Dalton Trans. 2014, 43, 2168–2175. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Di Bella, S. An Unprecedented Structural Interconversion in Solution of Aggregate Zinc(II) Salen Schiff-Base Complexes. Inorg. Chem. 2012, 51, 8409–8418. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. Supramolecular Aggregates of Defined Stereochemical Scaffolds: Aggregation/Deaggregation in Schiff-Base Zinc(II) Complexes Derived from Enantiopure trans-1,2-Diaminocyclohexane. Inorg. Chem. 2016, 55, 10320–10328. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Forte, G.; Consiglio, G.; Failla, S.; Di Bella, S. Aggregates of Defined Stereochemical Scaffolds: A Study in Solution of a Zinc(II) Schiff Base Complex Derived from the Enantiopure trans-1,2-Cyclopentanediamine. Inorg. Chem. 2017, 56, 14206–14213. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. Supramolecular Aggregation of a New Substituted Bis(salicylaldiminato)zinc(II) Schiff-Base Complex Derived from trans-1,2-Diaminocyclohexane. Inorganics 2018, 6, 8. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Cacciola, S.; Maccarrone, G.; Failla, S.; Di Bella, S. Dinuclear zinc(II) salen-type Schiff-base complexes as molecular tweezers. Dalton Trans. 2020, 49, 5121–5133. [Google Scholar] [CrossRef]
- Dalla Cort, A.; De Bernardin, P.; Forte, G.; Yafteh Mihan, F. Metal–salophen-based receptors for anions. Chem. Soc. Rev. 2010, 39, 3863–3874. [Google Scholar] [CrossRef]
- Yin, H.-Y.; Tang, J.; Zhang, J.-L. Introducing Metallosalens to Biological Studies: The Renaissance of Traditional Coordination Complexes. Eur. J. Inorg. Chem. 2017, 2017, 5085–5093. [Google Scholar] [CrossRef]
- Dalla Cort, A.; Mandolini, L.; Pasquini, C.; Rissanen, K.; Russo, L.; Schiaffino, L. Zinc–salophen complexes as selective receptors for tertiary amines. New J. Chem. 2007, 31, 1633–1638. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Sensitive Fluorescent Detection and Lewis Basicity of Aliphatic Amines. J. Phys. Chem. A 2011, 115, 14325–14330. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Colorimetric Discrimination between Important Alkaloid Nuclei Mediated by a Bis-Salphen Chromophore. Org. Lett. 2008, 10, 3311–3314. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Di Bella, S. Highly Sensitive Fluorescent Probe for Detection of Alkaloids. Tetrahedron 2011, 67, 9446–9449. [Google Scholar] [CrossRef]
- Puglisi, R.; Ballistreri, F.P.; Gangemi, C.M.A.; Toscano, R.M.; Tomaselli, G.A.; Pappalardo, A.; Trusso Sfrazzetto, G. Chiral Zn–salen complexes: A new class of fluorescent receptors for enantiodiscrimination of chiral amines. New J. Chem. 2017, 41, 911–915. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, L.; Bandeira, N.A.G.; Bo, C.; Kleij, A.W. Highly Efficient Chirality Transfer from Diamines Encapsulated within a Self-Assembled Calixarene-Salen Host. Chem. Eur. J. 2015, 21, 7144–7150. [Google Scholar] [CrossRef] [PubMed]
- Escárcega-Bobadilla, M.V.; Salassa, G.; Martínez Belmonte, M.; Escudero-Adán, E.C.; Kleij, A.W. Versatile Switching in Substrate Topicity: Supramolecular Chirality Induction in Di- and Trinuclear Host Complexes. Chem. Eur. J. 2012, 18, 6805–6810. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Lewis basicity of relevant monoanions in a non-protogenic organic solvent using a zinc(II) Schiff-base complex as reference Lewis acid. Dalton Trans. 2017, 46, 11608–11614. [Google Scholar] [CrossRef]
