Enhancing NO Uptake in Metal-Organic Frameworks via Linker Functionalization. A Multi-Scale Theoretical Study
Abstract
1. Introduction
2. Methodology
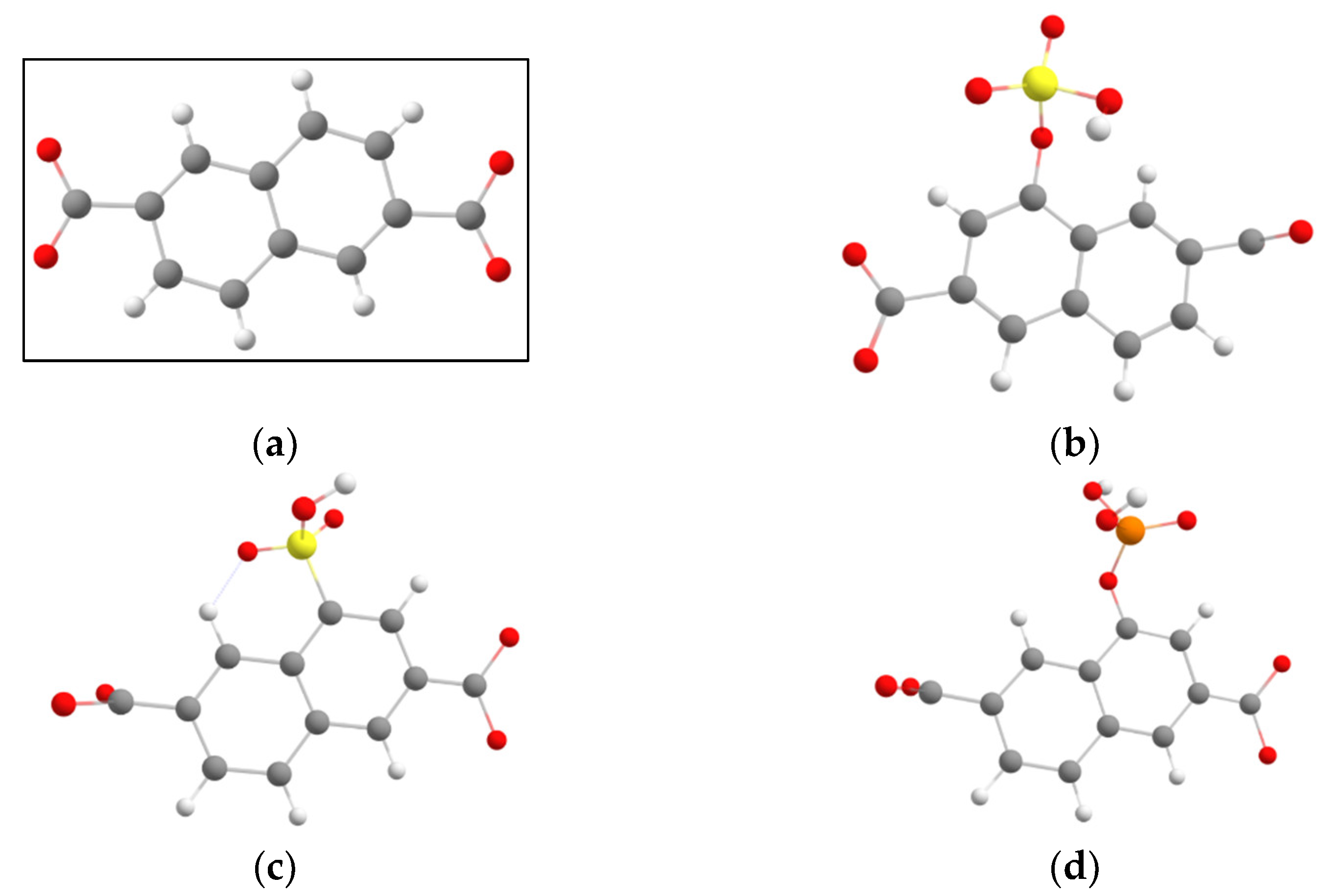
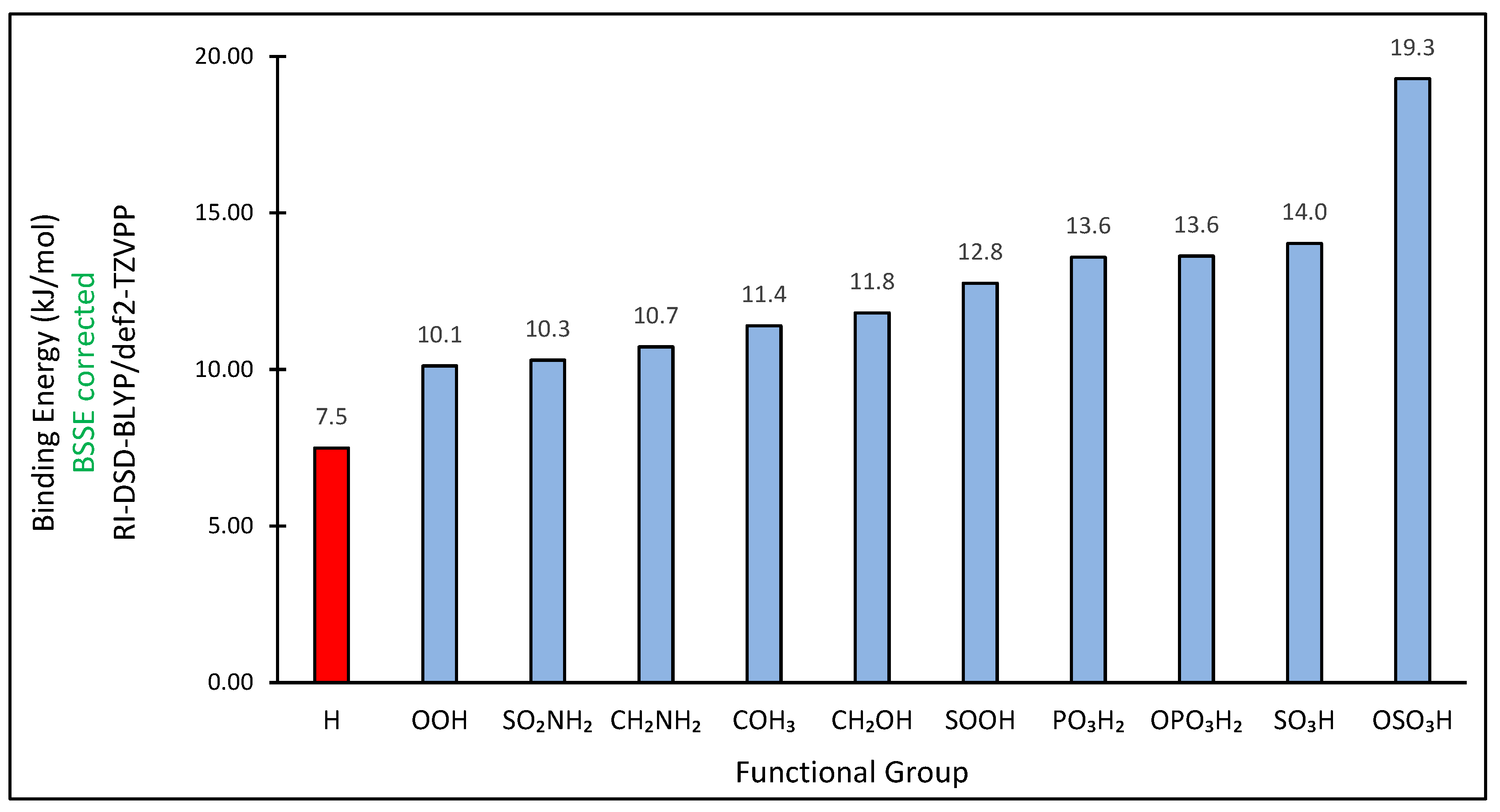
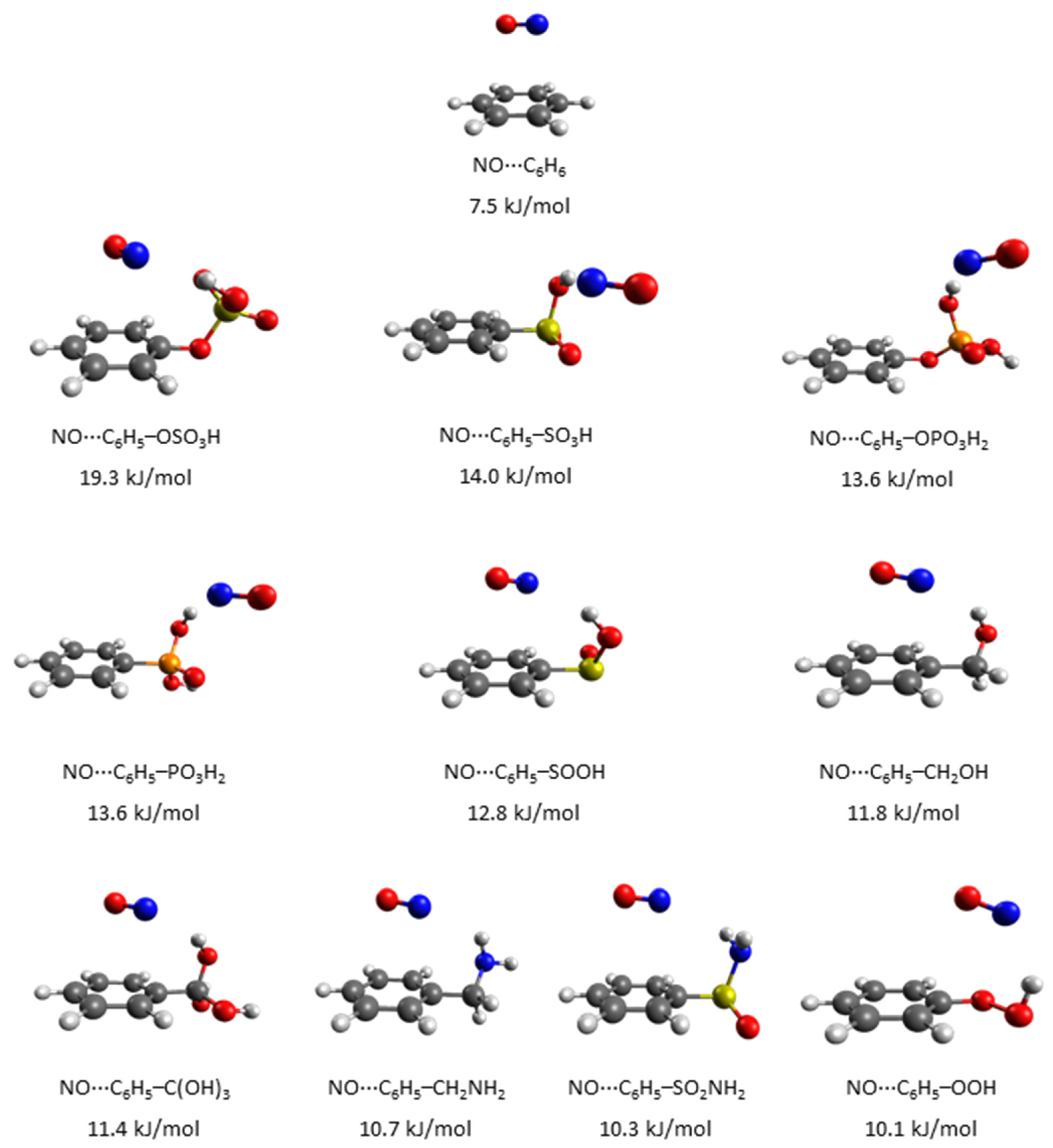
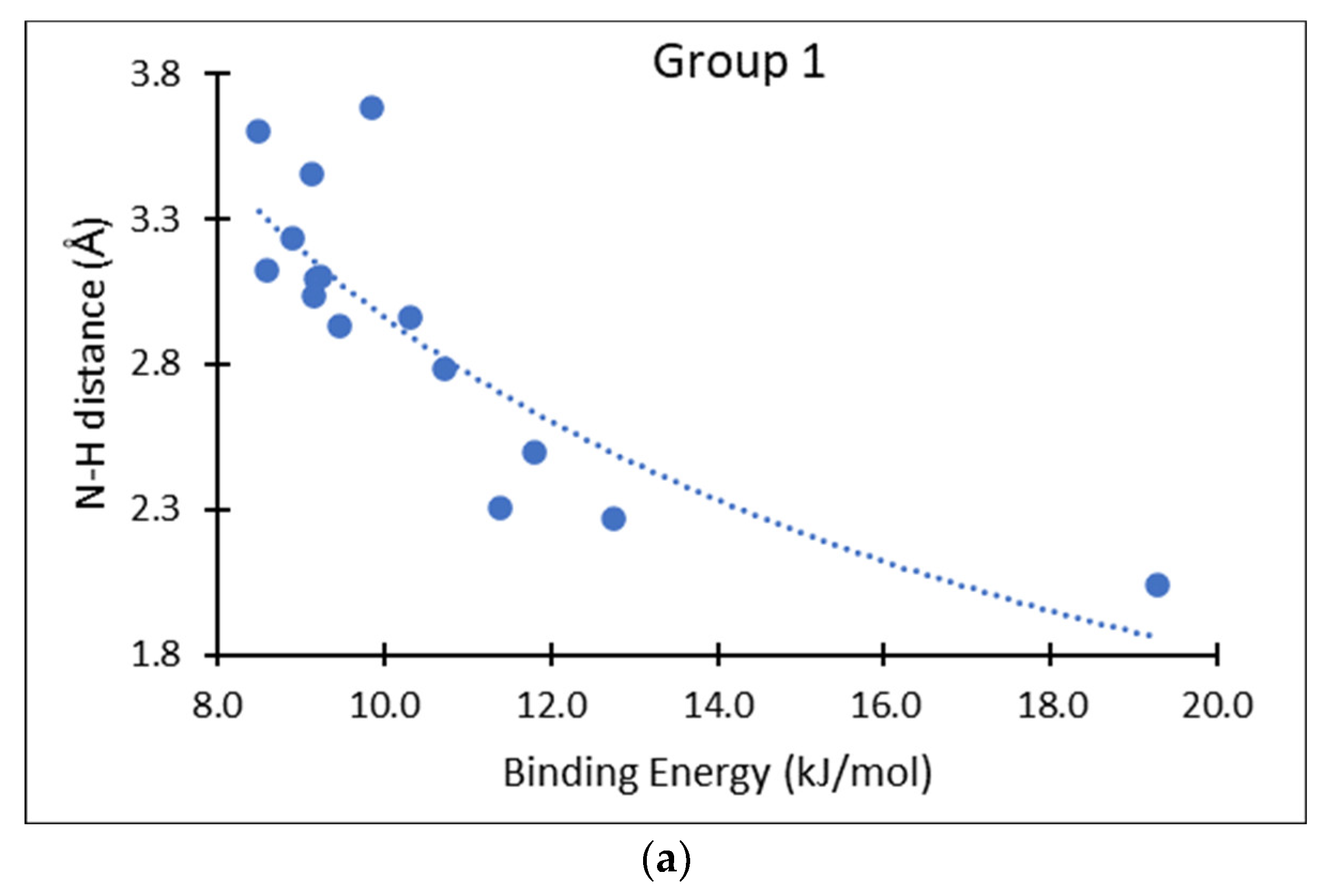
