Crystallization- and Metal-Driven Selection of Discrete Macrocycles/Cages and Their Metallosupramolecular Polymers from Dynamic Systemic Networks
Abstract
- -
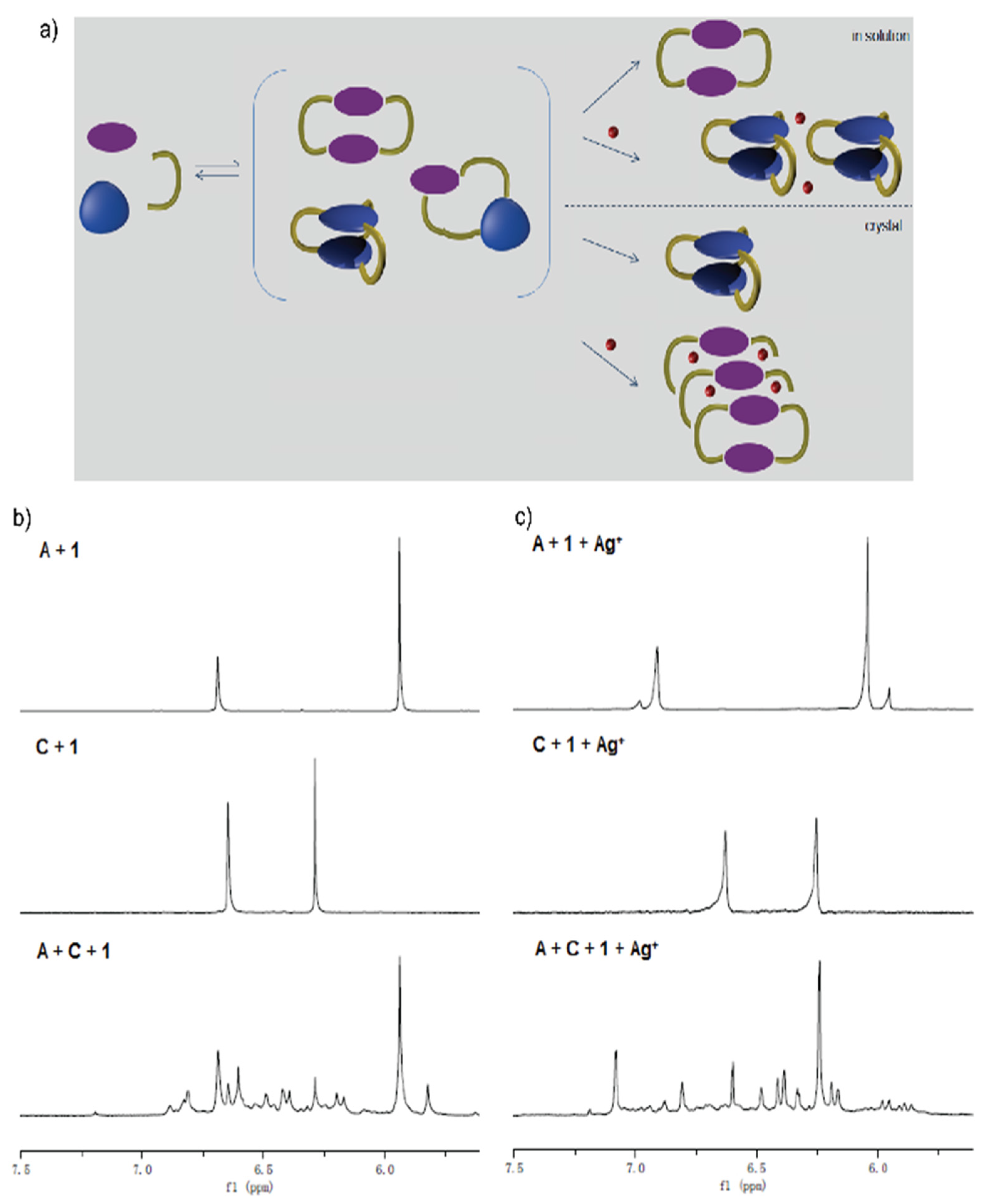
- L1: dialdehyde A with diamines 1 and 2;
- -
- L2: diamine 1 with aldehydes A and C.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehn, J.-M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 2007, 36, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L. (Ed.) Dynamic Combinatorial Chemistry. In Drug Discovery, Bioorganic Chemistry and Materials Science; John Wiley and Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Reek, J.N.H.; Otto, S. (Eds.) Dynamic Combinatorial Chemistry; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Barboiu, M. (Ed.) Constitutional Dynamic Chemistry; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; Volume 322. [Google Scholar]
- Goor, O.J.G.M.; Hendrikse, S.I.S.; Dankers, P.Y.W.; Meijer, E.W. From supramolecular polymers to multicomponent biomaterials. Chem. Soc. Rev. 2017, 46, 6621–6637. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Lehn, J.-M.; Meijer, E.W.; Matyjaszewski, K. From precision polymers to complex materials and systems. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Liu, B.; Thayumanavan, S. Substituent effects on the pH sensitivity of acetals and ketals and their correlation with encapsulation stability in polymeric nanogels. J. Am. Chem. Soc. 2017, 139, 2306–2317. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, G.; Tao, W.; Ma, Y.; Zhang, X.; He, F.; Pan, J.; Mei, L.; Pan, G. A drug-self-gated mesoporous antitumor nanoplatform based on pH-sensitive dynamic covalent bond. Adv. Funct. Mater. 2017, 27, 1605985. [Google Scholar] [CrossRef]
- Zhang, Y.; Barboiu, M. Constitutional Dynamic Materials—Toward Natural Selection of Function. Chem. Rev. 2016, 116, 809–834. [Google Scholar] [CrossRef]
- Kassem, S.; van Leeuwen, T.; Lubbe, A.S.; Wilson, M.R.; Feringa, B.L.; Leigh, D.A. Artificial molecular motors. Chem. Soc. Rev. 2017, 46, 2592–2621. [Google Scholar] [CrossRef]
- Foy, J.T.; Li, Q.; Goujon, A.; Colard-Itté, J.-R.; Fuks, G.; Moulin, E.; Schiffmann, O.; Dattler, D.; Funeriu, D.P.; Giuseppone, N. Dual-light control of nanomachines that integrate motor and modulator subunits. Nat. Nanotechnol. 2017, 12, 540–545. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, L.; Ramström, O. Double parallel dynamic resolution through lipase-catalyzed asymmetric transformation. Chem. Commun. 2013, 49, 1805–1907. [Google Scholar] [CrossRef]
- Zhang, Y.; Vongvilai, P.; Sakulsombat, M.; Fischer, A.; Ramström, O. Asymmetric Synthesis of Substituted Thiolanes through Domino Thia-Michael–Henry Dynamic Covalent Systemic Resolution using Lipase Catalysis. Adv. Synth. Catal. 2014, 356, 987–992. [Google Scholar] [CrossRef]
