Application of Elicitors at Two Maturation Stages of Vitis vinifera L. cv Monastrell: Changes in Skin Cell Walls
Abstract
:1. Introduction
2. Materials and Methods
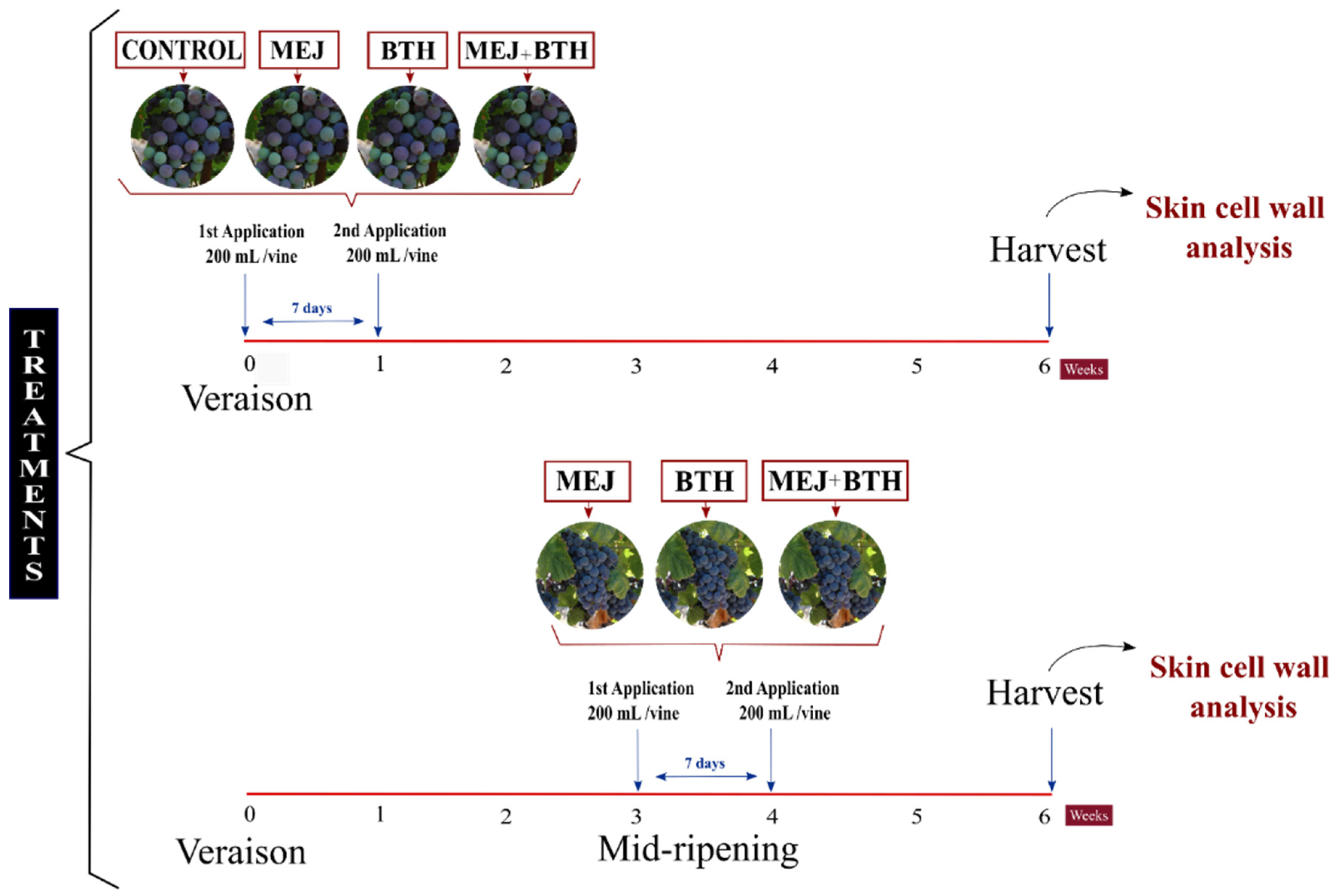
2.1. Experimental Design
2.2. Reagents and Standards
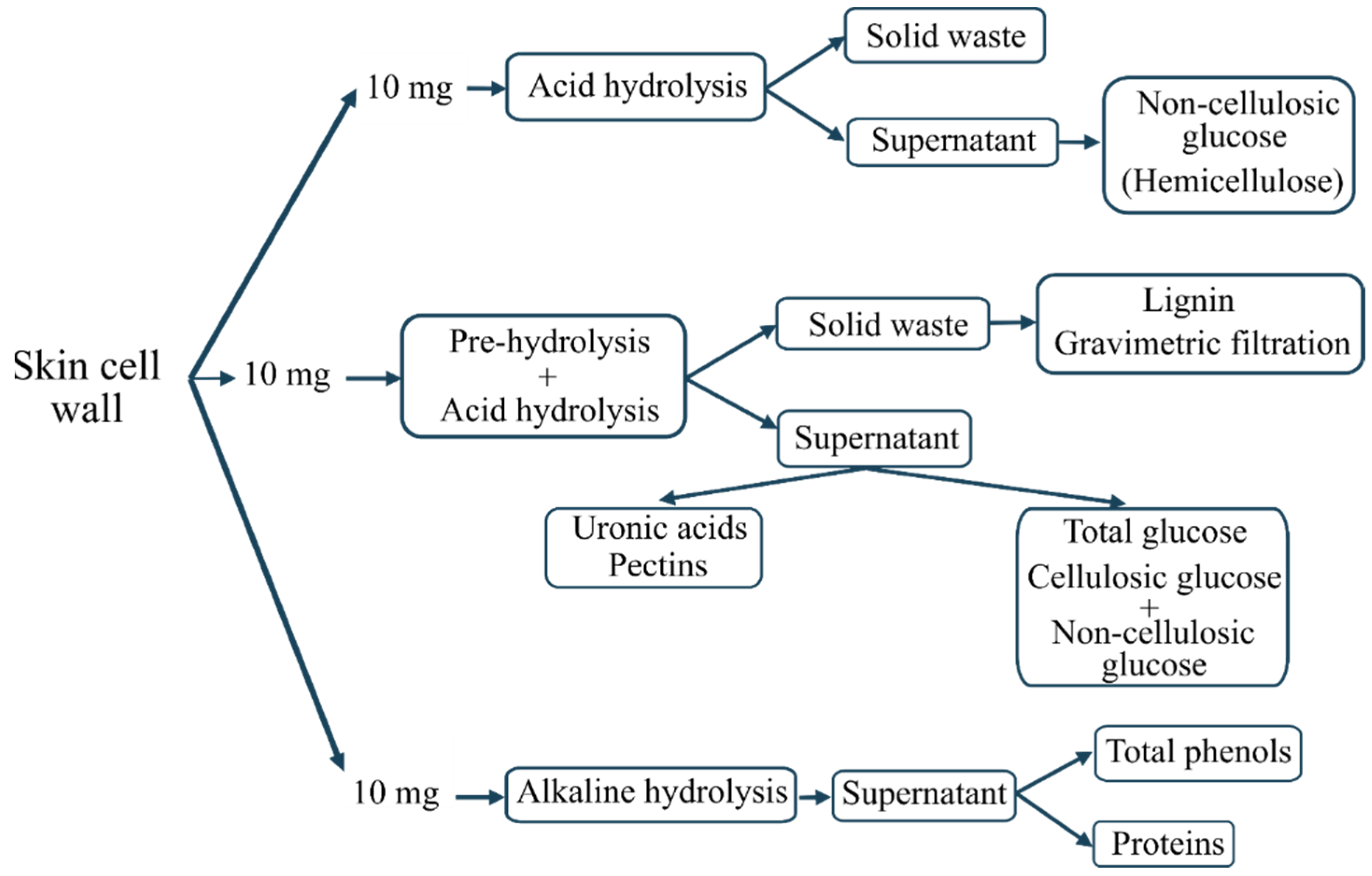
2.3. Isolation of Skin Cell Wall (SCW)
2.4. Skin Cell Wall (SCW) Composition
3. Results and Discussion
3.1. Isolation of Skin Cell Wall (SCW)
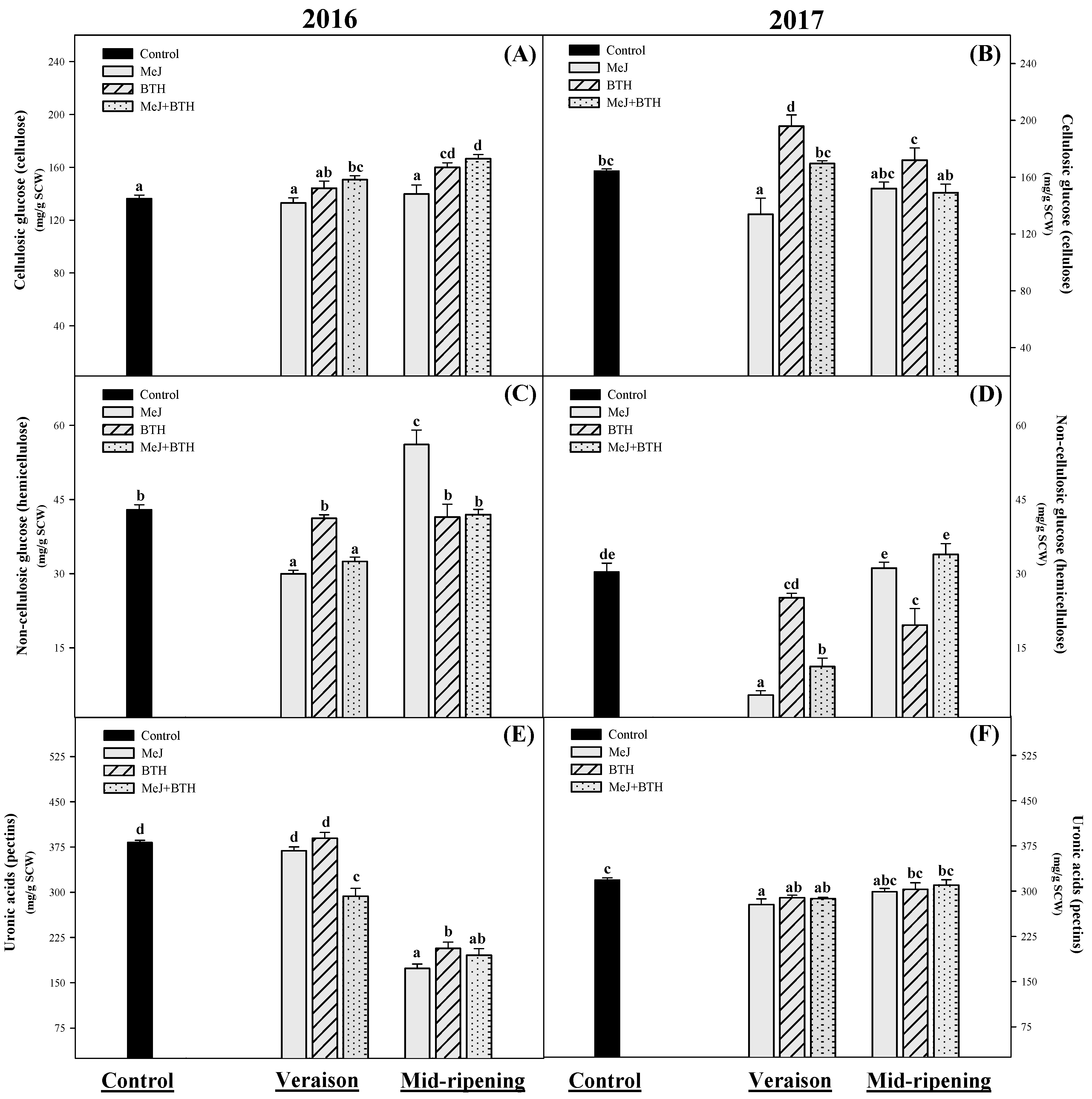
3.2. Carbohydrate Composition of Cell Walls (Cellulosic Glucose, Non-Cellulosic Glucose and Uronic Acids)
3.2.1. Cellulosic Glucose (Cellulose)
3.2.2. Non-Cellulosic Glucose (Hemicellulose)
3.2.3. Uronic Acids (Pectins)
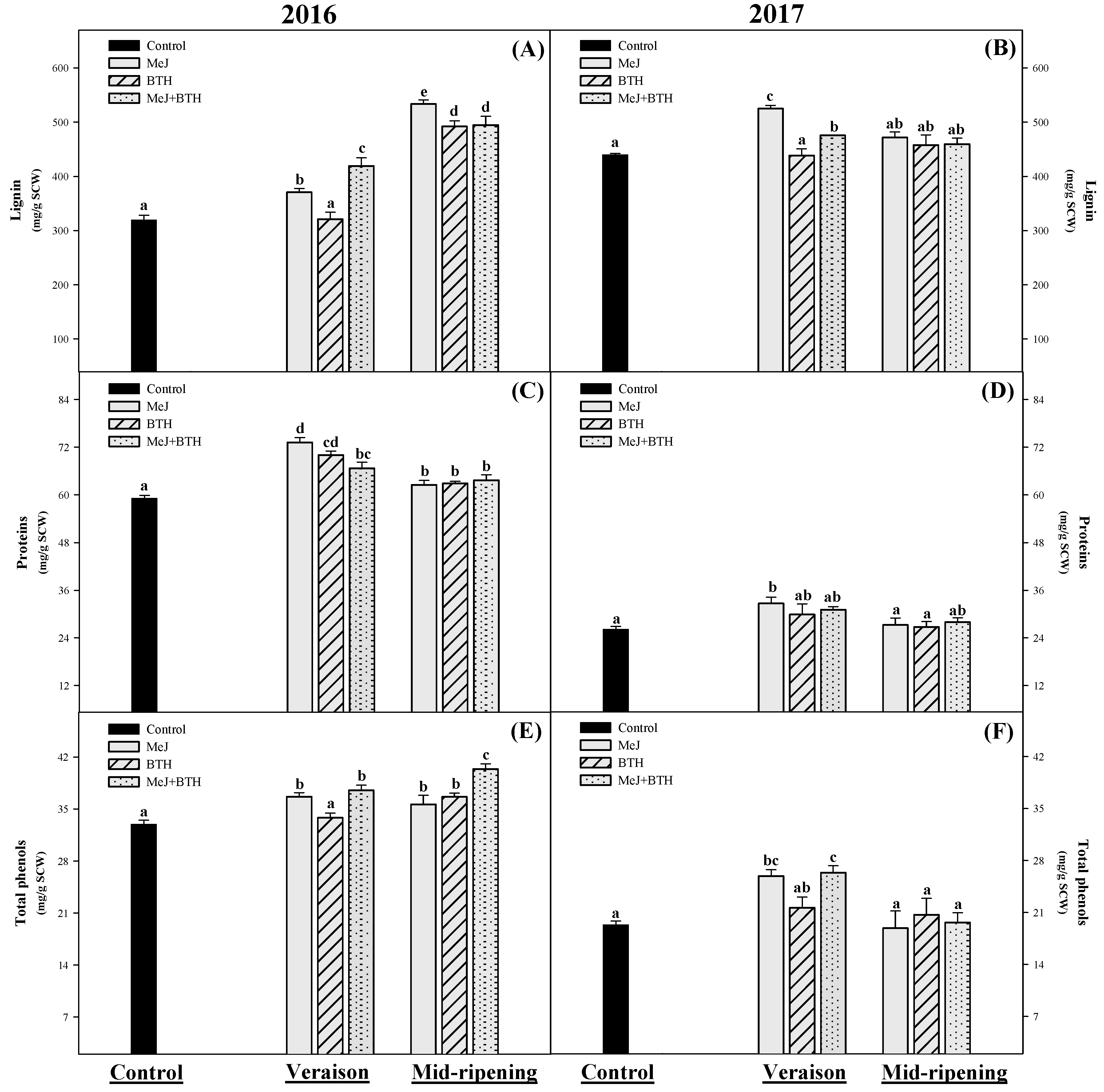
3.3. Lignin, Proteins and Total Phenols
3.3.1. Lignin
3.3.2. Proteins
3.3.3. Total Phenols
3.4. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ochoa-Velasco, C.E.; Avila-Sosa, R.; Navarro-Cruz, A.R.; López-Malo, A.; Palou, E. Biotic and Abiotic Factors