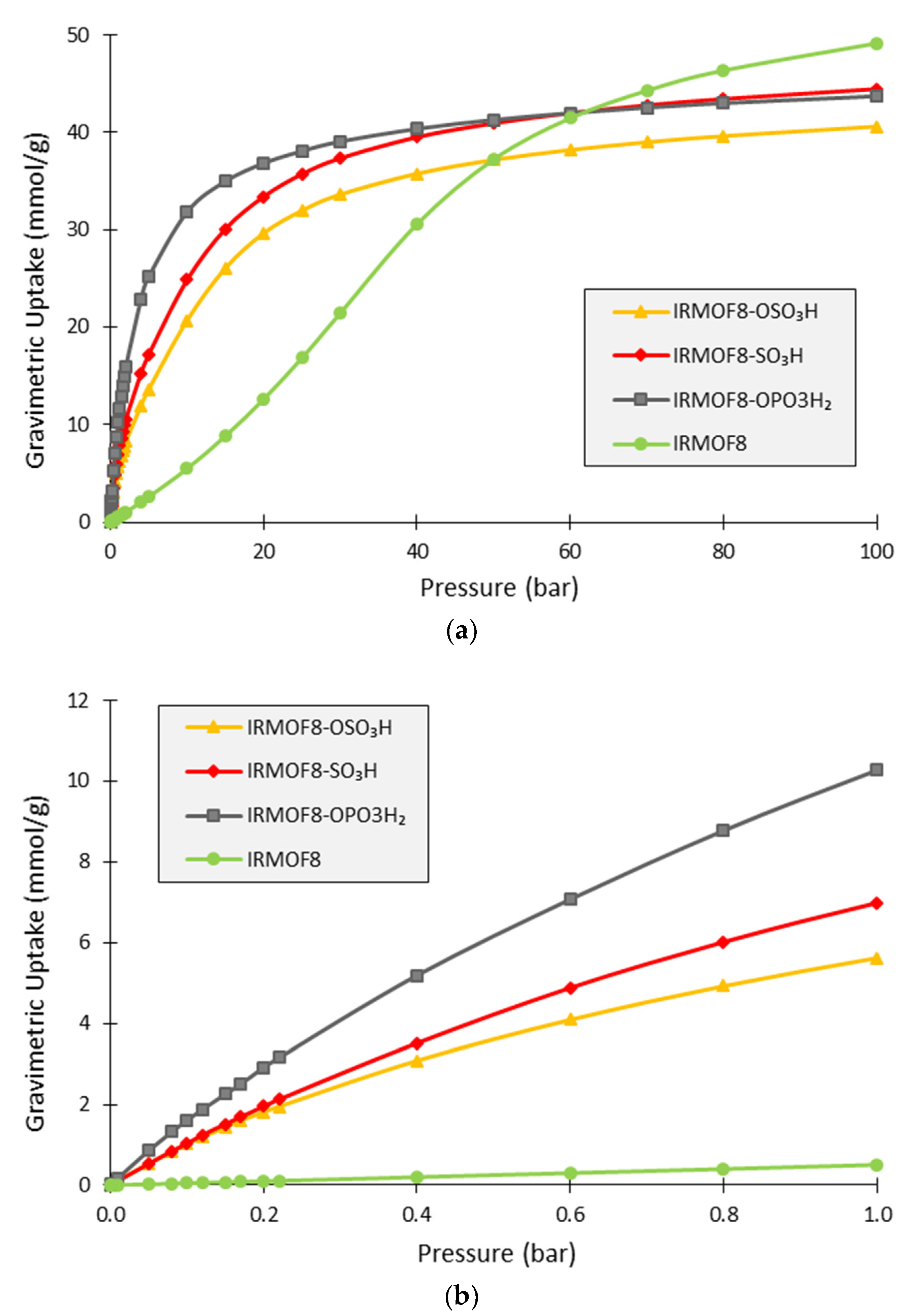
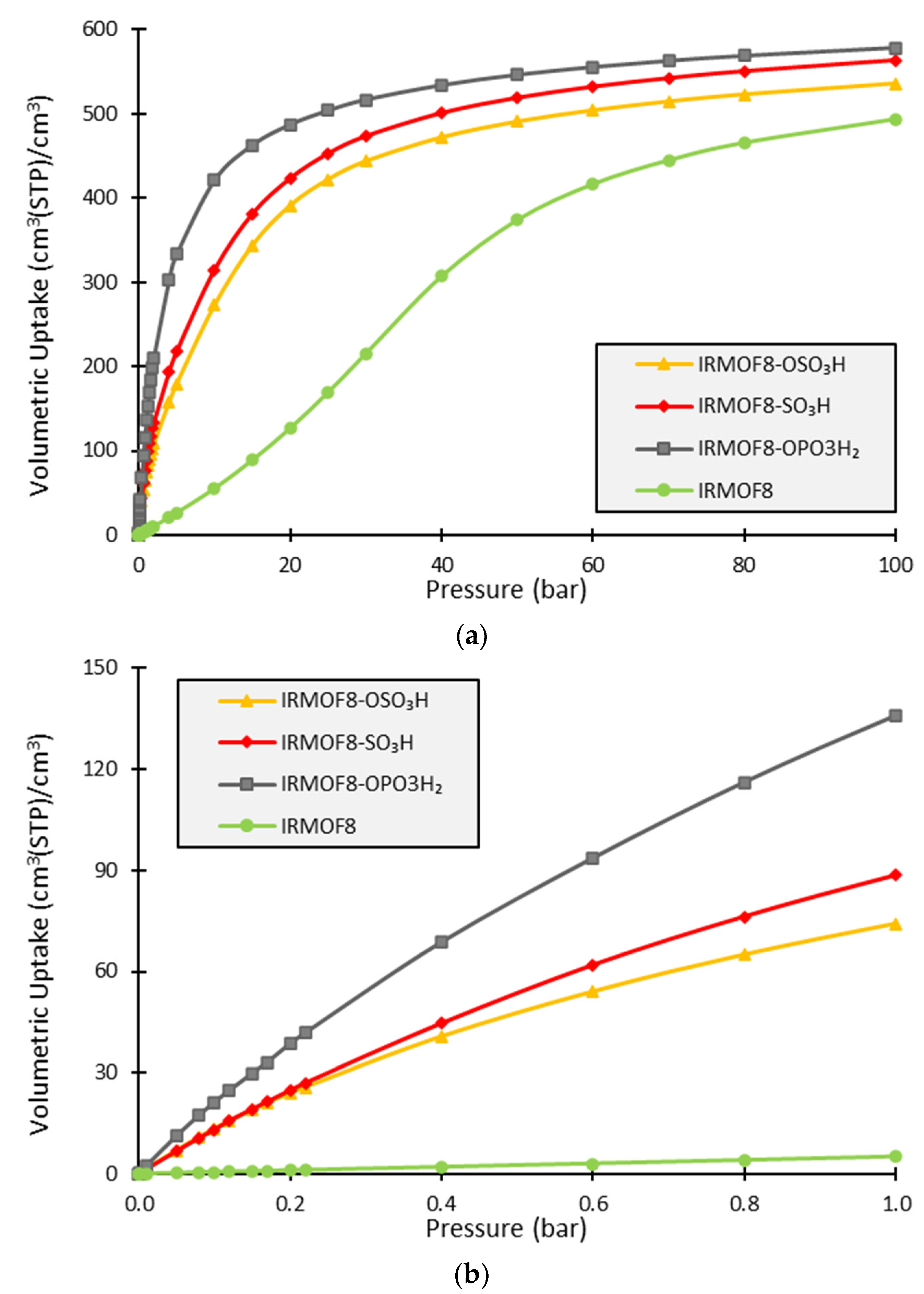
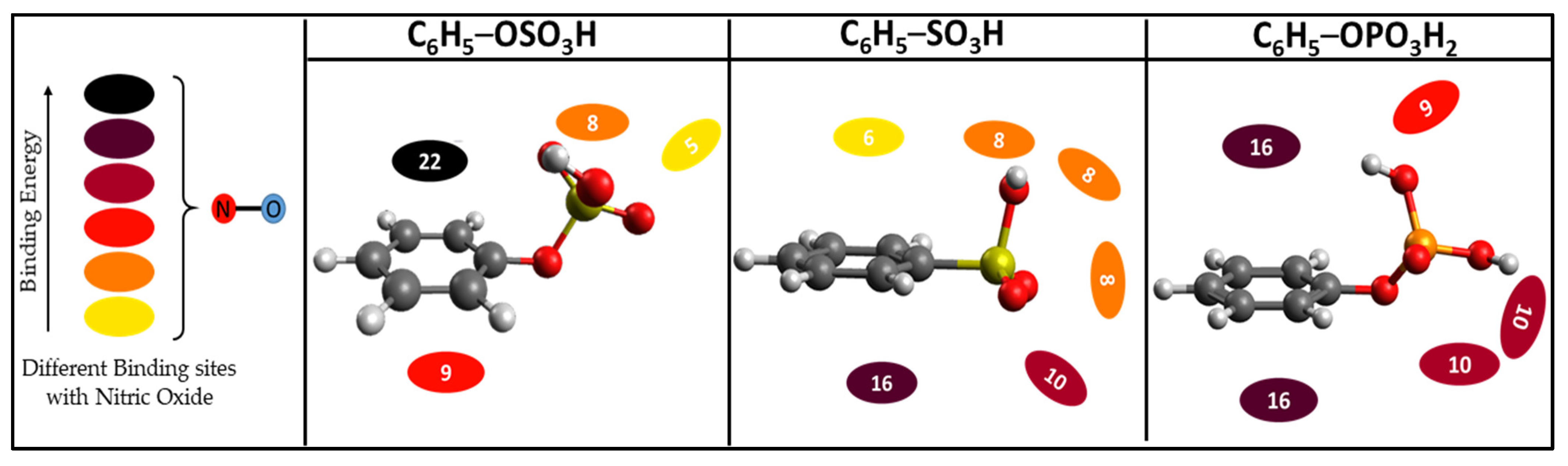
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, S.C.; Douglas Smoot, L. Modeling of nitrogen oxides formation and destruction in combustion systems. Prog. Energy Combust. Sci. 2000, 26, 417–458. [Google Scholar] [CrossRef]
- European Environment Agency. Nitrogen Oxides (NOx) Emissions. Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea-32-nitrogen-oxides-nox-emissions-1/assessment.2010-08-19.0140149032-3 (accessed on 12 July 2022).