- Clever, G.H.; Punt, P. Cation–anion arrangement patterns in self-assembled Pd2L4 and Pd4L8 coordination cages. Acc. Chem. Res. 2017, 50, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Fujita, D.; Ueda, Y.; Sato, S.; Mizuno, N.; Kumasaka, T.; Fujita, M. Self-assembly of tetravalent Goldberg polyhedra from 144 small components. Nature 2016, 540, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Danon, J.J.; Krüger, A.; Leigh, D.A.; Lemonnier, J.; Stephens, A.J.; Vitorica-yrezabal, I.J.; Woltering, S.L. Braiding a molecular knot with eight crossings. Science 2017, 355, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Ferrando-Soria, J.; Pineda, E.M.; Tuna, F.; Vitorica-Yrezabal, I.J.; Knappke, C.; Ujma, J.; Muryn, C.A.; Timco, G.A.; Barran, P.E.; et al. Making hybrid [n]-rotaxanes as supramolecular arrays of molecular electron spin qubits. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Wang, X.; Li, Y.; Song, B.; Bolarinwa, O.; Reese, R.A.; Zhang, T.; Wang, X.; Cai, J.; et al. Supersnowflakes: Stepwise self-assembly and dynamic exchange of rhombus star-shaped supramolecules. Am. Chem. Soc. 2017, 139, 8174–8185. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Krzyaniak, M.D.; Owczarek, M.; Ferris, D.P.; Wasielewski, M.R.; Stoddart, J.F. A Boat-Shaped Tetracationic Macrocycle with a Semiconducting Organic Framework. Angew. Chem. 2017, 129, 5889–5894. [Google Scholar] [CrossRef]
- Munkhbat, O.; Garzoni, M.; Raghupathi, K.R.; Pavan, G.M.; Thayumanavan, S. Role of aromatic interactions in temperature-sensitive amphiphilic supramolecular assemblies. Langmuir 2017, 32, 2874–2881. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. [Google Scholar] [CrossRef]
- Greb, L.; Mutlu, H.; Barner-kowollik, C.; Lehn, J. Photo-and metallo-responsive N-alkyl α-bisimines as orthogonally addressable main-chain functional groups in metathesis polymers. J. Am. Chem. Soc. 2016, 138, 1142–1145. [Google Scholar] [CrossRef]
- Ji, Q.; Lirag, R.C.; Miljanic, O.S. Kinetically controlled phenomena in dynamic combinatorial libraries. Chem. Soc. Rev. 2014, 43, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Kovaricek, P.; Lehn, J.-M.; Samorì, P. Dynamic covalent chemistry of bisimines at the solid/liquid interface monitored by scanning tunnelling microscopy. Nat. Chem. 2014, 6, 1017–1023. [Google Scholar]
- Zhang, Y.; Ramström, O. Thiazolidinones Derived from Dynamic Systemic Resolution of Complex Reversible-Reaction Networks. Chem. Eur. J. 2014, 20, 3288–3291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, L.; Ramström, O. Constitutional dynamic chemistry for bioactive compounds. In Supramolecular Systems in Biomedical Fields; Schneider, H.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 397–418. [Google Scholar]
- Dhers, S.; Holub, J.; Lehn, J.-M. Coevolution and ratiometric behaviour in metal cation-driven dynamic covalent systems. Chem. Sci. 2017, 8, 2125–2130. [Google Scholar] [CrossRef]