- Cano, M.; Rodríguez, L.; Lima, J.C.; Pina, F.; Dalla Cort, A.; Pasquini, C.; Schiaffino, L. Specific Supramolecular Interactions between Zn2+-Salophen Complexes and Biologically Relevant Anions. Inorg. Chem. 2009, 48, 6229–6235. [Google Scholar] [CrossRef]
- Sabaté, F.; Giannicchi, I.; Acóna, L.; Dalla Cort, A.; Rodríguez, L. Anion selectivity of Zn-salophen receptors: Influence of ligand substituents. Inorg. Chim. Acta 2015, 434, 1–6. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Anselmo, D.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Dimetallic Activation of Dihydrogen Phosphate by Zn(salphen) Chromophores. Eur. J. Inorg. Chem. 2010, 2010, 4611–4616. [Google Scholar] [CrossRef]
- Dalla Cort, A.; Bernardin, P.; Schiaffino, L. A New Water Soluble Zn-salophen Derivative as a Receptor for α-Aminoacids: Unexpected Chiral Discrimination. Chirality 2009, 21, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Jurček, O.; Cametti, M.; Pontini, M.; Kolehmainena, E.; Rissanen, K. A Zinc-Salophen/Bile-Acid Conjugate Receptor Solubilized by CTABr Micelles Binds Phosphate in Water. Org. Biomol. Chem. 2013, 11, 4585–4590. [Google Scholar] [CrossRef]
- Strianese, M.; Milione, S.; Maranzana, A.; Grassi, A.; Pellecchia, C. Selective detection of ATP and ADP in aqueous solution by using a fluorescent zinc receptor. Chem. Commun. 2012, 48, 11419–11421. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Pellecchia, C. Interaction of monohydrogensulfide with a family of fluorescent pyridoxal-based Zn(II) receptors. Dalton Trans. 2018, 47, 17392–17400. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F.; Contal, E.; Wantz, G.; Gigmes, D. Photoluminescence of Zinc Complexes: Easily Tunable Optical Properties by Variation of the Bridge Between the Imido Groups of Schiff Base Ligands. Eur. J. Inorg. Chem. 2014, 2014, 4186–4198. [Google Scholar] [CrossRef]
- Hai, Y.; Chen, J.-J.; Zhao, P.; Lv, H.; Yu, Y.; Xu, P.; Zhang, J.-L. Luminescent zinc salen complexes as single and two-photon fluorescence subcellular imaging probes. Chem. Commun. 2011, 47, 2435–2437. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cai, Y.-B.; Jing, J.; Zhang, J.-L. Unravelling the Correlation Between Metal Induced Aggregation and Cellular Uptake/Subcellular Localization of Znsalen: An Overlooked Rule for Design of Luminescent Metal Probes. Chem. Sci. 2015, 6, 2389–2397. [Google Scholar] [CrossRef]
- Brissos, R.; Ramos, D.; Lima, J.C.; Yafteh Mihan, F.; Borràs, M.; de Lapuente, J.; Dalla Cort, A.; Rodríguez, L. Luminescent Zinc Salophen Derivatives: Cytotoxicity Assessment and Action Mechanism Studies. New J. Chem. 2013, 37, 1046–1055. [Google Scholar] [CrossRef]
- Giannicchi, I.; Brissos, R.; Ramos, D.; de Lapuente, J.; Lima, G.C.; Dalla Cort, A.; Rodríguez, L. Substituent Effects on the Biological Properties of Zn-Salophen Complexes. Inorg. Chem. 2013, 52, 9245–9253. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Guarnieri, D.; Lamberti, M.; Landi, A.; Peluso, A.; Pellecchia, C. Fluorescent salen-type Zn(II) complexes as probes for detecting hydrogen sulfide and its anion: Bioimaging applications. Inorg. Chem. 2020, 59, 15977–15986. [Google Scholar] [CrossRef]
- Munzi, G.; Consiglio, G.; Failla, S.; Di Bella, S. Binding Properties of a Dinuclear Zinc(II) Salen-Type Molecular Tweezer with a Flexible Spacer in the Formation of Lewis Acid-Base Adducts with Diamines. Inorganics 2021, 9, 49. [Google Scholar] [CrossRef]