to Increase Bioactive Compounds in Fruits and Vegetables. In Food Bioconversion; Elsevier Science Publishing Co, Inc.: Amsterdam, The Netherlands, 2017; Volume 2, pp. 317–349. ISBN 9780128114131. [Google Scholar]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Methyl jasmonate treatment to increase grape and wine phenolic content in Tempranillo and Graciano varieties during two growing seasons. Sci. Hortic. 2018, 240, 378–386. [Google Scholar] [CrossRef]
- Jensen, J.S.; Demiray, S.; Egebo, M.; Meyer, A.S. Prediction of wine color attributes from the phenolic profiles of red grapes (Vitis vinifera). J. Agric. Food Chem. 2008, 56, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Alba, V.; Masi, G.; Caputo, A.R.; Tarricone, L. Effect of two exogenous plant growth regulators on the color and quality parameters of seedless table grape berries. Food Res. Int. 2019, 108667. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, Y.; Romero-Cascales, I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Martínez-Cutillas, A.; Gómez-Plaza, E. Increasing bioactive phenolic compounds in grapes: Response of six Monastrell grape clones to benzothiadiazole and methyl jasmonate treatments. Am. J. Enol. Vitic. 2013, 64, 459–465. [Google Scholar] [CrossRef]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lin, L. Elicitor-like effects of low-energy ultrasound on plant (Panax ginseng) cells: Induction of plant defense responses and secondary metabolite production. Appl. Microbiol. Biotechnol. 2002, 59, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hammond-Kosack, K.E.; Jones, J.D.G. Resistance gene-dependent plant defense responses. Plant Cell 1996, 8, 1773–1791. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of plants and microbial cell systems. Biotechnol. Appl. Biochem. 2003, 37, 91. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Improving phenolic and chromatic characteristics of Monastrell, Merlot and Syrah wines by using methyl jasmonate and