- European Environment Agency. Air Pollution Sources. Available online: https://www.eea.europa.eu/themes/air/air-pollution-sources-1 (accessed on 6 June 2022).
- Mannucci, P.M.; Harari, S.; Martinelli, I.; Franchini, M. Effects on health of air pollution: A narrative review. Intern. Emerg. Med. 2015, 10, 657–662. [Google Scholar] [CrossRef]
- Air Pollution. Available online: https://www.who.int/data/gho/data/themes/theme-details/GHO/air-pollution (accessed on 6 June 2022).
- World Bank. The Global Health Cost of PM2.5 Air Pollution: A Case for Action Beyond 2021; World Bank: Washington, DC, USA, 2022; ISBN 978-1-4648-1816-5. [Google Scholar]
- US EPA. What Is Acid Rain? Available online: https://www.epa.gov/acidrain/what-acid-rain (accessed on 6 June 2022).
- US EPA. Effects of Acid Rain. Available online: https://www.epa.gov/acidrain/effects-acid-rain (accessed on 6 June 2022).
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Batten, S.R.; Robson, R. Interpenetrating Nets: Ordered, Periodic Entanglement. Angew. Chem. Int. Ed. Engl. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Férey, G. Some suggested perspectives for multifunctional hybrid porous solids. Dalton Trans. 2009, 23, 4400–4415. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.; Wöll, C. Functionalized Coordination Space in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2008, 47, 8164–8168. [Google Scholar] [CrossRef]
- Kitagawa, S.; Matsuda, R. Chemistry of coordination space of porous coordination polymers. Coord. Chem. Rev. 2007, 251, 2490–2509. [Google Scholar] [CrossRef]
- Kepert, C.J. Advanced functional properties in nanoporous coordination framework materials. Chem. Commun. 2006, 7, 695–700. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal–organic frameworks—Prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Lin, J.; Ho, W.; Qin, X.; Leung, C.; Au, V.K.; Lee, S. Metal–Organic Frameworks for NOx Adsorption and Their Applications in Separation, Sensing, Catalysis, and Biology. Small 2022, 18, 2105484. [Google Scholar] [CrossRef]
- Xiao, B.; Wheatley, P.S.; Zhao, X.; Fletcher, A.J.; Fox, S.; Rossi, A.G.; Megson, I.L.; Bordiga, S.; Regli, L.; Thomas, K.M.; et al. High-Capacity Hydrogen and Nitric Oxide Adsorption and Storage in a Metal−Organic Framework. J. Am. Chem. Soc. 2007, 129, 1203–1209. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Eubank, J.F.; Wuttke, S.; Xiao, B.; Wheatley, P.S.; Bazin, P.; Lavalley, J.-C.; Daturi, M.; Vimont, A.; De Weireld, G.; et al. Nitric Oxide Adsorption and Delivery in Flexible MIL-88(Fe) Metal–Organic Frameworks. Chem. Mater. 2013, 25, 1592–1599. [Google Scholar] [CrossRef]
- Livas, C.G.; Tylianakis, E.; Froudakis, G.E. Enhancing of CO Uptake in Metal–Organic Frameworks by Linker Functionalization: A Multi-Scale Theoretical Study. Chemistry 2022, 4, 43. [Google Scholar] [CrossRef]
- Raptis, D.; Livas, C.; Stavroglou, G.; Giappa, R.M.; Tylianakis, E.; Stergiannakos, T.; Froudakis, G.E. Surface Modification Strategy for Enhanced NO2 Capture in Metal–Organic Frameworks. Molecules 2022, 27, 3448. [Google Scholar] [CrossRef]
- Frysali, M.G.; Klontzas, E.; Tylianakis, E.; Froudakis, G.E. Tuning the interaction strength and the adsorption of CO2 in metal organic frameworks by functionalization of the organic linkers. Microporous Mesoporous Mater. 2016, 227, 144–151. [Google Scholar] [CrossRef]