- Munzi, G.; Failla, S.; Di Bella, S. Highly selective and sensitive colorimetric/fluorometric dual mode detection of relevant biogenic amines. Analyst 2021, 146, 2144–2151. [Google Scholar] [CrossRef]
- Ikbal, S.A.; Sakata, Y.; Akine, S. A chiral spirobifluorene-based bis(salen) zinc(II) receptor towards highly enantioselective binding of chiral carboxylates. Dalton Trans. 2021, 50, 4119–4123. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Consiglio, G.; Munzi, G.; Failla, S.; Di Bella, S. Deaggregation properties and transmetalation studies of a zinc(II) salen-type Schiff-base complex. Dalton Trans. 2022, 51, 11859–11867. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Munzi, G.; Di Bella, S. A simple approach based on transmetalation for the selective and sensitive colorimetric/fluorometric detection of copper(II) ions in drinking water. New J. Chem. 2022, 46, 18018–18024. [Google Scholar] [CrossRef]
- Hui, J.K.-H.; Yu, Z.; MacLachlan, M.J. Supramolecular Assembly of Zinc Salphen Complexes: Access to Metal-Containing Gels and Nanofibers. Angew. Chem. Int. Ed. 2007, 46, 7980–7983. [Google Scholar] [CrossRef]
- Hui, J.K.-H.; MacLachlan, M.G. Metal-containing nanofibers via coordination chemistry. Coord. Chem. Rev. 2010, 254, 2363–2390. [Google Scholar] [CrossRef]
- Hui, J.K.-H.; Yu, Z.; Mirfakhrai, T.; MacLachlan, M.J. Supramolecular Assembly of Carbohydrate-Functionalized Salphen−Metal Complexes. Chem. Eur. J. 2009, 15, 13456–13465. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.K.-H.; MacLachlan, M.J. Fibrous Aggregates from Dinuclear Zinc(II) Salphen Complexes. Dalton Trans. 2010, 39, 7310–7319. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Failla, S.; Malandrino, G.; Di Bella, S. Controlling the Molecular Self-Assembly into Nanofibers of Amphiphilic Zinc(II) Salophen Complexes. J. Phys. Chem. C 2013, 117, 15335–15341. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Malandrino, G.; Di Bella, S. Self-Assembled Nanostructures of Amphiphilic Zinc(II) Salophen Complexes: Role of The Solvent on their Structure and Morphology. Dalton Trans. 2014, 43, 10208–10214. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mondal, P.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Zinc(II)-salphen complexes bearing long alkoxy side arms: Synthesis, solvent dependent aggregation, and spacer group substituent effect on mesomorphism and photophysical property. J. Mol. Liq. 2017, 246, 290–301. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mondal, P.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Induction of Mesomorphism through Supramolecular Assembly in Metal Coordination Compounds of “salphen”-Type Schiff Bases: Photoluminescence and Solvatochromism. Eur. J. Inorg. Chem. 2016, 2016, 4604–4614. [Google Scholar] [CrossRef]
- Bhattacharjee, C.R.; Das, G.; Mondal, P.; Prasad, S.K.; Rao, D.S.S. Novel Green Light Emitting Nondiscoid Liquid Crystalline Zinc(II) Schiff-Base Complexes. Eur. J. Inorg. Chem. 2011, 2011, 1418–1424. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bhattacharjee, C.R.; Mondal, P.; Prasad, S.K.; Rao, D.S.S. Synthesis and aggregation behaviour of luminescent mesomorphic zinc(II) complexes with ‘salen’ type asymmetric Schiff base ligands. Dalton Trans. 2015, 44, 7477–7488. [Google Scholar] [CrossRef]