benzothiadiazole. J. Int. Sci. Vigne Vin. 2017, 51, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the phenolic compound content of grapes by preharvest application of abcisic acid and a combination of methyl jasmonate and benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Martínez-Moreno, A.; Gil-Muñoz, R. Elicitors and pre-fermentative cold maceration: Effects on polyphenol concentration in Monastrell grapes and wines. Biomolecules 2019, 9, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Crespo-Villegas, O.; Garde-Cerdán, T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Gozzo, F. Systemic acquired resistance in crop protection: From nature to a chemical approach. J. Agric. Food Chem. 2003, 51, 4487–4503. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole enhances resveratrol and anthocyanin biosynthesis in grapevine, meanwhile improving resistnce to Botrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Gómez-Martínez, J.C.; Martínez-Jiménez, J.A.; Gil-Muñoz, R. Application of elicitors in two ripening periods of Vitis vinifera L. cv Monastrell: Influence on anthocyanin concentration of grapes and wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef]
- Pellerin, P.; Cabanis, J.C. Los glúcidos. In Enología: Fundamentos Científicos y Tecnológicos; Flanzy, C., Ed.; AMV Ediciones, Ediciones Mundi-Prensa: Madrid, Spain, 2000; pp. 66–96. ISBN 84-7114-859-5. [Google Scholar]
- Doco, T.; Williams, P.; Pauly, M.; O’Neill, M.; Pellerin, P. Polysaccharides from grape berry cell walls. Part II. Structural characterization of the xyloglucan polysaccharides. Carbohydr. Polym. 2003, 53, 253–261. [Google Scholar] [CrossRef]
- Hanlin, R.L.; Hrmova, M.; Harbertson, J.F.; Downey, M.O. Review: Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust. J. Grape Wine Res. 2010, 16, 173–188. [Google Scholar] [CrossRef]
- SIAM Sistema de Información Agrario de Murcia. Available online: http://siam.imida.es/apex/f?p=101:46:5540041647757103 (accessed on 20 December 2019).
