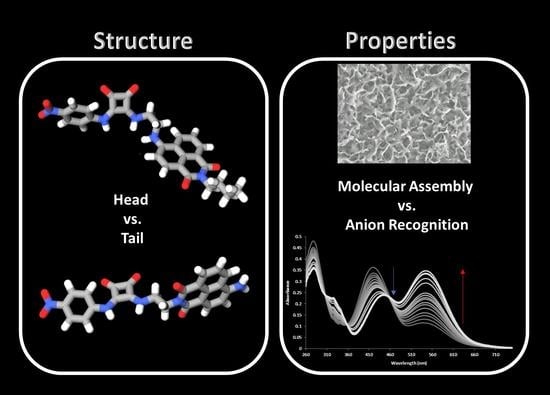
Head vs. Tail Squaramide–Naphthalimide Conjugates: Self-Assembly and Anion Binding Behaviour
Abstract
:1. Introduction
2. Materials and Methods
General Experimental Methods
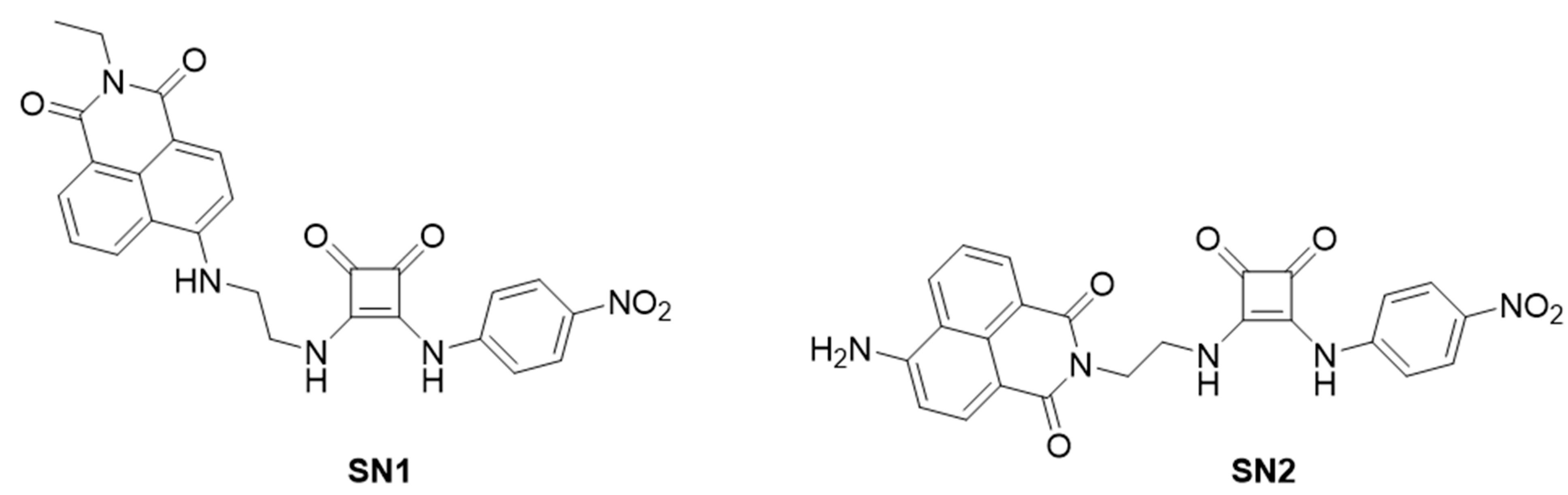
- 6-((2-Aminoethyl)amino)-2-ethyl-1H-benzo[de]isoquinoline-1,3(2H)-dione (2)
- 3-Ethoxy-4-((4-nitrophenyl)amino)cyclobut-3-ene-1,2-dione (3)
- 2-Ethyl-6-((2-((2-((4-nitrophenyl)amino)-3,4-dioxocyclobut-1-en-1l)amino)ethyl)amino)-1H-benzo[de]isoquinoline-1,3(2H)-dione (SN1)
- Tert-butyl(2-(6-nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl)carbamate (5)
- 2-(2-Aminoethyl)-6-nitro-1H-benzo[de]isoquinoline-1,3(2H)-dione (6)
- 6-Amino-2-(2-aminoethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (7)
- 6-Amino-2-(2-((2-((4-nitrophenyl)amino)-3,4-dioxocyclobut-1-en-1-yl)amino)ethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (SN2)
3. Results and Discussion
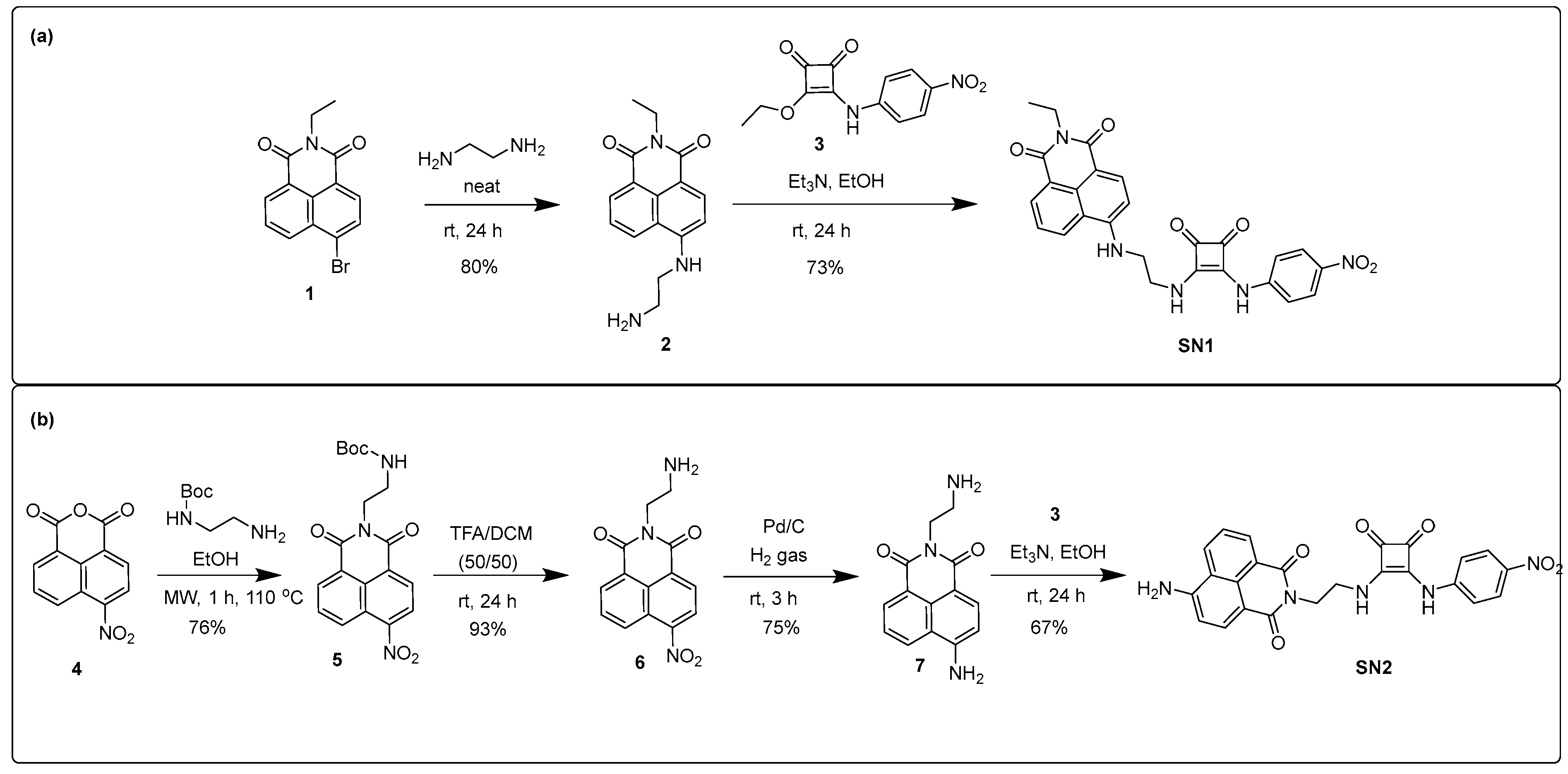
3.1. Synthesis
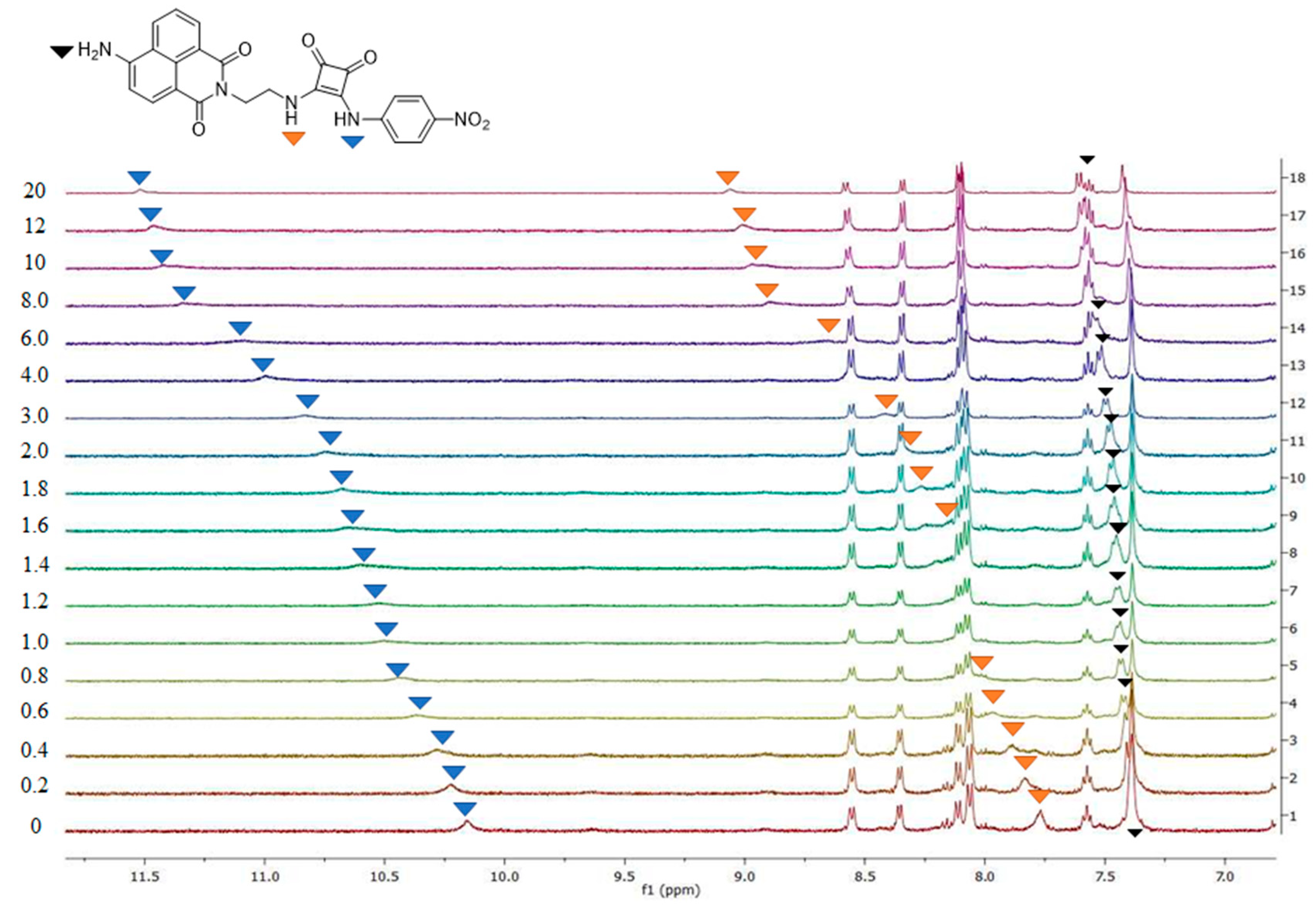
3.2. Variable-Temperature NMR and SEM Analysis
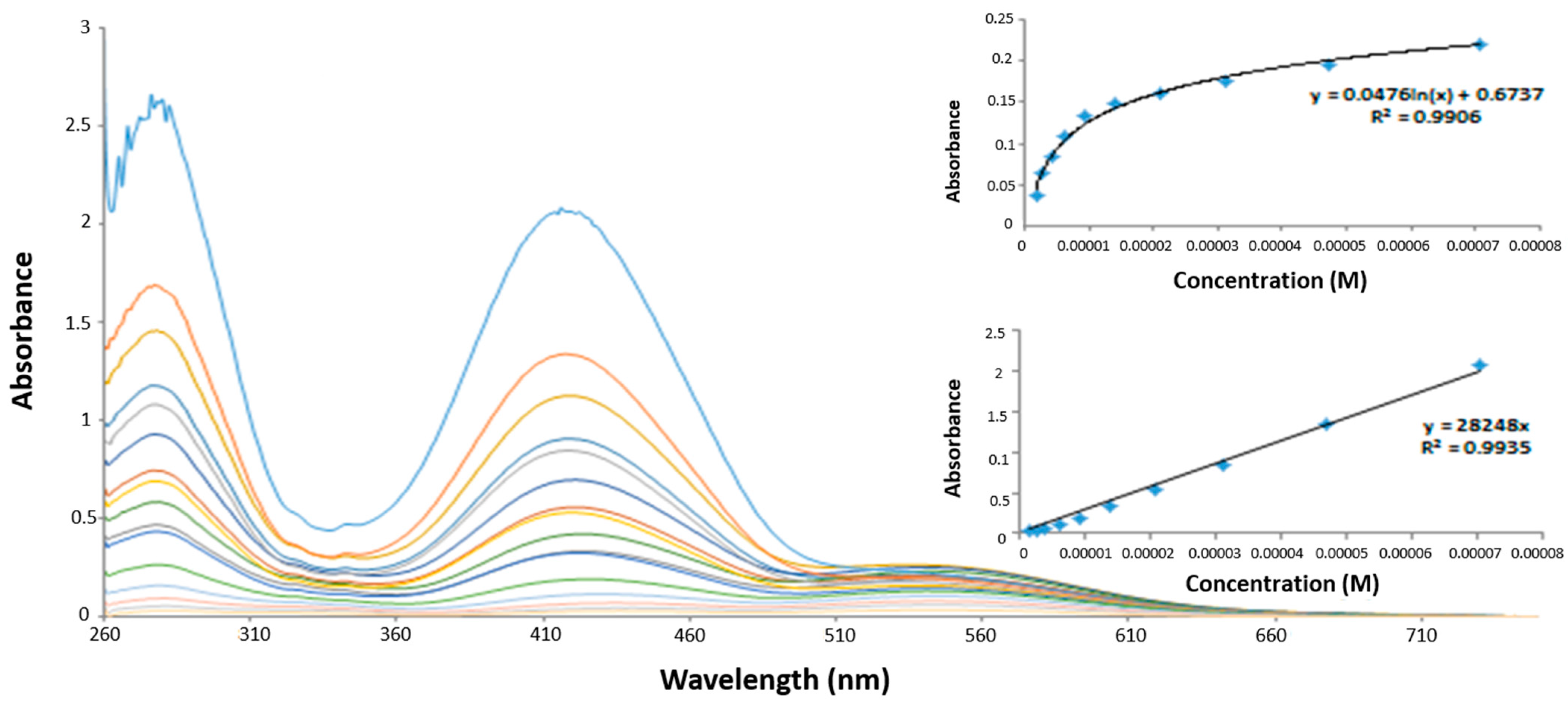
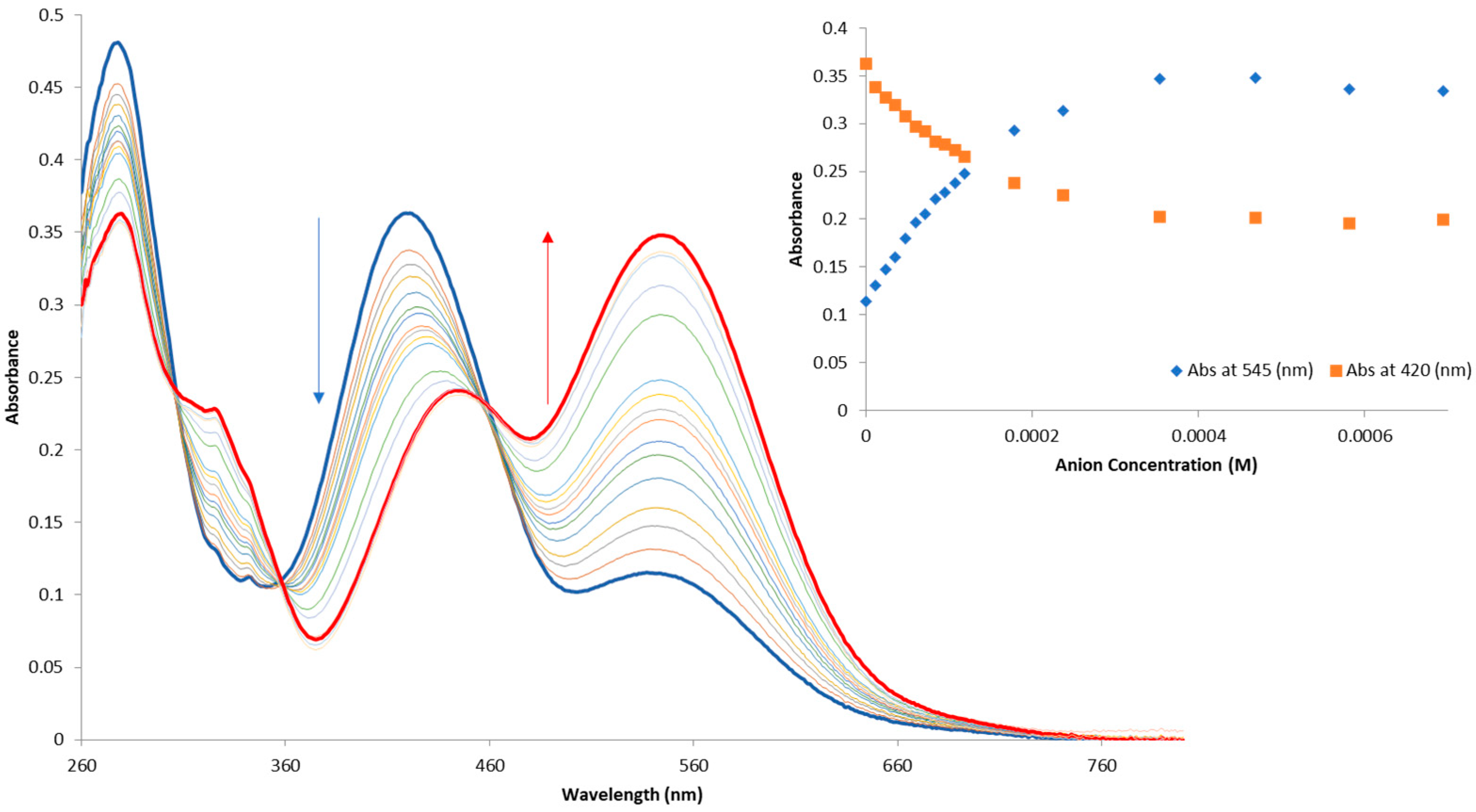
3.3. Anion Recognition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marchetti, L.A.; Kumawat, L.K.; Mao, N.; Stephens, J.C.; Elmes, R.B.P. The Versatility of Squaramides: From Supramolecular Chemistry to Chemical Biology. Chem 2019, 5, 1398–1485. [Google Scholar] [CrossRef]
- Ian Storer, R.; Aciro, C.; Jones, L.H. Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef]