- Giappa, R.M.; Tylianakis, E.; Di Gennaro, M.; Gkagkas, K.; Froudakis, G.E. A combination of multi-scale calculations with machine learning for investigating hydrogen storage in metal organic frameworks. Int. J. Hydrogen Energy 2021, 46, 27612–27621. [Google Scholar] [CrossRef]
- Stergiannakos, T.; Klontzas, E.; Tylianakis, E.; Froudakis, G.E. Enhancement of CO2 Adsorption in Magnesium Alkoxide IRMOF-10. J. Phys. Chem. C 2015, 119, 22001–22007. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Kozuch, S.; Gruzman, D.; Martin, J.M.L. DSD-BLYP: A General Purpose Double Hybrid Density Functional Including Spin Component Scaling and Dispersion Correction. J. Phys. Chem. C 2010, 114, 20801–20808. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy—Physical Chemistry Chemical Physics (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2005/cp/b508541a (accessed on 7 June 2022).
- Skylaris, C.-K.; Gagliardi, L.; Handy, N.C.; Ioannou, A.G.; Spencer, S.; Willetts, A. On the resolution of identity Coulomb energy approximation in density functional theory. J. Mol. Struct. THEOCHEM 2000, 501–502, 229–239. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Laaksonen, L. A graphics program for the analysis and display of molecular dynamics trajectories. J. Mol. Graph. 1992, 10, 33–34, 24. [Google Scholar] [CrossRef]
- Bergman, D.L.; Laaksonen, L.; Laaksonen, A. Visualization of solvation structures in liquid mixtures. J. Mol. Graph. Model 1997, 15, 301–306, 328–333. [Google Scholar] [CrossRef]
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef]
- Jones, J.E.; Chapman, S. On the determination of molecular fields.—II. From the equation of state of a gas. Proc. R. Soc. Lond. A 1924, 106, 463–477. [Google Scholar] [CrossRef]
- Yang, J.; Ren, Y.; Tian, A.; Sun, H. COMPASS Force Field for 14 Inorganic Molecules, He, Ne, Ar, Kr, Xe, H2, O2, N2, NO, CO, CO2, NO2, CS2, and SO2, in Liquid Phases. J. Phys. Chem. B 2000, 104, 4951–4957. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Lorentz, H.A. Ueber die Anwendung des Satzes vom Virial in der kinetischen Theorie der Gase. Ann. Phys. 1881, 248, 127–136. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livas, C.G.; Tylianakis, E.; Froudakis, G.E. Enhancing NO Uptake in Metal-Organic Frameworks via Linker Functionalization. A Multi-Scale Theoretical Study. Chemistry 2022, 4, 1300-1311. https://doi.org/10.3390/chemistry4040086
Livas CG, Tylianakis E, Froudakis GE. Enhancing NO Uptake in Metal-Organic Frameworks via Linker Functionalization. A Multi-Scale Theoretical Study. Chemistry. 2022; 4(4):1300-1311. https://doi.org/10.3390/chemistry4040086
Chicago/Turabian StyleLivas, Charalampos G., Emmanuel Tylianakis, and George E. Froudakis. 2022. "Enhancing NO Uptake in Metal-Organic Frameworks via Linker Functionalization. A Multi-Scale Theoretical Study" Chemistry 4, no. 4: 1300-1311. https://doi.org/10.3390/chemistry4040086
APA StyleLivas, C. G., Tylianakis, E., & Froudakis, G. E. (2022). Enhancing NO Uptake in Metal-Organic Frameworks via Linker Functionalization. A Multi-Scale Theoretical Study. Chemistry, 4(4), 1300-1311. https://doi.org/10.3390/chemistry4040086