- Pyrlin, S.V.; Hine, N.D.M.; Kleij, A.W.; Ramos, M.M.D. Self-assembly of bis-salphen compounds: From semiflexible chains to webs of nanorings. Soft Matter 2018, 14, 1181–1194. [Google Scholar] [CrossRef]
- Di Bella, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D. Zinc(II) as a versatile template for efficient dipolar and octupolar second-order nonlinear optical molecular materials. Inorganics 2018, 6, 133. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Malandrino, G.; Di Bella, S. Phase Transition and Vapochromism in Molecular Assemblies of a Polymorphic Zinc(II) Schiff-Base Complex. Inorg. Chem. 2014, 53, 9771–9777. [Google Scholar] [CrossRef]
- Mirabella, S.; Oliveri, I.P.; Ruffino, F.; Maccarrone, G.; Di Bella, S. Low-cost chemiresistive sensor for volatile amines based on a 2D network of a zinc(II) Schiff-base complex. Appl. Phys. Lett. 2016, 109, 143108. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Malandrino, G.; Mirabella, S.; Di Bella, S. Vapochromic and chemiresistive characteristics of a nanostructured molecular material composed of a zinc(II)-salophen complex. Dalton Trans. 2018, 47, 15977–15982. [Google Scholar] [CrossRef]
- Piccinno, M.; Angulo-Pachón, C.A.; Ballester, P.; Escuder, B.; Dalla Cort, A. Rational Design of a Supramolecular Gel Based on a Zn(II)-salophen Bis-dipeptide Derivative. RSC Adv. 2016, 6, 57306–57309. [Google Scholar] [CrossRef]
- Song, X.; Yu, H.; Yan, X.; Zhang, Y.; Miao, Y.; Ye, K.; Wang, Y. A luminescent benzothiadiazole-bridging bis(salicylaldiminato)zinc(II) complex with mechanochromic and organogelation properties. Dalton Trans. 2018, 47, 6146–6155. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Song, X.; Mu, X.; Wang, Y. Mechanochromic luminescence based on a phthalonitrile-bridging salophen zinc(II) complex. New J. Chem. 2019, 43, 15886–15891. [Google Scholar] [CrossRef]
- Kurz, H.; Hörner, G.; Weser, O.; Li Manni, G.; Weber, B. Quenched Lewis Acidity: Studies on the Medium Dependent Fluorescence of Zinc(II) Complexes. Chem. Eur. J. 2021, 27, 15159–15171. [Google Scholar] [CrossRef] [PubMed]
- Kurz, H.; Hils, C.; Timm, J.; Hörner, G.; Greiner, A.; Marschall, R.; Schmalz, H.; Weber, B. Self-Assembled Fluorescent Block Copolymer Micelles with Responsive Emission. Angew. Chem. Int. Ed. 2022, 61, e202117570. [Google Scholar] [CrossRef]
- Kojima, T.; Nakanishi, T.; Honda, T.; Harada, R.; Shiro, M.; Fukuzumi, S. Impact of Distortion of Porphyrins on Axial Coordination in (Porphyrinato)zinc(II) Complexes with Aminopyridines as Axial Ligands. Eur. J. Inorg. Chem. 2009, 2009, 727–734. [Google Scholar] [CrossRef]
- KC, C.B.; D’Souza, F. Design and photochemical study of supramolecular donor–acceptor systems assembled via metal–ligand axial coordination. Coord. Chem. Rev. 2016, 322, 104–141. [Google Scholar] [CrossRef]
- Salter, M.H.; Reibenspies, J.H.; Jones, D.B.; Hancock, R.D. Lewis Acid Properties of Zinc(II) in Its Cyclen Complex. The Structure of [Zn(Cyclen)(S=C(NH2)2](ClO4)2 and the Bonding of Thiourea to Metal Ions. Some Implications for Zinc Metalloenzymes. Inorg. Chem. 2005, 44, 2791–2797. [Google Scholar] [CrossRef]
- Linder, D.P.; Vinson, B.; Parks, R.; Crisp, A.; McAdoo, A.G.; Ebel, J.P.; Hoang, T.; Smith, H.; Oliver, A.G.; Hubin, T.J. Zinc-based cyclens containing pyridine and cross-bridges: X-ray and DFT structures, Lewis acidity, gas-phase acidity, and pKa values. Polyhedron 2022, 223, 115941. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).