- Saez Talens, V.; Englebienne, P.; Trinh, T.T.; Noteborn, W.E.M.; Voets, I.K.; Kieltyka, R.E. Aromatic Gain in a Supramolecular Polymer. Angew. Chem. Int. Ed. 2015, 54, 10502–10506. [Google Scholar] [CrossRef] [Green Version]
- Saez Talens, V.; Davis, J.; Wu, C.-H.; Wen, Z.; Lauria, F.; Gupta, K.B.S.S.; Rudge, R.; Boraghi, M.; Hagemeijer, A.; Trinh, T.T.; et al. Thiosquaramide-Based Supramolecular Polymers: Aromaticity Gain in a Switched Mode of Self-Assembly. J. Am. Chem. Soc. 2020, 142, 19907–19916. [Google Scholar] [CrossRef]
- Rostami, A.; Colin, A.; Li, X.Y.; Chudzinski, M.G.; Lough, A.J.; Taylor, M.S. N,N′-Diarylsquaramides: General, High-Yielding Synthesis and Applications in Colorimetric Anion Sensing. J. Org. Chem. 2010, 75, 3983–3992. [Google Scholar] [CrossRef]
- Edwards, S.J.; Valkenier, H.; Busschaert, N.; Gale, P.A.; Davis, A.P. High-Affinity Anion Binding by Steroidal Squaramide Receptors. Angew. Chem. Int. Ed. 2015, 54, 4592–4596. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Zhang, M.; Deng, C.; Guan, Y.; Gong, J.; Zhu, D.; Pan, Y.; Jiang, J.; Wang, L. Novel calix[4]arene-based receptors with bis-squaramide moieties for colorimetric sensing of anions via two different interaction modes. Tet. Lett. 2013, 54, 796–801. [Google Scholar] [CrossRef]
- Jagleniec, D.; Siennicka, S.; Dobrzycki, Ł.; Karbarz, M.; Romański, J. Recognition and Extraction of Sodium Chloride by a Squaramide-Based Ion Pair Receptor. Inorg. Chem. 2018, 57, 12941–12952. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Milani, M. The Squaramide versus Urea Contest for Anion Recognition. Chem. Eur. J. 2010, 16, 4368–4380. [Google Scholar] [CrossRef]