- De Vries, J.A.; Rombouts, F.M.; Voragen, A.G.J.; Pilnik, W. Comparison of the structural features of apple and citrus pectic substances. Carbohydr. Polym. 1984, 4, 89–101. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; López-Roca, J.M.; Gómez-Plaza, E.; Ros-García, J.M. Application and comparison of four selected procedures for the isolation of cell-wall material from the skin of grapes cv. Monastrell. Anal. Chim. Acta 2010, 660, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Castro-López, L.; Gómez-Plaza, E.; Ortega-Regules, A.; Lozada, D.; Bautista-Ortín, A.B. Role of cell wall deconstructing enzymes in the proanthocyanidin-cell wall adsorption-desorption phenomena. Food Chem. 2016, 196, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W. Colorimetric determination of hexuronic acids in plant materials. Anal. Chem. 1979, 51, 936–941. [Google Scholar] [CrossRef]
- Theander, O.; Aman, P. Studies on dietary-fibers. Analysis and chemical characterization of water-soluble and water-insoluble dietary-fibers. J. Agric. Res. 1979, 9, 97–106. [Google Scholar]
- Rustioni, L.; Cola, G.; Maghradze, D.; Abashidze, E.; Argiriou, A.; Aroutiounian, R.; Brazão, J.; Chipashvili, R.; Cocco, M.; Cornea, V.; et al. Description of the Vitis vinifera L. Phenotypic variability in eno-carpological traits by a Euro-Asiatic collaborative network among ampelographic collections. Vitis J. Grapevine Res. 2019, 58, 37–46. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. The composition of cell walls from grape marcs is affected by grape origin and enological technique. Food Chem. 2015, 167, 370–377. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; Du Toit, W.J. Investigating the relationship between grape cell wall polysaccharide composition and the extractability of phenolic compounds into Shiraz wines. Part I: Vintage and ripeness effects. Food Chem. 2019, 278, 36–46. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. A first approach towards the relationship between grape skin cell-wall composition and anthocyanin extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Revilla, G.; Zarra, I. Fisiología vegetal: Introducción a las células de las plantas: Membranas y pared. In Fundamentos de Fisiología Vegetal; Azcón-Bieto, J., Talón, M., Eds.; McGraw-Hill: Barcelona, Spain, 2003; pp. 3–22. ISBN 978-84-481-5168-3. [Google Scholar]
- Apolinar-Valiente, R. Pared Celular de uva y Polisacáridos de Vinos de Distinta Procedencia, Elaborados Mediante Tecnologías Enzimáticas y de Frío. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2011. [Google Scholar]
- Keegstra, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, M.; Röhring, H.; Schmidt, J.; Walden, R.; Schell, J. Cell signalling by oligosaccharides. Trends Plant Sci. 1997, 2, 111–115. [Google Scholar] [CrossRef]
- Pellerin, P.; Doco, T.; Vidal, S.; Williams, P.; Brillouet, J.; O’Neill, M. Structural characterization of red wine rhamnogalacturonan II. Carbohydr. Res. 1996, 290, 183–197. [Google Scholar] [CrossRef]
- Doco, T.; Brillouet, J.M.; Moutounet, M. Evolution of grape (Carignan noir cv.) and yeast polysaccharides during fermentation and post-maceration. Am. J. Enol. Vitic. 1996, 47, 108–110. [Google Scholar]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Boudet, A.M. Lignins and lignification: Selected issues. Plant Physiol. Biochem. 2000, 38, 81–96. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Hartley, R.D.; Harris, P.J.; Curzon, E.H. Linkage of p-coumaroyl and feruloyl groups to cell-wall polysaccharides of barley straw. Carbohydr. Res. 1986, 148, 71–85. [Google Scholar] [CrossRef]
- Showalter, A.M. Structure and function of plant cell wall proteins. Plant Cell 1993, 5, 9–23. [Google Scholar] [CrossRef]
- Cassab, G.I.; Varner, J.E. Cell wall proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 321–353. [Google Scholar] [CrossRef]