- Kulchat, S.; Chaur, M.N.; Lehn, J.-M. Kinetic selectivity and thermodynamic features of competitive imine formation in dynamic covalent chemistry. Chem. Eur. J. 2017, 23, 11108–11118. [Google Scholar] [CrossRef]
- Barboiu, M.; Dumitru, F.; Legrand, Y.; Van Der Lee, A. Self-sorting of equilibrating metallosupramolecular DCLs via constitutional crystallization. Chem. Commun. 2009, 16, 2192–2194. [Google Scholar] [CrossRef]
- Angelin, M.; Vongvilai, P.; Fischer, A.; Ramström, O. Crystallization-Driven Asymmetric Synthesis of Pyridine-β-nitroalcohols via Discovery-Oriented Self-Resolution of a Dynamic System. Eur. J. Org. Chem. 2010, 33, 6315–6318. [Google Scholar] [CrossRef]
- Kocsis, I.; Dumitrescu, D.; Legrand, Y.; Van Der Lee, A.; Grosu, I.; Barboiu, M. Self-sorting of dynamic metallosupramolecular libraries (DMLs) via metal-driven selection. Chem. Commun. 2014, 50, 2621–2623. [Google Scholar] [CrossRef]
- Zhang, Y.; Legrand, Y.M.; Van Der Lee, A.; Barboiu, M. Ligand- and Metal-Driven Selection of Flexible Adaptive Dynamic Host Receptors. Eur. J. Org. Chem. 2016, 10, 1825–1828. [Google Scholar] [CrossRef]
- Gilles, A.; Barboiu, M. Highly selective artificial K+ channels: An example of selectivity-induced transmembrane potential. J. Am. Chem. Soc. 2016, 138, 426–432. [Google Scholar] [CrossRef]
- CrysAlisPro. Rikagu Oxford Diffraction; Agilent Technologies Inc.: Oxfordshire, UK, 2012. [Google Scholar]
- Lee, A. Charge flipping for routine structure solution. J. Appl. Crystallogr. 2013, 46, 1306–1315. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP–a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. Crystals suite of programs. J. Appl. Crystallogr. 2003, 36, 1487. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; van der Lee, A.; Barboiu, M. Crystallization- and Metal-Driven Selection of Discrete Macrocycles/Cages and Their Metallosupramolecular Polymers from Dynamic Systemic Networks. Chemistry 2022, 4, 1281-1287. https://doi.org/10.3390/chemistry4040084
Zhang Y, van der Lee A, Barboiu M. Crystallization- and Metal-Driven Selection of Discrete Macrocycles/Cages and Their Metallosupramolecular Polymers from Dynamic Systemic Networks. Chemistry. 2022; 4(4):1281-1287. https://doi.org/10.3390/chemistry4040084
Chicago/Turabian StyleZhang, Yan, Arie van der Lee, and Mihail Barboiu. 2022. "Crystallization- and Metal-Driven Selection of Discrete Macrocycles/Cages and Their Metallosupramolecular Polymers from Dynamic Systemic Networks" Chemistry 4, no. 4: 1281-1287. https://doi.org/10.3390/chemistry4040084
APA StyleZhang, Y., van der Lee, A., & Barboiu, M. (2022). Crystallization- and Metal-Driven Selection of Discrete Macrocycles/Cages and Their Metallosupramolecular Polymers from Dynamic Systemic Networks. Chemistry, 4(4), 1281-1287. https://doi.org/10.3390/chemistry4040084