- Busschaert, N.; Kirby, I.L.; Young, S.; Coles, S.J.; Horton, P.N.; Light, M.E.; Gale, P.A. Squaramides as Potent Transmembrane Anion Transporters. Angew. Chem. Int. Ed. 2012, 51, 4426–4430. [Google Scholar] [CrossRef]
- Picci, G.; Kubicki, M.; Garau, A.; Lippolis, V.; Mocci, R.; Porcheddu, A.; Quesada, R.; Ricci, P.C.; Scorciapino, M.A.; Caltagirone, C. Simple squaramide receptors for highly efficient anion binding in aqueous media and transmembrane transport. Chem. Commun. 2020, 56, 11066–11069. [Google Scholar] [CrossRef]
- Singh, A.; Torres-Huerta, A.; Vanderlinden, T.; Renier, N.; Martínez-Crespo, L.; Tumanov, N.; Wouters, J.; Bartik, K.; Jabin, I.; Valkenier, H. Calix[6]arenes with halogen bond donor groups as selective and efficient anion transporters. Chem. Commun. 2022, 58, 6255–6258. [Google Scholar] [CrossRef]
- Marchetti, L.A.; Kramer, T.; Elmes, R.B.P. Amidosquaramides-a new anion binding motif with pH sensitive anion transport properties. Org. Biomol. Chem. 2022, 20, 7056–7066. [Google Scholar] [CrossRef]
- Ko, S.-K.; Kim, S.K.; Share, A.; Lynch, V.M.; Park, J.; Namkung, W.; Van Rossom, W.; Busschaert, N.; Gale, P.A.; Sessler, J.L.; et al. Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells. Nat. Chem. 2014, 6, 885–892. [Google Scholar] [CrossRef]
- Busschaert, N.; Park, S.-H.; Baek, K.-H.; Choi, Y.P.; Park, J.; Howe, E.N.W.; Hiscock, J.R.; Karagiannidis, L.E.; Marques, I.; Félix, V.; et al. A synthetic ion transporter that disrupts autophagy and induces apoptosis by perturbing cellular chloride concentrations. Nat. Chem. 2017, 9, 667–675. [Google Scholar] [CrossRef]