- Cosgrove, D. Paredes celulares: Estructura, biogénesis y expansión. In Fisiología Vegetal; Taiz, L., Zeiger, E., Eds.; Publicaciones de la Universitat Jaume I: Castelló de la Plana, Spain, 2006; Volume II, pp. 587–631. ISBN 978-84-8021-601-2. [Google Scholar]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Lau, J.M.; McNeil, M.; Darvill, A.G.; Albersheim, P. Structure of the backbone of rhamnogalacturonan I, a pectic polysaccharide in the primary cell walls of plants. Carbohydr. Res. 1985, 137, 111–125. [Google Scholar] [CrossRef]
- Ortega-Regules, A. Antocianos, Taninos y Composición de la Pared Celular en Distintas Variedades de uva. Evolución Durante la Maduración e Implicaciones Tecnológicas. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2006. [Google Scholar]
- Brisson, L.F.; Tenhaken, R.; Lamb, C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 1994, 6, 1703–1712. [Google Scholar] [CrossRef] [Green Version]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
| Year | Parameter | Control | Veraison | Mid-Ripening | ||||
|---|---|---|---|---|---|---|---|---|
| MeJ | BTH | MeJ + BTH | MeJ | BTH | MeJ + BTH | |||
| 2016 | Fresh skin (g) | 7.6 ± 1.1 a | 8.1 ± 0.1 a | 7.9 ± 0.1 a | 7.7 ± 0.3 a | 7.0 ± 0.6 a | 7.2 ± 0.4 a | 7.1 ± 0.4 a |
| SCW (mg/g fresh skin) | 116 ± 6 ab | 112 ± 10 ab | 106 ± 8 a | 115 ± 9 ab | 127 ± 7 b | 120 ± 8 ab | 132 ± 7 b | |
| 2017 | Fresh skin (g) | 7.4 ± 1 a | 7.4 ± 0.7 a | 7.4 ± 0.9 a | 8.1 ± 0.6 a | 8.3 ± 0.8 a | 7.6 ± 0.8 a | 7.7 ± 0.6 a |
| SCW (mg/g fresh skin) | 88 ± 7 a | 94 ± 6 a | 93 ± 6 a | 94 ± 6 a | 100 ± 6 a | 102 ± 6 a | 95 ± 4 a | |
| Factor | Cell Wall Components | ||||||
|---|---|---|---|---|---|---|---|
| Cellulosic Glucose (Cellulose) | Non-Cellulosic Glucose (Hemicellulose) | Uronic Acids (Pectins) | Lignin | Proteins | Total Phenols | ||
| Year (Y) | 2016 | 146 ± 14 a | 40 ± 8 b | 295 ± 89 a | 414 ± 84 a | 66 ± 5 b | 36 ± 2 b |
| 2017 | 162 ± 22 b | 22 ± 11 a | 298 ± 18 a | 467 ± 33 b | 29 ± 4 a | 22 ± 4 a | |
| Time of Application (TA) | Veraison | 153 ± 23 a | 27 ± 13 a | 326 ± 46 b | 414 ± 71 a | 49 ± 20 a | 29 ± 7 a |
| Mid-ripening | 156 ± 15 a | 36 ± 12 b | 254 ± 59 a | 482 ± 34 b | 44 ± 18 a | 28 ± 10 a | |
| Treatments (T) | Control | 150 ± 15 ab | 37 ± 7 a | 351 ±+ 35 b | 379 ± 66 a | 43 ± 18 a | 26 ± 7 a |
| MeJ | 139 ± 15 a | 29 ± 18 a | 287 ± 70 a | 471 ± 69 b | 48 ± 21 a | 29 ± 8 a | |
| BTH | 168 ± 23 c | 32 ± 11 a | 297 ± 69 a | 427 ± 71 ab | 47 ± 20 a | 28 ± 8 a | |
| MeJ + BTH | 158 ± 12 bc | 29 ± 12 a | 277 ± 46 a | 460 ± 35 b | 46 ± 19 a | 30 ± 9 a | |
| Interactions | Y × T | ns | * (3%) | * (12%) | ns | ns | ns |
| Y × TA | ns | ns | *** (29%) | *** (17%) | ns | ** (2%) | |
| T × TA | ns | *** (21%) | * (9%) | ** (7%) | ** (1%) | ns | |
| Y × T × TA | * (8%) | * (2%) | * (12%) | * (8%) | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Application of Elicitors at Two Maturation Stages of Vitis vinifera L. cv Monastrell: Changes in Skin Cell Walls. Chemistry 2022, 4, 98-111. https://doi.org/10.3390/chemistry4010008
Paladines-Quezada DF, Moreno-Olivares JD, Fernández-Fernández JI, Bleda-Sánchez JA, Gil-Muñoz R. Application of Elicitors at Two Maturation Stages of Vitis vinifera L. cv Monastrell: Changes in Skin Cell Walls. Chemistry. 2022; 4(1):98-111. https://doi.org/10.3390/chemistry4010008
Chicago/Turabian StylePaladines-Quezada, Diego F., Juan D. Moreno-Olivares, José I. Fernández-Fernández, Juan A. Bleda-Sánchez, and Rocío Gil-Muñoz. 2022. "Application of Elicitors at Two Maturation Stages of Vitis vinifera L. cv Monastrell: Changes in Skin Cell Walls" Chemistry 4, no. 1: 98-111. https://doi.org/10.3390/chemistry4010008
APA StylePaladines-Quezada, D. F., Moreno-Olivares, J. D., Fernández-Fernández, J. I., Bleda-Sánchez, J. A., & Gil-Muñoz, R. (2022). Application of Elicitors at Two Maturation Stages of Vitis vinifera L. cv Monastrell: Changes in Skin Cell Walls. Chemistry, 4(1), 98-111. https://doi.org/10.3390/chemistry4010008