- Duke, R.M.; Veale, E.B.; Pfeffer, F.M.; Kruger, P.E.; Gunnlaugsson, T. Colorimetric and fluorescent anion sensors: An overview of recent developments in the use of 1,8-naphthalimide-based chemosensors. Chem. Soc. Rev. 2010, 39, 3936–3953. [Google Scholar] [CrossRef] [Green Version]
- Gunnlaugsson, T.; Davis, A.P.; Glynn, M. Fluorescent photoinduced electron transfer (PET) sensing of anions using charge neutral chemosensors. Chem. Commun. 2001, 2556–2557. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Davis, A.P.; O’Brien, J.E.; Glynn, M. Synthesis and photophysical evaluation of charge neutral thiourea or urea based fluorescent PET sensors for bis-carboxylates and pyrophosphate. Org. Biomol. Chem. 2005, 3, 48–56. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Kruger, P.E.; Jensen, P.; Tierney, J.; Ali, H.D.P.; Hussey, G.M. Colorimetric "naked eye" sensing of anions in aqueous solution. J. Org. Chem. 2005, 70, 10875–10878. [Google Scholar] [CrossRef]
- Pfeffer, F.M.; Buschgens, A.M.; Barnett, N.W.; Gunnlaugsson, T.; Kruger, P.E. 4-Amino-1,8-naphthalimide-based anion receptors: Employing the naphthalimide N-H moiety in the cooperative binding of dihydrogenphosphate. Tet. Lett. 2005, 46, 6579–6584. [Google Scholar] [CrossRef]
- Duke, R.M.; Gunnlaugsson, T. Selective fluorescent PET sensing of fluoride (F-)using naphthalimide-thiourea and -urea conjugates. Tet. Lett. 2007, 48, 8043–8047. [Google Scholar] [CrossRef]
- Parkesh, R.; Lee, T.C.; Gunnlaugsson, T. Highly selective 4-amino-1,8-naphthalimide based fluorescent photoinduced electron transfer (PET) chemosensors for Zn(II) under physiological pH conditions. Org. Biomol. Chem. 2007, 5, 310–317. [Google Scholar] [CrossRef]
- Ali, H.D.P.; Kruger, P.E.; Gunnlaugsson, T. Colorimetric ‘naked-eye’ and fluorescent sensors for anions based on amidourea functionalised 1,8-naphthalimide structures: Anion recognition via either deprotonation or hydrogen bonding in DMSO. New J. Chem. 2008, 32, 1153–1161. [Google Scholar] [CrossRef]
- Elmes, R.B.P.; Turner, P.; Jolliffe, K.A. Colorimetric and Luminescent Sensors for Chloride: Hydrogen Bonding vs Deprotonation. Org. Lett. 2013, 15, 5638–5641. [Google Scholar] [CrossRef] [Green Version]
- Busschaert, N.; Elmes, R.B.P.; Czech, D.D.; Wu, X.; Kirby, I.L.; Peck, E.M.; Hendzel, K.D.; Shaw, S.K.; Chan, B.; Smith, B.D.; et al. Thiosquaramides: pH switchable anion transporters. Chem. Sci. 2014, 5, 3617–3626. [Google Scholar] [CrossRef]
- Elmes, R.B.P.; Jolliffe, K.A. Amino acid-based squaramides for anion recognition. Supramol. Chem. 2014, 27, 321–328. [Google Scholar] [CrossRef]
- Elmes, R.B.P.; Yuen, K. K.Y. ; Jolliffe, K.A. Sulfate-Selective Recognition by Using Neutral Dipeptide Anion Receptors in Aqueous Solution. Chem. Eur. J. 2014, 20, 7373–7380. [Google Scholar] [CrossRef]
- Elmes, R.B.P.; Busschaert, N.; Czech, D.D.; Gale, P.A.; Jolliffe, K.A. pH switchable anion transport by an oxothiosquaramide. Chem. Commun. 2015, 51, 10107–10110. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Hartley, A.; Turner, P.; Elmes, R.B.P.; Jolliffe, K.A. Macrocyclic squaramides: Anion receptors with high sulfate binding affinity and selectivity in aqueous media. Chem. Sci. 2016, 7, 4563–4572. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, L.A.; Mao, N.; Krämer, T.; Kitchen, J.A.; Elmes, R.B.P. A long wavelength colourimetric chemosensor for fluoride. Supramol. Chem. 2018, 30, 795–805. [Google Scholar] [CrossRef]
- Masilamani, G.; Batchu, H.; Amsallem, D.; Bedi, A. Novel Series of Diaminoanthraquinone-Based π-Extendable Building Blocks with Tunable Optoelectronic Properties. ACS Omega 2022, 7, 25874–25880. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, L.K.; Abogunrin, A.A.; Kickham, M.; Pardeshi, J.; Fenelon, O.; Schroeder, M.; Elmes, R.B.P. Squaramide—Naphthalimide Conjugates as “Turn-On” Fluorescent Sensors for Bromide Through an Aggregation-Disaggregation Approach. Front. Chem. 2019, 7, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Tomooka, C.S.; Moore, H.W. An Efficient General Synthesis of Squarate Esters. Synth. Commun. 1997, 27, 2177–2180. [Google Scholar] [CrossRef]
- Roke, D.; Wezenberg, S.J.; Feringa, B.L. Molecular rotary motors: Unidirectional motion around double bonds. Proc. Natl. Acad. Sci. USA 2018, 115, 9423–9431. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Meng, L.; Li, Z.; Mao, Y.; Lan, H.; Chen, L.; Fan, Y.; Yi, T. Large Red-Shifted Fluorescent Emission via Intermolecular π–π Stacking in 4-Ethynyl-1,8-naphthalimide-Based Supramolecular Assemblies. Langmuir 2014, 30, 11753–11760. [Google Scholar] [CrossRef]
- Collado-Fregoso, E.; Zugazagoitia, J.S.; Plaza-Medina, E.F.; Peon, J. Excited-State Dynamics of Nitrated Push−Pull Molecules: The Importance of the Relative Energy of the Singlet and Triplet Manifolds. J. Phys. Chem. A 2009, 113, 13498–13508. [Google Scholar] [CrossRef]
- Adair, L.D.; Trinh, N.; Vérité, P.M.; Jacquemin, D.; Jolliffe, K.A.; New, E.J. Synthesis of Nitro-Aryl Functionalised 4-Amino-1,8-Naphthalimides and Their Evaluation as Fluorescent Hypoxia Sensors. Chem. Eur. J. 2020, 26, 10064–10071. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, M.; Wu, L.; Guan, Y.; Pan, Y.; Jiang, J.; Lin, C.; Wang, L. Squaramide-based tripodal receptors for selective recognition of sulfate anion. Chem. Commun. 2013, 49, 2025–2027. [Google Scholar] [CrossRef]
- Prohens, R.; Tomàs, S.; Morey, J.; Deyà, P.M.; Ballester, P.; Costa, A. Squaramido-based receptors: Molecular recognition of carboxylate anions in highly competitive media. Tet. Lett. 1998, 39, 1063–1066. [Google Scholar] [CrossRef]
- Prohens, R.; Martorell, G.; Ballester, P.; Costa, A. A squaramide fluorescent ensemble for monitoring sulfate in water. Chem. Commun. 2001, 1456–1457. [Google Scholar] [CrossRef]
- Piña, M.N.; Soberats, B.; Rotger, C.; Ballester, P.; Deyà, P.M.; Costa, A. Selective sensing of competitive anions by non-selective hosts: The case of sulfate and phosphate in water. New J. Chem. 2008, 32, 1919–1923. [Google Scholar] [CrossRef]
- Delgado-Pinar, E.; Rotger, C.; Costa, A.; Pina, M.N.; Jimenez, H.R.; Alarcon, J.; Garcia-Espana, E. Grafted squaramide monoamine nanoparticles as simple systems for sulfate recognition in pure water. Chem. Commun. 2012, 48, 2609–2611. [Google Scholar] [CrossRef] [PubMed]
- Brynn Hibbert, D.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.J.; Pfeffer, F.M.; Thordarson, P. Determining binding constants from 1H NMR titration data using global and local methods: A case study using [n]polynorbornane-based anion hosts. Supramol. Chem. 2012, 24, 585–594. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abogunrin, A.A.; Healy, S.A.; Fenelon, O.; Elmes, R.B.P. Head vs. Tail Squaramide–Naphthalimide Conjugates: Self-Assembly and Anion Binding Behaviour. Chemistry 2022, 4, 1288-1299. https://doi.org/10.3390/chemistry4040085
Abogunrin AA, Healy SA, Fenelon O, Elmes RBP. Head vs. Tail Squaramide–Naphthalimide Conjugates: Self-Assembly and Anion Binding Behaviour. Chemistry. 2022; 4(4):1288-1299. https://doi.org/10.3390/chemistry4040085
Chicago/Turabian StyleAbogunrin, Anthony A., Stephen A. Healy, Orla Fenelon, and Robert B. P. Elmes. 2022. "Head vs. Tail Squaramide–Naphthalimide Conjugates: Self-Assembly and Anion Binding Behaviour" Chemistry 4, no. 4: 1288-1299. https://doi.org/10.3390/chemistry4040085
APA StyleAbogunrin, A. A., Healy, S. A., Fenelon, O., & Elmes, R. B. P. (2022). Head vs. Tail Squaramide–Naphthalimide Conjugates: Self-Assembly and Anion Binding Behaviour. Chemistry, 4(4), 1288-1299. https://doi.org/10.3390/chemistry4040085